Abstract
Background:
The dose of certain cell types in allografts affects engraftment kinetics and clinical outcomes after allogeneic stem cell transplantation (SCT). Hence, the present study investigated the association of cell compositions in allografts with outcomes after unmanipulated haploidentical SCT (haplo-SCT) for patients with acquired severe aplastic anemia (SAA).
Methods:
A total of 131 patients with SAA who underwent haplo-SCT were retrospectively enrolled. Cell subsets in allografts were determined using flow cytometry. To analyze the association of cellular compositions and outcomes, Mann–Whitney U nonparametric tests were conducted for patient age, sex, weight, human leukocyte antigen mismatched loci, ABO-matched status, patient ABO blood type, donor-recipient sex match, donor-recipient relationship, and each graft component. Multivariate analysis was performed using logistic regression to determine independent influence factors involving dichotomous variables selected from the univariate analysis.
Results:
A total of 126 patients (97.7%) achieved neutrophil engraftment, and 121 patients (95.7%) achieved platelet engraftment. At 100 days after transplantation, the cumulative incidence of II–IV acute graft-versus-host disease (GVHD) was 32.6%. After a median follow-up of 842 (range: 124–4110) days for surviving patients, the cumulative incidence of total chronic GVHD at 3 years after transplantation was 33.7%. The probability of overall survival at 3 years was 83.0%. Multivariate analysis showed that higher total doses of CD14+ (P = 0.018) and CD34+ cells (P < 0.001) were associated with a successful platelet engraftment. A successful platelet was associated with superior survival (P < 0.001). No correlation of other cell components with outcomes was observed.
Conclusions:
These results provide evidence and explain that higher doses of CD34+ and CD14+ cells in haploidentical allografts positively affect platelet engraftment, contributing to superior survival for patients with SAA.
Keywords: Aplastic Anemia, CD14+ Monocyte, CD34+ Cell, Haplo-Stem Cell Transplantation, Outcome
摘要
背景:
在异基因造血干细胞移植(allogeneic stem cell transplantation,allo-SCT)中,回输的特定移植物组分的数量影响患者造血重建和移植预后。因此,我们研究了在接受单倍型造血干细胞移植(haploidentical stem cell transplantation,Haplo-SCT)的重型再生障碍性贫血(severe aplastic anemia,SAA)患者中,回输的各种移植物组分与移植预后的关系。
方法:
我们的研究回顾性分析了131例接受Haplo-SCT的SAA患者,利用流式细胞术检测患者回输的移植物中各细胞亚群,利用非参数检验分析患者年龄、患者性别、供受者HLA不合位点、供受者ABO血型相合情况、患者ABO血型、供受者性别关系、供受者关系以及各移植物组分数量和移植预后的关系,利用logistic回归分析进行多因素分析。
结果:
在131例患者中,126例(97.7%)患者获得粒细胞植入,121例(95.7%)患者获得血小板植入。患者移植后100天的II-IV度急性移植物抗宿主病(graft-versus-host disease,GVHD)累积发生率为32.6%。所有存活患者的中位随访时间为842(124-4110)天,三年的慢性GVHD累积发生率为33.7%,3年总生存率(overall survival,OS)为83.0%。多因素分析显示,回输高剂量CD14+细胞(P=0.018)和CD34+细胞(P<0.001)的患者有更好的血小板植入率;而血小板植入的患者有更好的OS(P<0.001)。我们的分析结果没有发现其他移植物组分与预后相关。
结论:
我们的研究结果表明,在接受Haplo-SCT的SAA患者中,回输高剂量的CD34+和CD14+细胞促进血小板植入,进而改善患者的总体生存。
INTRODUCTION
Allogeneic stem cell transplantation (allo-SCT), including haploidentical SCT (Haplo-SCT), human leukocyte antigen (HLA)-matched sibling donor transplantation (MSDT) and unrelated donor transplantation (MUDT), provides a potentially curable method for patients with hematological diseases. Hematopoietic recovery is one of the key steps to a successful transplantation.[1] The dose of certain cell types in allografts affects engraftment kinetics and clinical outcomes after allo-SCT.[2,3,4,5,6,7,8] A number of studies have demonstrated that the association of higher CD34+ cell doses in allografts with the rapid engraftment of platelets in patients with hematological malignancies who underwent either MSDT, MUDT, or haplo-SCT.[4,5,7,8,9] The previous studies have also reported a correlation of higher CD34+ cell doses and better overall survival (OS).[3,10,11,12] Except for CD34+ cells, Dong et al.[13] reported that higher CD3+ cell doses in haploidentical allografts were associated with improved OS without producing more severe grades of graft-versus-host disease (GVHD). In HLA-mating sibling and unrelated transplant settings, Reshef et al.[14] observed the relationship of higher CD8+ cell doses with improved survival. Furthermore, Luo et al. observed the correlation of a lower CD4/CD8 ratio in bone marrow grafts with superior survival and reduced risk of acute GVHD.[9] These and other studies[2,3,5,6,7,8,10,13,14,15] suggest that it might be desirable to infuse optimal hematopoietic and immune compositions to patients with hematological malignancies to improve transplant outcomes.
Currently, few studies have focused on the association of cell subsets in allografts with outcomes after allo-SCT for patients with acquired severe acquired aplastic anemia (SAA). Islam et al. observed that an infusion of <2 × 106/kg of CD34+ cells was associated with an increased incidence of graft failures, higher incidence of bacterial infections, and delay in the engraftment of neutrophils in patients with acquired SAA who underwent MSDT and MUDT.[16] In recent years, Haplo-SCT has been an alternative source for SAA either in unmanipulated haplo-SCT based on granulocyte colony-stimulating factor (G-CSF) and anti-thymocyte globulin (ATG)-induced immune tolerance or haplo-SCT with post cyclophosphamide.[17,18] A multicenter study demonstrated that treating SAA patients with haplo-SCT could achieve comparable outcomes to those receiving MSDT.[19] However, the association of immune components in allografts with outcomes for patients with SAA who received haploidentical allografts remains unknown. Therefore, we analyzed 131 patients with acquired SAA to investigate the association of the dose of immune components in a mixture allografts of G-peripheral blood (PB) and G-bone marrow (BM) with outcomes for patients with acquired SAA.
