Abstract
Background
Epicardial fat tissue (EAT) acts as brown adipose tissue and protects the heart and coronary arteries against hypothermia. Recent studies demonstrated that EAT is a source of both anti-inflammatory and atherogenic cytokines. In this study, our aim was to investigate the association of vertical, horizontal, and area measurements of EAT thickness and their association with coronary artery disease, diastolic function, and myocardial performance index in patients who underwent coronary angiography.
Material/Methods
The study population consisted of patients who presented to our outpatient clinic with chest pain and whose non-invasive stress tests were positive between June 2015 and July 2017. Echocardiographic examinations were performed prior to the angiography. Coronary angiograms were performed using Judkins method from the femoral artery.
Results
Mean vertical thickness of EAT was 0.6 cm in patients with CAD and 0.46 cm in those without CAD (p=0.0001). Mean horizontal length of EAT was 2.91 cm in patients with CAD and was 2.41 cm in the subjects without CAD (p=0.001). ROC analysis showed 81% sensitivity and 53% specificity for a cut-off value of 0.45, and 67% sensitivity and 71% specificity for a cut-off value of 0.55 for EAT vertical (cm). Multivariate analysis showed that EAT is an independent risk factor for coronary artery disease.
Conclusions
Echocardiography is an inexpensive routine assessment for most patients. EAT thickness determined by echocardiography may be a useful indicator of increased CAD risk, but not diastolic dysfunction, of the left ventricle.
MeSH Keywords: Coronary Artery Disease; Echocardiography; Heart Failure, Diastolic
Background
Epicardial fat tissue (EAT) is located over the myocardium and is enclosed by visceral pericardium. It accounts for almost 15% of the total heart weight [1]. EAT acts as brown adipose tissue and protects the heart and coronary arteries against hypothermia with non-shivering thermogenesis [2]. It functions as a buffer for trauma and tension generated by arterial pulse and is a protective framework for cardiac autonomic nerves and ganglia [3]. Above all, EAT is a metabolically active immunological organ with a potential of cellular cross-talk with myocardium. Although direct evidence is lacking, under physiological conditions, EAT may store and release of fatty acids for local energy demands of the myocardium and arterial wall, which may also help to avoid lipid accumulation in the myocytes, known as “lipotoxicity” [4]. Previous studies demonstrated that EAT is a source of both anti-inflammatory and atherogenic cytokines such as tumor necrosis factor (TNF-α), monocyte chemoattractant protein-1 (MCP-1), interleukin-6, neuronal growth factor (NGF), resistin, visfatin, omentin, leptin, plasminogen activator inhibitor (PAI-1), angiotensinogen, and anti-inflammatory and antiatherogenic adipokines such as adiponectin and adrenomedullin. Expression of TNF-α, interleukin 1, interleukin 6, visfatin, PAI-1, and MCP-1 were also found to be increased in EAT samples obtained during cardiac surgery [5–13]. Inflammatory infiltration of this tissue was also shown in CAD. Since there is no fascia separating EAT and myocardium, and these 2 tissues share the same microcirculation, cytokines may directly diffuse into the myocardium or arterial route through vaso vasorums [4]. Therefore, EAT has been considered to have an important role in cardiovascular physiology and in the pathogenesis of disease.
In this study, we investigated the association of vertical, horizontal, and area measurements of EAT thickness and their association with coronary artery disease, diastolic function, and myocardial performance index in patients who recently underwent invasive coronary angiography.
Material and Methods
We screened 108 patients during the study between June 2015 and July 2017. Ninety-seven of them met the inclusion criteria and were enrolled in the study. The study population consisted of patients who presented to our outpatient clinic with chest pain and whose non-invasive stress tests were positive. All participants underwent coronary angiography for the first time. Exclusion criteria included history of coronary angiogram, acute coronary syndromes, pericardial effusion in echocardiography, suboptimal image quality in echocardiography, any rhythm other than sinus, and history of chronic renal failure. Echocardiography of each patient was performed by 2 echocardiographers. The measurements of both echocardiographers were performed independently in a period less than 24 h in order to avoid changes in clinical conditions. The intraobserver and interobserver variabilities were calculated from 3 repeated measurements on recorded images within 20 days after the first analysis. A total of 3 interobserver assessments were performed during the measurements. Three repeated measurements for each patient were used for calculating intraobserver and interobserver variability. The interclass correlation coefficient (ICC) was used for estimating the strength of concordance. Table 1 indicates ICC values of each echocardiographic parameter.
