Abstract
T cells serve an important role in the destruction of tumor cells and clearing of foreign pathogens. Previous studies have suggested that the T cell immune response of tumor-bearing patients is significantly lower than that of healthy people, and the principal reason for this is lymphocytopenia, which is caused by repeated cycles of chemotherapy. In addition to lymphocytopenia, the present study revealed that cytotoxic chemotherapy also weakens the homing ability of T cells to the T-cell zone of the spleen, which decreases the possibility of encounters between antigen-specific T cells and dendritic cells presenting the appropriate antigen, thereby weakening the immune response of T cells. These changes are attributed to the lower expression of C-C motif chemokine ligand 21 (CCL21) and C-C motif chemokine ligand 19 (CCL19) in the spleen of secondary lymphoid organs (SLOs). Finally, the present study identified that chemotherapy affects the function and survival of fibroblastic reticular cells in SLOs, which are the main source of CCL21 and CCL19. These observations aid us in further understanding the mechanism that is responsible for the decreased T cell immune response following repeated cycles of chemotherapy.
Keywords: chemotherapy, T cell, C-C motif chemokine ligand 21, C-C motif chemokine ligand 19, spleen
Introduction
Chemotherapy is a major therapeutic tool for a diverse range of tumor types and serves an irreplaceable role in the majority of them. Tumor-bearing patients usually present with a reduction in immunity following repeated chemotherapy, and almost half of them die from an infection prior to the occurrence of cancer invasion or organ failure (1–4). Patients who develop severe infection or have succumbed to mortality following chemotherapy are identified as being immunodeficient (5,6). Therefore, further clarification of the effects of chemotherapy on the immune system in vivo may aid in uncovering the specific molecular mechanisms underlying the downregulation of the immune response in tumor-bearing patients (7).
As is well established, the immune response in vivo affects the anti-infection and antitumor immune ability in vivo. T cells serve an important role in the killing of tumor cells and the clearing of foreign pathogens (8,9). A previous study suggested that the T cell immune response of tumor-bearing patients is significantly lower than that of non-tumor bearing patients or healthy controls (8). The main reason is that patients with malignant tumors often receive repeated cycles of chemotherapy that induce severe lymphocytopenia (1). Furthermore, there are abundant immune negative regulatory cells in tumor-bearing patients (8).
However, to the best of our knowledge, there is no report regarding the effects of chemotherapeutic drugs on secondary lymphoid organs (SLOs). It has previously been established that the steps of T cell activation are as follows: Directional chemo attraction to SLOs and encounter with dendritic cells (DCs) and activation, which is the premise for the generation of the T cell immune response (10). A modest immune response of T cells may be generated only after enough T cells are chemoattracted by chemokines to SLOs (11,12). Reportedly, decreasing the count of T cells homing to SLOs would weaken the immune response of T cells (11).
The C-C chemokine receptor 7 on the surfaces of T cells may be chemoattracted by the SLO-secreted C-C motif chemokine ligand 21 (CCL21) and C-C motif chemokine ligand 19 (CCL19) (13). Stromal cells are the major type of cells that secrete CCL21 and CCL19 in SLOs (12). The stromal cells in the spleen may be divided into four types depending on the expression of gp38 and cluster of differentiation 31 (CD31). In particular, the gp38 +CD31-fibroblastic reticular cells (FRCs) are the main contributor that secretes CCL21 and CCL19. FRCs are the major stromal cells in the T-cell zone. FRCs also account for the largest proportion (≤40%) of splenic stromal cells (14).
Immune cells are orderly distributed in different areas of SLOs. The T cells are primarily activated in the T-cell zone, where the main type of cells are FRCs (15,16). These FRCs serve roles in the structures of SLOs. Specifically, the abundant extracellular matrix (ER-TR7) secreted by FRCs forms 3D reticular supports for the crawling, contact and interaction between T cells and DCs (16). Notably, FRCs secrete chemokines CCL21 and CCL19 that recruit and chemoattract T cells, and as reported, CCL21−/− T cells in mice are inaccessible to the T-cell zone (17). Furthermore, the number of T cells in the T-cell zone of lymph nodes is significantly reduced in CCL21−/− and CCR7−/− mice, leading to a significant reduction of the immune response in T cells (3,4).
In addition to killing tumor cells, chemotherapy is also able to kill cells of other tissues without any selectivity. The stromal FRCs of SLOs are extremely critical for the homing of T cells and they affect the immune response by regulating the homing of T cells (18). Therefore, the aim of the present study was to investigate how chemotherapeutic drugs affect the homing of T cells to SLOs in addition to the underlying molecular mechanisms, and to further understand the specific molecular mechanism of the way in which chemotherapy weakens the immune response in vivo.
Materials and methods
Ethics statement
All animal experiments were approved by the Animal Ethical and Experimental Committee of the Third Military Medical University (TMMU, Chongqing, China). All animal surgeries were performed under anesthesia with sodium pentobarbital and all efforts were made to minimize suffering.
