Abstract
Correction for ‘Synthesis and evaluation of nuciferine and roemerine enantiomers as 5-HT2 and α1 receptor antagonists’ by Hui Li Heng et al., Med. Chem. Commun., 2018, DOI: 10.1039/c7md00629b.
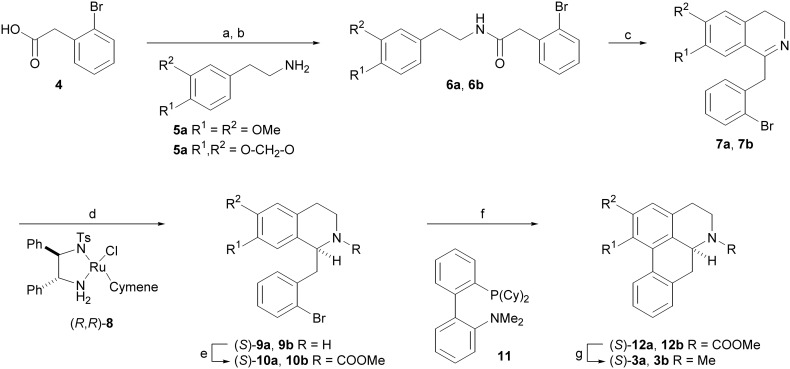
The authors regret that Scheme 1 showed the wrong structure for 12a, 12b, 3a and 3b. Please find below the corrected scheme.
Scheme 1.
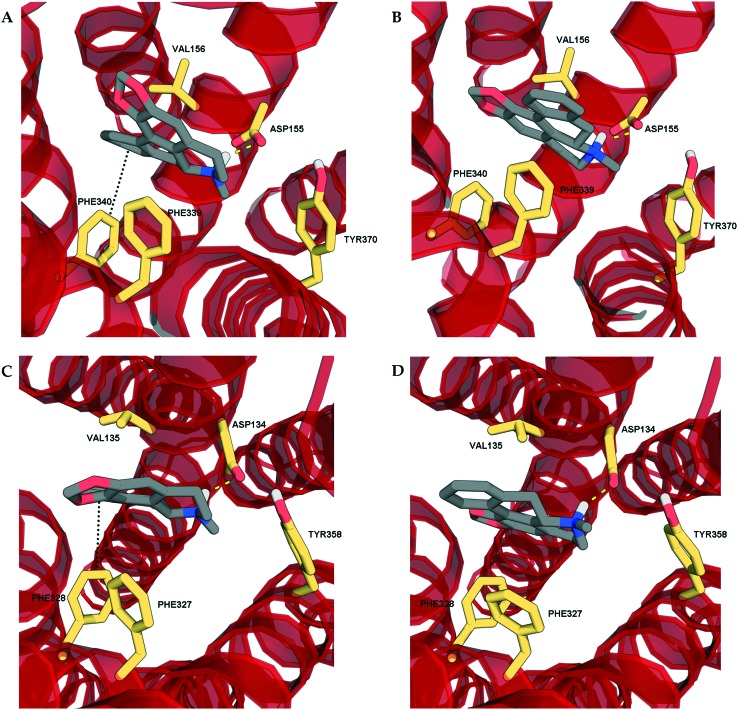
In addition, there were some errors in the numbering of two of the amino acids in Fig. 4, panels C and D. Please find below the corrected figure, including corrected caption.
Fig. 4. The docking poses of the two enantiomers of roemerine in complex with the 5-HT2A and 5-HT2C receptors. A and C. The poses of (R)-roemerine enable a π–π interaction with Phe340/328 as depicted by the black dotted lines. B and D. The poses of (S)-roemerine do not allow a π–π interaction with Phe340/328. For the purpose of clarity, only the principal binding residues are depicted and some of the transmembrane helices are not shown.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.