Table 1.
The interclass correlation coefficient (ICC) values of measured echocardiographic parameters.
| Interobserver ICC (concordance) | Intraobserver ICC (concordance) | |
|---|---|---|
| E | 81.2% (good) | 80.3% (good) |
| e’ | 72.6% (good) | 69.9% (moderate) |
| E/A | 78.9% (good) | 77.7% (good) |
| E/e’ | 68.7% (moderate) | 77.7% (good) |
| Tei Index | 73.6% (good) | 81.8% (good) |
| EAT vertical (cm) | 94.3% (very good) | 92.7% (very good) |
| EAT horizontal (cm) | 93.4% (very good) | 91.8% (very good) |
| EAT area (cm2) | 92.1% (very good) | 92.9% (very good) |
EAT – Epicardial adipose tissue thickness.
On admission, detailed medical histories were obtained from the patients, including age, gender, history of diabetes, history of hypertension, smoking status, family history of premature CAD, and medications. All patients with hypertension and/or dyslipidemia and/or diabetes mellitus were treated according to the current European Society of Cardiology Guideline recommendations and there were no significant differences between groups in terms of drug type.
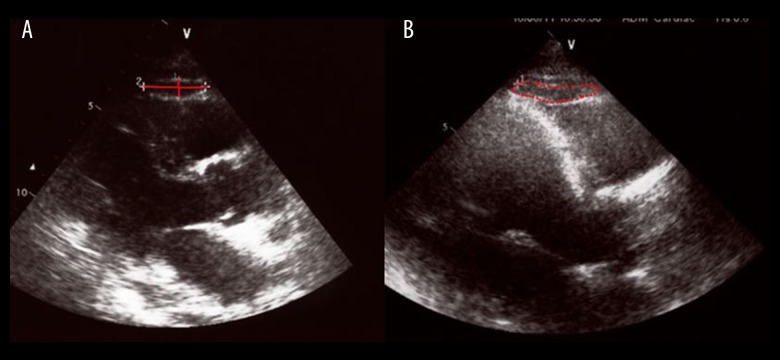
Prior to the angiography, blood samples were withdrawn for biochemical analysis and echocardiographic examinations were performed. All measurements were performed with the subjects in the left lateral decubitus position by echocardiography using a Vivid 7 Doppler echocardiographic unit (GE Vingmed Ultrasound, Horten, Norway) with 2.5-MHz probe. EAT thickness, horizontal width, and area were measured on the free wall of the right ventricle from the parasternal long-axis view (Figure 1). E and A waves were recorded by pulsed wave Doppler evaluations and placing the sample volume on the tips of mitral valve. Pulsed wave TDI was performed at the septal mitral annulus, and myocardial and annular peak diastolic early (e’) and late (a’) velocities were recorded. From the apical 5-chamber view with the pulsed Doppler sample in the LV outflow tract, isovolumic relaxation time (IRT) was measured as the time from closing of the aortic valve to the opening of the mitral valve. Then, isovolumic contraction time (ICT) was measured as the time from the closing of the mitral valve to the opening of the aortic valve, and ejection time (ET) was measured as the time between the opening and the closing of the aortic valve. Finally, Tei index (myocardial performance index) was calculated using the formula: ICT+IRT/ET. Diastolic dysfunction was defined as E/e’ >10, independent of parameters such as age, gender, abdominal obesity, hypertension, and diabetes.
Figure 1.
Measurements of epicardial fat by echocardiography (A: vertical and horizontal measurements, B: area).
Coronary angiography was performed using Judkins method from the femoral artery. Studies performed in this regard defined coronary artery disease (CAD) as 50% or more stenosis in at least 1 epicardial coronary artery; thus, such patients were allocated to the CAD group, whereas patients who had less than 50% stenosis were allocated to the control group. Thus, this cut-off value of 50% or more coronary artery stenosis increased the sensitivity of the current study.
Blood inflammatory markers such as hsCRP, serum interleukin 6 (IL-6), plasma alpha-1-antichymotrypsin, and homocysteine could not be studied due to excessive costs of these markers and lack of financial resources.
The study was approved by our Local Ethics Committee, and written informed consent was obtained from each participant.
