Abstract
The γ-secretase inhibitor blocks Notch activity by preventing its cleavage at the cell surface. In the present study, the effect of the γ-secretase inhibitor on the viability of gastric cancer cells when administered in combination with cisplatin was investigated, with particular focus on CD44highLgr-5high cancer cells. The four gastric cancer cell lines, MKN45, MKN74, SC-6-JCK and SH-10-TC, were used for the experiments. In the MTT assay, treatment with 25 µM dipeptide γ-secretase inhibitor (DAPT) alone did not affect cell proliferation in any of the four cell lines. Gastric cancer cells subjected to combination treatment with DAPT and cisplatin exhibited decreased viability when compared with those treated with cisplatin alone. Flow cytometry was performed to evaluate the expression of cluster of differentiation (CD)-44 and leucine-rich repeat-containing G-protein coupled receptor 5 (Lgr-5), two cancer stem cell markers in gastric cancers. Treatment with cisplatin alone significantly increased the proportion of CD44highLgr-5high cells. However, the addition of DAPT to cisplatin reduced the CD44highLgr-5high fraction, suggesting that DAPT reduced the number of gastric cancer cells. In conclusion, the present study demonstrated the synergistic effects of DAPT in combination with cisplatin by decreasing the survival of gastric cancer cells. In addition, combination treatment with DAPT reduced the number of CD44highLgr-5high cells, which are thought to exhibit cancer stem cell properties. These results highlight the therapeutic potential of DAPT in gastric cancer treatment.
Keywords: cancer stem cell, γ-secretase inhibitor, gastric cancer, Notch pathway
Introduction
Gastric cancer remains one of the leading causes of cancer mortality (1). Although new anti-tumor drugs, such as molecular target therapeutic agents, have been developed and used for the treatment of gastric cancers, disease progression often occurs even during chemotherapy. Resistance to chemotherapy is a major obstacle in the therapeutic management of gastric cancer. Several possible mechanisms responsible for chemoresistance have been proposed. First, the cancer stem cell theory hypothesizes that cancer stem cells develop tumorigenic properties and resistance to chemotherapeutic agents over time (2–5). Cancer stem cells possess self-renewal properties and are capable of differentiating into multiple cell types, which in turn promotes clonal tumor initiation and long-term clonal repopulation potential within the tumor. Furthermore, these cancer stem cells can exhibit intrinsic or acquired chemo-resistance, which are mediated by multiple mechanisms, including the formation of tumor spheroids, protection by a vascular niche, residence in the quiescent state, overexpression of transporter proteins associated with drug efflux and detoxification, and activation of antiapoptotic signaling pathways (2). Thus, based on the cancer stem cell concept, the development of new strategies targeting these cells is expected to improve outcomes of gastric cancer patients.
The Notch pathway is involved in various developmental and homeostatic processes, cell proliferation regulation, cell fate, differentiation, and cell death (6). The Notch pathway plays a role in juxtacrine interactions, during which intercellular signals are transmitted to adjacent responding cells. The Notch cascade is mediated by Notch ligands and receptors, as well as the intracellular domain of the Notch receptor (NICD). The interactions between Notch ligands and their receptors initiate γ-secretase-dependent cleavage of the Notch receptor and leads to the release of NICD, which then translocates into the nucleus to regulate gene expression and other effector molecules. Several studies have reported that Notch signaling is associated with various types of cancers (7,8). For example, Notch1 mRNA levels were demonstrated to be upregulated in gastric cancer tissues. Moreover, Notch2 receptor, Notch ligands, Jagged-1, Jagged-2, DLL-1, and DLL-3, and the major downstream targets of Notch signaling, namely, HES-1, and HES-2, were also significantly upregulated in gastric cancer tissues relative to normal gastric tissues (9). Another study reported that HES-1 can suppress the transcription of genes associated with differentiation of gastrointestinal epithelial cells. Thus, the above findings suggested that the canonical Notch signaling pathway contributes to the maintenance of cancer stem cell properties during gastric cancer carcinogenesis (10,11). Conversely, inhibition of the Notch pathway can reduce the cancer stem cell population and eventually lead to improved chemotherapy efficacy.
