Abstract
The epithelial-mesenchymal transition (EMT) serves critical roles in the migration, invasion and metastasis of human cancer cells. This process is initiated by regulation of E-cadherin expression by the major inducers of EMT. Previous studies reported that osteopontin (OPN) is essential for hepatocellular carcinoma (HCC) metastasis as it facilitates the EMT in HCC. However, the role and clinical significance of OPN as an EMT regulator in HCC remains unknown. The present study revealed that OPN regulated the expression of Twist by activating RAC serine/threonine-protein kinase (Akt), a critical EMT regulator. Interfering with the phosphoinositide 3-kinase (PI3K)/Akt pathway may suppress the expression of Twist enhanced by OPN. Increased Twist levels in HCC were associated with poor survival and tumor recurrence in patients with HCC following surgery. A significant association was observed between OPN expression and Twist levels in HCC, and a combination of these two parameters was revealed to be a more powerful predictor of poor patient prognosis. The findings of the present study indicate that Twist serves an notable role in OPN-mediated metastasis of HCC through activation of the PI3K/Akt pathway. Twist may be a potential therapeutic target for the prevention of HCC metastasis in patients exhibiting high OPN expression.
Keywords: osteopontin, Twist, hepatocellular carcinoma, metastasis, prognosis
Introduction
Hepatocellular carcinoma (HCC) is one of the most common types of cancer and primarily occurs in South Africa and Asian countries (1,2). According to statistical analyses, the incidence of liver cancer has increased in the majority of countries over the past 5 years (1). China alone accounted for ~50% of cases of newly diagnosed liver cancer and mortality in 2012 (2). Although curative resection is beneficial for the long-term survival of patients with HCC, the prognosis of these patients remains poor owing to the high rate of metastasis and recurrence (3,4). Therefore, identifying more accurate prognostic biomarkers of HCC is of great clinical value for the understanding HCC and to develop novel therapeutic strategies.
Osteopontin (OPN), a secreted glycosylated phosphoprotein encoded by the secreted phosphoprotein 1 gene, has been implicated as being a major mediator and potential therapeutic target of cancer metastasis (5,6). A previous study has demonstrated that OPN is a ligand that binds to αvβ integrins or receptors of the cluster of differentiation 44 family to promote cell adhesion, extracellular matrix degradation, and the prevention of apoptosis, angiogenesis and indolent tumor growth (7). Furthermore, OPN has been identified as a key inducer of tumor invasion and metastasis (8,9).
The epithelial-mesenchymal transition (EMT) serves a major role in tumor metastasis, a developmental process whereby E-cadherin, an epithelial marker, is downregulated and vimentin and N-cadherin, which are mesenchymal markers, are upregulated (10–12). Additionally, the EMT results in reduced epithelial cell intercellular adhesion and causes these cells to acquire fibroblastoid properties, thereby improving the ability of cells to migrate. Therefore, the EMT is important for the development, invasion and metastasis potential of cancer.
Twist, a major EMT regulator, is essential for tumor metastasis (13,14). In the process of metastasis, Twist serves a crucial role in the EMT by downregulating E-cadherin and β-catenin, and by regulating cell motility, invasiveness and metastasis (14–17). Furthermore, Twist expression was observed to be increased in different types of tumor, including prostate cancer (17), melanoma (18), pediatric osteosarcoma (19), T-cell lymphoma (20), gastric cancer (21), breast carcinoma (22) and HCC (23,24). Previous studies have proven that Twist and Snail, but not Slug, are major EMT inducers in HCC, and that Twist serves a major role in hepatitis C-associated HCC, unlike Snail (25).
A recent study demonstrated that OPN promotes EMT of HCC (8). The present study examined the role and clinical significance of OPN on the EMT regulator, Twist, in HCC cell lines and tumor tissues. OPN was revealed to regulate the expression of Twist, a major regulator of HCC metastasis. Furthermore, interfering with the phosphoinositide 3-kinase (PI3K)/RAC serine/threonine-protein kinase (Akt) pathway may suppress the expression of Twist enhanced by OPN. Therefore, we hypothesize that OPN and Twist may serve as synergistic prognostic biomarkers and therapeutic targets for HCC.
Materials and methods
Patients and follow-up
A total of 374 patients, including 306 males and 68 females (age range, 30–70 years, median age 55 years) with HCC, underwent a hepatectomy at the Liver Cancer Institute, Zhongshan Hospital, Fudan University (Shanghai, China) by the same surgical team between March 2004 and December 2006. In addition, these patients had not received any neo-adjuvant or adjuvant treatments, but did undergo a pathological examination and complete follow-up. Formalin-fixed, paraffin-embedded tissues, which included 374 HCC patient tissues and 192 matched adjacent non-tumor tissues were used to organize a tissue microarray (TMA) for immunohistochemistry (IHC) studies. The clinicopathological characteristics of patients whose tissues were used for the TMA are summarized in Table I. The present study was approved by the Research Ethics Committee of Zhongshan Hospital, Fudan University (Shanghai, China) and written informed consent was obtained from each patient. Follow-up was completed in May 2013. The follow-up procedures and treatment modalities following relapse are described in previous studies (26–28). To diagnose recurrence, α-fetoprotein (AFP) levels were analyzed and computed tomography (CT) and/or magnetic resonance imaging (MRI) scans were performed. Patient mortality and disease recurrence were used as endpoints and the endpoints included the overall survival (OS) time and the time to recurrence (TTR). The OS time was defined as the interval between the dates of surgery and mortality. The TTR was defined as the time between surgery and the first report of intrahepatic or distant recurrence or the last follow-up for patients who had not experienced recurrence at the time of mortality (patients who had succumbed to other causes were not included) (29). The TTR was recorded at the date of mortality or the last follow-up (30,31).
Table I.