Mice
A total of 80 6-week-old female C57BL/6 mice with a mean weight of 20 g were purchased from the Center of Experimental Animals of TMMU (Chongqing, China). The mice were maintained in a 25°C, 55% humidity, 0.03% CO2, light-regulated space with 12-h day and night cycles. They were fed a normal mouse chow and water ad libitum according to the TMMU Guidelines for Animal Experiments (SPF). All animal experimental protocols used in the present study were performed in accordance with the Institutional Guidelines for Animal Experiments. The mice were randomly divided into two groups (n=5 in each group). The control group was treated with intraperitoneal (i.p.) injection of phosphate-buffered saline (PBS), which was sterilized under high pressure. The test group received chemotherapeutic drugs [i.p., 4 mg/kg cisplatin, 100 mg/kg gemcitabine or 100 mg/kg fluorouracil (5-FU)] on days 0, 7 and 14, as previously reported (19–21). A total of 6 days following the 14-day medication, the mice were administered with carboxyfluorescein succinimidyl ester (CFSE)-marked 5×106 naive T cells marked by carboxyfluorescein succinimidyl ester (CFSE) via the caudal vein, which were isolated from the spleen of C57BL/6 mice by anti-CD3 microbeads. After 8 h, the mice were sacrificed by cervical dislocation under anesthesia and were sent for evaluation of relevant indices (22).
Adoptive transfers and cell migration in vivo
For T cell isolation, naive T cells were isolated using anti-CD3 magnetic beads (cat. no. 130-094-973; Miltenyi Biotec, Bergisch Gladbach, Germany), according to the manufacturer's protocol. Briefly, a single-cell suspension of C57BL/6 mice spleen was obtained. CD3+ T cell MicroBead Cocktail was added into single cell suspension and incubated for 10 min at 4°C. The cell suspension was placed into a column in the magnetic field of a MACS Separator. A 5 ml buffer volume was added to the column for immediate flush of the magnetically labeled CD3+ naive T cells (11,23). The purified T cells were labeled with 4 M CFSE (Molecular Probes; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 5×106 cells were transferred into each mouse via the tail vein. After 8 h, the mice were sacrificed and the spleens were removed for flow cytometry or microscopic analysis (22,23). The counts of CFSE+ cells in the lymph nodes and spleen of white pulp (WP) regions were assessed.
Flow cytometry
To detect homing ability of T cells, the spleens were collected 8 h following transfer of CFSE+ T cells, and were digested with 1 mg/ml type-I collagenase at 37°C for 30 min (or for another 30 min if digestion was not complete). Next, unicellular suspensions were collected and added with 500 µl of aseptic red blood cell lysis buffer (cat. no. C3702; Beyotime Institute of Biotechnology, Haimen, China) for lysis at 37°C for 4 min. An appropriate amount of flow cytometry dilution [2% standard fetal bovine serum (FBS) 5 mM MEDTA; cat. no. C0232; Beyotime Institute of Biotechnology] was added to dilute the cells to 1×106/ml, followed by detection of proportion of CFSE+ cells by using a FACSCanto (BD Biosciences, Franklin Lakes, NJ, USA) cytometer and analyzed by FlowJo version 10 (FlowJo LLC, Ashland, OR, USA) (14). For cell sorting, stromal cells were blocked by TreStain fcX™ (cat. no. 101320; dilution, 1:100; BioLegend, Inc., San Diego, CA, USA) for 10 minutes on ice prior to incubate with CD31 (cat. no. 102409; dilution, 1:100; BioLegend, Inc., San Diego, CA, USA) and gp38 (cat. no. 127415; dilution, 1:100; BioLegend, Inc.) which were diluted in PBS at 30 min on ice, washed twice by PBS and were sorted using a high-speed cell sorter (FACSAria; BD Biosciences).
Immunofluorescence microscopy
Spleens were immediately frozen in OCT (TissueTek, VWR International, Randor, PA, USA). The cryostat sections (10 µm) were fixed in ice-cold acetone for 10 min, prior to being incubated with the following antibodies at 4°C overnight: Anti-mouse ER-TR7 (cat. no. sc-73355; dilution, 1:100; Santa Cruz Biotechnology Inc., Dallas, TX, USA), and anti-mouse gp38 (cat. no. AF3244; dilution, 1:200; R&D Systems, Inc., Minneapolis, MN, USA); CCL21 (cat. no. AF457; dilution, 1:200; R&D Systems, Inc.). The primary antibodies were detected by fluorescein isothiocyanate (FITC)-conjugated anti-rat (cat. no. ZF-0315; dilution 1:100; OriGene Technologies, Inc., Beijing, China) or Cy3-conjugated anti-goat IgG (cat. no. A0502; dilution 1:200; Beyotime Institute of Biotechnology) at 37°C for 30 min. Average values of fluorescence were analyzed on LAS AF Lite version 4.0. (Leica Microsystems GmbH, Wetzlar, Germany). For in situ TUNEL assay, following staining by surface marker gp38 as above, Enhanced Immunostaining Permeabilization Buffer (cat. no. P0097; Beyotime Institute of Biotechnology) was added at room temperature for 5 min. Following washing three times with PBS, TUNEL detection regent (cat. no. C1089; Beyotime Institute of Biotechnology) was added at 37°C for 60 min and then washed three times with PBS. Stained cells were visualized using an Olympus confocal microscope (Olympus Corporation, Tokyo, Japan) equipped with a Leica camera (Leica Microsystems GmbH, Wetzlar, Germany). Positive cells were counted in each 10 randomly selected fields per section at a magnification of ×200.