METHODS
Ethical approval
This study met the guidelines of the Helsinki Declaration of 1975 and was approved by the Ethics Committee of Peking University People's Hospital (No. 2016PHB178-01). Informed consent was obtained from all patients or their guardians and donors.
Patients
A total of 131 patients with acquired SAA underwent haplo-HSCT at our institution were enrolled in the present retrospective study between January 2006 and December 2016. All patients were diagnosed with SAA based on the UK treatment guidelines.[20] Donor selection and HLA typing were performed as previously described.[21,22]
Conditioning regimen
All patients were uniformly treated with a myeloablative regimen as previously described.[23,24,25] The conditioning regimen included a combination of intravenous busulfan (0.8 mg/kg 4 times daily on days −7 and −6), cyclophosphamide (50 mg/kg once daily on day −5 to −2), and rabbit anti-thymocyte globulin (ATG [Sangstat, Lyon, France]; 2.5 mg/kg once daily for 4 consecutive days, days −5 to −2).
Mobilization protocol
Donor mobilization was conducted as previously described.[26] The G-CSF was subcutaneously administered at a dose of 5 μg/kg donor weight per day from day −3 until the last day of graft collection for 5 or 6 consecutive days. Each donor received G-CSF at approximately the same time every day. The donor weight included in the calculation was assessed during health examination for accuracy.
Graft-versus-host disease prophylaxis and treatment
All patients received immunosuppressive agents, including cyclosporine A, mycophenolate mofetil and short-term methotrexate, to prevent GVHD, and acute GVHD was treated according to previous reports.[23,25,27]
Bone marrow and peripheral blood stem cell allograft collection
On the 4th day of G-CSF administration, the donors underwent a procedure on the posterior iliac crest using epidural anesthesia to harvest BM grafts (G-BM). During the operation, the previously prepared autologous red blood cells (RBCs) were transfused back into the donors. Due to a major ABO blood group incompatibility, RBCs and/or plasma was removed from some bone marrow grafts before readministration to the recipients. Since these processes might lead to mononuclear cells (MNC) loses, we only collected data before processing.[26]
For the peripheral blood stem cell (G-PB) collection, leukapheresis was commenced on the 5th day after G-CSF conditioning using a COBE Spectra (Spectra LRS; COBE BCT Inc., Lakewood, CO, USA) at a planned rate of 80 ml/min. The leukapheresis was initiated within 4 h after the last injection of G-CSF. The total target MNC count from the bone marrow and the PB was 4–6 × 108 cells/kg recipient body weight. If the MNC count was less than the collection target, a second leukapheresis was completed on the 6th day after the administration of another dose of G-CSF.
Daily complete blood cell counts were obtained, and serum chemistry was tested during G-CSF administration to determine the mobilization states and physical condition of donors.
Flow cytometry evaluation of graft composition
Fresh samples from G-BM and G-PB were stained with MoAb for flow cytometry analysis of the following surface antigens: CD45, CD3, CD4, CD8, CD14, and CD34. The absolute dose of graft compositions was calculated as a percentage of CD34+ cells, CD3+ T cells, CD3+ CD4+ T cells, CD3+ CD8+ T cells, CD3+ CD4- CD8- T cells, and monocytes (MO), labeled with a four-color Ab cocktail within the side scatter low and CD45+ lymphocyte gate multiplied by the total MNC and divided by the actual donor weight to determine the cell dose per kg.[26,28] Acquisition and analyses were performed using CellQuest software (Becton-Dickinson, San Jose, CA, USA).
Definitions of engraftment and outcome measures
SAA was defined as marrow cellularity of <25% with any two of the following: neutrophils <0.5 × 109/L, platelets <20 × 109/L, and reticulocyte count <20 × 109/L. The patients were categorized as “very severe” if, in addition, the neutrophil count was <0.2 × 109/L.[29,30] Neutrophil engraftment was defined as the first of three consecutive days with an absolute neutrophil count (ANC) ≥0.5 × 109/L, and platelet engraftment was defined as the day the platelet count met or exceeded 20 × 109/L without transfusion for a week.[25]
The clinical endpoints were OS, transplant-related mortality (TRM), acute GVHD, and chronic GVHD. Hematological outcomes were assessed regarding neutrophil engraftment and platelet engraftment. All outcomes mentioned above were defined as previously described.[23,31] The endpoint of the last follow-up for all surviving patients was April 31, 2017.
Statistical analysis
Median value and ranges are reported for continuous variables and proportions for categorical variables. The ratio of CD4/CD8 introduced to display the distribution of T-cell subsets was brought into calculation. The Kolmogorov–Smirnov test was used to estimate the normality of variables, and variables consistent with a normal distribution were analyzed using Student's t-test. To analyze the association of cellular compositions and outcomes, Mann–Whitney U nonparametric tests were conducted for patient age, sex, weight, HLA mismatched loci, ABO-matched status, patient ABO blood type, donor-recipient sex match, donor-recipient relationship, and each graft component. Multivariate analysis was performed using logistic regression with a forward selection procedure to determine independent influence factors involving dichotomous variables selected from the univariate analysis. Ninety-five percent confidence intervals (CIs) were calculated with log transformation. P < 0.1 was defined as significant in the univariate analysis, while the P < 0.05 was defined as significant in the multivariate analysis. Calculations were performed using the SPSS 16.0 statistical package (SPSS Inc., Chicago, IL, USA).
RESULTS
Patient characteristics
The characteristics and outcomes of 131 patients with acquired SAA are outlined in Table 1. The median age of the patients was 16 years, ranging from 2 to 45 years old, with a predominance of males (53.4%). The median patient weight was 52 kg. The median and range of cellular compositions, including CD34+ cells, CD3+ T cells, CD3+ CD4+ T cells, CD3+ CD8+ T cells, CD3+ CD4- CD8- T cells, and CD14+ monocytes and the ratio of CD4/CD8 in total grafts are displayed in Table 2.