Association between Twist expression levels and clinicopathological characteristics in HCC patients.
| Twist expression, n | |||
|---|---|---|---|
| Variable | Low (n=204) | High (n=170) | P-value |
| Sex | 0.687 | ||
| Female | 39 | 29 | |
| Male | 165 | 141 | |
| Age, years | 0.467 | ||
| ≤50 | 98 | 89 | |
| >50 | 106 | 81 | |
| HBsAg | 0.543 | ||
| No | 16 | 10 | |
| Yes | 188 | 160 | |
| ALT, U/l | 0.498 | ||
| ≤75 | 185 | 150 | |
| >75 | 19 | 20 | |
| Liver cirrhosis | 0.745 | ||
| No | 24 | 18 | |
| Yes | 180 | 152 | |
| AFP, ng/ml | 0.830 | ||
| ≤20 | 74 | 64 | |
| >20 | 130 | 106 | |
| Tumor size, cm | 0.613 | ||
| ≤5 | 158 | 136 | |
| >5 | 46 | 34 | |
| Tumor number | 0.052a | ||
| Single | 190 | 166 | |
| Multiple | 14 | 4 | |
| Tumor capsule | 0.677 | ||
| Complete | 112 | 89 | |
| None | 92 | 81 | |
| Vascular invasion | 0.013 | ||
| No | 153 | 107 | |
| Yes | 51 | 63 | |
| Tumor differentiation | 0.633 | ||
| I/II | 155 | 125 | |
| III/IV | 49 | 45 | |
| BCLC stage | 0.157 | ||
| 0 and A | 59 | 38 | |
| B and C | 145 | 132 | |
HBsAg, hepatitis B surface antigen; AFP, α-fetoprotein; ALT, alanine amino transferase; BCLC, Barcelona Clinic Liver Cancer.
Fisher's exact tests, and χ2 tests for all other analyses.
TMA and IHC
The resected specimens (2–3 mm) were fixed in 10% formalin for four days at room temperature, and send to Pathology department of zhongshan hospital. The construction of the TMA (in collaboration with Shanghai Biochip Co., Ltd., Shanghai, China) and IHC were performed as described previously (32). Immunostaining was performed on TMA slides using a two-step process according to the manufacturer's protocols. Following deparaffinization, 4 µm sections were rehydrated in a descending alcohol series and subjected to antigen retrieval by microwaving in 0.01 mol/l sodium citrate (pH 6) for 10 min. When microwaving, sodium citrate was boiled at a ~100°C and sections placed in it, followed by the temperature ~30-40°C for 10 min, followed by the sections being allowed to cool naturally to room temperature. Then, sections were washed using phosphate buffered saline (PBS).
Sections were incubated at 4°C overnight with monoclonal antibodies against OPN (dilution, 1:100; cat no. ab8448; Abcam, Cambridge, UK) and Twist (dilution, 1:100; cat no. ab50581; Abcam). Immunostaining was performed using ChemMate DAKO EnVision Detection kit, Peroxidase/DAB, Rabbit/Mouse (cat no. GK500705; Dako; Agilent Technologies, Inc., Santa Clara, CA, USA), according the manufacturer's protocol. Subsequently, the sections were counterstained with hematoxylin at room temperature until the microscopic observation of sections were discoloration, used PBS to rinse and soaked it for 2 min, then mounted in dimethyl benzene. Negative controls were included in all assays and were treated identically but with the primary antibodies omitted. An optical microscope was used at a magnification ×200.
The intensity of staining was scored manually (0, no staining; 1, weak staining; 2, moderate; and 3, strong staining) by two independent experienced pathologists. Tumor cells in 5 randomly selected fields were scored based on the proportion of positively stained cells (0–100%). The final IHC scores were determined by multiplying the intensity scores and the proportion scores of the positive cells. Expression levels of OPN and Twist in all 374 samples were quantified. ‘High’ vs. ‘low’ OPN and Twist expression was defined according to the cut-off values of OPN and Twist level, which were defined as the median of the cohort. To evaluate the combined influence of OPN and Twist on the prognosis of patients, the 374 patients with HCC were separated into four groups: Group I, patients with low OPN and low Twist expression (n=117); Group II, patients with high OPN and low Twist expression (n=65); Group III, patients with low OPN and high Twist expression (n=87); and Group IV, patients with high OPN and high Twist expression (n=105).
Cell lines and plasmids
Three human HCC cell lines with various metastatic potentials, MHCC97-L, MHCC97-H and HCC-LM3, and the human non-transformed hepatic L-02 cell line, were used in the present study. MHCC97-L, MHCC97-H and HCC-LM3 with stepwise increasing metastatic potential were established from the same parent human HCC cell line at the Liver Cancer Institute, Fudan University (Shanghai, China). They have a genetically identical background (33,34). The L-02 cells were obtained from American Type Culture Collection (Manassas, VA, USA). These cell lines were routinely maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS; both Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in a humidified incubator containing 5% CO2.
Expression vectors for OPN and short hairpin RNA targeted at OPN (shOPN), as well as methods of cell transfection, were constructed as previously described (8). Twist small interfering RNA (siRNA) constructs were obtained from Sigma-Aldrich; Merck KGaA. The sequences of the primers used were as follows: Twist-siRNA forward, 5-gatccGCTGAGCAAGATTCAGACCttcaagagaGGTCTGAATCTTGCTCAGCttttta-3 and reverse, 3-gCGACTCGTTCTAAGTCTGGaagttctctCCAGACTTAGAACGAGTCGaaaaattcga-5 and Twist-siRNA-scramble forward, 5-gatccCGGTAACACAGACTGCAGTttcaagagaACTGCAGTCTGTGTTACCGttttta-3 and reverse, 3-gGCCATTGTGTCTGACGTCAaagttctctGCCATTGTGTCTGACGTCAaaaaattcga-5. Recombinant plasmids were prepared as described previously (34). Then, 10 µg plasmids were transfected into the MHCC-97L cells using lipofectamine 2000 (cat no. 11668019; Thermo Fisher Scientific, Inc.). Subsequently, the cells were collected after 24 h and cells were cleaved to extract protein for western blot analysis.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from HCLM3, MHCC97H, MHCC97L and L02 cell lines (that had not undergone transfection) and frozen tumor specimens using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA (1 µg) was reverse transcribed using PrimeScript® Reverse Transcriptase Master mix (Takara Bio, Inc., Otsu, Japan) according to the manufacturer's protocols. The sequences of the primers used were as follows: Twist forward, 5-GTCCGCAGTCTTACGAGGAG-3 and reverse, 5-GCTTGAGGGTCTGAATCTTGCT-3; and β-actin forward, 5-CATGTACGTTGCTATCCAGGC-3 and reverse, 5-CTCCTTAATGTCACGCACGAT-3. An AceQ® qPCR SYBR Green Master mix kit (Vazyme, Piscataway, NJ, USA) was used for qPCR. β-actin was used as the reference gene. Amplification and detection were perfor-med using the ABI PRISM® 7900HT Sequence Detection system (Applied Biosystems; Thermo Fisher Scientific, Inc.). Thermocycling conditions were as follows: 50°C for 2 min (required for optimal AmpErase UNG activity Applied Biosystems; Thermo Fisher Scientific, Inc.), template denaturation at 95°C for 10 min, 40 cycles of denaturation at 95°C for 15 sec, and combined primer annealing/elongation at 60°C for 1 min. The Twist level was normalized to β-actin to yield a 2−ΔΔCq value for relative expression of Twist (35).
