Abstract
The present study aimed to identify key pathways and genes in the pathogenesis of lung cancer. The GSE10072 dataset was downloaded from the Gene Expression Omnibus database. Protein-protein interaction data were collected from Human Protein Reference Database, and 201 pathways were downloaded from the Kyoto Encyclopedia of Genes and Genomes database. Signaling network impact analysis was performed to identify enriched pathways, followed by the construction of a pathway-pathway crosstalk network. Benzopyrene was used to treat normal human lung cells at concentrations of 0.01, 0.1, 1 and 10 µM, and cell viability was measured. Furthermore, growth arrest and DNA damage inducible β (GADD45B), p53, cyclin B, Akt and nuclear factor (NF)-κB protein levels were also measured via western blotting. Impact analysis identified 11 enriched lung cancer-associated KEGG pathways, including ‘complement and coagulation cascades’, ‘ECM-receptor interaction’, ‘P53 signaling pathway’, ‘cell adhesion molecules’ and ‘focal adhesion’. In addition, cell cycle, ‘drug metabolism-cytochrome P450’, ‘metabolic pathways’, ‘pathways in cancer’, ‘focal adhesion’ and ‘antigen processing and presentation’ were central in the pathway-pathway cross-talk network. Furthermore, the upregulated gene GADD45B was associated with three of the pathways, including an activated pathway (‘MAPK signaling pathway’) and two repressed pathways (‘cell cycle’ and ‘P53 pathway’). Western blotting demonstrated that the expression of NF-κB, Akt and GADD45B increased over time in lung cells treated with benzopyrene, whereas the expression levels of cyclin B and P53 decreased. In conclusion, GADD45B may contribute to lung carcinogenesis via affecting the MAPK, P53 signaling and cell cycle pathways.
Keywords: lung cancer, signaling pathway, pathway-pathway crosstalk, cell viability, protein expression
Introduction
Lung cancer is one of the most common types of cancer (1). Lung cancer has two main histological types: Non-small cell lung cancer (NSCLC; 80.4%) and SCLC (16.8%) (2); however, the underlying mechanisms for the development of lung cancer are not yet completely characterized.
Accumulated genetic abnormalities are associated with cancer. Somatic mutations in a number of genes, including epidermal growth factor receptor (EGFR), P53, KRAS, BRAF, Erb-B2 receptor tyrosine kinase 2 (ERBB2), MET, serine/threonine kinase 11, PIK3CA and Parkin RBR E3 ubiquitin protein ligase, have been identified in patients with lung cancer (3,4). Gene amplifications, including of EGFR, ERBB2, MET, PIK3CA and NK2 homeobox 1, have also been detected in lung cancer (5). A number of single nucleotide polymorphisms (SNPs) are associated with lung cancer susceptibility, including in interleukin-1, cytochrome P450, a 5′SNP in the ERCC excision repair 6, chromatin remodeling factor gene and SNPs in the nicotinic acetylcholine receptor gene cluster on chromosome 15q25.1 (6–9). Genetic abnormalities have been identified in numerous pathways, including the Notch (10), EGFR (11), PI3K (12), phosphatase and tensin homolog/phospho-Akt/P53 (13), mitogen-activated protein kinase (MAPK) (14) and cell cycle pathways.
In the past decade, there has been a pervasive application of high-throughput molecular technologies, including microarrays, in lung cancer research (15–17), which has greatly enriched the knowledge of the pathogenesis of the disease, and may potentially provide markers for the prognosis and targeted therapy of lung cancer. By enrolling 105 subjects in an Environment And Genetics in Lung cancer Etiology study (http://dceg.cancer.gov/eagle), Landi et al (18) produced a microarray dataset that included 107 expression values from tumor (n=58) and non-tumor tissues (n=49) from 74 subjects (non-smokers, n=20; former smokers, n=26; current smokers, n=28). Using this microarray analysis, 122 genes were identified that were differentially expressed between the tumor and non-tumor samples. In addition, several crucial smoking-associated genes and pathways were identified in the study, and a number of these, including Nima related kinase 2 and TTK protein kinase, were experimentally validated; however, the relationship between these genes and pathways were not considered in the original study.
In the present study, based on the microarray dataset produced by Landi et al (18), GSE10072, a novel pathway-pathway crosstalk approach was employed to identify pathways and genes that may have critical roles in the pathogenesis of lung cancer, and a number of the identified genes were experimentally validated.
Materials and methods
Source of pathway and microarray data
Protein-protein interaction data were downloaded from the Human Protein Reference Database (http://www.hprd.org/), and 201 lung cancer pathways were downloaded from the Kyoto Encyclopedia of Genes and Genomes (KEGG, http://www.kegg.jp/) database (19,20) using ‘lung cancer’ as the search term.
The raw data of the gene expression profile dataset GSE10072 in the ‘.CEL’ format were downloaded from the Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo/).
Identification of differentially expressed genes (DEGs)
The raw downloaded data were preprocessed and normalized using the R/Bioconductor package Affy with the Robust Multichip Average method for single-channel Affymetrix chips (21). A one-way analysis of variance was applied to each probe set to identify those that significantly changed expression level over time, as previously described (22). P<0.05 was considered to indicate a statistically significant result; the raw P-value was adjusted with the Bonferroni method (23).
Impact analysis
The pathway impact analysis as described by Draghici et al (24) was adopted, which considers the statistical significance of the enrichment of KEGG pathways, while also considering other crucial factors, including the magnitude of expression change for each gene, the topology of the signaling pathway, and the interactions between signaling pathways.
Construction of pathway-pathway crosstalk network
A hyper geometric distribution framework was applied to evaluate the significance of all non-empty intersections between two pathways, as previously described (25): Fisher's exact test computed the probability, p* using hyper geometric distribution with the parameters (S, NG, N).
