Abstract
Introduction
Lung cancer is the most common cancer worldwide. Latest guidelines from the College of American Pathologist and the European society of medical oncologists indicate anaplastic lymphoma kinase (ALK) rearrangement testing is standard practice. Historically, diagnostics for ALK used fluorescence in situ hybridisation (FISH); however, immunohistochemical (IHC) assays are becoming common practice. Unfortunately, recent assessment of current practice indicated that not all patients who should be tested for ALK translocation are undergoing ALK testing.
Methods
From a series of European and Israeli labs, we collected patients with discordant IHC and FISH testing, which were subsequently treated with ALK-targeted therapy, for discussion of the question, to treat or not to treat?
Results
Our study may support ALK IHC testing as a better predictor of response to targeted therapy provided that the labs implement controlled preanalytical procedures, use correct clone, run protocols on automated staining platforms and validate using external quality assessments.
Keywords: pathology, ALK, FISH, ISH
Key questions.
What is already known about this subject?
ALK immunohistochemical (IHC) and fluorescence in situ hybridisation (FISH) discordance continues to be a challenge.
Current clinical practice reveals ALK testing is not conducted in all patient samples with lung adenocarcinoma and mixed lung cancers with an adenocarcinoma component.
What does this study add?
Patients who were ALK FISH negative but IHC positive show complete or partial responses to ALK-targeted therapy.
How might this impact on clinical practice?
Our observational study indicates validated ALK IHC as primary testing benefits access to therapy.
Introduction
Histopathological subtyping and molecular profiling of patients with lung cancer is now part of routine practice. After the discovery of EGFR-activating mutations and their impact on EGFR-tyrosine kinase inhibitor (TKI) efficacy1 a decade ago, ALK is the second type of genetic alteration to be routinely screened and with the highest response rate among all targeted therapies. While present in less than 2%–7% of patients with advanced non-squamous non-small cell lung cancer (nsq NSCLC),1 the presence of an ALK rearrangement predicts a better overall response rate and progression-free survival for patients with advanced nsq NSCLC receiving crizotinib, a first-generation ALK inhibitor, over chemotherapy.1 As recommended by the College of American Pathologists, the International Association for the Study of Lung Cancer and the Association for Molecular Pathology, ALK testing should be performed in all patients with lung adenocarcinoma (ADC) or mixed lung cancer with an ADC component, regardless their clinical characteristics.1 Unfortunately, assessment of current practices reveals an unmet need.
Assessment of ALK rearrangement was initially based exclusively on fluorescence in situ hybridisation (FISH) with the use of an ALK break-apart probe, as FISH was the methodology used in the initial studies that demonstrated improved clinical response of patients with ALK-rearranged tumours to treatment with crizotinib.1 2 Nevertheless, the use of FISH as a unique selection method has some technical and practical drawbacks. FISH remains time-consuming and cost-consuming (ie, scoring guidelines require two pathologists to score borderline cases), possibly limiting the number of patients tested rapidly. Moreover, not all pathology labs are equipped to perform FISH. ALK immunohistochemical (IHC) assays may provide a cost-effective alternative technique for screening of ALK rearrangements.1 Therefore, many scientific societies recommend the use of IHC as a screening tool for ALK rearrangement.2 3 The proposed algorithm is that all IHC-positive and doubtful cases should undergo FISH testing for confirmation. However, current practice proposes a testing algorithm limited the reflex testing to the doubtful or equivocal cases. In the recent paper from von Laffert et al,4 it was described that if the validated IHC test is either unequivocal positive or negative, no further reflex testing for ALK is required before putting the patient under ALK-targeted therapy. For the IHC equivocal cases, reflex testing has to be conducted to further evaluate the ALK status by FISH or ideally more sensitive and specific methodologies like next generation sequencing (NGS). However, multicentre testing shows that ALK protein expression cannot be regarded as the method of choice unless standardisation in IHC protocols is applied, and the test is properly validated.1 3 5 6
Regardless of these considerations, ALK FISH/IHC discordance occurs in routine practice1 for both biological or technical reasons and, unfortunately for those patients, the decision of whether to treat with ALK-targeted therapies remains unclear. There have been sporadically reported cases of patients with a discordant ALK testing status that have experienced positive response under ALK TKI therapy. Recently, Shan et al 7 reported IHC-positive, FISH-negative cases with significant response to crizotinib. However, due to the low rate of ALK gene rearrangements and therefore ALK fusion proteins, those reports are rare to date. Therefore, should these patients be treated or not? Here, we gather such discordant cases from several institutes and look at the response of those patients under an ALK-targeted therapy to see if we could address this question.