In the present study, we investigated the effects of γ-secretase inhibitor, which effectively blocks Notch activity by preventing its cleavage at the cell surface, on the viability of gastric cancer cells when administered in combination with cisplatin. Our experiments focused on CD44highLgr-5high cancer cells, which represent a cancer stem cell-like population.
Materials and methods
Cell lines and reagents
The human gastric adenocarcinoma cell lines MKN45 and MKN74 were purchased from the RIKEN BioResource Center (Ibaraki, Japan). Cells were grown in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), penicillin (5,000 U/ml), streptomycin (5 mg/ml) and amphotericin B (250 µg/ml) at 37°C in a balanced air humidified incubator with 5% CO2 atmosphere. SC-6-JCK and SH-10-TC were procured from Cell Resource Center for Biomedical Research, Cell Bank, Institute of Development, Aging and Cancer, Tohoku University (Sendai, Japan). Cells were grown in RPMI-1640 medium supplemented with 10% heat-inactivated FBS, penicillin (5,000 U/ml), streptomycin (5 mg/ml), and amphotericin B (250 µg/ml) at 37°C in a balanced air humidified incubator with 5% CO2 atmosphere.
Cisplatin was purchased from Wako (no. 3039-20093; Tokyo, Japan). Dipeptide γ-secretase inhibitor (DAPT, γ-secretase inhibitor IX, N-[N-(3,5-difluorophenacetyl)-L-alanyl]-sphenylglycine t-butyl ester) was purchased from Merck KGaA (CALBIOCHEM; no. 565770, Darmstadt, Germany) and dissolved in dimethyl sulfoxide (DMSO).
MTT assay
Inhibition of cellular proliferation was measured by the modified MTT [3-(4,5 dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide] assay, which distinguishes live cells based on their ability to convert thiazolyl blue to dark blue formazan. Cells were seeded into 24-well culture plates at a density of approximately 10,000 cells/well. After 24 h, cells were added with 500 µl of medium with or without cisplatin and DAPT. MTT assay was performed after 72 h of incubation. In this experiment, the culture media were not replaced nor added with DAPT and/or cisplatin for 72 h. After treatment, each well was added with 50 µl of MTT and incubated at 37°C for 1 h. Subsequently, wells were added with 400 µl of DMSO for 30 min at room temperature to solubilize the formazan product. The absorbance at 570 nm was measured using MULTISKAN GO (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Western blot analysis
Approximately 200,000 cells were seeded into each well of six-well culture plates. After 24 h, cells were treated with 2 ml of medium/well and 16 µl of DMSO (control) or 50 µM DAPT. Cells were harvested after 24, 48, and 72 h and analyzed via western blot analysis. Antibodies targeting NICD (no. AP21093a; Abgent, Inc., San Diego, CA, USA), β-actin (no. 4967; Cell Signaling Technology, Inc., Danvers, MA, USA), and HES-1 (no. ab49170; Abcam, Cambridge, UK) were used as primary antibodies. HRP-conjugated anti-rabbit IgG antibody (no. 7074; Cell Signaling Technology, Inc.) was used as secondary antibody.
Next, we investigated protein expression in the presence of DAPT and/or cisplatin. Approximately 200,000 cells were seeded into each well of six-well culture plates. After 24 h of incubation, cells were treated with 2 ml of medium/well with or without cisplatin and DAPT for 72 h. Antibodies targeting NICD, β-actin, and HES-1 were used as primary antibodies. HRP-conjugated anti-rabbit IgG antibody was used as secondary antibody, as described above.
Flow cytometry assay
Approximately 1.0×106 cells were seeded into a 100-mm plate and cultured in 10 ml of medium with or without cisplatin and DAPT for 72 h. Cells were assessed using a MACS Quant Analyzer (Miltenyi Biotec, Bergisch Gladbach, Germany). Fluorescein isothiocyanate-conjugated anti-CD44 (no. 130-095-195; Miltenyi Biotec) and allophycocyanin-conjugated anti-Lgr-5 antibodies (no. 130-100-854; Miltenyi Biotec) were used as markers for gastric cancer stem cells. The voltage settings were adjusted in histograms of the control samples that were treated with DMSO alone and the visual peak of the population was set as 10°. We used 10° as the threshold point. For example, for CD44 expression analysis, cells with fluorophore brightness above 10° were judged as CD44high, and those below 10° were judged as CD44low.
