Abstract
We aimed at describing antimicrobial usage patterns throughout livestock production cycles, and comparing them across three countries from Northern, Central and Southern Europe. Given the difficulties to collect such detailed usage data, an expert opinion was deemed the most appropriate study design. This study provides new insights into the time periods and indications for which specific antimicrobial substances are used in different livestock sectors.
Veterinary experts (n=67) from different livestock sectors (broilers, pigs, dairy cattle and veal/fattening calves) and countries (Denmark, Portugal and Switzerland) replied to a questionnaire focusing on the time periods in the production cycle when antimicrobial substances were administered, and the respective indications for treatment.
Our results showed that for several antimicrobials, between-country and within-country variations exist regarding the temporal distributions of treatments and indications for use. These differences were also true for several critically important antimicrobials, which is a matter of concern. Furthermore, differences between countries were also evident regarding the antimicrobial substances licensed.
Based on our results, it is recommended to establish and promote treatment guidelines, invest in the prevention of diseases during critical moments of the production cycle and target undifferentiated use of antimicrobials. Moreover, discrepancies between countries should be further investigated to better understand the factors underlying the identified patterns and to distinguish prudent from non-prudent use. The results can inform decision-making with the aim to foster antimicrobial prudent use in the veterinary setting and, therefore, protect public health from the threat of antimicrobial resistance.
Keywords: antimicrobial use, livestock, antimicrobial resistance, international comparison, veterinarians, treatment indications
Introduction
Antimicrobial resistance is nowadays a topic of much discussion and of undeniable relevance for public health.1–3 Part of the resistance burden in humans is attributable to antimicrobial use (AMU) in livestock production, however its contribution has not yet been quantified.4 5 To preserve the efficacy of these substances, prudent AMU in human and veterinary medicine is widely advocated.6 7 Moreover, authorities are promoting a reduction of AMU in the veterinary field both at the national and international levels.2 8–11
A profound understanding of the underlying drivers and reasons of AMU in livestock production is fundamental for a successful reduction of antimicrobial consumption without jeopardising animal health, welfare and productivity. Nevertheless, comparison of AMU across countries has often been limited to comparing the total amount of active substance per animal population, because detailed data on AMU are only available for few countries. A key aspect that has not been thoroughly investigated relates to the patterns of use of specific antimicrobial substances. These patterns harbour essential information about how, when and for which reasons veterinarians use these compounds. Such patterns can also provide an insight on educational aspects, animal health issues, management practices, private standards or legislative regulations that need to be further considered in awareness-raising campaigns or in the identification of targeted measures. A better understanding of AMU patterns is of help to pinpoint specific factors that should be addressed by interventions, such as the development of inexpensive, easy-to-use vaccines or, disease eradication.
Furthermore, it is known that AMU estimates based on sales data vary greatly across Europe, in terms of the overall amounts sold per kilogram of animal biomass, and in the relative use of different antimicrobial classes and routes of administration.12 13 Potential dissimilarities in the patterns of AMU (eg, using a given substance for different indications or at different time points of the production cycle) should partly explain these variations.
The role of veterinarians on AMU is of central importance. First, the prescribing responsibility lies with the veterinarian. Secondly, veterinary practitioners often advise producers on farm management practices, including biosecurity and vaccination strategies that might have an impact in animal health and, therefore, affect AMU at the farm level. Hence, harvesting the knowledge acquired through practical work experience in specific livestock production systems from specialised veterinarians provides an opportunity to gain insights into AMU and reasons for specific AMU patterns in livestock.
The objectives of this study were to: (1) investigate the temporal AMU patterns throughout the production cycles and the indications for specific antimicrobial substances, within each of four common livestock sectors; (2) to compare the results obtained between the countries. The findings of this study shall allow us to identify potential targets for interventions to foster prudent AMU in livestock.
Materials and methods
Collecting such detailed data on AMU patterns and indications for treatment would be very difficult. Therefore, an expert opinion study was considered the most appropriate design. Veterinarians enrolled in this study were based in Denmark, Portugal and Switzerland, and declared clinical expertise and practical experience in at least one of the following livestock sectors: broilers, pigs, dairy cattle or veal/fattening calves. The selection of these countries allowed the inclusion of a Northern, Central and Southern European country, as well as countries with different levels of antimicrobial sales (below European average: Denmark and Switzerland; above European average: Portugal).12 The production cycle of fattening calves (slaughtered around 10–12 months of age) was targeted in Denmark and Portugal. For Switzerland, veterinarians with experience in the veal calf industry (animals slaughtered around six months of age) were contacted, due to the importance of this production system and its contribution to the overall antimicrobial consumption in the country.14
Participants
University departments working on animal production and clinics, as well as veterinarians and farmers’ associations, were contacted to identify potential participants, who were regarded as being particularly knowledgeable about animal health and AMU in the sector. Moreover, enrolled veterinarians were asked to suggest additional participants. This process, the so-called ‘snowball’ nomination, is frequently used to create an expert panel.15 We aimed to have between five and nine experts in each stratum, as this was previously described as an acceptable size for expert panels. Panel sizes can be extended when experts are inquired separately, but given the characteristics of the questionnaire and the difficulties to find adequate participants we limited the number of experts to the above-mentioned range.15
Participating veterinarians were asked to provide answers considering the AMU practices in the country. All experts were assured anonymity.
Questionnaire
Questionnaires were prepared in English using MS Excel16 and consisted of the following structures:
Personal details—with emphasis on the professional activity of the participants.