Table 1.
Patient characteristics and outcomes
| Characteristics | Value |
|---|---|
| Patient number | 131 |
| Sex (male/female) | 70/61 |
| Age (years) | 16 (2–45) |
| Weight (kg) | 52 (13–86) |
| HLA-A, B, DR mismatched loci | |
| 0 | 1 (0.8) |
| 1 | 4 (3.1) |
| 2 | 28 (21.4) |
| 3 | 98 (74.8) |
| ABO matched | |
| Matched | 74 (56.5) |
| Major mismatched | 21 (16.0) |
| Minor mismatched | 27 (20.6) |
| Bidirect mismatched | 9 (6.9) |
| Donor-recipient sex match | |
| Male-male | 52 (39.7) |
| Male-female | 42 (32.1) |
| Female-male | 19 (14.5) |
| Female-female | 18 (13.7) |
| Donor-recipient relation | |
| Patients-child | 104 (79.4) |
| Child-patients | 4 (3.1) |
| Sibling-sibling | 23 (17.6) |
| Acute GVHD (grades) | |
| II | 30 (22.9) |
| III | 6 (4.6) |
| IV | 6 (4.6) |
| Chronic GVHD (n = 33) | |
| Clinical extensive | 4 (4.5) |
| Engraftment (days) | |
| Neutrophil engraftment | 12 (10–31) |
| Platelet engraftment | 16 (7–276) |
| Engraftment (yes or no) | |
| Neutrophil engraftment | 126 (97.7) |
| Platelet engraftment | 121 (95.7) |
| PBC counts on 30th day posttransplantation | |
| Lymphocyte (×109/L) | 0.35 (0–6.15) |
| Neutrophil (×109/L) | 0.54 (0–2.40) |
| Survival | |
| Alive | 110 (83.0) |
| Dead | 21 (17.0) |
| Median follow-up for surviving patients (days) | 842 (124–4110) |
Values are shown as n, n (%), or median (range). GVHD: Graft-versus-host disease; PBC: Peripheral blood cell; HLA: Human leukocyte antigen.
Table 2.
Graft product component characteristics
| Cell type | Value |
|---|---|
| MNC (×108/kg) | 9.560 (5.713–20.336) |
| Lymphocytes (×106/kg) | 337.524 (76.675–964.222) |
| CD34+ cells (×106/kg) | 2.951 (0.691–16.912) |
| CD3+T cells (×106/kg) | 238.311 (53.481–647.556) |
| CD3+ CD4+ T cells (×106/kg) | 131.957 (26.836–387.449) |
| CD3+ CD8+ T cells (×106/kg) | 80.136 (22.811–219.172) |
| CD3+ CD4- CD8- T cells (×106/kg) | 14.280 (1.894–99.231) |
| CD14+ monocytes (×106/kg) | 174.444 (28.114–430.964) |
| CD4/CD8 ratio | 1.62 (0.66–5.17) |
Data are shown as median (range). Kg: Kilogram of donor. MNC: Mononuclear cells.
Transplant outcomes
A total of 126 patients (97.7%) achieved neutrophil engraftment and 121 patients (95.7%) achieved platelet engraftment. At 100 days after transplantation, the cumulative incidence of II–IV and III–IV acute GVHD was 32.6% (95% CI, 25–41%) and 9.3% (95% CI, 3–15%), respectively. After a median follow-up of 842 (range 124 to 4110) days for surviving patients, the cumulative incidence of total and moderate-to-severe chronic GVHD at 3 years after transplantation was 33.7% (95% CI, 24–44%) and 4.5% (95% CI, 1–9%), respectively. The cumulative incidence of TRM at 3 years was 17.0% (95% CI, 11–23%). The probability of OS at 3 years was 83.0% (95% CI, 77–89%).
Association of cell components in allografts with hematopoietic reconstitution
As shown in Table 1, the median time of neutrophil engraftment and platelet engraftment was 12 days (range: 10 to 31 days) and 16 days (range: 7–276 days), respectively.
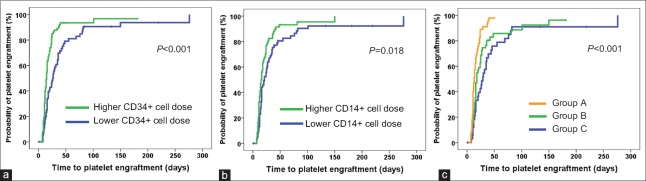
Univariate analysis showed that a higher total CD34+ cell dose in allografts was associated with neutrophil engraftment (P = 0.082); however, multivariate analysis did not demonstrate this association. In additional, in univariate analysis, higher total doses of CD34+ cells (P < 0.001), CD14+ cells (P = 0.018), and lymphocytes (P = 0.019) had positive effects on platelet engraftment, respectively, while in multivariate analysis, higher total doses of CD34+ cells (hazard ratio [HR] 1.891, 95% CI 1.297–2.757, P = 0.001) and CD14+ cells (HR 1.451, 95% CI 1.005–2.095, P = 0.047) were positively associated with rapid platelet engraftment. Therefore, the patients were classified into three subgroups according to CD34+ and CD14+ cell doses, including cases receiving higher CD14+ and higher CD34+ cells (Group A), cases receiving either lower CD14+ and higher CD34+ cells or lower CD34+ and higher CD14+ cells (Group B), and cases receiving lower CD14+ and lower CD34+ cells (Group C). The cumulative incidence of platelet engraftment of Group A was 98% (95% CI, 94–100%), which was significantly higher than that of Groups B (96% [95% CI, 89–100%], P = 0.003) and C (91% [95% CI, 81–100%], P < 0.001). Multivariate analysis showed that a higher total dose of CD34+ cells accompanied with a higher total dose of CD14+ cells in mixture allografts was associated with a higher incidence of platelet engraftment [Table 3 and Figure 1]. However, there were no association of other immune cell compositions, including total CD3+ T cell dose, CD3+ CD4+ T cell dose, CD3+ CD8+ T cell dose, CD3+ CD4- CD8- T cell dose, and CD4/CD8 ratio, with neutrophil and platelet engraftment.