To calculate statistical differences with respect to percentages of CD44high or Lgr-5high cells between samples, approximately 5.0×105 cells were seeded into 6-well plates and cultured in 2 ml/well of medium with or without cisplatin and DAPT. Three wells were used for each condition. After 72 h, cells were labeled with anti-CD44 and anti-Lgr-5 antibodies and assessed using a MACS Quant Analyzer.
Statistical analysis
Data are presented as the mean ± standard deviation of three independent experiments. Statistical analyses were performed using JMP 14.0.0 software (SAS Institute, Inc., Cary, NC, USA), and one-way analysis of variance followed by a Tukey-Kramer post hoc test for multiple comparisons. P<0.05 was considered to indicate a statistically significant difference.
Results
Seventy-two-hour exposure to DAPT suppresses downstream targets of Notch signaling
Results of western blot analysis revealed that NICD and HES-1, the primary downstream targets of Notch signaling, were downregulated for at least 72 h in MKN45 cells (Fig. 1). Therefore, cells were analyzed after 72 h of exposure to the reagents in the subsequent analyses.
Figure 1.
Expression of NICD and the downstream protein HES1 in MKN45 cells. MKN45 cells were treated with 50 µM DAPT for 24, 48 and 72 h. Then, cells were lysed and evaluated via western blot analysis using antibodies targeting NICD and HES1. Antibodies against β-actin were used to verify the equal loading of cellular proteins. Representative blots of >3 independent experiments are presented. NICD, intracellular domain of the Notch receptor; HES1, Hes family basic helix-loop-helix transcription factor 1; DAPT, dipeptide γ-secretase inhibitor.
Exposure to 0.5 µg/ml cisplatin reduces gastric cancer cell number
We determined the cisplatin concentration that reduced the number of gastric cancer cells by 20 to 30%. In MKN45 cells, the results of MTT assay showed that treatment with 0.1, 0.5, and 1.0 µg/ml cisplatin resulted in 7.0, 20.0 and 37.8% reduction in the number of gastric cancer cells relative to control samples, respectively (Fig. 2A). Similarly, in MKN 74 cells, 0.1, 0.5 and 1.0 µg/ml cisplatin resulted in 12.2, 25.1 and 41.9% reduction in the number of gastric cancer cells, respectively (Fig. 2B). Therefore, 0.5 µg/ml cisplatin was used in subsequent experiments.
Figure 2.
Effect of CDDP in (A) MKN45 and (B) MKN74 cells. Cells were treated with 0.1, 0.5 or 1.0 µg/m cisplatin for 72 h. Then, cells were lysed and cell proliferation was measured via the modified MTT assay. *P<0.05, as indicated. Data are presented as the mean ± standard deviation of three independent experiments. CDDP, cisplatin.
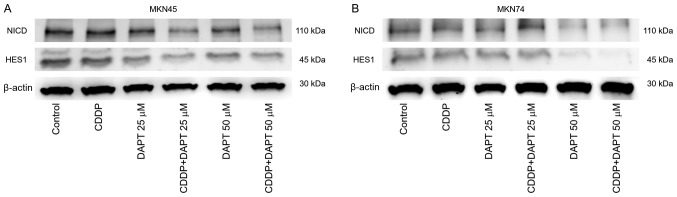
Combined treatment with cisplatin and DAPT downregulates both Notch and HES-1
We investigated the effect of DAPT in combination with 0.5 µg/ml cisplatin. Results of western blot analysis showed that treatment with cisplatin alone did not affect NICD or HES-1 in both MKN45 and MKN74 cells (Fig. 3). In MKN74 cells, a higher concentration of 25 µM DAPT did not produce evident changes in NICD and HES-1 levels, whereas 50 µM DAPT downregulated both Notch and HES-1 (Fig. 3B). In MKN45 cells, treatment with 25 or 50 µM DAPT alone slightly reduced the protein levels of NICD and HES-1, but downregulation was more evident after combined treatment with cisplatin and 25 or 50 µM DAPT (Fig. 3A).
Figure 3.