- Patterns of usage—this part was replicated for all the substances and was further subdivided into the following sections:
- Pattern of usage in sows versus fattening pigs—this question only targeted pig experts with the objective of estimating the relative proportion of treatments in sows compared with fattening pigs.
- Pattern of usage at the age class level—experts were asked to indicate the relative proportion of treatments for given age classes (eg, piglets, weaners, finishers for pig production; calves, heifers, dairy cows for cattle production). These age classes corresponded to a certain range of days, which were defined a priori through expert consultation. These periods varied slightly between countries due to country-specific production characteristics (eg, earlier weaning in Danish pig production). Details on the age class definitions can be found in the web application created to present the results of the study (https://lpgcarmo.shinyapps.io/eeii/).
- Indications at the age class level—experts were asked to indicate the relative proportions of treatments for different organ systems for the age classes described above. The organ system list included: gastrointestinal, mammary gland, musculoskeletal, neurological, reproductive, respiratory, septicaemia/multiple organs, skin/ocular and urinary.
- Pattern of usage for specific age periods—for pigs, dairy cattle and fattening calves (Denmark and Portugal) four time intervals were developed within each age class. These age periods were determined a priori through expert consultation and varied slightly between countries. Experts were asked to give the relative proportion of treatments for each one of these periods.
It should be stressed that questions on the patterns and indications for treatment focused on the amount of treatment performed rather than the amount of antimicrobials used.
To determine which active pharmaceutical ingredients (hereafter called substances) should be investigated in each country/livestock sector, data were collected on the substances licensed per country/animal species.17–19 Oral and parenteral substances were considered separately. Due to the high number of available active ingredients, β-lactamase sensitive penicillins were grouped under ‘penicillins’. In addition, sulfonamides and trimethoprim products were grouped under ‘sulfonamides/trimethoprim’. Lincomycin and spectinomycin were considered together irrespectively of being present in an individual or combination product. Finally, substances subjected to restrictions on use in Denmark (fluoroquinolones and third/fourth-generation cephalosporins) were not included in the questionnaire for Danish experts due to their limited use.20 For dairy cattle, two distinct categories were created for lactation and drying-off treatments. In these two categories, the pattern of AMU was determined irrespectively of the substances.
Questionnaires were pretested with four experts (one for each livestock sector) in September 2015. As no major modifications were suggested, three completed pretesting questionnaires were included in the analysis. The fourth expert who participated in the pretesting provided oral feedback, thus, no answers were used in the analysis.
The data collection took place between October 2015 and March 2016. Potential experts were contacted and if they agreed to participate, detailed information about the study was provided and the questionnaires were sent via electronic mail. Reminders followed around one and two months after the questionnaire was initially sent. Experts were rewarded with a bottle of wine (Portugal and Switzerland) or a gift card (Denmark). The questionnaires are available in the web application.
Analysis
Data management and preparation for analysis was conducted in MS Excel.16 Data were analysed with the statistical software R, V.3.3.1.21
When returned questionnaires included missing values, experts were contacted to provide the values at fault. In case experts did not reply to this request, incomplete entries were imputed using the following rule: (A) for numerical variables, the answers of experts from that country for that same substance were used to calculate the mean; (B) for categorical variables, the most common answer from experts from that country for that same substance was introduced. If an expert did not reply any question for a given substance, no values were imputed. Incomplete entries were only imputed when the expert replied to the questions for a given substance, but certain values were missing.
The proportion of treatments per age class or age period was summarised for each combination of country/livestock sector/substance using the mean of the estimates from the participating experts. Experts who did not provide any answers to a given substance were excluded from the mean calculation of that substance.
Given that age classes and age periods represented time intervals of different lengths, results were also summarised as the relative percentage of treatments per day. This was calculated by dividing the mean proportion of treatment of an age period by the length of the age period in days.
Prtx/day: proportion of treatments per day; a: a given age period; n: number of experts answering the question; d: duration of a given time period.
Results on the indications for treatment were summarised per age class and as a total (for all age classes), by calculating the mean of the estimates provided by the experts.
To be able to visualise the results interactively and for the entire set of country/livestock sector/substance combinations, a web application was developed using the R package ‘Shiny’.22 It can be accessed through the following link: https://lpgcarmo.shinyapps.io/eeii/.
Results
Experts’ information
The median number of years of clinical experience of participating veterinarians was 17 (IQR: 10–25). The percentage of participants working at university institutes was 19 per cent. In addition, 40 per cent of the respondents worked in mixed practices, meaning that they conducted clinical work with more than one animal species.
Six of the 67 participants (four Danish veterinarians—two from dairy, one from pig and one from the fattening calf industry—and two Portuguese veterinarians—one from the broiler and one from the pig industry) were not practising clinical veterinary medicine when this study was conducted. The answers from these experts were still included in the analysis because of the participants’ prior extensive clinical expertise. Moreover, their professional activities (related to livestock production and animal health) allowed these experts to have an up-to-date overview on national production practices.
Product availability
The antimicrobial substances authorised for each livestock species varied between the countries (for details see web application). It should be noted that due to the grouping of certain substances and the elimination of substances with use limimations in Denmark, the presented values should only be interpreted as an approximation of the total number of active pharmaceutical ingredients licensed in each country.
For broilers, Switzerland only had four substances licensed (one of them was licensed but not available on the Swiss market) while in Denmark and Portugal, 10 and 16 substances were licensed, respectively. In the other livestock sectors, fewer combinations of substances/route of administration were licensed in Denmark (dairy: 23, fattening calves: 24, pigs: 26) compared with Switzerland (dairy: 29, veal calves: 29, pigs: 30) and Portugal (dairy: 33, fattening calves: 33, pigs: 34).