Detection of protein by western blot analysis
Cells lysates were prepared as described previously (8). RIPA lysis buffer (cat no. P0013E; Beyotime Institute of Biotechnology, Haimen, China) was used for lysis. Protein concentrations were measured using a Bicinchoninic Acid Assay kit (Pierce; Thermo Fisher Scientific, Inc.). A total of 40 ug/ul of protein was separated using SDS-PAGE (5% concentration gel and 10% separation gel), according to protein mass and transferred onto polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA, USA). 5% skimmed milk was used to block polyvinylidene fluoride membranes at room temperature for one h. Proteins were then incubated with primary antibodies against OPN (cat no. sc-21742; dilution, 1:500; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and Twist (cat no. ab50581; dilution, 1:1,000; Abcam) at 4°C overnight. A mouse anti-human monoclonal antibody against GAPDH (cat no. 8884; dilution, 1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA) was used as an internal control. In addition, primary antibodies against Akt (cat no. ab8805; dilution, 1:1,000; Abcam), p-Akt (cat no. ab38449; dilution, 1:1,000; Abcam), matrix metalloproteinase 2 (MMP2) (cat no. ab37150; dilution, 1:1,000; Abcam) and urokinase (uPA) (cat no. ab82220; dilution, 1:1,000; Abcam) were used to analyze the mechanism of EMT. Secondary antibodies were goat anti-rabbit IgG (dilution, 1:5,000; cat no. ab6721; Abcam) and goat anti-mouse IgG (dilution, 1:5,000; cat no. ab6789; Abcam). We used the ECL Western Blotting Detection Kit (Thermo Fisher Scientific, Inc) to detect immobilized specific antigens in chemiluminescent Western blots through horseradish peroxidase (HRP) labeled antibodies. The bands were quantified using ImageJ v.2.0 software (National Institutes of Health, Bethesda, MD, USA). In order to improve the accuracy of the present study, each experiment was repeated ≥3 times.
Chemicals
LY294002 (cat no. s1105; Selleck Chemicals, Houston, TX, USA), which is able to inhibit Akt activation to assess whether PI3K/Akt signaling was involved in OPN-mediated metastasis. MHCC 97L cells with OPN overexpression were harvested after 1 h incubation with 50 umol/l PI3K/Akt inhibitor LY294002 (Selleck Chemicals) to suppress Akt activation, and collected cells to extract protein for western blot analysis.
Statistical analysis
Statistical analyses were performed using SPSS 15.0 (SPSS, Inc., Chicago, IL, USA). The Kaplan-Meier method was used to create survival and recurrence curves and to estimate OS and TTR. The significance of OS and TTR was determined using the log-rank test. Fisher's exact and χ2 tests were used to demonstrate clinicopathological association. Univariate and multivariate analyses were performed using Cox's proportional hazards model. Values are expressed as the mean ± standard deviation. All statistical tests were two-sided and P<0.05 was considered to indicate a statistically significant difference. For OPN or Twist density, the cut-off for the definition of subgroups was the median value. Samples were separated into two groups for each analysis. The first group was comprised of HCC with OPN and/or Twist levels exceeding the median value, and the second group comprised the rest. Each data set was analyzed separately.
Results
OPN enhances Twist expression in HCC cell lines
Recent studies have demonstrated that OPN may induce EMT in HCC (8,36). To determine the effects of OPN on the EMT regulator Twist in HCC metastasis, the expression of Twist was analyzed in HCC cell lines with downregulated or upregulated expression of OPN. Twist expression was elevated when OPN was overexpressed in the MHCC-97L cell line, which is usually not highly metastatic and exhibits decreased levels of OPN (Fig. 1A). However, downregulation of OPN in highly metastatic the HCC MHCC97-H and HCC-LM3 cell lines markedly reduced Twist expression (Fig. 1B). Therefore, we hypothesized that OPN may serve an important role in Twist expression and therefore, may affect the metastasis of liver cancer.
Figure 1.
OPN enhances Twist expression in HCC cell lines. (A) Western blot analysis demonstrates the effect of OPN overexpression on the expression of Twist in HCC cell lines. (B) Western blot analysis demonstrates the effect of knockdown of OPN on the expression of Twist in HCC cell lines. (C) Top panel represents the mRNA expression of Twist by RT-qPCR, and the bottom panel is the protein expression of Twist by western blot analysis. The mRNA levels of Twist in HCC cell lines with different metastatic potentials, as determined by reverse transcription-quantitative polymerase chain reaction. Western blot analysis also demonstrated that Twist expression was increased in human HCC cell lines with increasing metastatic potentials. (D) EMT-associated markers were detected in the MHCC-97L-OPN cell line transfected with siTwist or scrambled siRNA. OPN, osteopontin; HCC, hepatocellular carcinoma; EMT, epithelial-mesenchymal transition; siRNA, small interfering RNA; siTwist, siRNA targeted at Twist; NC, negative control; shOPN, short hairpin RNA targeted at OPN.
To evaluate the association between OPN, Twist and HCC metastasis, Twist levels were detected in a panel of human HCC cell lines with different metastatic potentials. Expression levels of Twist protein and mRNA were substantially increased in three established HCC cell lines compared with the non-transformed hepatic L-02 cell line (Fig. 1C). Additionally, the expression levels of Twist in the highly metastatic HCC MHCC97-H and HCC-LM3 cell lines, which exhibit increased levels of OPN, were much higher than that in the HCC MHCC97-L cell line, which is not highly metastatic and exhibits a low expression of OPN. These data indicated that expression of Twist is upregulated in HCC cell lines and that this increased expression is positively associated with the malignant phenotype of HCC cells.
The present study also aimed to determine whether or not Twist is involved in OPN-induced EMT. EMT markers were detected in the lowly metastatic HCC MHCC97-L cell line, which exhibits stable overexpression of OPN when transfected with a siRNA targeted at Twist (siTwist) or scrambled siRNA. Knockdown of Twist significantly reduced the expression of N-cadherin and increased the expression of E-cadherin (Fig. 1D). These results demonstrated that Twist is required for OPN-driven EMT.