ELISA
Tissues were harvested from mice and placed in PBS, supplemented with 1% bovine serum albumin, and 1 mM phenylmethylsulfonyl fluoride (PMSF; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Subsequently, the tissues were homogenized. CCL21 (catalog no. DY457; R&D Systems, Inc., Minneapolis, MN, USA) and CCL19 (cat. no. DY440; R&D Systems, Inc.) were detected using a DuoSet ELISA development kit, according to the manufacturer's protocol.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
PCR primer pairs, including their specific target genes, orientation and sequence were as follows: CCL21 forward, 5′-CCCTGGACCCAAGGCAGT-3′ and reverse, 5′-GGCTTAGAGTGCTTCCGGG-3′; CCL19 forward, 5′-CTGCCTCAGATTATCTGCCAT-3′ and reverse, 5′-TCATTAGCACCCCCCAGAGT-3′; and β-actin forward, 5′-CCTGAGGCTCTTTTCCAGCC-3′ and reverse, 5′-AGAGGTCTTTACGGATGTCAACGT-3′ (11). Whole spleens were placed in TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and total RNA was extracted according to the manufacturer's protocol. RNA template was reverse-transcribed using a PrimeScript RT Master Mix kit (Takara Biotechnology Co., Ltd., Dalian, China) for 30 min at 16°C, 30 min at 42°C, 5 min at 85°C and maintained at 4°C. RT-qPCR was performed for total chemokine mRNA using SYBRGreen (Takara Bio Inc., Otsu, Japan) and detected using a Bio-Rad RT-qPCR analyzer (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The thermocycling conditions were as follows: 95°C for 30 sec, then 40 cycles of 95°C for 5 sec, 59°C for 34 sec, then 95°C for 15 sec, 60°C for 60 sec, and 95°C for 15 sec. Expression was quantified by 2−ΔΔCq with β-actin as a reference (24), according to the manufacturer's protocol (Applied Biosystems; Thermo Fisher Scientific, Inc.).
Stromal cell culture and cell death investigation
Spleens were digested as described earlier (1 mg/ml I-type collagenase; digestion at 37°C for 30 min), prior to being counted and plated at a concentration of 5×105 cells/cm2 in a 6-well plate. The cell culture medium was α-MEM (Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with 10% batch-tested, low-Ig FBS and 1% penicillin/streptomycin. Plates were washed by PBS after 24 h to remove non-adheren T cells. After 5 days, mouse cell cultures primarily contained lymphatic endothelial cells (LECs) and FRCs. The gp38+CD31− FRCs were selected using a flow cytometer: A total of 5×105 cells were cultured for 24 h, followed by addition of the corresponding chemotherapeutic drug: 4 µM cisplatin, 2 µM gemcitabine or 2 mg/ml 5-FU. Following co-culture for 24 h in a humidified incubator at 37°C with 5% CO2, the cell death of FRCs was evaluated using Annexin V/7-AAD labeling, according to the manufacturer's protocol (Beyotime Institute of Biotechnology) and detected by using a FACSCanto (BD Bioscience) and analyzed by FlowJo version 10 (FlowJo LLC).
Statistical analysis
Multiple group comparisons were performed using a one-way analysis of variance, followed by Dunnett's multiple comparison test on GraphPad Prism software version 5.0 (GraphPad Software, Inc., La Jolla, CA, USA) and the data are presented as the mean ± standard error of the mean. The mice were randomly grouped (n=5 in each group). All experiments were repeated a minimum of three times. P<0.05 was considered to indicate a statistically significant difference.