All analyses were performed using SPSS for Windows (version 17.0, SPSS, Chicago, Illinois, USA). Quantitative variables are expressed as mean value ±SD for continuous variables and median and minimum-maximum levels for continuous variables with abnormal distribution. Comparison of continuous values between 2 groups was performed by means of independent-samples t test. Comparison of continuous variables with abnormal distribution between 2 groups was performed by Mann-Whitney U test. Categorical variables were compared by the chi-square test and Pearson test used for correlation parametric variables. A 2-tailed p-value <0.05 was considered statistically significant. The cut-off value of epicardial fat thickness for predicting CAD estimated by receiver operating characteristic (ROC) curve analysis and the sensitivity and specificity at that point were determined.
Results
The study population consisted of 97 patients (mean age 59±10.5 years; 49 men). Baseline clinical characteristics of the patients are presented in Table 2. The diagnosis of coronary artery disease was made in 48 subjects (49.5%) among the whole study population, who recently underwent coronary angiography in our hospital.
Table 2.
Comparisons of demographic and laboratory findings between two groups.
| CAD (+) (n=48) | CAD (−) (n=49) | p Value | |
|---|---|---|---|
| Age (Mean ±SD) | 61.6±10.5 | 56.6±10.1 | 0.021* |
| Male (n,%) | 31 (63.3) | 18 (36.7%) | 0.006* |
| Weight (kg) | 73.4±12.6 | 72.4±10.3 | 0.69 |
| Height (m) | 1.63±0.08 | 1.62±0.08 | 0.61 |
| Waist circumference (WC) (cm) | 97.6±11 | 97.2±11.5 | 0.83 |
| Hip circumference (HC) (cm) | 96.1±7.5 | 99.3±9.5 | 0.06 |
| WC/HC | 1.01±0.06 | 0.97±0.06 | 0.009* |
| BMI (kg/m2) | 27.3±3.8 | 27.4±4.3 | 0.98 |
| Obesity (≥30 kg/m2) [n(%)] | 13 (46.5) | 15 (53.5) | 0.65 |
| Overweigt (25–29.9 kg/m2) [n(%)] | 23 (54.7) | 19 (45.3) | 0.65 |
| Normal weight (<24.9 kg/m2) [n(%)] | 12 (44.5) | 15 (55.5) | 0.65 |
| Diabetes mellitus (n,%) | 24 (50.0) | 13 (26.5) | 0.017* |
| Smoker (n,%) | 22 (45.8) | 12 (24.4) | 0.027* |
| Chronic kidney disease (n,%) | 4 (8.3) | 3 (6.1) | 0.41 |
| Family history of CAD | 14 (29.1) | 11 (22.4) | 0.74 |
| Hypertension (n,%) | 29 (60.4) | 19 (38.7) | 0.03* |
| Total cholesterol (mg/dl) | 201.9±42.6 | 200.5±37 | 0.90 |
| LDL-C (mg/dl) | 121.6±35 | 122.1±32.6 | 0.65 |
| HDL-C (mg/dl) | 43.0±12.9 | 49.9±12.7 | 0.009* |
| Triglyceride (mg/dl) | 201.5±141 | 145.5±84.2 | 0.013* |
| Uric acid level (mg/dl) | 8.4±0.9 | 5.6±0.7 | 0.03* |
| Number of diseased coronary artery | |||
| 1 vessel | 18 | – | |
| 2 vessel | 15 | – | |
| 3 vessel | 15 | – | |
| Coronary lesion type | |||
| Type A | 25 | – | |
| Type B | 41 | – | |
| Type C | 30 | – | |
CAD – coronary artery disease; WC – waist circumference; HC – hip circumference; BMI – Body mass index; LDL-C – low density lipoprotein cholesterol; HDL-C – high density lipoprotein cholesterol
p>0.05 considered as statistically significant.