Table 3.
Univariate and multivariate analysis of factors associated with outcomes of patients with SAA who underwent haploidentical allografts
| Covariates | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Overall survival | ||||||
| Platelet engraftment (yes or no) | 0.031 | 0.012–0.080 | <0.001 | 0.031 | 0.012–0.080 | <0.001 |
| Neutrophil engraftment (yes or no) | 0.032 | 0.011–0.094 | <0.001 | |||
| Acute GVHD III–IV (yes or no) | 3.790 | 1.385–10.370 | 0.009 | |||
| Ratio of CD4/CD8 in allografts | 2.262 | 0.911–5.613 | 0.078 | |||
| Platelet engraftment (yes or no) | ||||||
| Higher total lymphocyte dose in allografts (×108/kg) | 1.552 | 1.074–2.243 | 0.019 | |||
| Groups classified according to total dose of CD14+ cell and CD34+ cell in allografts* | ||||||
| Group A | 2.666 | 1.641–4.331 | <0.001 | 2.666 | 1.641–4.331 | <0.001 |
| Group B | 1.359 | 0.856–2.158 | 0.193 | 1.359 | 0.856–2.158 | 0.193 |
| Group C | 1 | 1 | ||||
| Neutrophil engraftment (yes or no) | ||||||
| Higher total CD34+ cell dose (×106/kg) | 1.370 | 0.961–1.954 | 0.082 | 0.080 | ||
All variables involved were dichotomous variables. *The dose of CD34+ cell and CD14+ cell more than 2.768 × 106/kg and 169.605 × 106/kg was recorded as higher variable and determined using ROC curve. total patients were classified into three subgroups according to CD34+ cell and CD14+ cell doses, including cases receiving higher CD14+ and higher CD34+ cells (Group A), cases receiving either lower CD14+ and higher CD34+ cells or lower CD34+ and higher CD14+ cells (Group B), and cases receiving lower CD14+ and lower CD34+ cells (Group C). SAA: Severe aplastic anemia; HR: Hazard ratio; CI: Confidence interval; GVHD: Graft-versus-host disease; ROC: Receiver operating characteristic.
Figure 1.
Correlation of platelet engraftment with the total dose of CD14+ and CD34+ cells in patients with acquired severe aplastic anemia after haploidentical allografts. (a) Kaplan–Meier analysis for correlation of platelet engraftment with the total dose of CD34+ cells. (b) Kaplan–Meier analysis for correlation of platelet engraftment with the total dose of CD14+ cell. (c) Kaplan–Meier analysis for correlation of platelet engraftment with the total dose of CD14+ and CD34+ cells. The dose of CD34+ and CD14+ cells more than 2.768 × 106/kg and 169.605 × 106/kg was recorded as higher variable and determined using ROC curve. Total patients were classified into three subgroups according to CD34+ and CD14+ cell doses, including cases receiving higher CD14+ and higher CD34+ cells (Group A, 46 patients), cases receiving either lower CD14+ and higher CD34+ cells or lower CD34+ and higher CD14+ cells (Group B, 51 patients), and cases receiving lower CD14+ and lower CD34+ cells (Group C, 34 patients). ROC: Receiver operating characteristic.
Association of cell components with graft-versus-host disease
For acute GVHD III–IV, univariate analysis showed that a higher total NC dose was associated with a low probability of acute GVHD (P = 0.089), and multivariate analysis showed no association of cell composition in allografts with acute GVHD. In addition, univariate and multivariate analysis showed no association of cell components in allografts with total and moderate-to-severe chronic GVHD.
Association of cell components with overall survival
The factors associated with OS after univariate analysis include neutrophil engraftment (P < 0.001), platelet engraftment (P < 0.001), a GVHD III-IV (P = 0.009), and ratio of CD4/CD8 in mixture allografts (P = 0.078). Multivariate analysis showed that platelet engraftment (P < 0.001) was the only predictive factor for OS (HR 0.031, 95% CI, 0.012–0.080, P < 0.001) [Table 3 and Figure 2]. No association of CD34+, CD14+ cells with OS were found after univariate and multivariate analysis.
Figure 2.
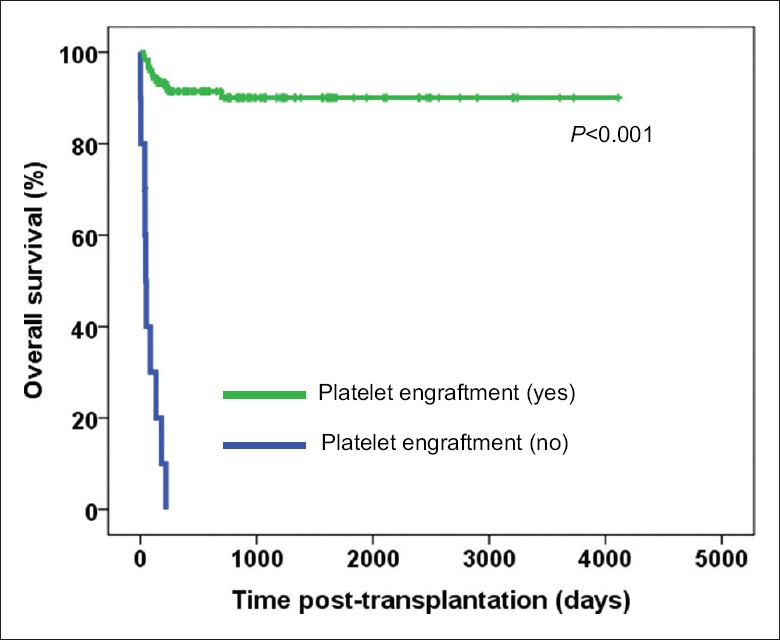
Kaplan–Meier analysis for correlation of overall survival and platelet engraftment in patients with acquired severe aplastic anemia after haploidentical allografts. Of the 131 patients, 121 patients achieved platelet engraftment while 10 patients did not achieve.