Expression of NICD and the downstream protein HES1 in (A) MKN45 and (B) MKN74 cells. Cells were treated with CDDP (0.5 µg/ml) and/or DAPT (25 or 50 µM) for 72 h. Then, cells were lysed and analyzed via immunoblot analysis using antibodies targeting NICD and HES1. Antibodies against β-actin were used to verify equal loading of cellular proteins. Representative blots of >3 independent experiments are presented. NICD, intracellular domain of the Notch receptor; HES1, Hes family basic helix-loop-helix transcription factor 1; DAPT, dipeptide γ-secretase inhibitor; CDDP, cisplatin.
Combined treatment with cisplatin and DAPT synergistically suppresses gastric cancer cell proliferation
MTT assay was performed to investigate cell proliferation in four gastric cancer cell lines, namely, MKN45, MKN74, SC-6-JCK, and SH-10-TC, in the presence of DAPT and/or cisplatin. Results showed that treatment with 25 µM DAPT alone did not affect cell proliferation in all four cell lines (Fig. 4). Combined treatment with DAPT and cisplatin decreased the number of gastric cancer cells than treatment with cisplatin alone. The difference reached statistical significance when the cells were treated with 50 µM DAPT and cisplatin in all four cell lines. The dose-dependent inhibitory effect of DAPT on cell proliferation when administered in combination with cisplatin was most apparent in the SC-6-JCK cell line, in which the number of gastric cancer cells was reduced by 30.2% upon treatment with 25 µM DAPT and by 58.7% upon treatment with 50 µM DAPT relative to samples treated with cisplatin alone.
Figure 4.
Proliferation in the four gastric cancer cell lines, (A) MKN45, (B) MKN74, (C) SC-10-TC and (D) SH-6-JCK upon combined treatment with DAPT and CDDP. Cells were treated with CDDP (0.5 µg/ml) and/or DAPT (25 or 50 µM) for 72 h. Then, cells were lysed and analyzed via MTT assay. *P<0.05 and **P<0.01, as indicated. Data are presented as the mean ± standard error of the mean of >3 independent experiments performed in triplicate. N.S., not significant; DAPT, dipeptide γ-secretase inhibitor; CDDP, cisplatin.
DAPT and cisplatin combination treatment reduces expression of stem cell markers CD44 and Lgr-5 more than cisplatin treatment alone
Fig. 5 shows the results of flow cytometry analysis. As described above, CD44 and Lgr-5 were used as cancer stem cell markers for gastric cancer. In untreated MKN45 cells, 18.2% of cells were positive for both CD44 and Lgr-5 (Fig. 5A, left above). Although treatment with cisplatin alone increased the number of cells positive for CD44 and Lgr-5 to 41.8% (Fig. 5A, right above), addition of DAPT to cisplatin significantly reduced the number of cells to 34.7% (Fig. 5A, right below). Similarly, in MKN74 cells, cisplatin treatment increased the number of cells positive for CD44 and Lgr-5 from 15.9 to 34.8% (Fig. 5B). Combined treatment with DAPT and cisplatin decreased the number of CD44-and Lgr-5-positive cells to 21.7%.
Figure 5.
Expression of CD44 and Lgr-5 in (A) MKN45, (B) MKN74, (C) SH-10-TC and (D) SC-6-JCK cells upon combined treatment with DAPT and CDDP. Cells were cultured with or without CDDP and DAPT for 72 h. Then, cells were analyzed via flow cytometry using anti-CD44 and anti-Lgr-5 antibodies. Representative plots from >3 independent experiments are presented. CD44, cluster of differentiation 44; Lgr-5, leucine-rich repeat-containing G-protein coupled receptor 5; DAPT, dipeptide γ-secretase inhibitor; CDDP, cisplatin.
Treatment with cisplatin alone increased the proportion of CD44highLgr-5high cells, whereas combination treatment with cisplatin and DAPT reduced the CD44highLgr-5high fraction. However, although DAPT treatment decreased the proportion of CD44high cells, its effects on Lgr-5high cells were not consistent. For instance, in MKN74 cells, combination treatment with DAPT and cisplatin (35.8%) reduced the number of Lgr-5high cells (CD44highLgr-5high cells plus CD44lowLgr-5high cells) compared to treatment with cisplatin alone (46.8%). The decrease in the number of Lgr-5high cells upon combination treatment with DAPT and cisplatin compared with that observed with cisplatin alone was more apparent in SC-6-JCK cells (84.6 to 68.8%). On the other hand, the DAPT treatment group showed a similar proportion of Lgr-5high cells (32.7%) as that of the control (no treatment) group (31.0%). Moreover, in MKN45 cells, the DAPT treatment group showed a higher number of Lgr-5high cells (40.4%) than that of the control group (33.7%). The proportion of Lgr-5high cells observed after combination treatment with DAPT and cisplatin (59.1%) was similar to that observed after cisplatin treatment (54.0%).