AMU patterns and indications
Overall, 0.4 per cent of numerical values and 0.1 per cent of categorical values were imputed.
The proportion of experts replying to the questions for each substance varied between countries/livestock sectors (Table 1). Treatment patterns and indications for all the substances can be consulted in the web application.
TABLE 1:
The proportion of experts answering the patterns and indications for use of each substance was calculated. Results stratified per livestock sector/country are summarised in the table using the median, 25% quantile and 75% quantile.
| Livestock sector | Country | Licensed substances (n) | Median (%) | 25% quantile (%) | 75% quantile (%) | Total number of experts |
| Broilers | Denmark | 10 | 67 | 42 | 100 | 3 |
| Broilers | Portugal | 16 | 75 | 50 | 100 | 4 |
| Broilers | Switzerland | 4 | 83 | 58 | 100 | 3 |
| Pigs | Denmark | 26 | 100 | 88 | 100 | 6 |
| Pigs | Portugal | 34 | 71 | 61 | 100 | 7 |
| Pigs | Switzerland | 31 | 56 | 38 | 75 | 8 |
| Dairy cattle | Denmark | 26 | 100 | 65 | 100 | 5 |
| Dairy cattle | Portugal | 35 | 86 | 71 | 100 | 7 |
| Dairy cattle | Switzerland | 31 | 75 | 56 | 100 | 8 |
| Fattening calves | Denmark | 24 | 63 | 31 | 75 | 4 |
| Fattening calves | Portugal | 33 | 71 | 57 | 86 | 7 |
| Veal calves | Switzerland | 29 | 60 | 40 | 80 | 5 |
Broilers
Oral sulfonamides/trimethoprim products are an example for which the treatment pattern was similar between the countries. Overall, there were more substances with a uniform pattern of use across age classes in Portugal than in the other two countries.
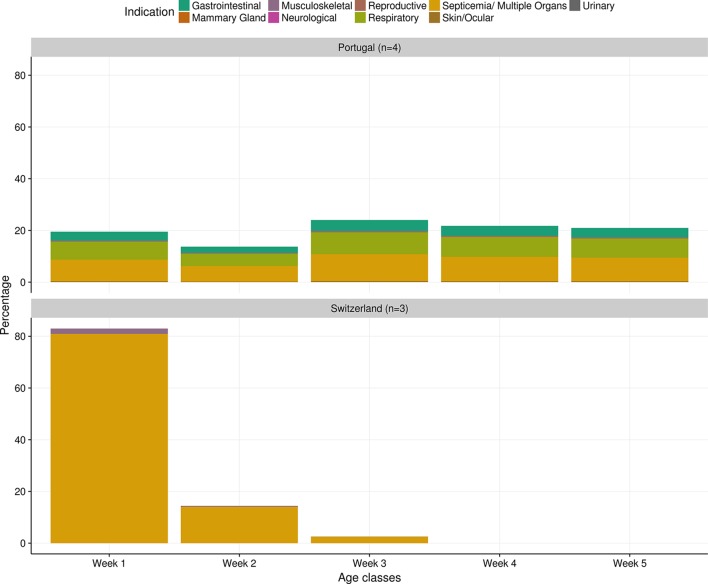
Differences between Portugal and Switzerland on the distribution and indication of oral enrofloxacin treatments were observed: in Switzerland, the majority of the treatments occurred in the first week and targeted ‘septicaemia/multiple organs’; while in Portugal the treatments were distributed rather uniformly over all age classes, with other indications also being considered (Fig 1). The variability in the answers of Swiss experts was smaller when compared with the Portuguese ones. ‘Septicaemia/Multiple Organs’ indication varied from a minimum of 97 per cent to a maximum of 100 per cent of oral enrofloxacin treatments. For that same indication, the estimates from Portuguese experts ranged from 0 to 87 per cent. Gastrointestinal treatments (minimum: 0 per cent; maximum: 29 per cent) and respiratory treatments (minimum: 14 per cent; maximum: 59 per cent) were also considered by Portuguese experts as frequent indications for the use of oral enrofloxacin in broilers.
FIG 1:
Indications for oral treatment with enrofloxacin in broilers. The proportion of treatments for each indication is depicted in different colours. Bars indicate the mean relative proportion of treatments with oral enrofloxacin in different age classes of the production cycle. n, number of answers.
Pigs
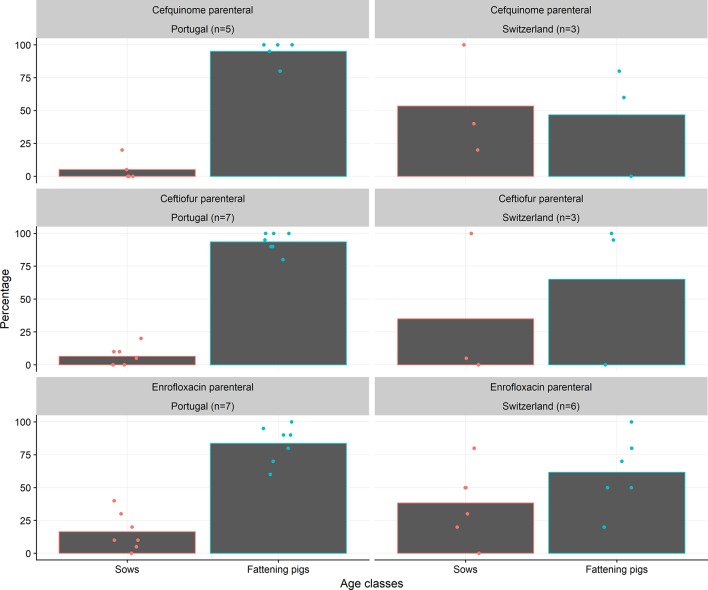
With respect to the proportion of treatments of sows versus fattening pigs (piglets, weaners and finishers), only few disparities were observed between countries. However, differences between the results for Portugal and Switzerland regarding the use of fluoroquinolones and third/fourth-generation cephalosporins deserve attention (Fig 2). In Portugal, the proportion of treatments in fattening pigs was higher than in Switzerland. It should also be noted that the variability of answers within each country was large.