DISCUSSION
Consistent with previous data,[16,19] we observed that a higher dose of CD34+ cells was associated with platelet engraftment in patients with SAA after unmanipulated haplo-SCT. We also observed that a higher CD14+ monocyte dose in mixed allografts of G-BM and G-PB was positively associated with platelet engraftment. Furthermore, the association of platelet engraftment with superior outcome was also observed in the present study. Therefore, these results provided evidence and a logical explanation that higher doses of CD34+ and CD14+ cells in haploidentical allografts positively affect platelet engraftment, which may contribute to superior survival for patients with SAA.
Available data on the relationship between CD34+ cell dose and engraftment after transplantation remain controversial. Islam et al.[16] demonstrated that the lower CD34+ cell dose of 2.0 × 106/kg instead of the median CD34+ level of 3.4 × 106/kg had a statistically significant effect on neutrophil engraftment (P = 0.046) but not on platelet engraftment (P = 0.63). In the present study, the higher total dose of CD34+ (≥2.768 × 106/kg) cells had a positive effect on the engraftment of platelet in multivariate analysis. Most studies have demonstrated that a higher CD34+ cell dose was associated with engraftment,[3,4,5,7,8,9,32] while a few studies showed no relationship between the CD34+ cell dose and engraftment.[10,12,33] The differences in transplant types (haploidentical blood and marrow transplantation or MUDT vs. haplo-SCT), conditioning regimen, and race may account for the different results. In summary, the results of the present study indicated that CD34+ cell dose is an important prognosis factor for engraftment after haplo-SCT in patients with SAA or hematological malignancies.[4,9,19]
Interestingly, in the present study, patients who received a higher dose of CD14+ monocytes had a higher probability of platelet engraftment. Monocytes are bone marrow-derived mononuclear cells that give rise to macrophages.[34,35] The previous studies have shown that monocyte/macrophages play an important role in bone homeostasis.[36,37] Winkler et al.[38] observed that bone marrow macrophages are pivotal to maintain the endosteal HSC niche. The macrophage component of bone marrow after bone marrow transplantation originated from the donor.[39,40] In addition, monocytes/macrophages were associated with the production of transforming growth factor-β (TGFβ) and platelet-derived growth factor (PDGF),[36] which together with the above findings suggests that macrophages are necessary for hematopoietic recovery. Therefore, CD14+ monocyte and CD34+ cells may synergistically enhance engraftment for patients with acquired SAA, whose microenvironment harbors defects.[41] Overall, these data suggest that higher doses of CD14+ and CD34+ cells in allografts could contribute to successful platelet engraftment, leading to superior OS.
Several studies have examined the relationships of immune components with outcomes in patients with hematological malignancies.[5,9,14,42,43,44,45] Fisher et al.[46] conducted a meta-analysis showing that high levels of regulatory T cells in the grafts were associated with improved OS, with a significant reduction in TRM and a reduced risk of acute GVHD. Xu et al.[47] suggested that a high CD4/CD8 ratio in allografts may predict adverse survival in patients with CML undergoing haplo-mismatched HSCT. Dong et al.[13] observed that a high number of CD3+ cells administered to patients resulted in a significantly better overall clinical outcome from haplo-SCT. However, in the present study, no correlation was found between the immune components (including CD3+ T cells, CD3+ CD4+ T cells, CD3+ CD8+ T cells, and CD3+ CD4- CD8- T cells and the ratio of CD4/CD8 in total allografts), and platelet engraftment, neutrophil engraftment, GVHD, or OS, potentially due to the differences in the conditioning regimen, GVHD prophylaxis, and underlying disease.
The present study has several limitations. First, it is a single-center study with few cases who failed to achieve hematopoietic recovery, a multicenter study with a large sample is needed to provide adequate statistical power. Second, the results were obtained from a Chinese population, and the possibility of extending these findings to other populations[48] remains to be investigated. Third, the optimal total dose of CD34+ cells and CD14+ monocytes in mixed grafts of G-PB and G-BM needs further investigation.
In summary, the findings of the present study suggest that both higher total doses of CD14+ and CD34+ cells in allografts may contribute to successful platelet engraftment in patients with acquired AA who underwent haplo-SCT. In addition, these data also indicated that successful platelet recovery was associated with superior survival. This information suggests that higher doses of CD34+ and CD14+ cells should be administered for the treatment of patients with acquired SAA by haploidentical allografts.
Financial support and sponsorship
This work was financially supported (in part) by a grant from the National Natural Science Foundation of China (No. 81470342).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank the faculty members of Peking University Institute of Hematology, who collected the samples and analyzed the flow cytometry data.