Our results also revealed that the Lgr-5high fraction was higher in the cisplatin treatment group than in the control group. However, the results of our study also suggested that unlike CD44 expression, γ-secretase inhibitor treatment did not directly modulate Lgr-5 expression in gastric cancer cells.
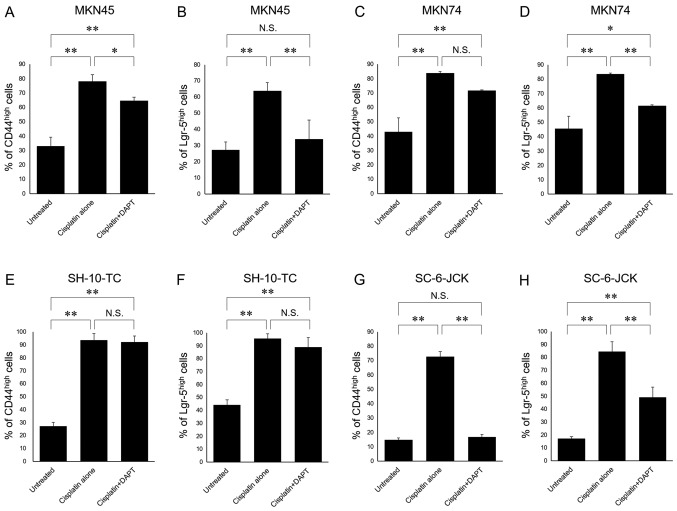
Fig. 6 shows results of statistical analysis with respect to percentages of CD44high cells and Lgr5high cells. In MKN45, MKN74, SH-10-TC, and SC-6-JCK cell lines, treatment with cisplatin alone significantly increased the proportions of both CD44high cells and Lgr5high cells. The proportions of CD44high cells and Lgr-5high cells observed after combination treatment with DAPT and cisplatin were significantly lower than those observed after treatment with cisplatin alone in MKN45, MKN74, and SC-6-JCK cell lines; however, this significant difference was not observed in CD44high MKN74 cells.
Figure 6.
Statistical analysis of CD44 and Lgr-5 expression in (A and B) MKN45, (C and D) MKN74, (E and F) SH-10-TC and (G and H) SC-6-JCK cells. Cells were cultured without cisplatin or DAPT (untreated controls), with cisplatin alone, or with DAPT and cisplatin for 72 h. Then, cells were analyzed via flow cytometry using anti-CD44 and anti-Lgr-5 antibodies. Three samples were used to calculate the mean ± standard deviation. *P<0.05 and **P<0.01, as indicated. N.S., not significant; CD44, cluster of differentiation 44; Lgr-5, leucine-rich repeat-containing G-protein coupled receptor 5; DAPT, dipeptide γ-secretase inhibitor.
Discussion
In the current study, we demonstrated that DAPT enhanced the cytotoxic effects of cisplatin in gastric cancer cells. Notably, although treatment with cisplatin alone increased the proportion of cells positive for cancer stem cell markers, combination treatment with DAPT and cisplatin reduced the proportion of such cells. The above results indicated that Notch signaling inhibition could induce enhanced chemotherapeutic effects when administered in combination with anti-tumor drugs by reducing the proportion of chemoresistant cancer stem cells relative to other tumor cells.
The therapeutic potential of γ-secretase inhibitors combined with 5-fluorouracil (FU) has been previously investigated (9). Lee et al (9) revealed that cbz-IL-CHO, a γ-secretase inhibitor I, reduced the proliferation of gastric cancer cells in vitro and in vivo when administered in combination with 5-FU. They demonstrated that γ-secretase inhibitor I mediated Akt signaling inhibition and subsequent apoptosis, G2/M arrest, and cell death in gastric cancer cells. Moreover, γ-secretase inhibitor I in combination with 5-FU promoted apoptosis of gastric cancer cells via activation of both the extrinsic and intrinsic apoptotic pathways. In the present study, we demonstrated that treatment of gastric cancer cell lines with DAPT, γ-secretase inhibitor IX, and cisplatin in combination was more effective than treatment with DAPT or cisplatin alone.