Where α was the number of DEGs in the pathway intersection; S, the number of DEGs in the pathway union; NG, the number of genes in the pathway intersection; and N, the number of genes in the pathway union.
The P-value to reject the null hypothesis with a probability of <p* was calculated using the sum of the probabilities with the same marginal totals, i.e.:
This procedure gave a two-tailed probability for a Fisher's exact test. P<0.05 was considered to indicate a statistically significant result, indicative of the association of two pathways.
Analysis of cross-talk between pathways
The state of a pathway was initially determined. A pathway was considered to be activated when it met the following criteria: i) Number of DEGs in the pathway >10; ii) Q-value [(number of upregulated DEGs in the pathway-number of downregulated DEGs in the pathway)/total number of genes in the pathway] >0.5. A repressed pathway met the two criteria: i) Number of DEGs in this pathway >10; ii) Q-value <-0.5. Next, pairs of pathways sharing common DEGs were identified, and these DEGs were listed.
Cell culture and treatment
Normal lung K562 cells were purchased from the American Type Culture Collection (Manassas, VA, USA). The cells were grown in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% heat-inactivated fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and 100 U/ml penicillin and streptomycin, in a humidified atmosphere with 5% CO2 at 37°C. To maintain drug resistance, adriamycin was supplemented at regular intervals for 2 weeks prior to any experiment. Benzopyrene was used to treat the cells at concentrations of 0.01, 0.1, 1 and 10 µM in the subsequent assays.
Measurement of cell viability
Cell proliferation was measured using an MTT assay. Cells (2×105 cells/ml) were seeded in 96-well plates with increasing concentrations of adriamycin, SNX-2112 and 17-AAG. After incubation at 37°C for 24, 48 and 72 h, 5 mg/ml MTT solution was added for incubation for 4 h. Then, 100 µl/well DMSO was added to solubilize the formazan crystals. Cell viability was assessed by measuring absorbance at 570 nm using a microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Western blot analysis
Cells were first lysed in lysis buffer (Sangon Biotech Co., Ltd., Shanghai, China) and then total protein was extracted. Followed by protein concentration was measured by the bicinchoninic acid method. Equal aliquots (20 µg protein per lane) of protein lysate were separated by 8–12% SDS-PAGE and transferred to a polyvinylidene difluoride membrane. Subsequent to blocking with 5% skimmed milk at 37°C for 1 h, Western blots were probed with primary antibodies against nuclear factor (NF)-κB (1:1,000; cat no. ab32360; Abcam, Cambridge, MA, USA), Akt (1:1,000; cat no. ab126811; Abcam), cyclin B (1:1,000; cat no. ab18221; Abcam), P53 (1:1,000; cat no. ab21985; Abcam), growth arrest and DNA damage inducible β (GADD45B) (1:1,000; cat no. ab128920; Abcam) and β-actin (1:1,000; cat no. ab6276; Abcam) overnight at 4°C. Three consecutive washes were performed for 10 min in PBS-Tween, followed by incubation with the alkaline phosphatase-conjugated goat anti-rabbit IgG secondary antibody (1:5,000; cat no. ab6722; Abcam) diluted in 5% skimmed milk at room temperature for 1 h. The immunoblots were visualized with enhanced chemiluminescence (GE Healthcare, Chicago, IL, USA) and autoradiography. The experiments were repeated three times, and the results were detected using the Image Lab software (version 4.1; Bio-Rad Laboratories, Inc.) on ChemiDoc MP imaging system (Bio-Rad Laboratories, Inc.).
Statistical analysis
All data were presented as the mean ± standard deviation. Statistical analysis was performed by one-way analysis of variance with a Bonferroni post hoc test in SPSS software (version 13.0; SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Identification of DEGs
Using a threshold of P<0.05, the genes with significantly differential expression were analyzed. A total of 1,763 DEGs were identified, of which 662 were associated with KEGG pathways.
Result of impact analysis
A total of 11 pathways were identified in the pathway impact analysis. Of these, ‘complement and coagulation cascades’, ‘ECM-receptor interaction’, ‘P53 signaling pathway’, ‘cell adhesion molecules (CAMs)’, ‘focal adhesion’ and ‘cell cycle’ were the top five pathways by impact factor value (Table I).
Table I.
Significantly enriched Kyoto Encyclopedia of Genes and Genomes pathways in GSE10072.
| Pathway | Impact factor | P-value |
|---|---|---|
| Complement and coagulation cascades | 17.258 | 2.08×10−7 |
| ECM-receptor interaction | 14.266 | 3.90×10−6 |
| P53 signaling pathway | 9.017 | 3.41×10−4 |
| Cell adhesion molecules | 70.734 | 4.42×10−4 |
| Focal adhesion | 9.283 | 1.17×10−3 |
| Cell cycle | 6.673 | 3.36×10−3 |
| Renin-angiotensin system | 5.778 | 8.30×10−3 |
| PPAR signaling pathway | 5.733 | 1.54×10−2 |
| TGF-beta signaling pathway | 6.352 | 2.24×10−2 |
| Leukocyte transendothelial migration | 114.039 | 2.34×10−2 |
| migration Tight junction | 6.133 | 4.85×10−2 |
Analysis of cross-talk between pathways
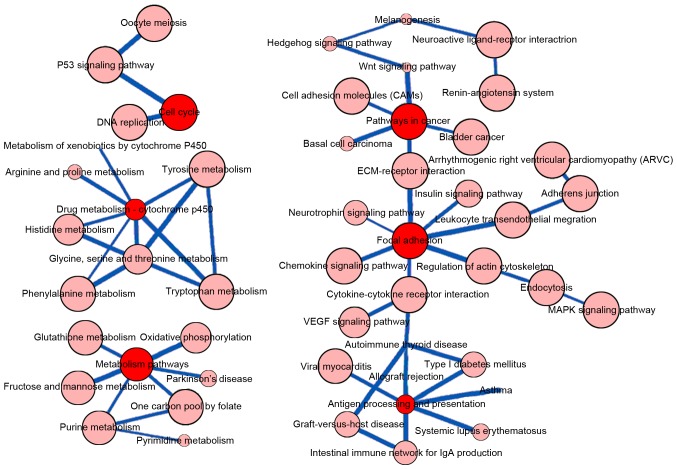
It was determined that the ‘cell cycle’, ‘drug metabolism-cytochrome P450’, ‘metabolic pathways’, ‘pathways in cancer’, ‘focal adhesion’ and ‘antigen processing and presentation’ KEGG pathways were central in the pathway-pathway crosstalk network (Fig. 1).