Mean body mass index (BMI) of the study group was 26.53±2.17 and there was a strong and positive relationship between BMI and EAT area (r=0.796, p=0.002). Mean vertical and horizontal thickness, and area of EAT according to body mass index categories in patients with or without CAD are presented in Table 3. ROC analysis showed 81% sensitivity and 53% specificity for a cut-off value of 0.45, and 67% sensitivity and 71% specificity for a cut-off value of 0.55 for EAT vertical (cm) (p<0.001 area under ROC curve: 0.831676) (Figure 2). Neutrophil/lymphocyte ratio was also significantly higher in patients with CAD compared to patients without CAD (7.2±3.1 versus 3.0±0.9; p<0.001). Regarding diastolic functions, E, e’, E/e’, and Tei index values were not correlated with EAT thickness. However, there was a weak but significant correlation between EAT thickness and E/A (Tables 4, 5). Multivariate analysis showed that EAT is an independent risk factor for coronary artery disease (OR: 1.5, 95% confidence interval (CI): 2.1246–18.0525) (Table 6). WC/HC, HDL, and triglyceride differences between groups did not reveal any correlations with EAT thickness (p=0.67, p=0.85, and p=0.43, respectively). Table 7 shows the comparison of EAT thickness according to SYNTAX (Synergy Between PCI With Taxus and Cardiac Surgery) score.
Table 3.
The comparison of EAT measurements between the patients with CAD and those without CAD in different Body Mass Index categories.
| Obesity (≥30 kg/m2) (n: 23) | Overweigt (25–29.9 kg/m2) (n: 35) | Normal weight (<24.9 kg/m2) (n: 39) | |
|---|---|---|---|
|
| |||
| CAD (+) vs. CAD (−) | CAD (+) vs. CAD (−) | CAD (+) vs. CAD (−) | |
| EAT vertical (cm) | 0.62±0.14 vs. 0.57±0.24 | 0.59±0.12 vs. 0.47±0.12 | 0.48±0.11 p: 0.1 vs. 0.44±0.23 |
| p: 0.03* | p: 0.02* | p: 0.132 | |
|
| |||
| EAT horizontal (cm) | 2.90±0.45 vs. 2.69±0.35 | 2.58±0.30 vs. 2.50±0.65 | 2.39±0.68 vs. 2.38±0.90 |
| p: 0.02* | p: 0.381 | p: 0.251 | |
|
| |||
| EAT area (cm2) | 1.54±0.48 vs. 1.38±0.58 | 1.20±0.30 vs. 1.10±0.58 | 1.15±0.62 vs. 1.21±0.42 |
| p: 0.01* | p: 0.323 | p: 0.412 | |
CAD – coronary artery disease; EAT – epicardial adipose tissue thickness.
p<0.05=statistically significant.
Figure 2.
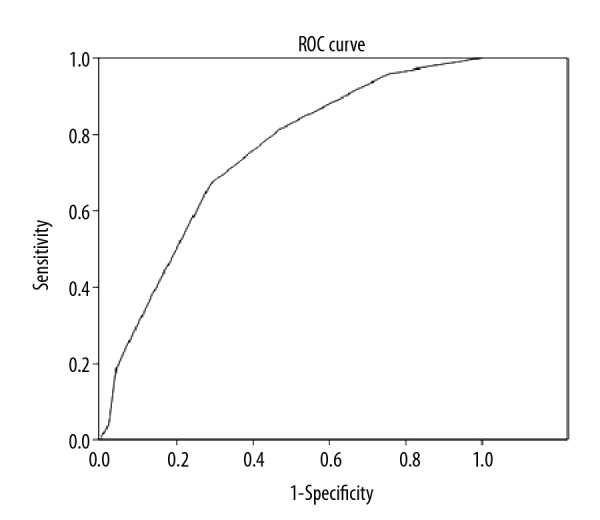
ROC analysis showed 67% sensitivity and 71% specificity for a cut-off value of 0.55 for EAT vertical (cm) (p<0.001 area under ROC curve: 0.831676).
Table 4.
Conventional echocardiographic measurements of the study groups.
| CAD (+) | CAD (−) | P value | |
|---|---|---|---|
| LA | 35.90±3.41 | 37.18±2.44 | 0.227 |
| LVED | 49.72±2.93 | 49.18±2.87 | 0.613 |
| LVES | 33.90±3.50 | 33.7±2.89 | 0.875 |
| IVS | 10.09±1.04 | 10.45±1.01 | 0.343 |
| PW | 9.45±1.36 | 9.86±1.16 | 0.377 |
LA – left atrium diameter, parasternal long axis; LVED – left ventricular end-diastolic diameter; LVES – left ventricular end-systolic diameter; IVS – interventricular septum thickness; PW – posterior wall thickness; all in milimeters.
p<0.05=statistically significant.
Table 5.
