Abstract
Carbon cycling responses of ecosystems to global warming will likely be stronger in cold ecosystems where many processes are temperature‐limited. Predicting these effects is difficult because air and soil temperatures will not change in concert, and will affect above and belowground processes differently. We disentangled above and belowground temperature effects on plant C allocation and deposition of plant C in soils by independently manipulating air and soil temperatures in microcosms planted with either Leucanthemopsis alpina or Pinus mugo seedlings. Daily average temperatures of 4 or 9°C were applied to shoots and independently to roots, and plants pulse‐labelled with 14 CO 2. We traced soil CO 2 and 14 CO 2 evolution for 4 days, after which microcosms were destructively harvested and 14C quantified in plant and soil fractions. In microcosms with L. alpina, net 14C uptake was higher at 9°C than at 4°C soil temperature, and this difference was independent of air temperature. In warmer soils, more C was allocated to roots at greater soil depth, with no effect of air temperature. In P. mugo microcosms, assimilate partitioning to roots increased with air temperature, but only when soils were at 9°C. Higher soil temperatures also increased the mean soil depth at which 14C was allocated. Our findings highlight the dependence of C uptake, use, and partitioning on both air and soil temperature, with the latter being relatively more important. The strong temperature‐sensitivity of C assimilate use in the roots and rhizosphere supports the hypothesis that cold limitation on C uptake is primarily mediated by reduced sink strength in the roots. We conclude that variations in soil rather than air temperature are going to drive plant responses to warming in cold environments, with potentially large changes in C cycling due to enhanced transfer of plant‐derived C to soils.
Keywords: air‐soil temperature interaction, growth, Leucanthemopsis alpina, photosynthesis, Pinus mugo, soil and root respiration
1. INTRODUCTION
In terrestrial ecosystems, most carbon (C) cycling processes are temperature‐sensitive, with lower rates observed at low temperature. Conversely, warming due to climate change is expected to accelerate C cycling, and these changes likely will be particularly large in cold ecosystem because these are most temperature‐limited in the first place (IPCC, 2013). The temperature sensitivity of isolated processes such as photosynthesis and respiration are relatively well studied (e.g., Davidson & Janssens, 2006; Yamori, Hikosaka, & Way, 2014). How these individual responses combine in complex natural ecosystems under realistic scenarios is less well understood, and the ultimate consequences of climate warming for future C cycling and ecosystem functioning therefore remain difficult to predict (Chapin et al., 2009).
Using meta‐analysis, Rustad, Campbell, Marion, and Norby (2001) compiled data from 20 studies that spanned a wide latitudinal and climatic range and found that, on average, biomass production increased by 19% under warming, with the largest values in cold ecosystems. Together with productivity, soil respiration and N mineralization also increased in many studies. The authors argued that plant productivity responses may have arisen from increased photosynthetic rates, longer growing seasons in studies with year‐round warming, and from improved plant N supply due to higher soil microbial activity and mineralization rates. More recent field experiments in alpine and artic conditions corroborate positive effects of warming on plant productivity (Dawes, Philipson, Fonti, & Bebi, 2015; Hudson, Henry, & Cornwell, 2011; Natali, Schuur, & Rubin, 2012; Sistla et al., 2013). For example, Sistla et al. (2013) found a 50% increase in aboveground vascular plant biomass in Alaskan tussock tundra after 14 years of experimental warming.
Soil microbial respiration exhibits a strong temperature dependency under controlled laboratory conditions (Kirschbaum, 1995). This temperature sensitivity is particularly large at low temperatures, suggesting the possibility of large ecosystem‐level C losses from cold ecosystem in which soil organic matter turns over only slowly. Warming thus may accelerate both primary production and decomposition, so that the ecosystem‐level consequences of these changes will depend on their relative magnitude (Crowther et al., 2016; Kirschbaum, 2000).
The above and belowground C cycle are closely coupled but they may experience different temperature regimes. While aboveground air and belowground soil temperatures are generally related, the degree of coupling depends on many factors including soil moisture (Ochsner, Horton, & Ren, 2001), insulation by snowpack (Maurer & Bowling, 2014), and the ratio of radiative to convective heat fluxes and their modifications by vegetation (Körner, 1998). Air and soil temperature are therefore expected to change differently with climate change (Jungqvist, Oni, Teutschbein, & Futter, 2014; Zhang, Chen, Smith, Riseborough, & Cihlar, 2005). It is therefore often unclear how future temperature regimes should most realistically be simulated (Hoch, 2013; Pumpanen, Heinonsalo, Rasilo, Villemot, & Ilvesniemi, 2012). Open‐top chambers are frequently used to passively warm ecosystems, however, they typically generate larger effects on air temperature than on soil temperature (Hobbie & Chapin, 1998; Hollister & Webber, 2006). In contrast, active warming systems such as buried heating cables predominantly warm soils (Hagedorn et al., 2010; Peterjohn, Melillo, & Steudler, 1994; Rustad & Fernandez, 1998). Overhead infrared lamps directly warm soil and canopy, with air warmed only indirectly (Kimball et al., 2008; Luo et al., 2010). Irrespective of the warming technique adopted, such studies will not allow to unequivocally separate effects of air and soil temperature unless these temperatures are manipulated independently.