Figure 1.
Network of pathway-pathway crosstalk for the GSE10072 dataset. The nodes represent each pathway, with the edges representing the crosstalk between the pathways. The thickness of the edges represents the strength of the pathway interactions. Red and pink represent primary and secondary nodes respectively.
Using the criteria defined for activated and repressed pathways, 2 pathways (‘cell cycle’ and ‘P53 signaling pathway’) were repressed, and 10 pathways were activated (Table II). The common DEGs between the pathways are listed in Table III. GADD45B was associated with three enriched pathways, including the activated ‘MAPK signaling pathway’ and the repressed ‘cell cycle’ and ‘P53 signaling pathway’ terms (Fig. 2).
Table II.
Status of the Kyoto Encyclopedia of Genes and Genomes pathways associated with the differentially expressed genes in lung cancer.
| DEGs | ||||
|---|---|---|---|---|
| Pathway | Upregulated | Downregulated | Both | Q-value |
| Cell cycle | 2 | 11 | 13 | −0.6923 |
| P53 signaling pathway | 2 | 8 | 10 | −0.6000 |
| Cytokine-cytokine receptor interaction | 13 | 3 | 16 | 0.6250 |
| Tight junction | 9 | 2 | 11 | 0.6364 |
| Neuroactive ligand-receptor interaction | 15 | 3 | 18 | 0.6667 |
| Chemokine signaling pathway | 11 | 2 | 13 | 0.6923 |
| Complement and coagulation cascades | 14 | 2 | 16 | 0.7500 |
| Axon guidance | 9 | 1 | 10 | 0.8000 |
| Vascular smooth muscle contraction | 12 | 1 | 13 | 0.8462 |
| MAPK signaling pathway | 13 | 1 | 14 | 0.8571 |
| Regulation of actin cytoskeleton | 11 | 0 | 11 | 1.0000 |
| Endocytosis | 10 | 0 | 10 | 1.0000 |
Table III.
Differentially expressed genes associated with multiple enriched Kyoto Encyclopedia of Genes and Genomes pathways.
| Pathway 1 | Pathway 2 | Gene IDs |
|---|---|---|
| Cytokine-cytokine receptor interaction | Chemokine signaling pathway | 6359, 1524, 2921, 2920, 6387 |
| Neuroactive ligand-receptor interaction | Vascular smooth muscle contraction | 1906, 10203, 1909, 185 |
| MAPK signaling pathway | Regulation of actin cytoskeleton | 2264, 6237, 2263 |
| MAPK signaling pathway | Endocytosis | 2264, 7048, 2263 |
| Regulation of actin cytoskeleton | Endocytosis | 2264, 2263, 8395 |
| Tight junction | Regulation of actin cytoskeleton | 10398, 6237, 4628 |
| Cytokine-cytokine receptor interaction | Neuroactive ligand-receptor interaction | 3953, 2690 |
| Cytokine-cytokine receptor interaction | Chemokine signaling pathway | 10563, 9547 |
| Tight junction | Vascular smooth muscle contraction | 10398, 4629 |
| Tight junction | MAPK signaling pathway | 6237, 10000 |
| Vascular smooth muscle contraction | Regulation of actin cytoskeleton | 10398, 4638 |
| Cell cycle | P53 signaling pathway | 4616 |
| Cell cycle | MAPK signaling pathway | 4616 |
| Chemokine signaling pathway | Axon guidance | 6387 |
| Chemokine signaling pathway | Vascular smooth muscle contraction | 115 |
| Chemokine signaling pathway | MAPK signaling pathway | 10000 |
| Chemokine signaling pathway | Regulation of actin cytoskeleton | 5295 |
| Chemokine signaling pathway | Endocytosis | 2869 |
| Cytokine-cytokine receptor interaction | Axon guidance | 6387 |
| Cytokine-cytokine receptor interaction | MAPK signaling pathway | 7048 |
| Cytokine-cytokine receptor interaction | Endocytosis | 7048 |
| Neuroactive ligand-receptor interaction | Complement and coagulation cascades | 728 |
| Neuroactive ligand-receptor interaction | Endocytosis | 154 |
| P53 signaling pathway | MAPK signaling pathway | 4616 |
| Tight junction | Chemokine signaling pathway | 10000 |
| Vascular smooth muscle contraction | MAPK signaling pathway | 5319 |
| Vascular smooth muscle contraction | MAPK signaling pathway | 5321 |
Figure 2.