FIG 2:
Mean proportion of treatments between sows and fattening pigs for parenteral use of cefquinome, ceftiofur and enrofloxacin. Points represent individual expert answers. n, number of answers.
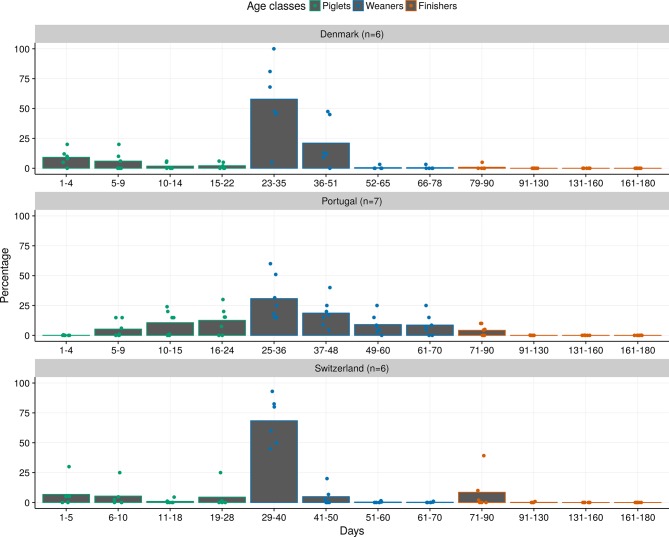
For most substances, treatment incidences peaked in the beginning of a production phase (first time period as piglet, weaner or finisher). Oral colistin constitutes an example: the pattern between the countries showed some similarities, peaking in the first time period in the weaning phase (Fig 3).
FIG 3.
Mean proportion of treatments with oral colistin across the pig production cycle. Bars indicate the mean relative proportion of treatments with oral colistin in different age periods of the production cycle. Points represent individual expert answers. n, number of answers.
The patterns of use of tetracycline substances also showed some similarities between countries. For chlortetracycline, both for Denmark and Switzerland, experts indicated that the majority of treatments occurred during the weaner stage. Nonetheless, it should be noted that Danish experts estimated a more uniform use during this production period, while Swiss participants considered that most treatments happened in the first 11 days after weaning. The treatment peak with parenteral oxytetracycline was estimated to happen in the beginning of the finisher stage in the three countries. However, for oral oxytetracycline treatments peaked in different production stages: in Denmark, most treatments occurred in the beginning of the weaner phase, whereas in Portugal the treatment peak happened at the start of the finisher phase.
Differences between Portugal and Switzerland with respect to the pattern of cephalosporins (parenteral ceftiofur) and fluoroquinolones (parenteral enrofloxacin and parenteral marbofloxacin) were observed. In general, the treatment pattern in Portugal was more uniformly distributed across the production cycle than in Switzerland, where it tended to peak in the first week of life. Some discrepancies on the treatment indications were also observed. Parenteral cefquinome is an example: in Portugal, most treatments targeted gastrointestinal diseases (mean: 52 per cent; minimum: 27 per cent, maximum: 80 per cent), while in Switzerland musculoskeletal problems were the main indication (mean: 87 per cent; minimum: 68 per cent, maximum: 97 per cent). Parenteral marbofloxacin constitutes another illustration: according to the experts, it is not used for respiratory diseases in Switzerland (mean: 0 per cent; minimum: 0 per cent, maximum: 0 per cent), contrarily to what was suggested by Portuguese experts (mean: 49 per cent; minimum: 0 per cent, maximum: 100 per cent).
Dairy cattle
For most substances, the majority of treatments occurred either during the calf phase or during the first period of lactation. Most mastitis treatments occurred in the first 2.5 months of lactation and no major differences in the pattern of AMU were detected between the countries.
The pattern of use of parenteral penicillins was similar between the three countries, with most treatments occurring during the adult phase of the animals. However, it should be noted that the relative proportion of treatments in Denmark during the calf and heifer phases was lower than in Portugal and Switzerland. With regard to the pattern of use of oral sulfonamides/trimethoprim, it is interesting to note that Swiss participants expected some of the treatments to occur after the calf phase; this was not indicated by Danish and Portuguese experts. Furthermore, the peak of treatments in Denmark and Portugal was estimated to happen in the first week of life, whereas in Switzerland it would occur later.
Regarding the indications of treatment during the calf phase, some differences were observed. For several substances (oral amoxicillin, parenteral danofloxacin and parenteral enrofloxacin) respiratory treatments had a higher preponderance in Switzerland than in Denmark and Portugal, where gastrointestinal indications had a higher importance.