OPN increases the expression of Twist via the PI3K/Akt signaling pathway
Previous studies have elucidated the mechanisms of metastasis induced by OPN, including the role of the mitogen-activated protein kinase, nuclear factor-κB and the PI3K/Akt pathways (9,37). The PI3K/Akt pathway is crucial in promoting invasion and metastasis and has been documented to be involved in the EMT of several types of human cancer (38–40). To assess whether PI3K/Akt signaling was involved in OPN-mediated metastasis, the present study examined the effect of OPN on the activation of PI3K/Akt. Phosphorylation of Akt was notably enhanced by OPN overexpression, whereas knockdown of OPN significantly decreased the phosphorylation of Akt (Fig. 2A and B). Consistently, suppression of Akt activation by the specific PI3K/Akt inhibitor LY294002 markedly attenuated the expression of OPN-induced Twist (Fig. 2C). These results indicated that the PI3K/Akt pathway is critical in the increased expression of Twist induced by OPN.
Figure 2.
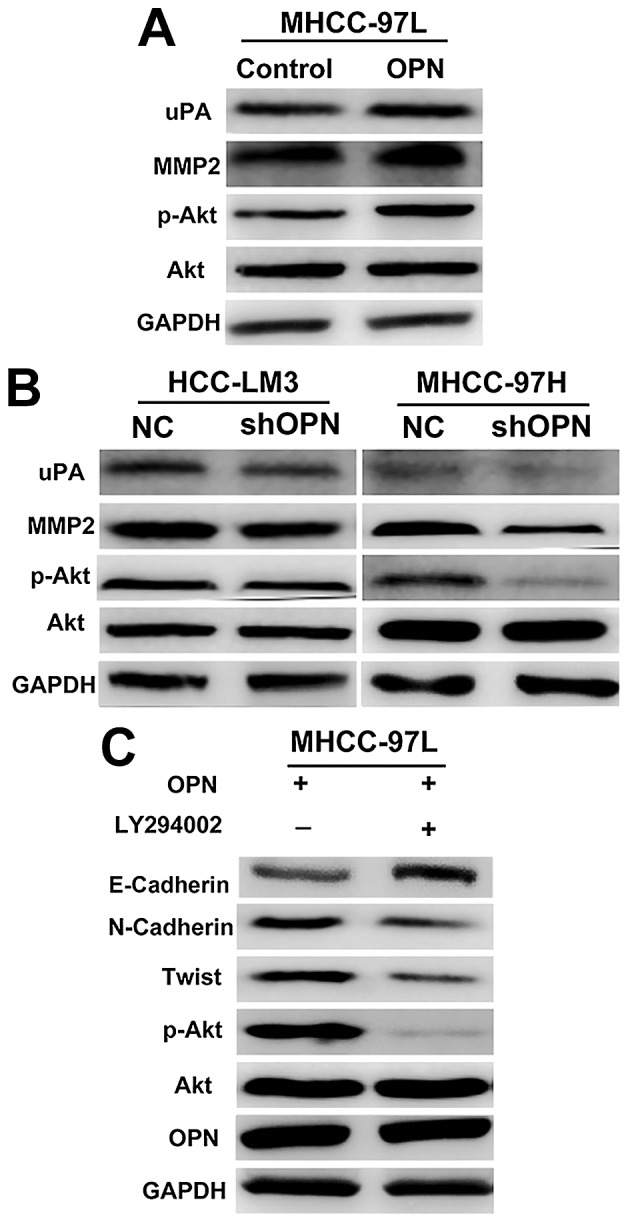
OPN induced the expression of Twist through the PI3K/Akt signaling pathway. (A) Western blot analysis demonstrates the effect of overexpression of OPN on the protein levels PI3K and Akt in HCC cells. (B) Western blot analysis depicting the effect of knockdown of OPN on the protein levels of PI3K and Akt in HCC cells. (C) Suppression of Akt activation by the specific PI3K/Akt inhibitor LY294002 markedly attenuated OPN-elicited EMT. OPN, osteopontin; PI3K, phosphoinositide 3-kinase; Akt, RAC serine/threonine-protein kinase; HCC, hepatocellular carcinoma; EMT, epithelial-mesenchymal transition; NC, negative control; uPA, urokinase; MMP2, matrix metalloproteinase 2; p-Akt, phosphorylated Akt; shOPN, short hairpin RNA targeted at OPN.
To investigate whether OPN mediated metastasis via the PI3K/Akt/Twist pathway, genes associated with metastasis, including MMP2 and uPA, were further investigated. As demonstrated in Fig. 2A, MMP2 and uPA were upregulated in MHCC-97L cells stably overexpressing OPN, but were markedly decreased in cells transfected with shOPN (Fig. 2B). Taken together, these results suggested that OPN activates PI3K/Akt signaling, which increases the expression of Twist and the metastasis of HCC cells.
OPN and Twist expression detected by TMA and IHC staining
To evaluate the potential role of Twist in HCC, the expression levels of Twist in 192 human HCC tissues were identified by IHC analyses. Higher Twist levels were detected in tumor tissues than in their paired non-cancerous liver tissues (Fig. 3A and B). The clinical significance of Twist was further investigated using TMAs containing HCC tissues from 374 patients. IHC staining revealed that high expression of Twist was significantly associated with the vascular invasion of HCC (P=0.013; Table I), whereas no significant association was observed between Twist density and other clinicopathological characteristics of HCC patients. Next, the association between OPN and Twist expression in human HCC tissues were analyzed. On the basis of the IHC results, TMA analysis of HCC specimens revealed that OPN expression was positively associated with Twist expression in the HCC samples (P<0.001; Fig. 3C and D).
Figure 3.
Twist expression detected by TMA and immunohistochemical staining. (A) Different expression statuses of Twist in tumor tissues and their paired non-cancerous liver tissues. (B) Immunohistochemical analyses demonstrated markedly increased Twist levels in tumor tissues compared with their paired non-cancerous liver tissues. *P<0.05, **P<0.01. (C) OPN and Twist, detected by immunohistochemical staining in consecutive sections of HCC tissues from the same patient. (D) OPN is significantly associated with Twist expression, as determined by immunohistochemistry in a TMA constructed from HCC tumor samples. **P<0.01. TMA, tissue microarray; OPN, osteopontin; HCC, hepatocellular carcinoma; IHC, immunohistochemistry.
Prognostic value of Twist in HCC patients
Using the integrated optical density median value as the cut-off value, the 374 HCC patients were divided into two groups. The OS time of HCC patients with high Twist expression was significantly lower than that of the patients with low Twist expression (P=0.041; Fig. 4A), whereas the TTR of the high-Twist-expression group was significantly higher than that of the low-Twist-expression group (P=0.017; Fig. 4B). The OS and TTR times of the patients in group I were markedly longer than those those of the patients in group IV (Fig. 4C and D).