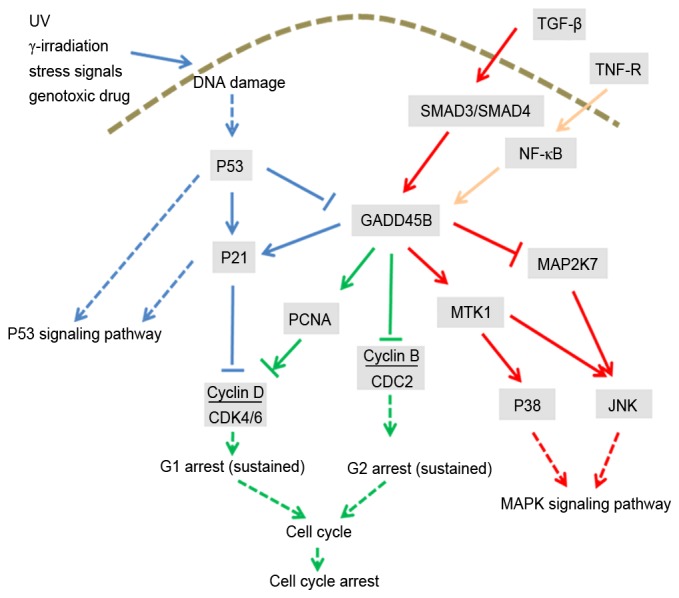
Schematic diagram of the pathway-pathway crosstalk via GADD45B. In this figure, the blue arrows represent the interaction with the P53 signaling pathway, the green arrows represent the interaction with the cell cycle pathway and the red arrows represent the interaction with the MAPK signaling pathway. Yellow arrows represent the interactions between GADD45B and genes that are not associated with ‘P53 signaling pathway’, ‘MAPK signaling pathway’ or ‘cell cycle’. Solid lines represent direct interactions; dashed lines represent indirect effects. GADD45B, growth arrest and DNA damage inducible β; UV, ultraviolet radiation; TGF-β, transforming growth factor-β; TNF-R, tumor necrosis factor receptor; NF-κB, nuclear factor-κB; CDK, cyclin-dependent kinase; CDC2, cyclin-dependent kinase 1; PCNA, proliferating cell nuclear antigen; MAPK, mitogen-activated protein kinase; MTK1, MAPK kinase kinase 4; MAP2K7, MAPK kinase 7; JNK, c-Jun N-terminal kinase.
Measurement of cell viability and western blot analysis
Benzopyrene treatment reduced the viability of lung cells at all concentrations from 72 h (Fig. 3). GADD45B, P53, cyclin, Akt and NF-κB protein levels were detected by Western blotting (Fig. 4). Western blotting demonstrated that the expression of NF-κB, Akt and GADD45B increased over time in lung cells treated with benzopyrene, whereas the expression of cyclin B and P53 was decreased (Fig. 5).
Figure 3.
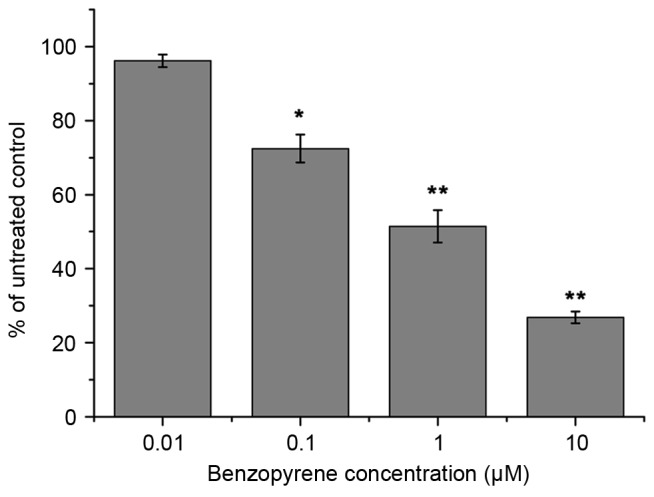
MTT assay of human bronchial epithelial cells cultured for 72 h with 0.01, 0.1, 1 or 10 µM benzopyrene. The data are presented as means ± standard deviation. *P<0.05 and **P<0.01 relative to the untreated control.
Figure 4.
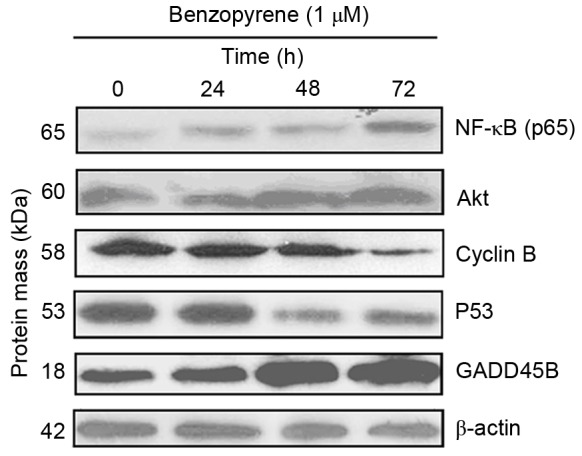
Identification of the GADD45B, P53, cyclin B, Akt and NF-κB protein expression levels using western blot analysis. GADD45B, growth arrest and DNA damage inducible β; NF-κB, nuclear factor-κB.
Figure 5.