Cisplatin and 5-FU are two of the major chemotherapeutic agents used for the treatment of gastric cancers. Acquired cisplatin resistance remains a serious concern in the management of gastric cancer patients. Notch signaling has been suggested to be involved in the development of cisplatin resistance (12,13). We speculated that cancer stem cells play critical roles in mediating cisplatin resistance, since they express drug transporters that counteract the cytotoxic effects of chemotherapeutic agents, as described above. Our flow cytometry results showed that the number of gastric cancer cells positive for CD44 and Lgr-5 increased upon treatment with cisplatin alone, whereas combined treatment with DAPT and cisplatin decreased the number of positive cells. The above results indicated that the chemoresistant fraction increased after cisplatin treatment and that DAPT partly altered the chemosensitivity of the cells. Although previous studies have shown the chemosensitivity induced by manipulating the Notch signaling pathway through inhibiting tumor stem cells (14–16), there have been limited reports on gastric cancer cells. Moreover, to our knowledge, this study is the first to investigate the effect of combination treatment with cisplatin and DAPT.
CD44 and Lgr-5 are recognized as surface markers of gastric cancer stem cells (17–23). Xi et al (24) investigated a total of 68 gastric cancer patients and revealed that positive staining for Lgr-5 was significantly associated with weaker response to chemotherapy and shorter survival periods than patients negative for Lgr-5. Li et al (21) revealed that CD44+ cells exhibited cancer stem cell characteristics. These cells showed higher Notch1 expression and were more chemoresistant than CD44- cells. In addition, treatment with DAPT inhibited cancer stem cell properties and suppressed the invasion and proliferation capabilities of CD44+ cells. Furthermore, the present study revealed that DAPT treatment augmented the cytotoxic effects of cisplatin and reduced the CD44highLgr-5high fraction in gastric cancer cell lines.
Du et al (11) investigated the association between Notch signaling and gastric cancer by conducting a meta-analysis based on 15 studies representing a total of 1,547 gastric cancer cases and 450 controls. Expression levels of Notch receptors (Notch1 and Notch2) and their ligands (DLL-4 and HES-1) were found to be significantly higher in gastric cancer tissues than in normal tissues. Notch signaling has been speculated to be involved in carcinogenesis and disease progression in gastric cancers. Moreover, protein levels of Notch1 and Jagged-1 have been established as adverse prognostic factors for gastric cancers (11,25). Thus, direct suppression of the Notch pathway serves as a promising strategy for the treatment of gastric cancers, as well as for enhancing the cytotoxicity of antitumor drugs.
Our study has several limitations. First, we investigated in vitro effects of DAPT in cultured cells. Further comprehensive studies on animals and clinical specimens are required to examine in vivo synergistic antitumor effects of DAPT. Second, although CD44 and Lgr-5 have been widely known and investigated as stem cell markers in gastric cancer cells (26,27), functional assays are needed to investigate how DAPT directly affects stemness. We would like to treat them as the subjects of our next studies.
In conclusion, our findings demonstrated the therapeutic potential of DAPT in the treatment of gastric cancer. Furthermore, combined treatment with DAPT with cisplatin showed enhanced chemotherapeutic effects, particularly in reducing the population of CD44highLgr-5high cells, which exhibit cancer stem cell-like properties. We hope the data obtained in this study will serve as useful reference for future research to overcome acquired cisplatin resistance during gastric cancer treatment.
Acknowledgements
The authors would like to thank Ms. Yuki Osaki (Department of Gastroenterology and Hepatology, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, Japan) for their technical assistance during the experiments.
Glossary
Abbreviations
- CDDP
cisplatin
- DAPT
dipeptide γ-secretase inhibitor
- DMSO
dimethyl sulfoxide
- NICD
intracellular domain of the Notch receptor
Funding
The present study was partially supported by Takeda Pharmaceutical Co., Ltd. (grant no. Takeda Research Support 2017).