Methods
Patient selection
In this study, 72 patients with ALK FISH/IHC discordant status were gathered from European and Israeli university hospitals. The patients included in the study cohort are routine patients who were responding to these following criteria: ALK testing status for both IHC and FISH (either positive or negative and always interpretable), treatment with any ALK-targeted therapies and clinical outcome (complete response, stable disease, partial response, progression, no response and death). Forty-six patients were excluded because of at least one biological or clinical missing data.
Cohort description
The remaining cohort consists of 26 patients (mean age 59 years; mean age 53 in FISH−/IHC+ and 64 years in FISH+/IHC− subgroups) and showed the same number of ALK IHC+/FISH− cases (n=13) and IHC−/FISH+ cases (n=13). The number of men and women was equal; however, the low number of cases does not provide sufficient information regarding potential bias in patient selection. Twenty-four cases were diagnosed as ADC; only one was reported with squamous cell carcinoma and one classified as not otherwise specified. Sixteen cases were primary tumours, seven were metastatic and three cases were not specified. Seventeen samples were collected as biopsies, seven as resection specimens (otherwise specified) and two were not specified. Twenty-three cases were screened for both EGFR and KRAS mutation. There was only one case with concurrent L858R EGFR mutation and one case harbouring a KRAS mutation.
ALK analysis
FISH and IHC assays were performed according the routine lab practices. IHC testing used two clones, clone 5A4 (Abcam) or D5F3 (Cell Signaling Technology (CST) or Ventana), demonstrating a high concordance between IHC overexpression and FISH translocation.1 3 5 Half of the IHC tests were performed using the D5F3 clone, 77% of them done using the Ventana ALK assay (Predilute D5F3 antibody, OptiView detection system on Benchmark system using Food and Drug Administration-approved protocol), 23% using the same clone, but in lab-developed format (concentrated Ab on Roche Benchmark XT instrument with ultraView detection kit). All remaining test were run with 5A4 clone from Abcam on Ventana Benchmark XT platform using the in-house validated protocol. No statistically significant difference was found in ALK IHC positivity related to IHC test used, although a trend of higher percentage of ALK FISH−/IHC+ was achieved with the D5F3 clone compared with 5A4 (8/13 vs 5/13, so 62% vs 38%). The scoring system used for IHC interpretation was either described as intensity driven (from 0 to 3+) for ALK 5A4 or CST D5F3 or provided with interpretation guidelines using a binary score (regardless of percentage of cell stained) in the Ventana procedure. FISH testing used an ALK break-apart dual colour probe from Abbott (Chicago, Illinois, USA) or Zytovision (Bremerhaven, Germany) according to the manufacturers’ instructions. A sample was considered positive for rearrangement if at least 15% of the nuclei showed split signals or isolated 3′ signals.