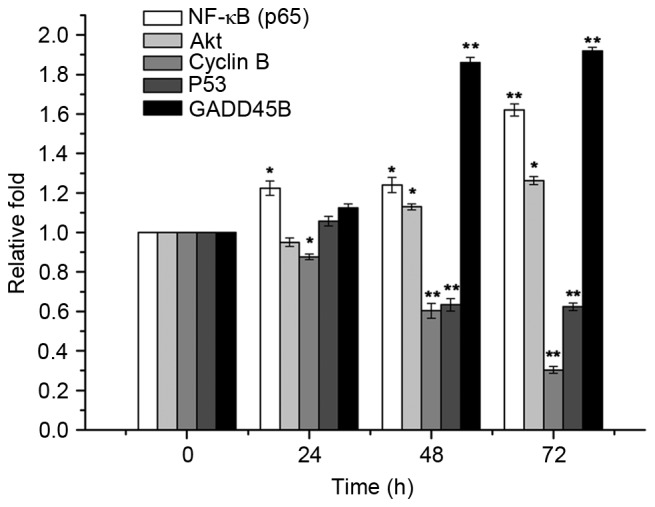
Quantification of the GADD45B, P53, cyclin B, Akt and NF-κB protein levels from the western blot analysis. *P<0.05 and **P<0.01 compared with 0 h. GADD45B, growth arrest and DNA damage inducible β; NF-κB, nuclear factor-κB.
Discussion
In the present study, significantly enriched pathways were identified with impact analysis, and a pathway-pathway crosstalk network was constructed. GADD45B was identified as a connection between a number of the enriched pathways, and experimental validation of the expression of this gene and associated pathways was performed.
In comparison to classical pathway analysis, impact analysis also considers the over-representation of DEGs in a given pathway and the abnormal perturbation of that pathway, as measured by the expression changes across the pathway topology (26). Previously, the roles of the P53 signaling pathway, cell adhesion, focal adhesion and the cell cycle in the pathogenesis of lung cancer have been confirmed and studied extensively (27,28). In contrast, the complement and coagulation cascades in this disease have been less reported. Corrales et al (29), reported the activation of the complement system by detecting the anaphylatoxin C5a, a potent immune mediator generated subsequent to complement activation in lung cancer cell lines. Plasminogen activator inhibitor (PAI) variants PAI-1 A15T and PAI-2 S413C influence the prognosis of patients with lung cancer (30), and PAI-1 has been demonstrated to inhibit the activation of the coagulation system (31). Levels of the erythrocyte complement receptor 1 were significantly lower in patients with small cell lung cancer (32). A previous study revealed that activated coagulation factor X inhibited the migration of lung cancer cells and may serve a key role in cell migration (33). Thus, it was indicated that the complement and coagulation cascades may also serve an important role in the pathogenesis of lung cancer.
The pathway-pathway cross-talk network indicated the central roles of ‘cell cycle’, ‘drug metabolism-cytochrome P450’, ‘metabolic pathways’, ‘pathways in cancer’, ‘focal adhesion’ and ‘antigen processing and presentation’; metabolism-associated pathways may therefore be particularly important in the pathogenesis of lung cancer. Tumor cells sustain high rates of glycolysis even in aerobic conditions to maintain their rapid growth; alterations in in primary metabolites (34) and glycolysis-associated enzymes, including hexokinase II and glyceraldehyde-3-phosphate dehydrogenase (35,36), have been identified in lung cancer. Additionally, the alteration of metabolic pathways may affect the efficacy of anti-tumor drugs, e.g. the glutathione metabolic pathway, which is involved in the detoxification or inactivation of platinum drugs (37). The cytochromes P450 are a group of enzymes that catalyze the oxidative biotransformation of the majority of drugs and other lipophilic xenobiotics (38).
In the present study, GADD45B, an upregulated gene, was determined to be a common link between three KEGG pathways, including the upregulated ‘MAPK signaling pathway’ and the repressed ‘cell cycle’ and ‘P53 signaling pathway’. It was therefore considered to potentially have an important role in the pathogenesis of lung cancer. GADD45B, encoding MyD118, is part of a highly conserved Gadd45 gene family with GADD45A, encoding GADD45, and GADD45G, encoding CR6. Gadd45 proteins have been implicated for their involvement in tumorigenesis (39) and age-associated pathologies (40); however, MyD118, GADD45 and CR6 are considered to have similar but not identical functions via different apoptotic and growth suppressive pathways (41). The MAPK signaling pathway has been confirmed to have an important role in the pathogenesis of lung cancer (42). In the present study, this signaling pathway was activated in patients with lung cancer. Gupta et al (43) demonstrated that GADD45B promotes cell survival via activation of the GADD45a-P38-NF-κB pathway and inhibition of the MAPK kinase 4-c-Jun N-terminal kinases (JNK) pathway. P38 and JNK are members of the MAPK family (44). TGF-β can activate Smad by phosphorylation (45). One previous study demonstrates that following the induction by TGF-β, Smad transcription factors activate P38 via GADD45B (46). Another study demonstrates that SMAD3 and SMAD4 can activate GADD45B following the induction by TGF-β (47). GADD45B and GADD45G are cyclin-dependent kinase 2/cyclin B1 kinase inhibitors, and thus function in in G2/M cell cycle arrest (48). This is in accord with the observation in the present study that the cell cycle pathway was repressed.
It was also identified in the present study that the P53 signaling pathway was repressed. Previously, Mi et al (49) observed the upregulation of GADD45B and the downregulation of P53 in human prostate cancer cells exposed to silvestrol. This signaling pathway has been reported to correlate with the radioresponse of NSCLC (13). GADD45A is a target gene of P53 (50). Lambert et al (51) reported the upregulation of GADD45B mRNA in Saos-2-His273 cells exposed to proline rich membrane anchor 1, a P53-reactivating agent.
Benzopyrene is a carcinogen particularly associated with lung cancer (52); therefore, the normal lung cells were treated with benzopyrene in the present study to investigate the process of lung carcinogenesis. The harmful effects of benzopyrene against cell viability were observed by an MTT assay at various concentrations. Western blotting demonstrated that the expression of NF-κB, Akt and GADD45B were increased over time in lung cells treated with benzopyrene, whereas the expression of cyclin B and P53 were decreased. This corresponded with the data that the AKT-MAPK pathway was activated via NF-κB, while the P53 pathway and cell cycle pathway were repressed; thus, GADD45B may contribute to lung carcinogenesis via activating the MAPK signaling pathway and repressing the P53 signaling and cell cycle pathways.