Veal/fattening calves
When compared with Danish experts, Portuguese veterinarians estimated a higher relative proportion of treatments in steers with certain substances, such as oral formulations of tilmicosin and tylosin, or parenteral formulations of florfenicol, gamithromycin, tildipirosin and tulathromycin. In these cases, the main indication for treatment in steers was respiratory disease.
For oral tylosin, the vast majority of calf treatments in Denmark were estimated to target gastrointestinal disease (mean: 90 per cent, minimum: 80 per cent, maximum: 100 per cent). In Portugal and Switzerland, the respiratory indication dominated the use of this substance in calves (Portugal—mean: 78 per cent, minimum: 45 per cent, maximum: 100 per cent; Switzerland—mean: 94 per cent, minimum: 79 per cent, maximum: 100 per cent). Differences between countries were observed regarding the indications of parenteral cefquinome and danofloxacin. For the latter, Swiss experts estimated a higher proportion of respiratory treatments in calves than Portuguese experts; for parenteral cefquinome a similar finding was detected, when comparing Switzerland with Denmark and Portugal.
Discussion
This expert opinion study demonstrates differences in the consumption patterns of antimicrobials in three European countries. These variations are evident in the timing, as well as in the indication of treatment for particular substances. Within-country variability was also observed, implying that there are differences in the pattern of AMU even within the same livestock sector/country.
Potential explanations for differences observed between the countries
Several factors can influence the indications and patterns of AMU and, therefore, contribute to the differences observed between the countries. Overall, these can be divided into three categories: (A) animal health and farm management; (B) socioeconomic factors; and (C) policy/market factors.
Disease prevalence and predisposing management practices/farming conditions are obvious factors to consider when interpreting the differences observed between the countries. An example of influencing management practices is animal transportation. For instance, veal calves in Switzerland are generally transported to a fattening farm between 25 and 35 days of age. This practice might explain the peak in the relative proportion of treatments with several substances in the age segment (7–60 days) that includes the above-mentioned period. Moreover, for multiple antimicrobials respiratory treatments were the primary indication in Swiss cattle, while in the other countries gastrointestinal problems prevailed. This might reflect differences in the prevalence of specific animal health problems and could therefore provide grounds to prioritise prevention/control measures.
Multiple socioeconomic factors can influence AMU. Veterinarians’ education might have an implication on the prescription practices11 and preference of practitioners for certain substances. The same experts who participated in this study were also asked to score potential measures to reduce the use of antimicrobials in livestock. Improving veterinarian’s education was considered as an impactful and feasible intervention for that purpose.23 Furthermore, the experience of veterinarians is often considered an important factor that shapes patterns of AMU.24–27 We hypothesise that, together with education, this could be a reason why most participants did not provide answers to all the substances included in the questionnaires (Table 1). Veterinarians might rely on a series of products that they use for specific diseases, without considering all available options. This reflects the need to further investigate and understand the impact of education on the prescription practices of veterinarians and guarantee that a similar level of awareness is attained, both in the undergraduate education and in the continuous education of European veterinarians.
On the policy/market side, it should be noted that product availability differs between countries and could also contribute to the variation in use patterns and indications observed in this study. The fact that only four antimicrobial substances are licensed for use in the Swiss broiler sector is an example of a restriction in substance availability that can potentially influence the choices of veterinarians. The Danish restrictions on the use of fluoroquinolones and third/fourth-generation cephalosporins is another example.28 29 The Weighted Animal Daily Doses strategy implemented as part of the Danish Yellow card benchmarking system—which allocates higher importance to third/fourth-generation cephalosporins, fluoroquinolones, colistin and tetracyclines—also constitutes an illustration of a policy that shapes the pattern of use of substances by influencing the selection of antimicrobials to be prescribed.30 Product prices can also influence veterinarians’ preferences, given that economic aspects often drive decisions in livestock farming.27 31 However, no information on the price differences between countries could be collected.
It is already known that the level of AMU, as well as the relative consumption of different antimicrobial classes and the preference for given routes of administration vary between countries.12 13 This can contribute to the variability between countries observed in our results, regarding the AMU temporal patterns and indications. In a comparison between antimicrobial consumption in Denmark and Switzerland, the largest difference in antimicrobial consumption (measured in mg/kg of biomass), both for pigs and cattle, was observed for sulfonamides/trimethoprim.13 In our results, it was also possible to observe differences between the two countries on the temporal distribution and indication for treatments with these substances. With the exception of adult dairy cattle, the vast majority of treatments with sulfonamides/trimethoprim in Denmark were specified for gastrointestinal problems, while in Switzerland, other indications were also considered.
Study limitations
Expert opinion is often used for modelling purposes,32 33 as well as to get an insight on stakeholders’ perspective over a topic.34–36 Expert opinion is an accepted method when data are unavailable or difficult to collect. Nevertheless, interpretation of the results should take into account that only a small knowledgeable sample of the population is being inquired. A limited number of participating experts might hamper adequate inferences about the true situation in the country and, therefore, extrapolations should be done with caution.
For the majority of the country/livestock sector strata, the number of experts was within the acceptable range.15 The recommended minimum number (5) of broiler experts fell short (Denmark: 3, Portugal: 4, Switzerland: 3). Nonetheless, it should be stressed that due to the centralised structure of the broiler production system in Europe, few veterinarians are responsible for the largest part of the countries’ production. This also implicates that practitioners from the same companies would provide similar answers, given the standardised procedures with respect to antimicrobial treatments. It should also be noted that experts were asked to answer the questionnaire based on the AMU practices in their country. However, their answers might have been biased by their personal experience.