Figure 4.
Prognostic value of Twist in HCC patients. Kaplan-Meier curves of (A) OS and (B) TTR in HCC patients expressing different levels of Twist. The patients with higher Twist levels exhibited significantly shorter OS and TTR times than patients with lower Twist levels. The patients of subgroup I had (C) the longest OS and (D) the lowest possibility of tumor recurrence, among the four subgroups, which were divided according to combinations of OPN and Twist expression (I, low OPN+low Twist; II, low OPN and high Twist; III, high OPN and low Twist; IV, high OPN and high Twist). HCC, hepatocellular carcinoma; OS, overall survival; TTR, time to recurrence; OPN, osteopontin.
Univariate and multivariate analyses of the prognostic value of Twist in HCC patients
To determine the prognostic value of Twist for HCC patients, univariate and multivariate analyses were performed on the clinicopathological characteristics and Twist expression levels of patients (Table II). Univariate analysis revealed that OPN expression, Twist expression, serum AFP level, hepatitis B surface antigen, tumor size, tumor capsulation, vascular invasion, Barcelona Clinic Liver Cancer stage (41) and tumor differentiation were significantly associated with the OS and TTR times of patients with HCC (Table II). However, no prognostic significance for OS or TTR was observed in association with the other characteristics, including sex, age, liver cirrhosis, ALT and tumor number. Individual characteristics that exhibited significance by univariate analysis were adopted as covariates in a multivariate Cox's proportional hazards model and combined variables were further analyzed. When OPN was combined with Twist, the combination of the two was a more potent independent prognostic indicator for OS (P=0.001) and TTR (P<0.001) than each factor alone.
Table II.
Univariate and multivariate analyses of factors associated with OS and TTR of HCC (n=374).
| OS | TTR | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Univariate analysis | ||||||
| OPN (high vs. low) | 1.52 | 1.13–2.04 | 0.005 | 1.42 | 1.09–1.86 | 0.009 |
| Twist (high vs. low) | 1.35 | 1.01–1.81 | 0.043 | 1.38 | 1.06–1.79 | 0.018 |
| Sex (female vs. male) | 1.06 | 0.72–1.55 | 0.779 | 1.13 | 0.76–1.70 | 0.548 |
| Age, years (>50 vs. ≤50) | 1.11 | 0.83–1.48 | 0.478 | 1.09 | 0.81–1.47 | 0.555 |
| ALT, U/l (≥75 vs. <75) | 1.09 | 0.68–1.73 | 0.722 | 1.04 | 0.65–1.68 | 0.874 |
| AFP, ng/ml (>20 vs. ≤20) | 1.66 | 1.21–2.28 | 0.002 | 1.47 | 1.08–2.02 | 0.016 |
| Liver cirrhosis (yes vs. no) | 1.42 | 0.85–2.38 | 0.179 | 1.58 | 0.93–2.68 | 0.092 |
| HBsAg (positive vs. negative) | 1.91 | 0.94–3.87 | 0.075 | 2.50 | 1.23–5.06 | 0.011 |
| Tumor size, cm (>5 vs. ≤5) | 1.64 | 1.18–2.29 | 0.003 | 1.55 | 1.09–2.21 | 0.015 |
| Tumor number (multiple vs. single) | 1.59 | 0.84–3.00 | 0.157 | 1.18 | 0.58–2.41 | 0.641 |
| Tumor capsule (none vs. complete) | 1.53 | 1.15–2.05 | 0.004 | 1.39 | 1.03–1.87 | 0.031 |
| Vascular invasion (yes vs. no) | 2.03 | 1.50–2.73 | <0.001 | 1.23 | 1.05–1.45 | 0.010 |
| BCLC stage (B and C vs. 0 and A) | 1.79 | 1.24–2.58 | 0.002 | 1.53 | 1.07–2.18 | 0.020 |
| Tumor differentiation (III–IV vs. I–II) | 1.57 | 1.14–2.16 | 0.006 | 1.43 | 1.02–2.00 | 0.037 |
| Combination of OPN and Twist | 0.006 | 0.004 | ||||
| II vs. I | 1.40 | 0.93–2.10 | 0.193 | 1.28 | 0.88–1.86 | 0.193 |
| III vs. I | 1.15 | 0.73–1.81 | 0.543 | 1.16 | 0.78–1.74 | 0.459 |
| IV vs. I | 1.92 | 1.31–2.82 | 0.001 | 1.87 | 1.32–2.65 | <0.001 |
| Multivariate analysis | ||||||
| AFP, ng/ml (>20 vs. ≤20) | 1.47 | 1.06–2.04 | 0.023 | 1.37 | 1.02–1.84 | 0.035 |
| Tumor size, cm (>5 vs. ≤5) | 1.43 | 1.00–2.04 | 0.051 | 1.47 | 1.02–2.10 | 0.088 |
| Tumor capsule (none vs. complete) | 1.45 | 1.08–1.95 | 0.013 | 1.38 | 1.06–1.80 | 0.018 |
| Vascular invasion (yes vs. no) | 1.43 | 1.01–2.02 | 0.042 | 1.30 | 0.95–1.79 | 0.104 |
| BCLC stage (B and C vs. 0 and A) | 1.38 | 0.90–2.09 | 0.138 | 1.46 | 1.00–2.11 | 0.048 |
| Tumor differentiation (III–IV vs. I–II) | 1.19 | 0.85–1.67 | 0.320 | 1.14 | 0.83–1.56 | 0.419 |
| Combination OPN and Twist | 0.038 | 0.019 | ||||
| II vs. I | 1.40 | 0.93–2.13 | 0.111 | 1.27 | 0.87–1.86 | 0.223 |
| III vs. I | 1.16 | 0.74–1.83 | 0.525 | 1.18 | 0.79–1.76 | 0.430 |
| IV vs. I | 1.77 | 1.19–2.65 | 0.005 | 1.77 | 1.23–2.54 | 0.002 |
Univariate and multivariate analyses were performed using Cox's proportional hazards regression model. Variables were adopted in multivariate analysis based on their prognostic significance by univariate analysis. CI, confidence interval; HR, hazard ratio; OS, overall survival; TTR, time to recurrence; OPN, osteopontin; ALT, alanine amino transferase; AFP, α-fetoprotein; HBsAg, hepatitis B surface antigen; BCLC, Barcelona Clinic Liver Cancer. I, low OPN and Twist; II, low OPN and high Twist; III, high OPN and low Twist; IV, high OPN and Twist.