Although a series of comprehensive bioinformatics analyses and validation experiments were performed, there were two major limitations in the present study: i) Only one lung cell line was used in the current study, which may not eliminate the heterogeneity of lung cancer; ii) knockdown of GADD45B was not performed, and the expressions of other proteins after GADD45B knockdown were not considered, which may weaken the regulatory associations between them.
In addition to ‘ECM-receptor interaction’, ‘P53 signaling pathway’, ‘cell adhesion molecules (CAMs)’, ‘focal adhesion’ and ‘cell cycle’, it was also identified that the ‘complement and coagulation cascades’ pathway may be associated with the pathogenesis of lung cancer; therefore, it was speculated that GADD45B may contribute to lung carcinogenesis via activating the MAPK signaling pathway, and repressing the P53 signaling and cell cycle pathways. Thus, the role of this gene in lung cancer should be studied further; GADD45B siRNA knockdown experiments will assist the further validation of this speculation.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
XJ and JL were involved in the conception and design of the research and drafting the manuscript. ZZ participated in the acquisition of data. RX performed the analysis and interpretation of data. YG was involved in the statistical analysis, XL participated in the design of the study and performed the statistical analysis. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.McGuire S. World cancer report 2014. Geneva, switzerland: World health organization, international agency for research on cancer, WHO Press, 2015. Adv Nutr. 2016;7:418–419. doi: 10.3945/an.116.012211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Travis WD, Travis LB, Devesa SS. Lung cancer. Cancer. 1995;75(Suppl 1):S191–S202. doi: 10.1002/1097-0142(19950101)75:1+<191::AID-CNCR2820751307>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 3.Ahrendt SA, Decker PA, Alawi EA, Zhu Yr YR, Sanchez-Cespedes M, Yang SC, Haasler GB, Kajdacsy-Balla A, Demeure MJ, Sidransky D. Cigarette smoking is strongly associated with mutation of the K-ras gene in patients with primary adenocarcinoma of the lung. Cancer. 2001;92:1525–1530. doi: 10.1002/1097-0142(20010915)92:6<1525::AID-CNCR1478>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 4.Naoki K, Chen TH, Richards WG, Sugarbaker DJ, Meyerson M. Missense mutations of the BRAF gene in human lung adenocarcinoma. Cancer Res. 2002;62:7001–7003. [PubMed] [Google Scholar]
- 5.Weir BA, Woo MS, Getz G, Perner S, Ding L, Beroukhim R, Lin WM, Province MA, Kraja A, Johnson LA, et al. Characterizing the cancer genome in lung adenocarcinoma. Nature. 2007;450:893–898. doi: 10.1038/nature06358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engels EA, Wu X, Gu J, Dong Q, Liu J, Spitz MR. Systematic evaluation of genetic variants in the inflammation pathway and risk of lung cancer. Cancer Res. 2007;67:6520–6527. doi: 10.1158/0008-5472.CAN-07-0370. [DOI] [PubMed] [Google Scholar]
- 7.Wenzlaff AS, Cote ML, Bock CH, Land SJ, Santer SK, Schwartz DR, Schwartz AG. CYP1A1 and CYP1B1 polymorphisms and risk of lung cancer among never smokers: A population-based study. Carcinogenesis. 2005;26:2207–2212. doi: 10.1093/carcin/bgi191. [DOI] [PubMed] [Google Scholar]
- 8.Lin Z, Zhang X, Tuo J, Guo Y, Green B, Chan CC, Tan W, Huang Y, Ling W, Kadlubar FF, et al. A variant of the Cockayne syndrome B gene ERCC6 confers risk of lung cancer. Hum Mutat. 2008;29:113–122. doi: 10.1002/humu.20610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amos CI, Wu X, Broderick P, Gorlov IP, Gu J, Eisen T, Dong Q, Zhang Q, Gu X, Vijayakrishnan J, et al. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat Genet. 2008;40:616–622. doi: 10.1038/ng.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westhoff B, Colaluca IN, D'Ario G, Donzelli M, Tosoni D, Volorio S, Pelosi G, Spaggiari L, Mazzarol G, Viale G, et al. Alterations of the Notch pathway in lung cancer; Proc Natl Acad Sci USA; 2009; pp. 22293–22298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leidner RS, Fu P, Clifford B, Hamdan A, Jin C, Eisenberg R, Boggon TJ, Skokan M, Franklin WA, Cappuzzo F, et al. Genetic abnormalities of the EGFR pathway in African American Patients with non-small-cell lung cancer. J Clin Oncol. 2009;27:5620–5626. doi: 10.1200/JCO.2009.23.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gustafson AM, Soldi R, Anderlind C, Scholand MB, Qian J, Zhang X, Cooper K, Walker D, McWilliams A, Liu G, et al. Airway PI3K pathway activation is an early and reversible event in lung cancer development. Sci Transl Med. 2010;2:26ra25. doi: 10.1126/scitranslmed.3000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung IL, Kang HJ, Kim KC, Kim IG. PTEN/pAkt/p53 signaling pathway correlates with the radioresponse of non-small cell lung cancer. Int J Mol Med. 2010;25:517–523. doi: 10.3892/ijmm_00000372. [DOI] [PubMed] [Google Scholar]