Footnotes
Edited by: Qiang Shi
REFERENCES
- 1.Sun YQ, He GL, Chang YJ, Xu LP, Zhang XH, Han W, et al. The incidence, risk factors, and outcomes of primary poor graft function after unmanipulated haploidentical stem cell transplantation. Ann Hematol. 2015;94:1699–705. doi: 10.1007/s00277-015-2440-x. doi: 10.1007/s00277-015-2440-x. [DOI] [PubMed] [Google Scholar]
- 2.Sohn SK, Kim JG, Kim DH, Lee NY, Suh JS, Lee KB, et al. Impact of transplanted CD34+ cell dose in allogeneic unmanipulated peripheral blood stem cell transplantation. Bone Marrow Transplant. 2003;31:967–72. doi: 10.1038/sj.bmt.1704042. doi: 10.1038/sj.bmt.1704042. [DOI] [PubMed] [Google Scholar]
- 3.Pulsipher MA, Chitphakdithai P, Logan BR, Leitman SF, Anderlini P, Klein JP, et al. Donor, recipient, and transplant characteristics as risk factors after unrelated donor PBSC transplantation: Beneficial effects of higher CD34+ cell dose. Blood. 2009;114:2606–16. doi: 10.1182/blood-2009-03-208355. doi: 10.1182/blood-2009-03-208355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu DH, Zhao XS, Chang YJ, Liu YK, Xu LP, Chen H, et al. The impact of graft composition on clinical outcomes in pediatric patients undergoing unmanipulated HLA-mismatched/haploidentical hematopoietic stem cell transplantation. Pediatr Blood Cancer. 2011;57:135–41. doi: 10.1002/pbc.23107. doi: 10.1002/pbc.23107. [DOI] [PubMed] [Google Scholar]
- 5.Lee HS, Park LC, Lee EM, Shin SH, Kim YS, Moon JH, et al. Predictive factors for rapid neutrophil and platelet engraftment after allogenic peripheral blood stem cell transplantation in patients with acute leukemia. Ann Hematol. 2013;92:1685–93. doi: 10.1007/s00277-013-1847-5. doi: 10.1007/s00277-013-1847-5. [DOI] [PubMed] [Google Scholar]
- 6.Lee JW, Kim SK, Jang PS, Chung NG, Jeong DC, Cho B, et al. Impact of CD34+ cell dose in children who receive unrelated PBSCT with in vivo T-cell depletion for hematologic malignancies. Bone Marrow Transplant. 2015;50:68–73. doi: 10.1038/bmt.2014.202. doi: 10.1038/bmt.2014.202. [DOI] [PubMed] [Google Scholar]
- 7.Bittencourt H, Rocha V, Chevret S, Socié G, Espérou H, Devergie A, et al. Association of CD34 cell dose with hematopoietic recovery, infections, and other outcomes after HLA-identical sibling bone marrow transplantation. Blood. 2002;99:2726–33. doi: 10.1182/blood.v99.8.2726. doi: 10.1182/blood.V99.8.2726. [DOI] [PubMed] [Google Scholar]
- 8.Beelen DW, Ottinger HD, Elmaagacli A, Scheulen B, Basu O, Kremens B, et al. Transplantation of filgrastim-mobilized peripheral blood stem cells from HLA-identical sibling or alternative family donors in patients with hematologic malignancies: A prospective comparison on clinical outcome, immune reconstitution, and hematopoietic chimerism. Blood. 1997;90:4725–35. [PubMed] [Google Scholar]
- 9.Luo XH, Chang YJ, Xu LP, Liu DH, Liu KY, Huang XJ, et al. The impact of graft composition on clinical outcomes in unmanipulated HLA-mismatched/haploidentical hematopoietic SCT. Bone Marrow Transplant. 2009;43:29–36. doi: 10.1038/bmt.2008.267. doi: 10.1038/bmt.2008.267. [DOI] [PubMed] [Google Scholar]
- 10.Lee SH, Lee MH, Lee JH, Min YH, Lee KH, Cheong JW, et al. Infused CD34+ cell dose predicts long-term survival in acute myelogenous leukemia patients who received allogeneic bone marrow transplantation from matched sibling donors in first complete remission. Biol Blood Marrow Transplant. 2005;11:122–8. doi: 10.1016/j.bbmt.2004.11.018. doi: 10.1016/j.bbmt.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 11.Kałwak K, Porwolik J, Mielcarek M, Gorczyńska E, Owoc-Lempach J, Ussowicz M, et al. Higher CD34(+) and CD3(+) cell doses in the graft promote long-term survival, and have no impact on the incidence of severe acute or chronic graft-versus-host disease after in vivo T cell-depleted unrelated donor hematopoietic stem cell transplantation in children. Biol Blood Marrow Transplant. 2010;16:1388–401. doi: 10.1016/j.bbmt.2010.04.001. doi: 10.1016/j.bbmt.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Gómez-Almaguer D, Gómez-Peña Á, Jaime-Pérez JC, Gómez-Guijosa MÁ, Cantú-Rodríguez O, Gutiérrez-Aguirre H, et al. Higher doses of CD34+ progenitors are associated with improved overall survival without increasing GVHD in reduced intensity conditioning allogeneic transplant recipients with clinically advanced disease. J Clin Apher. 2013;28:349–55. doi: 10.1002/jca.21278. doi: 10.1002/jca.21278. [DOI] [PubMed] [Google Scholar]
- 13.Dong L, Wu T, Zhang MJ, Gao ZY, Lu DP. CD3+ cell dose and disease status are important factors determining clinical outcomes in patients undergoing unmanipulated haploidentical blood and marrow transplantation after conditioning including antithymocyte globulin. Biol Blood Marrow Transplant. 2007;13:1515–24. doi: 10.1016/j.bbmt.2007.09.007. doi: 10.1016/j.bbmt.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Reshef R, Huffman AP, Gao A, Luskin MR, Frey NV, Gill SI, et al. High graft CD8 cell dose predicts improved survival and enables better donor selection in allogeneic stem-cell transplantation with reduced-intensity conditioning. J Clin Oncol. 2015;33:2392–8. doi: 10.1200/JCO.2014.60.1203. doi: 10.1200/JCO.2014.60.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi Y, Lee JH, Kim SD, Kim DY, Lee JH, Seol M, et al. Prognostic implications of CD14 positivity in acute myeloid leukemia arising from myelodysplastic syndrome. Int J Hematol. 2013;97:246–55. doi: 10.1007/s12185-013-1266-3. doi: 10.1007/s12185-013-1266-3. [DOI] [PubMed] [Google Scholar]