Correlation of echocardiographic measurements regarding diastolic functions and vertical epicardial adipose tissue thickness.
| Vertical EAT | ||
|---|---|---|
| Correlation | P | |
| E | −0.303 | 0.054 |
| e’ | −0.251 | 0.109 |
| E/A | −0.418 | 0.006* |
| E/e’ | 0.107 | 0.504 |
| Tei Index | 0.194 | 0.213 |
EAT – epicardial adipose tissue thickness. E – peak mitral flow velocity of early rapid filling wave; A – late filling velocity of e’: Tissue Doppler velocity averaged from septal annulus.
p<0.05=statistically significant.
Table 6.
Role of epicardial fat measurement and other parameters on coronary artery disease.
| Control group coefficients (r) | P value | |
|---|---|---|
| EAT | 0.36 | 0.001* |
| DM | 0.10 | 0.28 |
| HT | 0.14 | 0.17 |
| Smoking | 0.26 | 0.01 |
| LDL (mg/dL) | −0.08 | 0.83 |
| Triglyceride (mg/dL) | 0.17 | 0.08 |
EAT – vertical measurement of epicardial fat; LDL – low density lipoprotein cholesterol; DM – diabetes mellitus; HT – hypertension.
p<0.05=statistically significant.
Table 7.
The comparison of EAT thickness according to SYNTAX score.
| High SYNTAX Score (>32) (n: 13) | Intermediate SYNTAX Score (23–32) (n: 15) | Low SYNTAX Score (<22) (n: 20) | |
|---|---|---|---|
| EAT vertical (cm) | 0.62±0.34 | 0.48±0.21 | 0.34±0.32 |
| p: 0.01* | p: 0.02* | p: 0.29 | |
|
| |||
| EAT horizontal (cm) | 2.75±0.45 | 2.39±0.44 | 2.45±0.70 |
| p: 0.002* | p: 0.38 | p: 0.23 | |
|
| |||
| EAT area (cm2) | 1.54±0.64 | 1.22±0.45 | 1.34±0.40 |
| p: 0.001* | p: 0.03* | p: 0.45 | |
EAT – epicardial adipose tissue thickness; SYNTAX – Synergy between PCI with Taxus and Cardiac Surgery.
p<0.05=statistically significant.
Discussion
The findings of the present study can be summarized as follows: First, EAT thickness, length, and area, measured on the free wall from the parasternal long-axis view is significantly associated with CAD. This association between the existence of CAD and EAT area and between EAT horizontal and vertical length was shown for the first time. Second, vertical thickness of 5.5 mm is 67% sensitive and 71% specific for the prediction of CAD. Third, regarding diastolic functions, EAT fat thickness is weakly but significantly correlated with E, e’, and E/A, but it is not correlated with E/e’ or myocardial performance index (Tei).
The association of EAT with CAD has been investigated in several studies. In the study of Chaowalit et al., they could not find a relationship between CAD and EAT [14]. However, this result is inconsistent with many other studies. Jeong et al. and Shemirani et al. found that EAT thickness is correlated with the severity of CAD [15,16]. Anh et al. found that EAT equal to or thicker than 3.0 mm was an independent factor of CAD in multiple logistic analysis [17]. Another study showed EAT thickness of equal to or thicker than 5.2 mm had 85% sensitivity and 81% specificity for predicting CAD [18]. In a study conducted by the same group, women who underwent coronary angiography and who had no obstructive coronary artery disease, in contrast to traditional risk factors for atherosclerosis, EAT was the only independent predictor of microvascular dysfunction [19]. Studies with computed tomography (CT) confirmed the relationship of EAT and coronary calcium scores or CAD [20–22]. Bachar et al. found that EAT thickness of 2.4 mm was the optimal cut-off point for the prediction of significant CAD in asymptomatic subjects. It has been clearly demonstrated that EAT has proinflammatory effects. The most accepted hypothesis is that epicardial fat is a metabolically active organ with endocrine and paracrine functions. Recent studies have demonstrated that adipose tissue produces various genes related to adipokine production, which have important roles in atherosclerosis [23–25]. Epicardial fat located next to the coronary vessels may possibly provoke the paracrine effects of epicardial adipokines, as part of underlying pathogenesis of CAD. In 2014, Mazurek et al. revealed that pericoronary adipose tissue activity was significantly correlated with the plaque burden and necrotic core component of coronary plaques [26].