- 14.Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K, Sougnez C, Greulich H, Muzny DM, Morgan MB, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–1075. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bremnes RM, Veve R, Gabrielson E, Hirsch FR, Baron A, Bemis L, Gemmill RM, Drabkin HA, Franklin WA. High-throughput tissue microarray analysis used to evaluate biology and prognostic significance of the E-cadherin pathway in non-small-cell lung cancer. J Clin Oncol. 2002;20:2417–2428. doi: 10.1200/JCO.2002.08.159. [DOI] [PubMed] [Google Scholar]
- 16.Yanaihara N, Caplen N, Bowman E, Seike M, Kumamoto K, Yi M, Stephens RM, Okamoto A, Yokota J, Tanaka T, et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9:189–198. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 17.Liu XG, Zhu WY, Huang YY, Ma LN, Zhou SQ, Wang YK, Zeng F, Zhou JH, Zhang YK. High expression of serum miR-21 and tumor miR-200c associated with poor prognosis in patients with lung cancer. Med Oncol. 2012;29:618–626. doi: 10.1007/s12032-011-9923-y. [DOI] [PubMed] [Google Scholar]
- 18.Landi MT, Dracheva T, Rotunno M, Figueroa JD, Liu H, Dasgupta A, Mann FE, Fukuoka J, Hames M, Bergen AW, et al. Gene expression signature of cigarette smoking and its role in lung adenocarcinoma development and survival. PLoS One. 2008;3:e1651. doi: 10.1371/journal.pone.0001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogata H, Goto S, Sato K, Fujibuchi W, Bono H, Kanehisa M. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 1999;27:29–34. doi: 10.1093/nar/27.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joshi-Tope G, Gillespie M, Vastrik I, D'Eustachio P, Schmidt E, de Bono B, Jassal B, Gopinath GR, Wu GR, Matthews L, et al. Reactome: A knowledgebase of biological pathways. Nucleic Acids Res. 2005;33:D428–D432. doi: 10.1093/nar/gki072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gautier L, Cope L, Bolstad BM, Irizarry RA. Affy-analysis of Affymetrix GeneChip data at the probe level. Bioinformatics. 2004;20:307–315. doi: 10.1093/bioinformatics/btg405. [DOI] [PubMed] [Google Scholar]
- 22.Górecki T, Smaga Ł. A comparison of tests for the one-way ANOVA problem for functional data. Computational Statistics. 2015;30:987–1010. doi: 10.1007/s00180-015-0555-0. [DOI] [Google Scholar]
- 23.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J Royal Statistical Society. Series B (Methodological) 1995:1–300. [Google Scholar]
- 24.Draghici S, Khatri P, Tarca AL, Amin K, Done A, Voichita C, Georgescu C, Romero R. A systems biology approach for pathway level analysis. Genome Res. 2007;17:1537–1545. doi: 10.1101/gr.6202607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Francesconi M, Remondini D, Neretti N, Sedivy JM, Cooper LN, Verondini E, Milanesi L, Castellani G. Reconstructing networks of pathways via significance analysis of their intersections. BMC Bioinformatics. 2008;9(Suppl 4):S9. doi: 10.1186/1471-2105-9-S4-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tarca AL, Draghici S, Khatri P, Hassan SS, Mittal P, Kim JS, Kim CJ, Kusanovic JP, Romero R. A novel signaling pathway impact analysis. Bioinformatics. 2009;25:75–82. doi: 10.1093/bioinformatics/btn577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhong G, Chen X, Fang X, Wang D, Xie M, Chen Q. Fra-1 is upregulated in lung cancer tissues and inhibits the apoptosis of lung cancer cells by the P53 signaling pathway. Oncol Rep. 2016;35:447–453. doi: 10.3892/or.2015.4395. [DOI] [PubMed] [Google Scholar]
- 28.Havel LS, Kline ER, Salgueiro AM, Marcus AI. Vimentin regulates lung cancer cell adhesion through a VAV2-Rac1 pathway to control focal adhesion kinase activity. Oncogene. 2015;34:1979–1990. doi: 10.1038/onc.2014.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corrales L, Ajona D, Rafail S, Lasarte JJ, Riezu-Boj JI, Lambris JD, Rouzaut A, Pajares MJ, Montuenga LM, Pio R. Anaphylatoxin C5a creates a favorable microenvironment for lung cancer progression. J Immunol. 2012;189:4674–4683. doi: 10.4049/jimmunol.1201654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pappot H, Pedersen AN, Brünner N, Christensen IJ. The complex between urokinase (uPA) and its type-1 inhibitor (PAI-1) in pulmonary adenocarcinoma: Relation to prognosis. Lung Cancer. 2006;51:193–200. doi: 10.1016/j.lungcan.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 31.McVey JH. Tissue factor pathway. Baillieres Best Pract Res Clin Haematol. 1999;12:361–372. doi: 10.1053/beha.1999.0030. [DOI] [PubMed] [Google Scholar]
- 32.Currie MS, Vala M, Pisetsky DS, Greenberg CS, Crawford J, Cohen HJ. Correlation between erythrocyte CR1 reduction and other blood proteinase markers in patients with malignant and inflammatory disorders. Blood. 1990;75:1699–1704. [PubMed] [Google Scholar]