Availability of data and materials
The data sets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
RK, MI, ET, KM, AO, HSa and TN performed the experiments. RK and MI analyzed the data and drafted the manuscript. MI, HSh, SH, DU, KT and HO designed and supervised the study. DU, KT and HO revised the manuscript for important intellectual content. All authors have read and approved the final version to be published.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Cancer Information Service, National Cancer Center, corp-author. http://ganjoho.jp/en/professional/statistics/table_download.html Cancer mortality from Vital Statistics in Japan (1958–2016) [Google Scholar]
- 2.Vinogradov S, Wei X. Cancer stem cells and drug resistance: The potential of nanomedicine. Nanomedicine (Lond) 2012;7:597–615. doi: 10.2217/nnm.12.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdullah LN, Chow EK. Mechanisms of chemoresistance in cancer stem cells. Clin Transl Med. 2013;2:3. doi: 10.1186/2001-1326-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colak S, Medema JP. Cancer stem cells-important players in tumor therapy resistance. FEBS J. 2014;281:4779–4791. doi: 10.1111/febs.13023. [DOI] [PubMed] [Google Scholar]
- 5.Dawood S, Austin L, Cristofanilli M. Cancer stem cells: Implications for cancer therapy. Oncology (Williston Park) 2014;28:1101–1107, 1110. [PubMed] [Google Scholar]
- 6.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: Cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 7.Abel EV, Kim EJ, Wu J, Hynes M, Bednar F, Proctor E, Wang L, Dziubinski ML, Simeone DM. The Notch pathway is important in maintaining the cancer stem cell population in pancreatic cancer. PLoS One. 2014;9:e91983. doi: 10.1371/journal.pone.0091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang R, Wang G, Song Y, Tang Q, You Q, Liu Z, Chen Y, Zhang Q, Li J, Li J, Muhammand S, Wang X. Colorectal cancer stem cell and chemoresistant colorectal cancer cell phenotypes and increased sensitivity to Notch pathway inhibitor. Mol Med Rep. 2015;12:2417–2424. doi: 10.3892/mmr.2015.3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee HW, Kim SJ, Choi IJ, Song J, Chun KH. Targeting Notch signaling by γ-secretase inhibitor I enhances the cytotoxic effect of 5-FU in gastric cancer. Clin Exp Metastasis. 2015;32:593–603. doi: 10.1007/s10585-015-9730-5. [DOI] [PubMed] [Google Scholar]
- 10.Katoh M. Dysregulation of stem cell signaling network due to germline mutation, SNP, Helicobacter pylori infection, epigenetic change and genetic alteration in gastric cancer. Cancer Biol Ther. 2007;6:832–839. doi: 10.4161/cbt.6.6.4196. [DOI] [PubMed] [Google Scholar]
- 11.Du X, Cheng Z, Wang YH, Guo ZH, Zhang SQ, Hu JK, Zhou ZG. Role of Notch signaling pathway in gastric cancer: A meta-analysis of the literature. World J Gastroenterol. 2014;20:9191–9199. doi: 10.3748/wjg.v20.i27.9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang ZP, Sun YL, Fu L, Gu F, Zhang L, Hao XS. Correlation of Notch1 expression and activation to cisplatin-sensitivity of head and neck squamous cell carcinoma. Ai Zheng. 2009;28:100–103. [PubMed] [Google Scholar]
- 13.Gu F, Ma Y, Zhang Z, Zhao J, Kobayashi H, Zhang L, Fu L. Expression of Stat3 and Notch1 is associated with cisplatin resistance in head and neck squamous cell carcinoma. Oncol Rep. 2010;23:671–676. doi: 10.3892/or_00000683. [DOI] [PubMed] [Google Scholar]