Discussion
It is well-known that liver cancer is associated with a high mortality and primarily occurs in less developed countries (1,2). Although patients with HCC exhibit significantly improved survival times following curative resection, the prognosis of these patients remains poor owing to tumor invasiveness and metastasis (42). Therefore, identifying more accurate prognostic biomarkers is of great clinical value, allowing for further understanding of HCC and the development of novel therapeutic strategies.
EMT is a major step in tumor metastasis (10,43). This process is regulated by major EMT regulators, including Twist, Snail and Slug, and is initiated by suppression of E-cadherin expression (10). Twist serves an important role through its regulation of E-cadherin expression in human cancer. The role of Twist in cancer metastasis was first reported in study on a breast cancer model, the results of which indicating that Twist induced EMT, resulting in the promotion of tumor invasion (13). Twist has been revealed to be associated with metastasis in various types of cancer, including HCC, through the induction of EMT changes and cancer invasiveness (23). The present study detected an association between Twist expression and OPN expression in HCC cell lines and in patients with HCC. The results of the present study demonstrated that Twist expression was induced by OPN in HCC. This finding was further confirmed using TMA, which revealed that OPN overexpression in HCC tumor tissues was associated with Twist expression. Additionally, Twist expression was observed in various HCC cell lines with different metastatic potentials. Twist was overexpressed in metastatic cell lines compared with non-metastatic primary cell lines. The TMA immunohistochemical assay performed in the present study also supported the hypothesis that Twist expression was positively associated with metastasis in HCC tumor tissues. Therefore, these data indicated that OPN serves a crucial role in the induction of HCC progression through the regulation of Twist expression.
A further important finding from the present study is that the hyper-activation of the PI3K/Akt signaling pathway is responsible for the progression of HCC cells induced by OPN. Twist is the most important transcription factor in the negative regulation of E-cadherin expression and the EMT of epithelial cells. Evidence indicates that Twist phosphorylation is predominantly regulated by the PI3K/Akt pathway (44). An increase in Akt signaling is a key tumor survival mechanism that promotes tumor metastatic processes. A previous study (45) have demonstrated that activated Akt serves a critical role in hematogenous intrahepatic metastasis in an orthotopic implantation model of HCC. The present study revealed that, through the activation of PI3K/Akt signaling induced by OPN, the level of Twist increased correspondingly, which led to the downregulation of E-cadherin. This concept was further supported by the observation that knockdown of OPN markedly reduced OPN-induced Akt activation. When HCC cell lines stably overexpressing OPN were incubated with LY294002, a PI3K inhibitor, the expression of Twist and N-cadherin was markedly reduced. Taken together, these results indicated that OPN activates PI3K/Akt/Twist signaling, which promotes the metastasis of HCC cells. To the best of our knowledge, the present study is the first to document a link among OPN, PI3K/Akt and Twist in human HCC.
Previous studies identified that OPN was associated with aggressive and metastatic HCC phenotypes, and with poor patient prognosis (46–48). The results of the present study indicated that patients with high Twist expression exhibit significantly shorter OS and TTR times than patients with low Twist expression. Additionally, according to univariate and multivariate Cox proportional hazards regression analyses, the predictive range of OPN expression levels combined with those of Twist was more sensitive than that of OPN alone for OS and TTR. Taken together, the results of the present study clearly demonstrate that a combination of OPN and Twist expression levels may serve as a powerful prognostic indicator of HCC.
In conclusion, the data presented in the current study revealed that Twist was markedly upregulated in HCC patients and that high expression of Twist was associated with poor patient prognosis. Additionally, the expression of Twist, as a major regulator of EMT, was regulated by OPN though the PI3K/Akt signaling pathway. These findings indicate that OPN and Twist may serve as synergistic indicators for HCC patients following curative resection. Therefore, Twist may be a potential therapeutic target to inhibit HCC metastasis in patients with high OPN expression.
Acknowledgements
Not applicable.
Funding
The present study was supported by the China National Natural Science Foundation (grant nos. 81772563, 81372647 and 81472672), the National Key Basic Research Program of China (grant no. 2013CB910500) and the China National Key Projects for Infectious Diseases (grant no. 2012ZX10002-012).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
XXY, YZ, XCZ and XMG designed and performed the experiments, analyzed data. CQW, YYS and WC participated in collecting the samples and correcting patient sample's following-up investigation data. XXY and YZ performed bioinformatics analyses. NR and LXQ participated in data interpretation and provided valuable discussions with regard to clinical correlates. QZD and HLJ designed and supervised the entire project, designed the experiments, and prepared the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Research Ethics Committee of Zhongshan Hospital, Fudan University (Shanghai, China). Clinical samples were collected from these patients after obtaining informed consent according to an established protocol approved by committee's regulations. The data did not contain any information that could lead to patient identification.
Patient consent for publication
Written informed consent for publication was obtained from all participants.