- 16.Islam MS, Anoop P, Datta-Nemdharry P, Sage D, Gordon-Smith EC, Turner D, et al. Implications of CD34+ cell dose on clinical and haematological outcome of allo-SCT for acquired aplastic anaemia. Bone Marrow Transplant. 2010;45:886–94. doi: 10.1038/bmt.2009.267. doi: 10.1038/bmt.2009.267. [DOI] [PubMed] [Google Scholar]
- 17.Xu LP, Jin S, Wang SQ, Xia LH, Bai H, Gao SJ, et al. Upfront haploidentical transplant for acquired severe aplastic anemia: Registry-based comparison with matched related transplant. J Hematol Oncol. 2017;10:25. doi: 10.1186/s13045-017-0398-y. doi: 10.1186/s13045-017-0398-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang YJ, Huang XJ. Haploidentical SCT: The mechanisms underlying the crossing of HLA barriers. Bone Marrow Transplant. 2014;49:873–9. doi: 10.1038/bmt.2014.19. doi: 10.1038/bmt.2014.19. [DOI] [PubMed] [Google Scholar]
- 19.Xu LP, Wang SQ, Wu DP, Wang JM, Gao SJ, Jiang M, et al. Haplo-identical transplantation for acquired severe aplastic anaemia in a multicentre prospective study. Br J Haematol. 2016;175:265–74. doi: 10.1111/bjh.14225. doi: 10.1111/bjh.14225. [DOI] [PubMed] [Google Scholar]
- 20.Marsh JC, Ball SE, Cavenagh J, Darbyshire P, Dokal I, Gordon-Smith EC, et al. Guidelines for the diagnosis and management of aplastic anaemia. Br J Haematol. 2009;147:43–70. doi: 10.1111/j.1365-2141.2009.07842.x. doi: 10.1111/j.1365-2141.2009.07842.x. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Chang YJ, Xu LP, Liu KY, Liu DH, Zhang XH, et al. Who is the best donor for a related HLA haplotype-mismatched transplant? Blood. 2014;124:843–50. doi: 10.1182/blood-2014-03-563130. doi: 10.1182/blood-2014-03-563130. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Fu HX, Liu DH, Xu LP, Zhang XH, Chang YJ, et al. Influence of two different doses of antithymocyte globulin in patients with standard-risk disease following haploidentical transplantation: A randomized trial. Bone Marrow Transplant. 2014;49:426–33. doi: 10.1038/bmt.2013.191. doi: 10.1038/bmt.2013.191. [DOI] [PubMed] [Google Scholar]
- 23.Lu DP, Dong L, Wu T, Huang XJ, Zhang MJ, Han W, et al. Conditioning including antithymocyte globulin followed by unmanipulated HLA-mismatched/haploidentical blood and marrow transplantation can achieve comparable outcomes with HLA-identical sibling transplantation. Blood. 2006;107:3065–73. doi: 10.1182/blood-2005-05-2146. doi: 10.1182/blood-2005-05-2146. [DOI] [PubMed] [Google Scholar]
- 24.Huang X, Liu D. Related HLA-mismatched/haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion: Observations of a single Chinese center. Clin Transpl. 2011:237–45. [PubMed] [Google Scholar]
- 25.Xu LP, Liu KY, Liu DH, Han W, Chen H, Chen YH, et al. A novel protocol for haploidentical hematopoietic SCT without in vitro T-cell depletion in the treatment of severe acquired aplastic anemia. Bone Marrow Transplant. 2012;47:1507–12. doi: 10.1038/bmt.2012.79. doi: 10.1038/bmt.2012.79. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Chang Y, Xu L, Zhang X, Huang X. Negative association of donor age with CD34+ cell dose in mixture allografts of G-CSF-primed bone marrow and G-CSF-mobilized peripheral blood harvests. Chin Med J. 2014;127:3597–601. doi: 10.3760/cma.j.issn.0366-6999.20141161. [PubMed] [Google Scholar]
- 27.Xiao-Jun H, Lan-Ping X, Kai-Yan L, Dai-Hong L, Huan C, Wei H, et al. HLA-mismatched/haploidentical hematopoietic stem cell transplantation without in vitro T cell depletion for chronic myeloid leukemia: Improved outcomes in patients in accelerated phase and blast crisis phase. Ann Med. 2008;40:444–55. doi: 10.1080/07853890801908903. doi: 10.1080/07853890801908903. [DOI] [PubMed] [Google Scholar]
- 28.Wang YT, Zhao XY, Zhao XS, Xu LP, Zhang XH, Wang Y, et al. The impact of donor characteristics on the immune cell composition of mixture allografts of granulocyte-colony-stimulating factor-mobilized marrow harvests and peripheral blood harvests. Transfusion. 2015;55:2874–81. doi: 10.1111/trf.13251. doi: 10.1111/trf.13251. [DOI] [PubMed] [Google Scholar]
- 29.Bacigalupo A, Hows J, Gluckman E, Nissen C, Marsh J, Van Lint MT, et al. Bone marrow transplantation (BMT) versus immunosuppression for the treatment of severe aplastic anaemia (SAA): A report of the EBMT SAA working party. Br J Haematol. 1988;70:177–82. doi: 10.1111/j.1365-2141.1988.tb02460.x. doi: 10.1111/j.1365-2141.1988.tb02460.x. [DOI] [PubMed] [Google Scholar]
- 30.Camitta BM, Thomas ED, Nathan DG, Santos G, Gordon-Smith EC, Gale RP, et al. Severe aplastic anemia: A prospective study of the effect of early marrow transplantation on acute mortality. Blood. 1976;48:63–70. [PubMed] [Google Scholar]