Given that air and soil temperature do not change synchronously, a central question is whether air or soil temperature is more important in controlling plant productivity and C allocation. Leaves and the photosynthetic apparatus are affected directly by air temperature. Indeed, CO2 assimilation can increase because of a direct stimulation of photosynthesis in warmer air (Berry & Bjorkman, 1980; Medlyn et al., 2002; Yamori et al., 2014). On the other hand, photosynthetic rates also are controlled by the activity of sinks for assimilates (Iglesias, Lliso, Tadeo, & Talon, 2002; Pammenter, Loreto, & Sharkey, 1993; Repo, Leinonen, Ryyppö, & Finér, 2004; Savitch, Gray, & Huner, 1997; Turnbull, Murthy, & Griffin, 2002). A high assimilate consumption rate often induces an up‐regulation of photosynthesis, whereas inactive sinks cause a down‐regulation which often is paralleled by an accumulation of surplus carbohydrates in leaves. It has been posited that, in cold environments, plant growth may be more temperature‐limited than photosynthesis per se (Grace, 2002; Hoch & Körner, 2009; Hoch, Popp, & Körner, 2002). Low soil temperature may be a particularly critical determinant of this effect because strongly reduced root growth has been found below approximately 6–7°C (Alvarez‐Uria & Körner, 2007; Schenker, Lenz, Körner, & Hoch, 2014). In cold environments, low rates of photosynthesis may thus be the result of a low belowground sink activity in the soil rather than of low air temperatures.
In this study, we aimed at disentangling effects of air and soil temperature on CO2 uptake, allocation of assimilates within plants, and the fate of rhizodeposits in soils. We used experimental microcosms to expose a herbaceous and a woody plant species naturally found at the alpine treeline to temperature treatments. We factorially combined air and soil temperature treatments (levels: 4 and 9°C); this temperature range was chosen because it reflects a range that frequently occurs in the native habitat of the investigated species. We pulse‐labelled plants with 14CO2 and followed the fate of labelled assimilates through the plant‐soil system using liquid scintillation counting and an autoradiographic technique that allows to map the small‐scale belowground distribution of 14C (Hagedorn, Bruderhofer, Ferrari, & Niklaus, 2015; Rime & Niklaus, 2017; Stiehl‐Braun, Powlson, Poulton, & Niklaus, 2011). We focused on the short‐term (~1 week) consequences of both acclimation to temperature and assimilate fate after labelling because we were interested in the relatively immediate mechanisms that govern C allocation. Specifically, we hypothesized that belowground temperature would be a more important determinant of C allocation than aboveground temperature.
2. METHODS
We independently manipulated air and soil temperature of microcosms containing seedlings of either Leucanthemopsis alpina (L., Heywood) or Pinus mugo (Turra). We selected these species because they are typical representatives of nonwoody and woody vegetation that occurs naturally at the tree line where vegetation is presumably shaped by temperature limitations. After an initial conditioning period, we traced the fate of assimilates by pulse‐labelling the microcosms with 14CO2.
2.1. Plant material and microcosm preparation
The preparation of plant material and microcosms is detailed in Supporting Information. All seeds material originated from locations in the Swiss Alps near the treeline (2,000–2,200 m a.s.l.). Plants were transferred to cylindrical microcosms (10 cm diameter × 15 cm length) filled with soil collected at a treeline site where both species co‐occur. Microcosms were kept for 7 months (L. alpina) and 16 months (P. mugo) in a glasshouse at day and night temperatures of 12–14 and 8–10°C, respectively, with a photoperiod of 15 hr. Microcosms were watered regularly and supplied with mineral fertilizer.
2.2. Experimental design
The temperature treatment we applied consisted of average target temperatures of 4 and 9°C. This manipulation was applied to air and soil separately, creating four distinct temperature combinations. When exposed to this treatment, L. alpina and P. mugo plants were 8 months and 4 years old, respectively. By then, the roots of both species filled the whole soil compartment. L. alpina had multiple, heavily branched shoots with lengths of up to 10 cm. The apical shoot of P. mugo reached 10–12 cm in height.