Competing interests
The authors have declared that no competing interests exist.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Yang Y, Nagano H, Ota H, Morimoto O, Nakamura M, Wada H, Noda T, Damdinsuren B, Marubashi S, Miyamoto A, Miyamoto A, et al. Patterns and clinicopathologic features of extrahepatic recurrence of hepatocellular carcinoma after curative resection. Surgery. 2007;141:196–202. doi: 10.1016/j.surg.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 4.Tang ZY, Ye SL, Liu YK, Qin LX, Sun HC, Ye QH, Wang L, Zhou J, Qiu SJ, Li Y, et al. A decade's studies on metastasis of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:187–196. doi: 10.1007/s00432-003-0511-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wai PY, Kuo PC. Osteopontin: Regulation in tumor metastasis. Cancer Metastasis Rev. 2008;27:103–118. doi: 10.1007/s10555-007-9104-9. [DOI] [PubMed] [Google Scholar]
- 6.Tuck AB, Chambers AF, Allan AL. Osteopontin overexpression in breast cancer: Knowledge gained and possible implications for clinical management. J Cell Biochem. 2007;102:859–868. doi: 10.1002/jcb.21520. [DOI] [PubMed] [Google Scholar]
- 7.McAllister SS, Gifford AM, Greiner AL, Kelleher SP, Saelzler MP, Ince TA, Reinhardt F, Harris LN, Hylander BL, Repasky EA, et al. Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell. 2008;133:994–1005. doi: 10.1016/j.cell.2008.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong QZ, Zhang XF, Zhao Y, Jia HL, Zhou HJ, Dai C, Sun HJ, Qin Y, Zhang WD, Ren N, et al. Osteopontin promoter polymorphisms at locus-443 significantly affect the metastasis and prognosis of human hepatocellular carcinoma. Hepatology. 2013;57:1024–1034. doi: 10.1002/hep.26103. [DOI] [PubMed] [Google Scholar]
- 9.Bhattacharya SD, Mi Z, Kim VM, Guo H, Talbot LJ, Kuo PC. Osteopontin regulates epithelial mesenchymal transition-associated growth of hepatocellular cancer in a mouse xenograft model. Ann Surg. 2012;255:319–325. doi: 10.1097/SLA.0b013e31823e3a1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang J, Weinberg RA. Epithelial-mesenchymal transition: At the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Wang SH, Wu XC, Zhang MD, Weng MZ, Zhou D, Quan ZW. Upregulation of H19 indicates a poor prognosis in gallbladder carcinoma and promotes epithelial-mesenchymal transition. Am J Cancer Res. 2015;6:15–26. [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Q, Han J, Fan J, Duan L, Guo M, Lv Z, Hu G, Chen L, Wu F, Tao X, et al. IL-17 induces EMT via Stat3 in lung adenocarcinoma. Am J Cancer Res. 2016;6:440–451. [PMC free article] [PubMed] [Google Scholar]
- 13.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Yang MH, Wu KJ. TWIST activation by hypoxia inducible factor-1 (HIF-1): Implications in metastasis and development. Cell Cycle. 2008;7:2090–2096. doi: 10.4161/cc.7.14.6324. [DOI] [PubMed] [Google Scholar]
- 15.Cheng GZ, Zhang W, Wang LH. Regulation of cancer cell survival, migration, and invasion by Twist: AKT2 comes to interplay. Cancer Res. 2008;68:957–960. doi: 10.1158/0008-5472.CAN-07-5067. [DOI] [PubMed] [Google Scholar]
- 16.Cheng GZ, Chan J, Wang Q, Zhang W, Sun CD, Wang LH. Twist transcriptionally up-regulates AKT2 in breast cancer cells leading to increased migration, invasion, and resistance to paclitaxel. Cancer Res. 2007;67:1979–1987. doi: 10.1158/0008-5472.CAN-06-1479. [DOI] [PubMed] [Google Scholar]
- 17.Kwok WK, Ling MT, Lee TW, Lau TC, Zhou C, Zhang X, Chua CW, Chan KW, Chan FL, Glackin C, et al. Up-regulation of TWIST in prostate cancer and its implication as a therapeutic target. Cancer Res. 2005;65:5153–5162. doi: 10.1158/0008-5472.CAN-04-3785. [DOI] [PubMed] [Google Scholar]
- 18.Hoek K, Rimm DL, Williams KR, Zhao H, Ariyan S, Lin A, Kluger HM, Berger AJ, Cheng E, Trombetta ES, et al. Expression profiling reveals novel pathways in the transformation of melanocytes to melanomas. Cancer Res. 2004;64:5270–5282. doi: 10.1158/0008-5472.CAN-04-0731. [DOI] [PubMed] [Google Scholar]
- 19.Entz-Werle N, Stoetzel C, Berard-Marec P, Kalifa C, Brugiere L, Pacquement H, Schmitt C, Tabone MD, Gentet JC, Quillet R, et al. Frequent genomic abnormalities at TWIST in human pediatric osteosarcomas. Int J Cancer. 2005;117:349–355. doi: 10.1002/ijc.21068. [DOI] [PubMed] [Google Scholar]
- 20.van Doorn R, Dijkman R, Vermeer MH, Out-Luiting JJ, van der Raaij-Helmer EM, Willemze R, Tensen CP. Aberrant expression of the tyrosine kinase receptor EphA4 and the transcription factor twist in Sezary syndrome identified by gene expression analysis. Cancer Res. 2004;64:5578–5586. doi: 10.1158/0008-5472.CAN-04-1253. [DOI] [PubMed] [Google Scholar]
- 21.Rosivatz E, Becker I, Specht K, Fricke E, Luber B, Busch R, Höfler H, Becker KF. Differential expression of the epithelial-mesenchymal transition regulators Snail, SIP1, and twist in gastric cancer. Am J Pathol. 2002;161:1881–1891. doi: 10.1016/S0002-9440(10)64464-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang J, Hou Y, Zhou M, Wen S, Zhou J, Xu L, Tang X, Du YE, Hu P, Liu M. Twist induces epithelial-mesenchymal transition and cell motility in breast cancer via ITGB1-FAK/ILK signaling axis and its associated downstream network. Int J Biochem Cell Biol. 2016;71:62–71. doi: 10.1016/j.biocel.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Lee TK, Poon RT, Yuen AP, Ling MT, Kwok WK, Wang XH, Wong YC, Guan XY, Man K, Chau KL, Fan ST. Twist overexpression correlates with hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clin Cancer Res. 2006;12:5369–5376. doi: 10.1158/1078-0432.CCR-05-2722. [DOI] [PubMed] [Google Scholar]
- 24.Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, Xu Y, Li YW, Tang ZY. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586–2593. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 25.Yang MH, Chen CL, Chau GY, Chiou SH, Su CW, Chou TY, Peng WL, Wu JC. Comprehensive analysis of the independent effect of twist and snail in promoting metastasis of hepatocellular carcinoma. Hepatology. 2009;50:1464–1474. doi: 10.1002/hep.23221. [DOI] [PubMed] [Google Scholar]
- 26.Marrero JA, Fontana RJ, Barrat A, Askari F, Conjeevaram HS, Su GL, Lok AS. Prognosis of hepatocellular carcinoma: Comparison of 7 staging systems in an American cohort. Hepatology. 2005;41:707–716. doi: 10.1002/hep.20636. [DOI] [PubMed] [Google Scholar]