Results
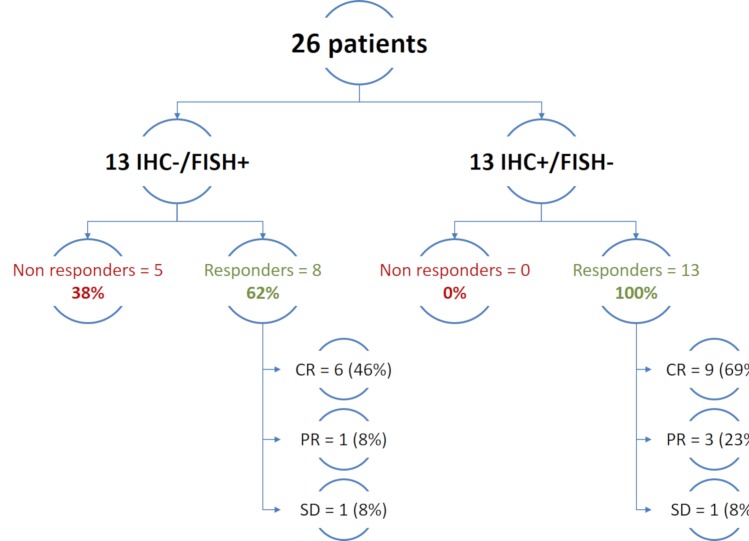
Our study reflects real-world practices in ALK testing, using several IHC and FISH methods. Twenty-five patients were treated with crizotinib, and one patient was treated with second-generation ALK-targeted therapy. The overall response rate was 100% in the FISH−/IHC+ group and 62% in the IHC−/FISH+ group (figure 1). Based on our cohort, in IHC+/FISH− cases, the answer to the question, to treat or not, is clearly yes. Additionally, two-thirds of the IHC−/FISH+ patients have experienced a significant response under ALK TKI, also suggesting an important benefit for these patients to be treated with ALK-targeted therapies. The observed response rate in the FISH+/IHC− of 62% is close to the observed response rate from the clinical trial with crizotinib (74%).3 This observed response rate may be influenced by false-positive cases reported by FISH. A recent study showed that concordance between IHC and FISH was dramatically improved by slight adjustment of the 15% cut-off.1 Alternatively, it may reflect the true biological features of the disease in regards to all possible oncogenic pathways that may be active to escape the ALK blockade. Lastly, it cannot be ruled out that RR may be improved with the use of more powerful ALK TKI than crizotinib, as suggested by the response experienced by one FISH+/IHC− patient under ceritinib after the first line crizotinib has failed.5 Given the low number of cases, our current study does not allow us to conclude between these hypotheses and further work should be conducted to investigate activation of any possible escape pathway.
Figure 1.
Patients response to ALK-targeted therapies according to their ALK discordant status. CR, complete response; FISH, fluorescence in situ hybridisation; IHC, immunohistochemical; PR, partial response; SD, stable disease.
Based on this small number of cases, it is difficult to challenge the Clinical Trial Assay standard; however, these results demonstrate the potential of an alternative screening strategy that may improve patients’ management. To date, performing both IHC and FISH testing gives pathologists the best chance at identify highest number of eligible patients. Following dual IHC and FISH testing, 21/26 patients have benefited from an ALK TKI therapy and have experienced a clinical response versus 13 when using sole IHC screening and 8 when using sole FISH screening. However, we are aware such a strategy might be difficult to implement in each lab. In addition, several papers show ALK FISH testing may miss a significant number of patients who could benefit from ALK therapies.5 The main drawbacks regarding FISH are tumour heterogeneity and difficulty in detecting small chromosomal inversions. Additionally, Martin et al 8 reported that FISH borderline cases were associated with different patterns of split signals. In addition, Peled et al 9 have shown that intron 19 deletion derived abnormal RNA editing with the abnormal cDNA, which was similar to the classic EML4-ALK fusion. This would be a possible explanation for the FISH−/IHC+ population in our cohort that all responded to any ALK-targeted therapies.
These study results indicate that all IHC+/FISH− have responded to ALK TKI therapy, in combination with data from van der Wekken10 that reported no response in 13 cases with FISH+/IHC− result may indicate that ALK IHC could be a better predictor of response to therapy. Interestingly, longitudinal study of external quality assessments (EQAs) highlights an improvement of ALK testing using IHC. This probably comes from careful training of pathologists over the years and also from the improvement of the clones and/or amplification systems used. Indeed, the two main drawbacks for IHC are the lack of internal controls and the fact that all clones and methodologies are not equivalent as the first results from several EQA on ALK IHC clearly show. Those technical reasons can explain the amount of IHC−/FISH+ reported (ie, generating false negative results by IHC due to technical problems). These issues could be addressed by implementing controlled preanalytical procedures and using optimal clones and protocols on automated staining platforms. Interpretation guidelines provided by the supplier and the use of a binary score (regardless of percentage of cell stained) likely played a significant role in recent improvements of the IHC procedure.