Results
Chemotherapy weakens the homing ability of T cells to the T-cell zone of spleen
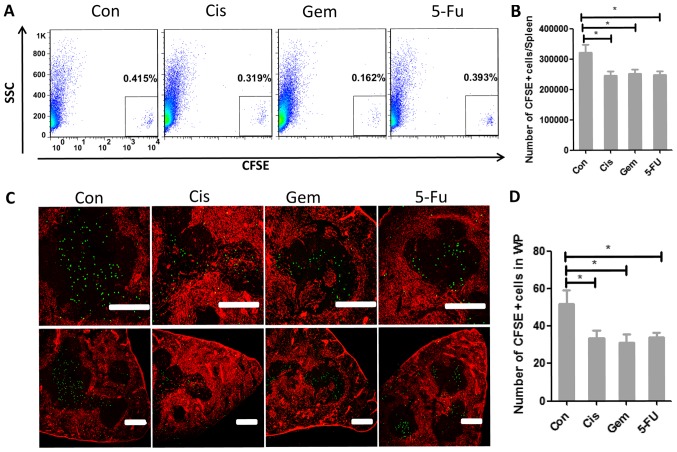
The generation of the T cell immune response requires rare antigen-specific T cells to encounter DCs that present the appropriate antigen. Such a spontaneous encounter is rare in vivo and only occurs in SLOs (15–18). An aim of the present study was to clarify whether chemotherapy affected the homing ability of T cells to the T-cell zone; therefore, the present study constructed a mouse model for repeated chemotherapy. At day 5 following the final administration of chemotherapy treatment, the CFSE-labeled T cells were retransfused. After 8 h, the proportion of CFSE+ T cells in the spleen was detected. These results illustrated that the proportion of CFSE+ T cells following chemotherapy dropped significantly (Fig. 1A and B). The homing ability of T cells to the spleen by immune fluorescence was also examined. After ascertaining its specific localization to the white pulps of spleen, the count of CFSE+ T cells in the splenic white pulps of mice was identified to be significantly reduced (Fig. 1C and D).
Figure 1.
Chemotherapy weakens the homing ability of T cells to the T-cell zone of the spleen. (A) Naive T lymphocytes were purified, labeled with CFSE and transferred into control mice or chemotherapy-treated mice (Cis, Gem or 5-FU) and 8 h later, the CFSE+ cells in the spleen were detected by flow cytometry. (B) The number of CFSE+ cells in different groups. (C) The localization of CFSE+ cells (green) in the spleen white pulps (ER-TR7; red) of control or test mice was ascertained 8 h later by immunofluorescence. Scale bar, 200 µm. (D) Counts of CFSE+ cells in the spleen white pulps of control or test mice. One-way analysis of variance, followed by Dunnett's multiple comparisons test, was used for all analyses. Error bars represent the standard error of the mean. *P<0.05. CFSE, carboxyfluorescein succinimidyl ester; Con, control; Cis, cisplatin; Gem, gemcitabine; 5-FU, fluorouracil; SCC, side scatter; WP, white pulps.
Splenic expression of CCL21 and CCL19 is downregulated following chemotherapy
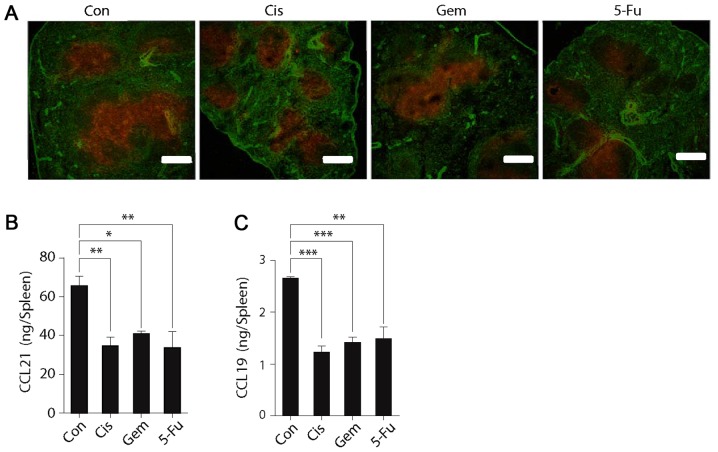
SLOs are able to express the chemokines CCL19 and CCL21 that attract CCR7+ T cells. In order to clarify why the homing ability of T cells was reduced following chemotherapy, the splenic expression of these two chemokines was investigated. The fluorescence results demonstrated that the splenic expression of CCL19 and CCL21 was significantly reduced following chemotherapy administration (Fig. 2A). The splenic expression of CCL21 and CCL19 was also measured using ELISA, which also indicated that expression was decreased following chemotherapy (Fig. 2B and C). These results further indicated that chemotherapy may downregulate the homing ability of T cells to the splenic SLOs, and that this is primarily caused by the downregulation of chemokines in splenic T cells.
Figure 2.