- 27.Llovet JM, Fuster J, Bruix J, Barcelona-Clinic Liver Cancer Group The Barcelona approach: Diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl. 2004;10(2 Suppl 1):S115–S120. doi: 10.1002/lt.20034. [DOI] [PubMed] [Google Scholar]
- 28.Sun HC, Zhang W, Qin LX, Zhang BH, Ye QH, Wang L, Ren N, Zhuang PY, Zhu XD, Fan J, Tang ZY. Positive serum hepatitis B e antigen is associated with higher risk of early recurrence and poorer survival in patients after curative resection of hepatitis B-related hepatocellular carcinoma. J Hepatol. 2007;47:684–690. doi: 10.1016/j.jhep.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 29.Qian YB, Zhang JB, Wu WZ, Fang HB, Jia WD, Zhuang PY, Zhang BH, Pan Q, Xu Y, Wang L, et al. P48 is a predictive marker for outcome of postoperative interferon-alpha treatment in patients with hepatitis B virus infection-related hepatocellular carcinoma. Cancer. 2006;107:1562–1569. doi: 10.1002/cncr.22206. [DOI] [PubMed] [Google Scholar]
- 30.Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology. 2008;48:1312–1327. doi: 10.1002/hep.22506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh PP, Shi Q, Foster NR, Grothey A, Nair SG, Chan E, Shields AF, Goldberg RM, Gill S, Kahlenberg MS, et al. Relationship between metformin use and recurrence and survival in patients with resected Stage III colon cancer receiving adjuvant chemotherapy: Results from north central cancer treatment group N0147 (Alliance) Oncologist. 2016;21:1509–1521. doi: 10.1634/theoncologist.2016-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu XD, Zhang JB, Zhuang PY, Zhu HG, Zhang W, Xiong YQ, Wu WZ, Wang L, Tang ZY, Sun HC. High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J Clin Oncol. 2008;26:2707–2716. doi: 10.1200/JCO.2007.15.6521. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Tian B, Yang J, Zhao L, Wu X, Ye SL, Liu YK, Tang ZY. Stepwise metastatic human hepatocellular carcinoma cell model system with multiple metastatic potentials established through consecutive in vivo selection and studies on metastatic characteristics. J Cancer Res Clin Oncol. 2004;130:460–468. doi: 10.1007/s00432-004-0564-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tian J, Tang ZY, Ye SL, Liu YK, Lin ZY, Chen J, Xue Q. New human hepatocellular carcinoma (HCC) cell line with highly metastatic potential (MHCC97) and its expressions of the factors associated with metastasis. Br J Cancer. 1999;81:814–821. doi: 10.1038/sj.bjc.6690769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 36.Dong Q, Zhu X, Dai C, Zhang X, Gao X, Wei J, Sheng Y, Zheng Y, Yu J, Xie L, et al. Osteopontin promotes epithelial-mesenchymal transition of hepatocellular carcinoma through regulating vimentin. Oncotarget. 2016;7:12997–13012. doi: 10.18632/oncotarget.7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun BS, Dong QZ, Ye QH, Sun HJ, Jia HL, Zhu XQ, Liu DY, Chen J, Xue Q, Zhou HJ, et al. Lentiviral-mediated miRNA against osteopontin suppresses tumor growth and metastasis of human hepatocellular carcinoma. Hepatology. 2008;48:1834–1842. doi: 10.1002/hep.22531. [DOI] [PubMed] [Google Scholar]
- 38.Fu J, Chen Y, Cao J, Luo T, Qian YW, Yang W, Ren YB, Su B, Cao GW, Yang Y, et al. p28GANK overexpression accelerates hepatocellular carcinoma invasiveness and metastasis via phosphoinositol 3-kinase/AKT/hypoxia-inducible factor-1α pathways. Hepatology. 2011;53:181–192. doi: 10.1002/hep.24015. [DOI] [PubMed] [Google Scholar]
- 39.Hua Z, Gu X, Dong Y, Tan F, Liu Z, Thiele CJ, Li Z. PI3K and MAPK pathways mediate the BDNF/TrkB-increased metastasis in neuroblastoma. Tumour Biol. 2016;37:16227–16236. doi: 10.1007/s13277-016-5433-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang C, Wang Y, Feng Y, Zhang Y, Ji B, Wang S, Sun Y, Zhu C, Zhang D, Sun Y. Gli1 promotes colorectal cancer metastasis in a Foxm1-dependent manner by activating EMT and PI3K-AKT signaling. Oncotarget. 2016;7:86134–86147. doi: 10.18632/oncotarget.13348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kikuchi L, Chagas AL, Alencar RSSM, Tani C, Diniz MA, D'Albuquerque LAC, Carrilho FJ. Adherence to BCLC recommendations for the treatment of hepatocellular carcinoma: Impact on survival according to stage. Clinics (Sao Paulo) 2017;72:454–460. doi: 10.6061/clinics/2017(08)01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huo TI, Lin HC, Huang YH, Wu JC, Chiang JH, Lee PC, Lee SD. The model for end-stage liver disease-based Japan Integrated Scoring system may have a better predictive ability for patients with hepatocellular carcinoma undergoing locoregional therapy. Cancer. 2006;107:141–148. doi: 10.1002/cncr.21972. [DOI] [PubMed] [Google Scholar]
- 43.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 44.Li CW, Xia W, Lim SO, Hsu JL, Huo L, Wu Y, Li LY, Lai CC, Chang SS, Hsu YH, et al. AKT1 Inhibits Epithelial-to-Mesenchymal transition in breast cancer through phosphorylation-dependent Twist1 degradation. Cancer Res. 2016;76:1451–1462. doi: 10.1158/0008-5472.CAN-15-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakanishi K, Sakamoto M, Yasuda J, Takamura M, Fujita N, Tsuruo T, Todo S, Hirohashi S. Critical involvement of the phosphatidylinositol 3-kinase/Akt pathway in anchorage-independent growth and hematogeneous intrahepatic metastasis of liver cancer. Cancer Res. 2016;62:2971–2975. [PubMed] [Google Scholar]
- 46.Huang H, Zhang XF, Zhou HJ, Xue YH, Dong QZ, Ye QH, Qin LX. Expression and prognostic significance of osteopontin and caspase-3 in hepatocellular carcinoma patients after curative resection. Cancer Sci. 2010;101:1314–1319. doi: 10.1111/j.1349-7006.2010.01524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ye QH, Qin LX, Forgues M, He P, Kim JW, Peng AC, Simon R, Li Y, Robles AI, Chen Y, et al. Predicting hepatitis B virus-positive metastatic hepatocellular carcinomas using gene expression profiling and supervised machine learning. Nat Med. 2003;9:416–423. doi: 10.1038/nm843. [DOI] [PubMed] [Google Scholar]
- 48.Zhang H, Ye QH, Ren N, Zhao L, Wang YF, Wu X, Sun HC, Wang L, Zhang BH, Liu YK, et al. The prognostic significance of preoperative plasma levels of osteopontin in patients with hepatocellular carcinoma. J Cancer Res Clin Oncol. 2006;132:709–717. doi: 10.1007/s00432-006-0119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.