- 33.Borensztajn K, Peppelenbosch MP, Spek CA. Coagulation Factor Xa inhibits cancer cell migration via LIMK1-mediated cofilin inactivation. Thromb Res. 2010;125:e323–e328. doi: 10.1016/j.thromres.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 34.Fan TW, Lane AN, Higashi RM, Farag MA, Gao H, Bousamra M, Miller DM. Altered regulation of metabolic pathways in human lung cancer discerned by (13)C stable isotope-resolved metabolomics (SIRM) Mol Cancer. 2009;8:41. doi: 10.1186/1476-4598-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koukourakis MI, Giatromanolaki A, Sivridis E, Tumour and Angiogenesis Research Group Lactate dehydrogenase isoenzymes 1 and 5: Differential expression by neoplastic and stromal cells in non-small cell lung cancer and other epithelial malignant tumors. Tumor Biol. 2003;24:199–202. doi: 10.1159/000074430. [DOI] [PubMed] [Google Scholar]
- 36.Koukourakis MI, Giatromanolaki A, Sivridis E, Gatter KC, Harris AL, Tumor and Angiogenesis Research Group Pyruvate dehydrogenase and pyruvate dehydrogenase kinase expression in non small cell lung cancer and tumor-associated stroma. Neoplasia. 2005;7:1–6. doi: 10.1593/neo.04373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang P, Ebbert JO, Sun Z, Weinshilboum RM. Role of the glutathione metabolic pathway in lung cancer treatment and prognosis: A review. J Clin Oncol. 2006;24:1761–1769. doi: 10.1200/JCO.2005.02.7110. [DOI] [PubMed] [Google Scholar]
- 38.Zanger UM, Turpeinen M, Klein K, Schwab M. Functional pharmacogenetics/genomics of human cytochromes P450 involved in drug biotransformation. Anal Bioanal Chem. 2008;392:1093–1108. doi: 10.1007/s00216-008-2291-6. [DOI] [PubMed] [Google Scholar]
- 39.Tamura RE, de Vasconcellos JF, Sarkar D, Libermann TA, Fisher PB, Zerbini LF. GADD45 proteins: Central players in tumorigenesis. Curr Mol Med. 2012;12:634–651. doi: 10.2174/156652412800619978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moskalev AA, Smit-McBride Z, Shaposhnikov MV, Plyusnina EN, Zhavoronkov A, Budovsky A, Tacutu R, Fraifeld VE. Gadd45 proteins: Relevance to aging, longevity and age-related pathologies. Ageing Res Rev. 2012;11:51–66. doi: 10.1016/j.arr.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Azam N, Vairapandi M, Zhang W, Hoffman B, Liebermann DA. Interaction of CR6 (GADD45gamma) with proliferating cell nuclear antigen impedes negative growth control. J Biol Chem. 2001;276:2766–2774. doi: 10.1074/jbc.M005626200. [DOI] [PubMed] [Google Scholar]
- 42.Hosokawa S, Toyooka S, Fujiwara Y, Tokumo M, Soh J, Takigawa N, Hotta K, Yoshino T, Date H, Tanimoto M, Kiura K. Comprehensive analysis of EGFR signaling pathways in Japanese patients with non-small cell lung cancer. Lung Cancer. 2009;66:107–113. doi: 10.1016/j.lungcan.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 43.Gupta M, Gupta SK, Hoffman B, Liebermann DA. Gadd45a and Gadd45b protect hematopoietic cells from UV-induced apoptosis via distinct signaling pathways, including p38 activation and JNK inhibition. J Biol Chem. 2006;281:17552–17558. doi: 10.1074/jbc.M600950200. [DOI] [PubMed] [Google Scholar]
- 44.Takekawa M, Tatebayashi K, Itoh F, Adachi M, Imai K, Saito H. Smad-dependent GADD45beta expression mediates delayed activation of p38 MAP kinase by TGF-beta. EMBO J. 2002;21:6473–6482. doi: 10.1093/emboj/cdf643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Macias MJ, Martin-Malpartida P, Massagué J. Structural determinants of Smad function in TGF-β signaling. Trends Biochem Sci. 2015;40:296–308. doi: 10.1016/j.tibs.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Howley BV, Hussey GS, Link LA, Howe PH. Translational regulation of Inhibin βA by TGFβ via the RNA-binding protein hnRNP E1 enhances the invasiveness of epithelial-to-mesenchymal transitioned cells. Oncogene. 2016;35:1725–1735. doi: 10.1038/onc.2015.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Major MB, Jones DA. Identification of a gadd45beta 3′enhancer that mediates SMAD3- and SMAD4-dependent transcriptional induction by transforming growth factor beta. J Biol Chem. 2004;279:5278–5287. doi: 10.1074/jbc.M311517200. [DOI] [PubMed] [Google Scholar]
- 48.Vairapandi M, Balliet AG, Hoffman B, Liebermann DA. GADD45b and GADD45g are cdc2/cyclinB1 kinase inhibitors with a role in S and G2/M cell cycle checkpoints induced by genotoxic stress. J Cell Physiol. 2002;192:327–338. doi: 10.1002/jcp.10140. [DOI] [PubMed] [Google Scholar]
- 49.Mi Q, Kim S, Hwang BY, Su BN, Chai H, Arbieva ZH, Kinghorn AD, Swanson SM. Silvestrol regulates G2/M checkpoint genes independent of p53 activity. Anticancer Res. 2006;26:3349–3356. [PubMed] [Google Scholar]
- 50.Zhan Q. Gadd45a, a p53-and BRCA1-regulated stress protein, in cellular response to DNA damage. Mutat Res. 2005;569:133–143. doi: 10.1016/j.mrfmmm.2004.06.055. [DOI] [PubMed] [Google Scholar]
- 51.Lambert JM, Moshfegh A, Hainaut P, Wiman KG, Bykov VJ. Mutant p53 reactivation by PRIMA-1MET induces multiple signaling pathways converging on apoptosis. Oncogene. 2010;29:1329–1338. doi: 10.1038/onc.2009.425. [DOI] [PubMed] [Google Scholar]
- 52.Denissenko MF, Pao A, Tang M, Pfeifer GP. Preferential formation of benzo[a]pyrene adducts at lung cancer mutational hotspots in p53. Science. 1996;274:430–432. doi: 10.1126/science.274.5286.430. [DOI] [PubMed] [Google Scholar]