Means were used to summarise the relative proportion of treatments in different age classes and age periods, even when the number of answers was limited and data were not normally distributed. Using medians, instead of means, could have resulted in proportions of treatments above or below 100 per cent in a production cycle.
Due to the large number of substances already included in the questionnaires, combination products and intramammary preparations were not assessed separately. Moreover, the analysis was performed at the level of substance/route of administration. However, the pattern of usage might differ between products, namely if the substance concentration varies. This should not have a large influence on the results of this study; however, the potential for bias should be acknowledged.
Finally, indications for treatment were grouped into organ systems. In certain cases, the choice of organ system might not be straightforward, which can introduce some bias (eg, in broilers, certain diseases can progress from gastrointestinal manifestations to septicaemia). In addition, aggregating indications at the level of organ system might have eventually masked some within-country and between-country differences on the specific indications for treatment.
Relevance and implications
Understanding the moment of use and the reason for treatment for each substance is an important piece of information that could support the development of tailored interventions to enhance prudent AMU and, thus, protect animal and public health. However, collecting detailed data on AMU patterns can be very challenging. Even automated systems, such as the Danish VetStat,37 collect data at the age class level, without further resolution.
Furthermore, the use of single standardised weights to calculate antimicrobial consumption has been shown to influence the results obtained.38 Combining the temporal patterns of treatment with weight distributions might enable a more precise calculation of AMU.
It should be stressed that no inferences can be made about the frequency of treatment, neither between substances nor between countries. This study focused on the distribution of treatments across the production cycle. No information was collected on the amount of antimicrobials used, and the results of this study were not combined with usage data. Nonetheless, in several occasions it was possible to observe a rather uniform distribution of treatments of oral antimicrobials throughout the entire production cycle. Such a distribution might indicate an undifferentiated or prophylactic AMU, which is undesirable and merits further investigation.
Due to the complexity of the topic and the characteristics of the data collected in this study, limited inferences can be made about prudent use of antimicrobials. Nonetheless, it is interesting to note that the first-line antimicrobials recommended by the Danish guidelines39 for treating common occurring gastrointestinal diseases in pigs were also suggested by the experts to be mainly used for gastrointestinal treatments. This, however, cannot guarantee that the guidelines are being followed, as indication data were collected at the organ system level and not at the pathogen level. Furthermore, the first-choice treatment was beyond the scope of the study. On the other hand, the large relative proportion of treatments with oral enrofloxacin in young Swiss broilers should be a matter of concern, given its negative effects on the bone and cartilage development.40 41 This might be related to the limited antimicrobial options available for Swiss broiler veterinarians; it must therefore be guaranteed that enrofloxacin is not used as a first-line antimicrobial due to the lack of other options.
The variability on the temporal distribution and indication of treatments observed between and within countries suggests the need to develop and promote guidelines. These should always take into consideration the needs and the expertise of veterinary practitioners. The variability detected for critically important antimicrobials, as defined by the World Health Organization,42 is of particular concern given that these compounds are of relevance for human medicine and should only be used in veterinary medicine as a last resource.
The distribution of treatments tended to peak in the beginning of the age periods, after animals were moved to a new production stage. This highlights the need to prevent AMU through specific measures (eg, reduced mixing of animals from different origins, promoting vaccination, improving biosecurity) to protect animals in those moments when their immunity is more compromised.
Lastly, the differences in the licensed substances in each country hamper a harmonised European strategy on the fight against AMU and antimicrobial resistance. Regulating antimicrobial licensing at European level might strengthen a common approach on the topic.
Despite the fact that this expert opinion study increases our knowledge about the allocation of antimicrobial treatments, both regarding their temporal distribution and the indications for use, this approach should not be seen as an equivalent replacement for good-quality AMU data. The improvement of data collection systems for livestock antimicrobial consumption should be continuously fostered.
Conclusions
To the best of our knowledge, this is the first time that AMU patterns are studied in such detail for a comprehensive set of substances. The between-country and within-country variations in the temporal patterns and indications for treatment reveal a need for treatment guidelines. Special attention should be given to critically important antimicrobials, by addressing the pathogens fostering their use and providing alternatives with similar levels of efficacy. Drivers of the observed differences (within and between countries) should be further investigated to elucidate tailored interventions that will boost prudent use. Moreover, targeted interventions to prevent the need for antimicrobials in the moments when livestock is particularly susceptible to disease are recommended.
Acknowledgments
We thank all the experts enrolled in this study for their availability to participate. We also thank our colleagues Aurélie Tschopp, Beat Thomann and Ranya Özcelik for their feedback on the manuscript.
Footnotes
Contributors: All the authors were involved in the conceptualisation of the paper. LPC drafted the questionnaire, which was reviewed and approved by LRN, LA, PMC, GS and IM. Data management and data analysis were performed by LPC and IB. YA participated in the management and analysis of data, as well as in the development of the web application. LRN, LA, PMdC, CM, GS and IM provided valuable expertise on the topic, namely on the interpretation of results related to the antimicrobial use patterns and indications in their countries. IM, GS and LRN were involved in the funding acquisition. IM and LRN were responsible for the doctoral candidate (LPC) supervision. LPC drafted the paper, which was reviewed and approved by all the authors.
Funding: This study was funded by the Swiss Federal Food Safety and Veterinary Office (project number 1.12.21) and the University of Copenhagen Research Center for Control of Antibiotic Resistance (http://uc-care.ku.dk/).
Competing interests: None declared.
Ethics approval: In accordance with the local legislation, no ethical approval was required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Data can be obtained from the corresponding author.