There is only limited data regarding the association between EAT and diastolic dysfunction. Iacobellis et al. showed that increased EAT thickness is correlated with impaired diastolic filling and atrial enlargement in morbidly obese patients [27]. They suggested that this correlation may be due to a mechanical effect of EAT in addition to local interactions with myocardium. Cavalcante et al. evaluated 110 patients who underwent cardiac computed tomography and transthoracic echocardiography [28]. They showed that EAT volume is an independent predictor of diastolic dysfunction in apparently healthy overweight patients, even after accounting for associated co-morbidities such as metabolic syndrome, hypertension, and subclinical CAD. They also suggested that measurement of EFV adds incrementally to the prediction of diastolic dysfunction, mean e’, and mean E/e’. In a study by Cetin et al., EAT was found to be significantly correlated with left atrial dimensions, diastolic dysfunction, and left ventricular mass in patients with untreated hypertension [29]. Konishi et al. also found that pericardial fat volumes were correlated with diastolic dysfunction, which was defined as E/e’ >10, independent of age, gender, abdominal obesity, hypertension, and diabetes [30]. However, in our study, EAT thickness was weakly correlated with E, e’, and E/A, but not with E/e’. Considering that the diagnosis of diastolic dysfunction is usually made during echocardiography, our study indicates that routine measurement of EAT would not give additional information for the prediction of diastolic dysfunction.
There are a few concerns about the evaluation of EAT with echocardiography. First, echocardiography may not be an accurate method with which to assess EAT thickness, since EAT has a 3-dimensional shape without a uniform distribution over the heart. However, in previous studies, echocardiographic EAT measurements showed a very good correlation with measurements with magnetic resonance imaging [31,32]. Echocardiography is a part of routine assessment for most of the patients and is an inexpensive and accessible imaging modality. Moreover, no radiation or contrast administration is needed, which makes it a more appropriate tool for risk stratification. However, studies evaluating the validity and differences of the landmarks used to quantify epicardial fat thickness are still needed [33]. Second, there is controversy about when to measure EAT. Although some authors claim that it should be measured in systole because of its compressibility, this is not widely accepted, and we obtained the measurements in diastole for the sake of concordance with previous studies [15,34].
There are several limitations of the present study, including the relatively small study population and evaluation of diastolic function with only E, A, E’, and A’. Similar to previous studies, in our study, coronary artery disease was defined as more 50% stenosis in at least 1 coronary artery. Although this is not an accurate measure of atherosclerosis severity due to the systemic nature of the disease, the aim of the present study was not to investigate the relationship of EAT thickness with the severity of atherosclerotic disease, but to rather to assess the presence of atherosclerosis. Finally, patients in the CAD group were significantly older compared to the normal coronary artery group. Mazzaccoli et al. showed that older subjects had thicker epicardial fat, indicating an increased cardiometabolic risk in the elderly [35]. Age may also be a confounding factor in our study.
Conclusions
Length and area EAT thickness measured on the free wall from the parasternal long-axis view are significantly associated with CAD. Echocardiography is an inexpensive routine assessment for most patients, and EAT thickness determined by echocardiography may provide a useful indicator of increased CAD risk, but not for diastolic dysfunction of the left ventricle. Further studies should be conducted with more diastolic parameters.