Conclusion
Currently, two targeted therapies for ALK are approved by the European Medical Association (EMA), crizotinib and ceritinib, and both require an ALK-positive test in tumour before eligibility for drug therapy. Validated FISH and IHC tests are currently available from manufacturers and can be used in accordance with the EMA test for approval of ALK inhibitor treatment. In this observational study, the response rate to ALK-targeted therapies if one of both tests is positive was significant enough to consider ALK therapy as an option. According to our observational study and evidence that the current phase III trials for the second generation ALK inhibitors are using a validated IHC test as the clinical trial assay, there is a strong suggestion to use IHC as a primary test for ALK testing. This global cohort study provides evidence that IHC is an ideal, robust and accurate testing procedure for ALK in NSCLC by using validated IHC assays and implementing quality assurance measures, including participation in EQA schemes.
Acknowledgments
The authors would like to thank Dr Claire Faure and Paula Toro for critical review of the manuscript and Laya Bhavaraju for manuscript management.
Footnotes
Contributors: Data collection study design revised the paper: all authors. Analyses of data drafted and revised the paper: GE.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors
Competing interests: GE and US are Roche employees. All other authors are currently or have in the past collaborated with Roche Diagnostics. There was no financial support or compensation provided for the design and the writting of this report.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Paez JG, Jänne PA, Lee JC, et al. . EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497–500. 10.1126/science.1099314 [DOI] [PubMed] [Google Scholar]
- 2. Rodig SJ, Mino-Kenudson M, Dacic S, et al. . Unique clinicopathologic features characterize ALK-rearranged lung adenocarcinoma in the western population. Clin Cancer Res 2009;15:5216–23. 10.1158/1078-0432.CCR-09-0802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Solomon BJ, Mok T, Kim DW, et al. . First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167–77. 10.1056/NEJMoa1408440 [DOI] [PubMed] [Google Scholar]
- 4. von Laffert M, Schirmacher P, Warth A, et al. . ALK-Testung beim nicht-kleinzelligen Lungenkarzinom (NSCLC): immunhistochemie (IHC) und/oder Fluoreszenz-in-situ-Hybridisierung (FISH)? Pneumologie 2016;70:277–81. 10.1055/s-0042-102626 [DOI] [PubMed] [Google Scholar]
- 5. Leighl NB, Rekhtman N, Biermann WA, et al. . Molecular testing for selection of patients with lung cancer for epidermal growth factor receptor and anaplastic lymphoma kinase tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the study of lung cancer/association for molecular pathology guideline. J Clin Oncol 2014;32:3673–9. 10.1200/JCO.2014.57.3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wynes MW, Sholl LM, Dietel M, et al. . An international interpretation study using the ALK IHC antibody D5F3 and a sensitive detection kit demonstrates high concordance between ALK IHC and ALK FISH and between evaluators. J Thorac Oncol 2014;9:631–8. 10.1097/JTO.0000000000000115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shan L, Jiang P, Xu F, et al. . BIRC6-ALK, a Novel fusion gene in ALK break-apart FISH-negative lung adenocarcinoma, responds to crizotinib. J Thorac Oncol 2015;10:e37–e39. 10.1097/JTO.0000000000000467 [DOI] [PubMed] [Google Scholar]
- 8. Martin V, Bernasconi B, Merlo E, et al. . ALK testing in lung adenocarcinoma: technical aspects to improve FISH evaluation in daily practice. J Thorac Oncol 2015;10:595–602. 10.1097/JTO.0000000000000444 [DOI] [PubMed] [Google Scholar]
- 9. Peled N, Palmer G, Hirsch FR, et al. . Next-generation sequencing identifies and immunohistochemistry confirms a novel crizotinib-sensitive ALK rearrangement in a patient with metastatic non-small-cell lung cancer. J Thorac Oncol 2012;7:e14–e16. 10.1097/JTO.0b013e3182614ab5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van der Wekken AJ, Pelgrim R, ’t Hart N, et al. . Dichotomous ALK-IHC is a better predictor for ALK inhibition outcome than traditional ALK-FISH in advanced non-small cell lung cancer. Clin Cancer Res 2017;23:4251–8. 10.1158/1078-0432.CCR-16-1631 [DOI] [PubMed] [Google Scholar]