Expression of CCL21 and CCL19 in the spleen was reduced following chemotherapy. (A) Spleen cryostat sections from control mice or test mice (treated with Cis, Gem or 5-FU) were stained for CCL21 (red) and fibroblast antibodies (ER-TR7; green), which were imaged by confocal microscopy. Scale bar, 200 µm. Expression of (B) CCL21 and (C) CCL19 in the spleens of control and test mice in tissue homogenates was further detected by ELISA. One-way analysis of variance, followed by Dunnett's multiple comparisons test, was used for all analyses. Error bars represent the standard error of the mean. *P<0.05, **P<0.01, ***P<0.001. n.s., not significant. Con, control; Cis, cisplatin; Gem, gemcitabine; 5-FU, fluorouracil, CCL21, C-C motif chemokine ligand 21; CCL19, C-C motif chemokine ligand 19.
Chemotherapy does not affect the homing ability of T cells to the lymph nodes or chemokine expression in the lymph nodes
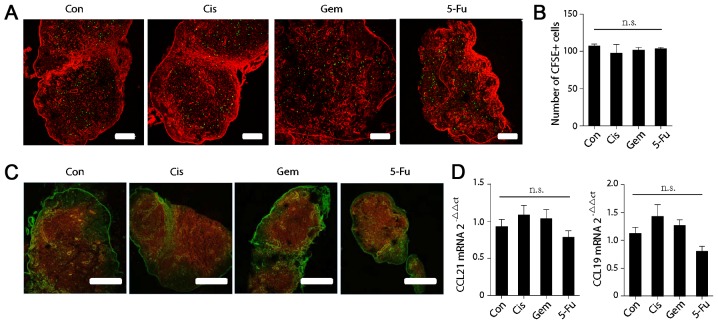
In addition to the spleen, another major constituent of SLOs are the lymph nodes. The homing ability of T cells to the lymphoids was further examined by immune fluorescence and it was revealed that the count of CFSE-positive T cells in the T cell zone of the lymphoids was not significantly altered following chemotherapy (Fig. 3A and B). Additionally, the expression of CCL21 and CCL19 in the lymph nodes was detected by immune fluorescence (Fig. 3C) and RT-qPCR (Fig. 3D), and no significant changes were identified following chemotherapy (Fig. 3C and D).
Figure 3.
Chemotherapy does not affect the homing ability of T cells to the lymph nodes or chemokine expression in the lymph nodes. (A) The localization of CFSE+ cells in the lymph nodes of control or test mice was ascertained 8 h after T cells transfer by immunofluorescence. Scale bar, 200 µm. (B) Counts of CFSE+ cells in the in the lymph nodes of control or test mice. (C) Lymph node cryostat sections from control or test mice were stained for CCL21 (red) and fibroblast antibodies (ER-TR7; green). (D) Chemokine expression in the lymph nodes of control or test mice was quantified by reverse transcription-quantitative polymerase chain reaction. One-way analysis of variance, followed by Dunnett's multiple comparisons test, was used for all analyses. Error bars represent the standard error of the mean. n.s., not significant. CCL21, C-C motif chemokine ligand 21; CCL19, C-C motif chemokine ligand 19; CFSE, carboxyfluorescein succinimidyl ester.
Chemotherapy affects the function and survival of FRCs
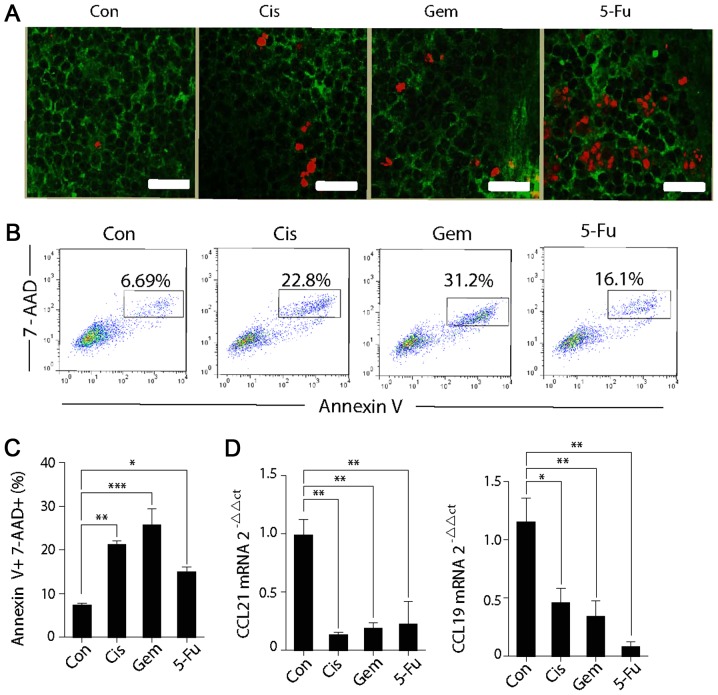
Certain anticancer agents, in addition to their direct cytotoxic effects on tumor cells, may also affect other normal cells. As chemokines, CCL21 and CCL19 are primarily secreted by gp38+ FRCs. Additionally, the present study investigated whether conventional anticancer agents were also able to affect FRCs. Immune fluorescence also demonstrated that the TUNEL expression of gp38+ FRCs in the T-cell zone were significantly higher following chemotherapy when compared with the control group, suggesting that chemotherapy induces the apoptosis of FRCs (Fig. 4A). Splenic stromal cells were cultured and the gp38+CD31− FRCs was selected. Following the successful culture, the FRCs with a specific chemotherapeutic drug were cultured for 24 h and cell death was detected. It is evident that the proportions of Annexin V+7-AAD+ cells following chemotherapeutic treatment are all significantly higher when compared with the control group (Fig. 4B and C). The FRCs were collected following co-culture with the chemotherapeutic drugs, and the mRNA levels of CCL21 and CCL19 were detected. The expression levels of the two chemokines were demonstrated to be significantly reduced (Fig. 4D). It may be deduced that chemotherapeutic drugs are able to induce injury to FRCs by causing cell death and weakening the chemokine-secreting ability. Therefore, the chemokine expression in the SLOs is downregulated, leading to the weakened homing ability of T cells to the spleen.