Footnotes
Source of support: Departmental sources
References
- 1.Corradi D, Maestri R, Callegari S, et al. The ventricular epicardial fat is related to the myocardial mass in normal, ischemic and hypertrophic hearts. Cardiovasc Pathol. 2004;13(6):313–16. doi: 10.1016/j.carpath.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Sacks HS, Fain JN, Holman B, et al. Uncoupling protein-1 and related messenger ribonucleic acids in human epicardial and other adipose tissues: Epicardial fat functioning as brown fat. J Clin Endocrinol Metab. 2009;94(9):3611–15. doi: 10.1210/jc.2009-0571. [DOI] [PubMed] [Google Scholar]
- 3.Sacks HS, Fain JN. Human epicardial fat: What is new and what is missing? Clin Exp Pharmacol Physiol. 2011;38(12):879–87. doi: 10.1111/j.1440-1681.2011.05601.x. [DOI] [PubMed] [Google Scholar]
- 4.Cherian S, Lopaschuk GD, Carvalho E. Cellular cross-talk between epicardial adipose tissue and myocardium in relation to the pathogenesis of cardiovascular disease. Am J Physiol Endocrinol Metab. 2012;303(8):E937–49. doi: 10.1152/ajpendo.00061.2012. [DOI] [PubMed] [Google Scholar]
- 5.Baker AR, Silva NF, Quinn DW, et al. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol. 2006;5:1. doi: 10.1186/1475-2840-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaldakov GN, Fiore M, Stankulov IS, et al. Neurotrophin presence in human coronary atherosclerosis and metabolic syndrome: A role for NGF and BDNF in cardiovascular disease? Progress Brain Res. 2004;146:279–89. doi: 10.1016/S0079-6123(03)46018-4. [DOI] [PubMed] [Google Scholar]
- 7.Kremen J, Dolinkova M, Krajickova J, et al. Increased subcutaneous and epicardial adipose tissue production of proinflammatory cytokines in cardiac surgery patients: Possible role in postoperative insulin resistance. J Clin Endocrinol Metab. 2006;91(11):4620–27. doi: 10.1210/jc.2006-1044. [DOI] [PubMed] [Google Scholar]
- 8.Cheng KH, Chu CS, Lee KT, et al. Adipocytokines and proinflammatory mediators from abdominal and epicardial adipose tissue in patients with coronary artery disease. Int J Obes (Lond) 2008;32(2):268–74. doi: 10.1038/sj.ijo.0803726. [DOI] [PubMed] [Google Scholar]
- 9.Fain JN, Sacks HS, Buehrer B, et al. Identification of omentin mRNA in human epicardial adipose tissue: comparison to omentin in subcutaneous, internal mammary artery periadventitial and visceral abdominal depots. Int J Obes (Lond) 2008;32(5):810–15. doi: 10.1038/sj.ijo.0803790. [DOI] [PubMed] [Google Scholar]
- 10.Iacobellis G, Pistilli D, Gucciardo M, et al. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine. 2005;29(6):251–55. doi: 10.1016/j.cyto.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Silaghi A, Achard V, Paulmyer-Lacroix O, et al. Expression of adrenomedullin in human epicardial adipose tissue: role of coronary status. Am J Physiol Endocrinol Metab. 2007;293(5):E1443–50. doi: 10.1152/ajpendo.00273.2007. [DOI] [PubMed] [Google Scholar]
- 12.Langheim S, Dreas L, Veschini L, et al. Increased expression and secretion of resistin in epicardial adipose tissue of patients with acute coronary syndrome. Am J Physiol Heart Circ Physiol. 2010;298(3):H746–53. doi: 10.1152/ajpheart.00617.2009. [DOI] [PubMed] [Google Scholar]
- 13.Mazurek T, Zhang L, Zalewski A, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108(20):2460–66. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 14.Chaowalit N, Somers VK, Pellikka PA, et al. Subepicardial adipose tissue and the presence and severity of coronary artery disease. Atherosclerosis. 2006;186(2):354–59. doi: 10.1016/j.atherosclerosis.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Jeong JW, Jeong MH, Yun KH, et al. Echocardiographic epicardial fat thickness and coronary artery disease. Circ J. 2007;71(4):536–39. doi: 10.1253/circj.71.536. [DOI] [PubMed] [Google Scholar]
- 16.Shemirani H, Khoshavi M. Correlation of echocardiographic epicardial fat thickness with severity of coronary artery disease-an observational study. Anadolu Kardiyol Derg. 2012;12(3):200–5. doi: 10.5152/akd.2012.061. [DOI] [PubMed] [Google Scholar]
- 17.Ahn SG, Lim HS, Joe DY, et al. Relationship of epicardial adipose tissue by echocardiography to coronary artery disease. Heart. 2008;94(3):e7. doi: 10.1136/hrt.2007.118471. [DOI] [PubMed] [Google Scholar]