- 14.Cui D, Dai J, Keller JM, Mizokami A, Xia S, Keller ET. Notch pathway inhibition using PF-03084014, a γ-secretase inhibitor (GSI), enhances the antitumor effect of docetaxel in prostate cancer. Clin Cancer Res. 2015;21:4619–4629. doi: 10.1158/1078-0432.CCR-15-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z, Da Silva TG, Jin K, Han X, Ranganathan P, Zhu X, Sanchez-Mejias A, Bai F, Li B, Fei DL, et al. Notch signaling drives stemness and tumorigenicity of esophageal adenocarcinoma. Cancer Res. 2014;74:6364–6374. doi: 10.1158/0008-5472.CAN-14-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiu M, Peng Q, Jiang I, Carroll C, Han G, Rymer I, Lippincott J, Zachwieja J, Gajiwala K, Kraynov E, et al. Specific inhibition of Notch1 signaling enhances the antitumor efficacy of chemotherapy in triple negative breast cancer through reduction of cancer stem cells. Cancer Lett. 2013;328:261–270. doi: 10.1016/j.canlet.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 17.Han ME, Jeon TY, Hwang SH, Lee YS, Kim HJ, Shim HE, Yoon S, Baek SY, Kim BS, Kang CD, Oh SO. Cancer spheres from gastric cancer patients provide an ideal model system for cancer stem cell research. Cell Mol Life Sci. 2011;68:3589–3605. doi: 10.1007/s00018-011-0672-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen T, Yang K, Yu J, Meng W, Yuan D, Bi F, Liu F, Liu J, Dai B, Chen X, et al. Identification and expansion of cancer stem cells in tumor tissues and peripheral blood derived from gastric adenocarcinoma patients. Cell Res. 2012;22:248–258. doi: 10.1038/cr.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang J, Zhang Y, Chuai S, Wang Z, Zheng D, Xu F, Zhang Y, Li C, Liang Y, Chen Z. Trastuzumab (herceptin) targets gastric cancer stem cells characterized by CD90 phenotype. Oncogene. 2012;31:671–682. doi: 10.1038/onc.2011.282. [DOI] [PubMed] [Google Scholar]
- 20.Xu G, Shen J, Ou Yang X, Sasahara M, Su X. Cancer stem cells: The ‘heartbeat’ of gastric cancer. J Gastroenterol. 2013;48:781–797. doi: 10.1007/s00535-012-0712-y. [DOI] [PubMed] [Google Scholar]
- 21.Li LC, Wang DL, Wu YZ, Nian WQ, Wu ZJ, Li Y, Ma HW, Shao JH. Gastric tumor-initiating CD44+ cells and epithelial-mesenchymal transition are inhibited by γ-secretase inhibitor DAPT. Oncol Lett. 2015;10:3293–3299. doi: 10.3892/ol.2015.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z, Liu C. Lgr5-positive cells are cancer-stem-cell-like cells in gastric cancer. Cell Physiol Biochem. 2015;36:2447–2455. doi: 10.1159/000430205. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Guo X, Zhang D, Fan Y, Qin L, Dong S, Zhang L. Upregulated miR-132 in Lgr5+ gastric cancer stem cell-like cells contributes to cisplatin-resistance via SIRT1/CREB/ABCG2 signaling pathway. Mol Carcinog. 2017;56:2022–2034. doi: 10.1002/mc.22656. [DOI] [PubMed] [Google Scholar]
- 24.Xi HQ, Cui JX, Shen WS, Wu XS, Bian SB, Li JY, Song Z, Wei B, Chen L. Increased expression of Lgr5 is associated with chemotherapy resistance in human gastric cancer. Oncol Rep. 2014;32:181–188. doi: 10.3892/or.2014.3207. [DOI] [PubMed] [Google Scholar]
- 25.Chang HH, Lee H, Hu MK, Tsao PN, Juan HF, Huang MC, Shih YY, Wang BJ, Jeng YM, Chang CL, et al. Notch1 expression predicts an unfavorable prognosis and serves as a therapeutic target of patients with neuroblastoma. Clin Cancer Res. 2010;16:4411–4420. doi: 10.1158/1078-0432.CCR-09-3360. [DOI] [PubMed] [Google Scholar]
- 26.Li XB, Yang G, Zhu L, Tang YL, Zhang C, Ju Z, Yang X, Teng Y. Gastric Lgr5(+) stem cells are the cellular origin of invasive intestinal-type gastric cancer in mice. Cell Res. 2016;26:838–849. doi: 10.1038/cr.2016.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watanabe T, Okumura T, Hirano K, Yamaguchi T, Sekine S, Nagata T, Tsukada K. Circulating tumor cells expressing cancer stem cell marker CD44 as a diagnostic biomarker in patients with gastric cancer. Oncol Lett. 2017;13:281–288. doi: 10.3892/ol.2016.5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.