Figure 4.
Cytotoxic chemotherapy affects the function and survival of FRCs. (A) Spleen cryostat sections from control mice or chemotherapy-treated mice (Cis, Gem or 5-FU) were stained for TUNEL (red) and gp38 (green), which were imaged by confocal microscopy. Scale bar, 75 µm. (B) FRCs were co-cultured with Cis, Gem or 5-FU in vitro for 24 h, and then the expression in Annexin V+7-AAD+ cells were detected via flow cytometry. (C) The percentages of Annexin V+7-AAD+ FRCs in different groups. (D) Chemokine expression in the FRCs after 24 h of chemotherapeutic culture in vitro was quantified by reverse transcription-quantitative polymerase chain reaction. One-way analysis of variance, followed by Dunnett's multiple comparisons test, was used for all analyses. Error bars represent the standard error of the mean. *P<0.05; **P<0.01; ***P<0.001; n.s., not significant. FRCs, fibroblastic reticular cells; Con, control; Cis, cisplatin; Gem, gemcitabine; 5-FU, fluorouracil CCL21, C-C motif chemokine ligand 21; CCL19, C-C motif chemokine ligand 19.
Discussion
In addition to killing tumor cells, chemotherapeutic drugs, when they reach the blood, are also able to kill other cells, including immune cells, in normal tissues without any selectivity. This is considered to be the main reason for the ability of chemotherapy to weaken immune ability in vivo (7,25,26).
SLOs serve a key role in the immune response. The generation of immune responses requires the encounter of rare antigen-specific T cells with DCs, presenting the appropriate antigen. The spontaneous encounter between them is rare in vivo and only occurs in the SLOs (4,27,28). However, to the best of our knowledge, no previous studies have detailed the effects of chemotherapy on SLOs. The results of the present study demonstrated that chemotherapy weakens the homing ability of T cells to the T-cell zone of the spleen. This change may decrease the probability of contact between antigen-specific T lymphocytes and antigen-presenting cells carrying corresponding antigens, thereby further weakening the T cell immune response in vivo.
Naive T cells express CCR7 while SLOs highly express its ligands CCL21 and CCL19 (3). This explains the effective homing ability of naive T cells to SLOs. The secretion of these chemokines in SLOs is not constant, but may be changed in different situations. For instance, upon infection, the reactivated T cells will secrete abundant interferon-γ, leading to the downregulated expression of CCL21 and CCL19, and the subsequent downregulation of the immune response of T cells upon the next infection (11,29,30). Additionally, splenic expression of CCL21 and CCL19 is also reduced in inflammatory melanomas; however, the reasons for this have yet to be elucidated. The results of the present study demonstrated that the splenic expression of CCL21 and CCL19 is reduced following chemotherapy and provided evidence that chemotherapy may downregulate the homing ability of T cells to the splenic SLOs, and that this is primarily caused by the downregulation of chemokines in splenic T cells.
SLOs contain several compartments characterized by specific resident stromal cells, and the most important compartments are the B-cell and T-cell zones (16). The B-cell zone is composed of follicular dendritic cells, which produce C-X-C chemokine ligand 13 to attract B cells. The T-cell zone (paracortex) is rich in FRCs that express the chemokine ligands CCL19 and CCL21 in order to attract naive T cells (31,32). The expression of CCL21 and CCL19 by FRCs in the splenic T cell zone and lymph nodes facilitates the effective interaction between DCs and T cells (17). In addition to killing tumor cells, chemotherapy, when reaching the blood, is also able to kill other normal tissue cells without any selectivity (6,7). The results of the present study demonstrated that chemotherapeutic drugs also induce injury to FRCs, causing cell death and weakening the ability of chemokine secretion. This also explains why the expression of chemokines in SLOs is reduced following chemotherapy and further suggests that, in addition to lymphocytopenia, chemotherapeutic drugs also affect the immune response of T cells through their impact on SLOs.