The air temperature treatment and the isotope label were applied with the help of a large acrylic chamber that contained up to eight microcosms (Supporting Information). We had only a single such chamber, and therefore applied the different temperature manipulations in sequential runs. The specific air temperature levels were assigned randomly to consecutive pairs of runs. In total, there were four runs with L. alpina (2012, April 30th–July 7th) and eight runs with P. mugo (2013, February 4th–June 1st), that is, there were two and four replicates for each air temperature. The soil temperature treatment was applied to groups of microcosms within the chamber. Chamber construction and temperature control are described in Supporting Information.
2.3. Temperature treatment and 14CO2 pulse‐labelling
The sides of the microcosms were insulated with 1 cm thick foam to restrict heat fluxes to the top and bottom surface and to prevent lateral gradients in soil temperature. Microcosms were well water‐supplied (60% water holding capacity (WHC), adjusted before the microcosms were placed in the chamber). The chamber was left slightly open to enable air exchange while still allowing for temperature regulation. The diurnal temperature amplitudes achieved were 6°C in air and 2°C in soils (Figure 1) and reflect typical growing‐season conditions found in the Swiss Alps at 2,200 m a.s.l. (Hagedorn et al., 2010). We regularly added water to compensate for water losses and re‐adjusted levels to 60% WHC after 7 days. Then, the chamber was sealed, and 14CO2 released by acidification of a Na2 14CO3 solution in a glass bulb through which chamber air was circulated. The chamber was kept closed for 24 hr during which CO2 concentrations were kept between 300 and 500 ppm (LI‐6200, Licor, Lincoln, NE) by releasing CO2 from unlabeled Na2CO3 when required. This procedure maximized 14CO2 uptake. Then, the chamber was opened, vented, and the temperature treatment continued for another 4 days before the microcosms were harvested destructively.
Figure 1.
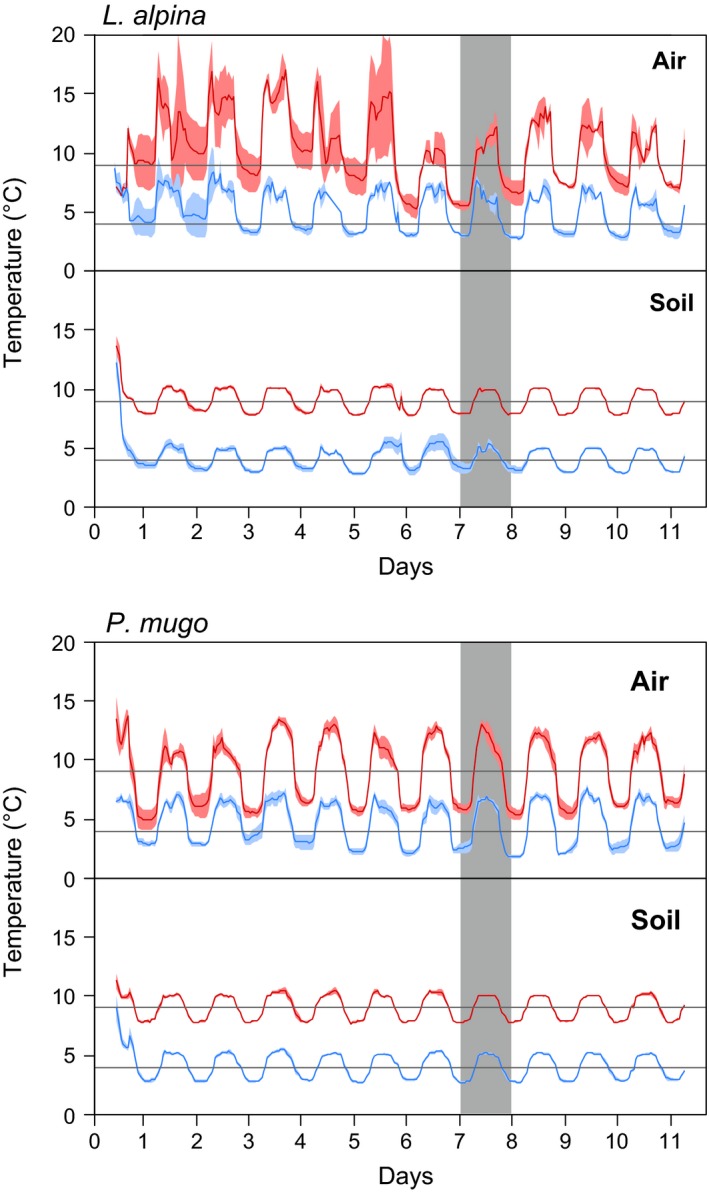
Air and soil temperature over the 11 days of experimental manipulation. Data show averages across blocks, with shaded areas indicating standard errors calculated using blocks as replicate (air temperature: n = 2 in L. alpina and n = 4 in P. mugo: soil temperature: n = 8 in L. alpina and n = 16 in P. mugo). The gray area indicates the day of 14C labelling. Horizontal lines indicate target average temperatures (4 and 9°C)
To label L. alpina microcosms, we released 57.5 kBq 14C per microcosm (but note that the amount of label was controlled at the chamber and not the microcosm level; Supporting Information for a Discussion). Because some of the measured fractions had very low labelling, we later used higher amounts when labelling P. mugo microcosms; specifically, we released 100 kBq 14C per microcosm, or 650 kBq when the chamber included microcosms for the autoradiographic analysis of soil sections (see below).