References
- 1.World Health Organization (WHO), 2016. United Nations high-level meeting on antimicrobial resistance http://www.who.int/antimicrobial-resistance/events/UNGA-meeting-amr-sept2016/en/ (accessed 21 Oct 2016).
- 2.European Commission, 2011. Communication from the commission on the European parliament and the council - action plan against the rising threats from antimicrobial resistance:1–15 http://ec.europa.eu/dgs/health_food-safety/docs/communication_amr_2011_748_en.pdf.
- 3.Food and Agriculture Organization of the United Nations (FAO), 2016. The FAO Action plan on antimicrobial resistance 2016-2020. Rome http://www.fao.org/3/a-i5996e.pdf.
- 4.Guardabassi L. Sixty years of antimicrobial use in animals: what is next? Vet Rec 2013;173:599–603. 10.1136/vr.f7276 [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Alvarez L, Dawson S, Cookson B, et al. Working across the veterinary and human health sectors. J Antimicrob Chemother 2012;67 Suppl 1:i37–i49. 10.1093/jac/dks206 [DOI] [PubMed] [Google Scholar]
- 6.European Commission, 2015. Commission notice - Guidelines for the prudent use of antimicrobials in veterinary medicine (2015/C 299/04):Off J Eur Union. 7–26 http://ec.europa.eu/health//sites/health/files/antimicrobial_resistance/docs/2015_prudent_use_guidelines_en.pdf. [Google Scholar]
- 7.World Organization for Animal Health (OIE). Chapter 6.9, 2016. Responsible and prudent use of antimicobial agents in veterinary medicine http://www.oie.int/index.php?id=169&L=0&htmfile=chapitre_antibio_use.htm.
- 8.Swiss Federal Food Safety and Veterinary Office, 2016. StAR programme. https://www.blv.admin.ch/blv/de/home/das-blv/strategien/nationale-strategie-antibiotikaresistenzen.html (accessed 13 Oct 2016).
- 9.Alban L, Dahl J, Andreasen M, et al. Possible impact of the "yellow card" antimicrobial scheme on meat inspection lesions in Danish finisher pigs. Prev Vet Med 2013;108:334–41. 10.1016/j.prevetmed.2012.11.010 [DOI] [PubMed] [Google Scholar]
- 10.The European Parliament, 2011. Public health threat of antimicrobial resistance http://www.europarl.europa.eu/sides/getDoc.do?pubRef=-//EP//TEXT+TA+P7-TA-2011-0473+0+DOC+XML+V0//EN.
- 11.EMA (European Medicines Agency) and EFSA (European Food Safety Authority). EMA and EFSA Joint Scientific Opinion on measures to reduce the need to use antimicrobial agents in animal husbandry in the European Union, and the resulting impacts on food safety (RONAFA). Efsa J 2017;15:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.European Medicines Agency (EMA), European Surveillance of Veterinary Antimicrobial Consumption (ESVAC). Sales of veterinary antimicrobial agents in 29 European countries in 2014: Trends across 2011 to 2014 - Sixth ESVAC report, 2016. http://www.ema.europa.eu/docs/en_GB/document_library/Report/2016/10/WC500214217.pdf. [Google Scholar]
- 13.Carmo LP, Nielsen LR, Alban L, et al. Comparison of Antimicrobial Consumption Patterns in the Swiss and Danish Cattle and Swine Production (2007–2013). Front Vet Sci 2017;4:1–11. 10.3389/fvets.2017.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lava M, Schüpbach-Regula G, Steiner A, et al. Antimicrobial drug use and risk factors associated with treatment incidence and mortality in Swiss veal calves reared under improved welfare conditions. Prev Vet Med 2016;126:121–30. 10.1016/j.prevetmed.2016.02.002 [DOI] [PubMed] [Google Scholar]
- 15.Gustafson LL, Gustafson DH, Antognoli MC, et al. Integrating expert judgment in veterinary epidemiology: example guidance for disease freedom surveillance. Prev Vet Med 2013;109:1–9. 10.1016/j.prevetmed.2012.11.019 [DOI] [PubMed] [Google Scholar]
- 16.Microsoft Corporation. Microsoft Excel TM. 2010.
- 17.Veterinærmedicinsk Industriforening (VIF), 2015. Medicin Til Dyr [Medicine to animals] http://www.medicintildyr.dk/ (accessed 10 Sep 2015).
- 18.Institut für Veterinärpharmakologie und -toxikologie, 2015. Veterinary Swiss Drug Compendium http://www.vetpharm.uzh.ch/perldocs/index_i.htm (accessed 10 Sep 2015).
- 19.Apifarma, 2015. Simposium Veterinário [Veterinary Symposium] http://www.apifarma.pt/simposiumvet/Paginas/Pesquisaavancada.aspx (accessed 10 Sep 2015).