In addition to the spleen, another major component of SLOs is lymph nodes. However, the results of the present study suggested that chemotherapy does not affect the homing ability of T cells to the lymph nodes or the chemokine expression in the lymph nodes. We hypothesized that this may be due to the difference in anatomic structures between the lymph nodes and the spleen. The spleen is rich in blood vessels; it filters the old red blood cells and directly interacts with pathogens, small grains and foreign antigens in the blood (22). As a result, it is hypothesized that the concentrations of chemotherapeutic drugs are higher in the spleen compared with concentrations in the lymph nodes. Therefore, chemotherapeutic drugs exert different effects on the chemokine-secretory ability of the spleen and the lymph nodes.
To conclude, cytotoxic chemotherapy may weaken the homing ability of T cells to the T-cell zone of the spleen. This is attributed to the reduced expression of CCL21 and CCL19 in the spleen of SLOs. Furthermore, the function and survival of FRCs in SLOs, which are the main source of CCL21 and CCL19, are also affected by cytotoxic chemotherapy, thereby explaining why the expression of CCL21 and CCL19 in the spleen is downregulated. These observations aid in improving understanding of the mechanism underlying the decreased T cell immune response following repeated cycles of chemotherapy.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Nature Science Foundation of China (grant nos. 81472648 and 81500089), the Research on The Basis and Frontier of Chongqing (grant no. cstc2016jcyjA0049).
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
BG and BZ designed the study. LL and LZ performed experiments and prepared the manuscript. JG, LZ and YY processed the data. CH was involved in the acquisition of data for the flow cytometry. CH and LZ were involved in the preparation and revision of this manuscript. All authors approved the final version of this manuscript.
Ethics approval and consent to participate
All animal experiments were approved by the Animal Ethical and Experimental Committee of the Third Military Medical University (TMMU, Chongqing, China). All animal surgeries were performed under anesthesia with sodium pentobarbital and all efforts were made to minimize suffering.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bodey GP. Infection in cancer patients. A continuing association. Am J Med. 1986;81:11–26. doi: 10.1016/0002-9343(86)90510-3. [DOI] [PubMed] [Google Scholar]
- 2.de Martel C, Franceschi S. Infections and cancer: Established associations and new hypotheses. Crit Rev Oncol Hematol. 2009;70:183–194. doi: 10.1016/j.critrevonc.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 3.Foerster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: Balancing immunity and tolerance. Nat Rev Immunol. 2008;8:362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 4.Foerster R, Schubel A, Breitfeld D, Kremmer E, Renner-Müller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/S0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 5.Brandman JR, Ruckdeschel JC, O'Donnell MR, Horton J. Small cell cancer of lung; rapid tumor necrosis leading to serious pulmonary infections after intensive chemotherapy. NY State J Med. 1981;81:1332–1334. [PubMed] [Google Scholar]
- 6.Vento S, Cainelli F, Ternesgen Z. Lung infections after cancer chemotherapy. Lancet Oncol. 2008;9:982–992. doi: 10.1016/S1470-2045(08)70255-9. [DOI] [PubMed] [Google Scholar]
- 7.Vento S, Cainelli F. Infections in patients with cancer undergoing chemotherapy: Aetiology, prevention, and treatment. Lancet Oncol. 2003;4:595–604. doi: 10.1016/S1470-2045(03)01218-X. [DOI] [PubMed] [Google Scholar]
- 8.De Visser KE, Schumacher TNM, Kruisbeek AM. CD8(+) T cell tolerance and cancer immunotherapy. J Immunother. 2003;26:1–11. doi: 10.1097/00002371-200301000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Marchant A, Goldman M. T cell-mediated immune responses in human newborns: Ready to learn? Clin Exp Immunol. 2005;141:10–18. doi: 10.1111/j.1365-2249.2005.02799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Day TA, Koch M, Nouailles G, Jacobsen M, Kosmiadi GA, Miekley D, Kuhlmann S, Jörg S, Gamradt P, Mollenkopf HJ, et al. Secondary lymphoid organs are dispensable for the development of T-cell-mediated immunity during tuberculosis. Eur J Immunol. 2010;40:1663–1673. doi: 10.1002/eji.201040299. [DOI] [PubMed] [Google Scholar]
- 11.Mueller SN, Hosiawa-Meagher KA, Konieczny BT, Sullivan BM, Bachmann MF, Locksley RM, Ahmed R, Matloubian M. Regulation of homeostatic chemokine expression and cell trafficking during immune responses. Science. 2007;317:670–674. doi: 10.1126/science.1144830. [DOI] [PubMed] [Google Scholar]