- 31.Chang YJ, Zhao XY, Xu LP, Zhang XH, Wang Y, Han W, et al. Donor-specific anti-human leukocyte antigen antibodies were associated with primary graft failure after unmanipulated haploidentical blood and marrow transplantation: A prospective study with randomly assigned training and validation sets. J Hematol Oncol. 2015;8:84. doi: 10.1186/s13045-015-0182-9. doi: 10.1186/s13045-015-0182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Remberger M, Törlén J, Ringdén O, Engström M, Watz E, Uhlin M, et al. Effect of total nucleated and CD34(+) cell dose on outcome after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2015;21:889–93. doi: 10.1016/j.bbmt.2015.01.025. doi: 10.1016/j.bbmt.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 33.Kim DH, Won DI, Lee NY, Sohn SK, Suh JS, Lee KB, et al. Non-CD34+ cells, especially CD8+ cytotoxic T cells and CD56+ natural killer cells, rather than CD34 cells, predict early engraftment and better transplantation outcomes in patients with hematologic malignancies after allogeneic peripheral stem cell transplantation. Biol Blood Marrow Transplant. 2006;12:719–28. doi: 10.1016/j.bbmt.2006.03.005. doi: 10.1016/j.bbmt.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 34.Huber R, Pietsch D, Günther J, Welz B, Vogt N, Brand K, et al. Regulation of monocyte differentiation by specific signaling modules and associated transcription factor networks. Cell Mol Life Sci. 2014;71:63–92. doi: 10.1007/s00018-013-1322-4. doi: 10.1007/s00018-013-1322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swirski FK, Hilgendorf I, Robbins CS. From proliferation to proliferation: Monocyte lineage comes full circle. Semin Immunopathol. 2014;36:137–48. doi: 10.1007/s00281-013-0409-1. doi: 10.1007/s00281-013-0409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pirraco RP, Reis RL, Marques AP. Effect of monocytes/macrophages on the early osteogenic differentiation of hBMSCs. J Tissue Eng Regen Med. 2013;7:392–400. doi: 10.1002/term.535. doi: 10.1002/term.535. [DOI] [PubMed] [Google Scholar]
- 37.Chang MK, Raggatt LJ, Alexander KA, Kuliwaba JS, Fazzalari NL, Schroder K, et al. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in viv o. J Immunol. 2008;181:1232–44. doi: 10.4049/jimmunol.181.2.1232. doi: 10.4049/jimmunol.181.2.1232. [DOI] [PubMed] [Google Scholar]
- 38.Winkler IG, Sims NA, Pettit AR, Barbier V, Nowlan B, Helwani F, et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010;116:4815–28. doi: 10.1182/blood-2009-11-253534. doi: 10.1182/blood-2009-11-253534. [DOI] [PubMed] [Google Scholar]
- 39.Simmons PJ, Przepiorka D, Thomas ED, Torok-Storb B. Host origin of marrow stromal cells following allogeneic bone marrow transplantation. Nature. 1987;328:429–32. doi: 10.1038/328429a0. doi: 10.1038/328429a0. [DOI] [PubMed] [Google Scholar]
- 40.Gordon MY. The origin of stromal cells in patients treated by bone marrow transplantation. Bone Marrow Transplant. 1988;3:247–51. [PubMed] [Google Scholar]
- 41.Holmberg LA, Seidel K, Leisenring W, Torok-Storb B. Aplastic anemia: Analysis of stromal cell function in long-term marrow cultures. Blood. 1994;84:3685–90. [PubMed] [Google Scholar]
- 42.Mohty M, Bagattini S, Chabannon C, Faucher C, Bardou VJ, Bilger K, et al. CD8+ T cell dose affects development of acute graft-vs-host disease following reduced-intensity conditioning allogeneic peripheral blood stem cell transplantation. Exp Hematol. 2004;32:1097–102. doi: 10.1016/j.exphem.2004.07.020. doi: 10.1016/j.exphem.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 43.Cao TM, Shizuru JA, Wong RM, Sheehan K, Laport GG, Stockerl-Goldstein KE, et al. Engraftment and survival following reduced-intensity allogeneic peripheral blood hematopoietic cell transplantation is affected by CD8+ T-cell dose. Blood. 2005;105:2300–6. doi: 10.1182/blood-2004-04-1473. doi: 10.1182/blood-2004-04-1473. [DOI] [PubMed] [Google Scholar]
- 44.Rezvani K, Mielke S, Ahmadzadeh M, Kilical Y, Savani BN, Zeilah J, et al. High donor FOXP3-positive regulatory T-cell (Treg) content is associated with a low risk of GVHD following HLA-matched allogeneic SCT. Blood. 2006;108:1291–7. doi: 10.1182/blood-2006-02-003996. doi: 10.1182/blood-2006-02-003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Zhao XY, Xu LP, Zhang XH, Han W, Chen H, et al. Lower incidence of acute GVHD is associated with the rapid recovery of CD4+CD25+CD45RA+ regulatory T cells in patients who received haploidentical allografts from NIMA-mismatched donors: A retrospective (development) and prospective (validation) cohort-based study. Oncoimmunology. 2016;5:e1242546. doi: 10.1080/2162402X.2016.1242546. doi: 10.1080/2162402X.2016.1242546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fisher SA, Lamikanra A, Dorée C, Gration B, Tsang P, Danby RD, et al. Increased regulatory T cell graft content is associated with improved outcome in haematopoietic stem cell transplantation: A systematic review. Br J Haematol. 2017;176:448–63. doi: 10.1111/bjh.14433. doi: 10.1111/bjh.14433. [DOI] [PubMed] [Google Scholar]
- 47.Xu LP, Luo XH, Chang YJ, Liu DH, Liu KY, Chen YH, et al. High CD4/CD8 ratio in allografts predicts adverse outcomes in unmanipulated HLA-mismatched/haploidentical hematopoietic stem cell transplantation for chronic myeloid leukemia. Ann Hematol. 2009;88:1015–24. doi: 10.1007/s00277-009-0728-4. doi: 10.1007/s00277-009-0728-4. [DOI] [PubMed] [Google Scholar]
- 48.Vasu S, Leitman SF, Tisdale JF, Hsieh MM, Childs RW, Barrett AJ, et al. Donor demographic and laboratory predictors of allogeneic peripheral blood stem cell mobilization in an ethnically diverse population. Blood. 2008;112:2092–100. doi: 10.1182/blood-2008-03-143677. doi: 10.1182/blood-2008-03-143677. [DOI] [PMC free article] [PubMed] [Google Scholar]