- 20.DANMAP, 2016. DANMAP 2015 - Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. https://www.danmap.org/~/media/Projekt%20sites/Danmap/DANMAP%20reports/DANMAP%20%202015/DANMAP%202015.ashx
- 21.R Core Team, 2016. R: A Language and Environment for Statistical Computing. https://www.r-project.org/
- 22.Chang W, Cheng J, Allaire JJ, et al. , 2017. shiny: Web Application Framework for R. https://cran.r-project.org/package=shiny
- 23.Carmo LP, Nielsen LR, Alban L, et al. Veterinary expert opinion on potential drivers and opportunities for changing antimicrobial usage practices in livestock in denmark, portugal, and switzerland. Front Vet Sci 2018;5:1–14. 10.3389/fvets.2018.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coyne LA, Latham SM, Williams NJ, et al. Understanding the culture of antimicrobial prescribing in agriculture: a qualitative study of UK pig veterinary surgeons. J Antimicrob Chemother 2016;71:3300–12. 10.1093/jac/dkw300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDougall S, Compton C, Botha N. Factors influencing antimicrobial prescribing by veterinarians and usage by dairy farmers in New Zealand. N Z Vet J 2017;65:1–9. 10.1080/00480169.2016.1246214 [DOI] [PubMed] [Google Scholar]
- 26.De Briyne N, Atkinson J, Pokludová L, et al. Factors influencing antibiotic prescribing habits and use of sensitivity testing amongst veterinarians in Europe. Vet Rec 2013;173:475 10.1136/vr.101454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Postma M, Speksnijder DC, Jaarsma AD, et al. Opinions of veterinarians on antimicrobial use in farm animals in Flanders and the Netherlands. Vet Rec 2016;179:68 10.1136/vr.103618 [DOI] [PubMed] [Google Scholar]
- 28.Aarestrup FM, Jensen VF, Emborg HD, et al. Changes in the use of antimicrobials and the effects on productivity of swine farms in Denmark. Am J Vet Res 2010;71:726–33. 10.2460/ajvr.71.7.726 [DOI] [PubMed] [Google Scholar]
- 29.Agersø Y, Aarestrup FM. Voluntary ban on cephalosporin use in Danish pig production has effectively reduced extended-spectrum cephalosporinase-producing Escherichia coli in slaughter pigs. J Antimicrob Chemother 2013;68:569–72. 10.1093/jac/dks427 [DOI] [PubMed] [Google Scholar]
- 30.Danish Veterinary and Food Administration, 2017. Special provisions for the reduction of the consumption of antibiotics in pig holdings (the yellow card initiative) https://www.foedevarestyrelsen.dk/english/SiteCollectionDocuments/Dyrevelfaerd%20og%20veterinaermedicin/Veterin%C3%A6rmedicin/Yellow%20Card,%20English%20version,%20180517.pdf (accessed 20 Jul 2017).
- 31.Speksnijder DC, Jaarsma AD, van der Gugten AC, et al. Determinants associated with veterinary antimicrobial prescribing in farm animals in the Netherlands: a qualitative study. Zoonoses Public Health 2015;62 Suppl 1:39–51. 10.1111/zph.12168 [DOI] [PubMed] [Google Scholar]
- 32.Scheetz MH, Bolon MK, Postelnick M, et al. Cost-effectiveness analysis of an antimicrobial stewardship team on bloodstream infections: a probabilistic analysis. J Antimicrob Chemother 2009;63:816–25. 10.1093/jac/dkp004 [DOI] [PubMed] [Google Scholar]
- 33.EFSA (European Food Safety Authority). Guidance on expert knowledge elicitation in food and feed safety risk assessment. Efsa J 2014;12:278. [Google Scholar]
- 34.Sikkens JJ, van Agtmael MA, Peters EJ, et al. Assessment of appropriate antimicrobial prescribing: do experts agree? J Antimicrob Chemother 2016;71:2980–7. 10.1093/jac/dkw207 [DOI] [PubMed] [Google Scholar]
- 35.Kuster K, Cousin ME, Jemmi T, et al. Expert opinion on the perceived effectiveness and importance of on-farm biosecurity measures for cattle and swine farms in switzerland. PLoS One 2015;10:e0144533–17. 10.1371/journal.pone.0144533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stebler N, Schuepbach-Regula G, Braam P, et al. Use of a modified Delphi panel to identify and weight criteria for prioritization of zoonotic diseases in Switzerland. Prev Vet Med 2015;121:165–9. 10.1016/j.prevetmed.2015.05.006 [DOI] [PubMed] [Google Scholar]
- 37.Stege H, Bager F, Jacobsen E, et al. VETSTAT-the Danish system for surveillance of the veterinary use of drugs for production animals. Prev Vet Med 2003;57:105–15. 10.1016/S0167-5877(02)00233-7 [DOI] [PubMed] [Google Scholar]
- 38.Pardon B, Catry B, Dewulf J, et al. Prospective study on quantitative and qualitative antimicrobial and anti-inflammatory drug use in white veal calves. J Antimicrob Chemother 2012;67:1027–38. 10.1093/jac/dkr570 [DOI] [PubMed] [Google Scholar]
- 39.Nielsen EO, Jorsal SE. Ny Vejledning om ordinering af antibiotika til svin [New guidelines for antimicrobial prescription for pigs] DVT, 2018. http://infolink2003.elbo.dk/DVT/dokumenter/doc/17794.pdf.
- 40.Holtom PD, Pavkovic SA, Bravos PD, et al. Inhibitory effects of the quinolone antibiotics trovafloxacin, ciprofloxacin, and levofloxacin on osteoblastic cells in vitro. J Orthop Res 2000;18:721–7. 10.1002/jor.1100180507 [DOI] [PubMed] [Google Scholar]
- 41.Maślanka T, Jaroszewski JJ, Mikołajczyk A, et al. Effect of increasing doses of enrofloxacin on chicken articular cartilage. Pol J Vet Sci 2009;12:21–33. [PubMed] [Google Scholar]
- 42.World Health Organization (WHO). Critically important antimicrobials human medicine - 5th rev. Geneva, 2017. http://apps.who.int/iris/bitstream/10665/255027/1/9789241512220-eng.pdf?ua=1 [Google Scholar]