- 18.Eroglu S, Sade LE, Yildirir A, et al. Epicardial adipose tissue thickness by echocardiography is a marker for the presence and severity of coronary artery disease. Nutr Metab Cardiovasc Dis. 2009;19(3):211–17. doi: 10.1016/j.numecd.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Sade LE, Eroglu S, Bozbas H, et al. Relation between epicardial fat thickness and coronary flow reserve in women with chest pain and angiographically normal coronary arteries. Atherosclerosis. 2009;204(2):580–85. doi: 10.1016/j.atherosclerosis.2008.09.038. [DOI] [PubMed] [Google Scholar]
- 20.Ding J, Kritchevsky SB, Harris TB, et al. The association of pericardial fat with calcified coronary plaque. Obesity (Silver Spring) 2008;16(8):1914–19. doi: 10.1038/oby.2008.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Vos AM, Prokop M, Roos CJ, et al. Peri-coronary epicardial adipose tissue is related to cardiovascular risk factors and coronary artery calcification in post-menopausal women. Eur Heart J. 2008;29(6):777–83. doi: 10.1093/eurheartj/ehm564. [DOI] [PubMed] [Google Scholar]
- 22.Mahabadi AA, Reinsch N, Lehmann N, et al. Association of pericoronary fat volume with atherosclerotic plaque burden in the underlying coronary artery: A segment analysis. Atherosclerosis. 2010;211(1):195–99. doi: 10.1016/j.atherosclerosis.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Ramazan Oncel C, Kucuk M. The value of epicardial adipose tissue thickness for cardiovascular risk stratification in hypertensive patients. Acta Cardiol Sin. 2017;33:559. doi: 10.6515/ACS20170220C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katlandur H, Ulucan S, Ozdil H, et al. Evaluation of echocardiographic epicardial fat thickness as a sign of cardiovascular risk in positive exercise test patients. Acta Cardiol Sin. 2016;32:684–89. doi: 10.6515/ACS20160110A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aydin E, Altin C, Sakallioglu O, et al. Epicardial adipose tissue thickness and carotid intima-media thickness in hemodialysis patients. Acta Cardiol Sin. 2017;33:266–72. doi: 10.6515/ACS20161023A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazurek T, Kochman J, Kobylecka M, et al. Inflammatory activity of pericoronary adipose tissue may affect plaque composition in patients with acute coronary syndrome without persistent ST-segment elevation: Preliminary results. Kardiol Pol. 2014;72(5):410–16. doi: 10.5603/KP.a2013.0320. [DOI] [PubMed] [Google Scholar]
- 27.Iacobellis G, Leonetti F, Singh N, Sharma MA. Relationship of epicardial adipose tissue with atrial dimensions and diastolic function in morbidly obese subjects. Int J Cardiol. 2007;115(2):272–73. doi: 10.1016/j.ijcard.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 28.Cavalcante JL, Tamarappoo BK, Hachamovitch R, et al. Association of epicardial fat, hypertension, subclinical coronary artery disease, and metabolic syndrome with left ventricular diastolic dysfunction. Am J Cardiol. 2012;110(12):1793–98. doi: 10.1016/j.amjcard.2012.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cetin M, Kocaman SA, Durakoglugil ME, et al. Effect of epicardial adipose tissue on diastolic functions and left atrial dimension in untreated hypertensive patients with normal systolic function. J Cardiol. 2013;61(5):359–64. doi: 10.1016/j.jjcc.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 30.Konishi M, Sugiyama S, Sugamura K, et al. Accumulation of pericardial fat correlates with left ventricular diastolic dysfunction in patients with normal ejection fraction. J Cardiol. 2012;59(3):344–51. doi: 10.1016/j.jjcc.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 31.Iacobellis G, Ribaudo MC, Assael F, et al. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: A new indicator of cardiovascular risk. J Clin Endocrinol Metab. 2003;88(11):5163–68. doi: 10.1210/jc.2003-030698. [DOI] [PubMed] [Google Scholar]
- 32.Iacobellis G, Assael F, Ribaudo MC, et al. Epicardial fat from echocardiography: A new method for visceral adipose tissue prediction. Obes Res. 2003;11(2):304–10. doi: 10.1038/oby.2003.45. [DOI] [PubMed] [Google Scholar]
- 33.Kim SJ, Kim HS, Jung JW, et al. Correlation between epicardial fat thickness by echocardiography and other parameters in obese adolescents. Korean Circ J. 2012;42(7):471–78. doi: 10.4070/kcj.2012.42.7.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelson MR, Mookadam F, Thota V, et al. Epicardial fat: An additional measurement for subclinical atherosclerosis and cardiovascular risk stratification? J Am Soc Echocardiogr. 2011;24(3):339–45. doi: 10.1016/j.echo.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 35.Mazzoccoli GDM, Greco A. Age-related changes of epicardial fat thickness. Biomedicine & Preventive Nutrition. 2012;2(1):38–41. [Google Scholar]