- 12.Zhao L, Liu L, Guo B, Zhu B. Regulation of adaptive immune responses by guiding cell movements in the spleen. Frontiers Microbiol. 2015;6 doi: 10.3389/fmicb.2015.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siegert S, Luther SA. Positive and negative regulation of T cell responses by fibroblastic reticular cells within paracortical regions of lymph nodes. Front Immunol. 2012;3:285. doi: 10.3389/fimmu.2012.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malhotra D, Fletcher AL, Astarita J, Lukacs-Kornek V, Tayalia P, Gonzalez SF, Elpek KG, Chang SK, Knoblich K, Hemler ME, et al. Transcriptional profiling of stroma from inflamed and resting lymph nodes defines immunological hallmarks. Nat Immunol. 2012;13:499–510. doi: 10.1038/ni.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cesta MF. Normal structure, function, and histology of the spleen. Toxicol Pathol. 2006;34:455–465. doi: 10.1080/01926230600867743. [DOI] [PubMed] [Google Scholar]
- 16.den Haan JM, Mebius RE, Kraal G. Stromal cells of the mouse spleen. Front Immunol. 2012;3:201. doi: 10.3389/fimmu.2012.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luther SA, Bidgol A, Hargreaves DC, Schmidt A, Xu Y, Paniyadi J, Matloubian M, Cyster JG. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J Immunol. 2002;169:424–433. doi: 10.4049/jimmunol.169.1.424. [DOI] [PubMed] [Google Scholar]
- 18.Bajenoff M, Egen JG, Koo LY, Laugier JP, Brau F, Glaichenhaus N, Germain RN. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. 2006;25:989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bepler G, Kusmartseva I, Sharma S, Gautam A, Cantor A, Sharma A, Simon G. RRM1 modulated in vitro and in vivo efficacy of gemcitabine and platinum in non-small-cell lung cancer. J Clin Oncol. 2006;24:4731–4737. doi: 10.1200/JCO.2006.06.1101. [DOI] [PubMed] [Google Scholar]
- 20.Barry MA, Behnke CA, Eastman A. Activation of programmed cell death (apoptosis) by cisplatin, other anticancer drugs, toxins and hyperthermia. Biochem Pharmacol. 1990;40:2353–2362. doi: 10.1016/0006-2952(90)90733-2. [DOI] [PubMed] [Google Scholar]
- 21.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rébé C, Ghiringhelli F. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–3061. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 22.Zhao L, Gao J, Li Y, Liu L, Yang Y, Guo B, Zhu B. Disrupted homeostatic cytokines expression in secondary lymph organs during HIV infection. Int J Mol Sci. 2016;17:413. doi: 10.3390/ijms17030413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao L, Chen J, Liu L, Gao J, Guo B, Zhu B. Essential role of TNF-alpha in development of spleen fibroblastic reticular cells. Cell Immunol. 2015;293:130–136. doi: 10.1016/j.cellimm.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Shitara K, Matsuo K, Oze I, Mizota A, Kondo C, Nomura M, Yokota T, Takahari D, Ura T, Muro K. Meta-analysis of neutropenia or leukopenia as a prognostic factor in patients with malignant disease undergoing chemotherapy. Cancer Chemother Pharmacol. 2011;68:301–307. doi: 10.1007/s00280-010-1487-6. [DOI] [PubMed] [Google Scholar]
- 26.Bruserud O, Bergheim J, Shammas FV, Nesthus I. Serum concentrations of tumour necrosis factor-alpha during chemotherapy-induced leukopenia in patients with acute leukaemia and bacterial infections. Leuk Res. 1994;18:415–421. doi: 10.1016/0145-2126(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 27.De Togni P, Goellner J, Ruddle NH, Streeter PR, Fick A, Mariathasan S, Smith SC, Carison R, Shonnick LP, Strauss-Schoenberger J, et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264:703–707. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- 28.Stranford S, Ruddle NH. Follicular dendritic cells, conduits, lymphatic vessels, and high endothelial venules in tertiary lymphoid organs: Parallels with lymph node stroma. Frontiers Immunol. 2012;3:350. doi: 10.3389/fimmu.2012.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bronte V, Pittet MJ. The spleen in local and systemic regulation of immunity. Immunity. 2013;39:806–818. doi: 10.1016/j.immuni.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao L, Liu L, Gao J, Yang Y, Hu C, Guo B, Zhu B. T lymphocytes maintain structure and function of fibroblastic reticular cells via lymphotoxin (LT)-B. BMC Immunol. 2014;15:33–33. doi: 10.1186/s12865-014-0033-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malhotra D, Fletcher AL, Turley SJ. Stromal and hematopoietic cells in secondary lymphoid organs: Partners in immunity. Immunol Rev. 2013;251:160–176. doi: 10.1111/imr.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turley SJ, Fletcher AL, Elpek KG. The stromal and haematopoietic antigen-presenting cells that reside in secondary lymphoid organs. Nat Rev Immunol. 2010;10:813–825. doi: 10.1038/nri2886. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.