2.4. Soil respiration
We collected soil respiration (CO2 and 14CO2) in the postlabelling phase of the experiment. In L. alpina microcosms, soil respiration was collected from hydrophobic gas‐permeable tubes (Accurel PP V8/2HF, Membrana GmbH, Wuppertal, Germany) installed horizontally at 5 and 10 cm depth, through which air (2 ml/min) was pumped. The collected CO2 was trapped in 0.5 M NaOH before the now CO2‐free air was circulated back to the tubing. We also trapped soil surface CO2 efflux but did not use these data because of technical difficulties. For the P. mugo microcosms that were labelled later we simplified the setup to a static microchamber (2.7 cm diameter × 6 cm length test tube inserted 3 cm into the ground) which contained a vial with 2 ml 1M NaOH.
NaOH solutions were replaced every 24 hr and the trapped CO2 quantified by acid titration after precipitation of carbonate with BaCl2, using phenolphthalein as indicator (Alef & Nannipieri, 1995). Trapped 14CO2 was determined by liquid scintillation counting of a 1 ml aliquot (TriCarb 2900, Packard BioScience, Meriden, CT; 4 ml Ultima Gold cocktail, Perkin Elmer, Waltham, MA).
2.5. Destructive harvest
Plant shoots were clipped at soil level. The microcosms used for autoradiographic imaging of belowground 14C distribution were immediately frozen and processed as described below. Soil and roots were collected from the remaining microcosms separately from 0–5, 5–10 and 5–15 cm depth sections. Roots were washed, the root‐free soil sieved (2 mm), and an aliquot stored at 4°C for microbial biomass determination. A separate root‐free soil aliquot was dried at 105°C for bulk 14C analysis. All plant material was dried at 75°C and weighed.
2.6. Analysis of plant and soil material
Dry plant and soil material was ground and 14C in samples quantified by liquid scintillation counting (LSC) of 14CO2 produced by dry combustion (Packard 307 sample oxidizer; 6 ml Carbosorb E mixed with 12 ml Permafluor E, Perkin Elmer).
Soil microbial C was determined by chloroform fumigation‐extraction (Vance, Brookes, & Jenkinson, 1987), with some modifications (Supporting Information). For L. alpina, 14C in microbial extracts was below the detection limit, most likely because of the lower amount of 14C applied during pulse‐labelling.
2.7. Autoradiography of soil sections
The frozen and structurally still intact belowground parts of the microcosms were freeze‐dried, embedded in epoxy resin, and a vertical soil section prepared that was oriented vertically through the center of the microcosm (Stiehl‐Braun et al., 2011; Supporting Information for details). This section was used to expose imaging plates that were then scanned at 200 μm resolution. We then recorded the depth distribution of the activity, excluding areas containing the highly‐labelled main root of P. mugo (Supporting Information Figure S5).
2.8. Statistical analyses
Given that response patterns differed among species and that these differences could not unequivocally be attributed to species identity because the species were labelled at different ontogenetic stages and times of the season, we analyzed these data sets separately. All data were analyzed by fitting linear models that reflected the hierarchical nature of the experimental design (aov function with error terms, http://www.r-project.org). The terms fitted were block, soil and air temperature (coded as two‐level factors), and the interaction of soil and air temperature. Block refers to replicates in time, that is, consecutive pairs of runs at low and high air temperature. The error terms fitted were run (the replicate for the air temperature treatment) and run × microcosm pair (the soil temperature treatment was randomly assigned to sets of two microcosms within the chamber). For the analysis of data available at the soil layer, the model was extended with interactions with soil depth, and the corresponding error terms (run × layer as error term for air temperature × depth, and run × microcosm pair × layer as error term for soil temperature × depth).
All dependent variables that quantified amounts of material (e.g., biomass, 14C in specific fractions) were log‐transformed prior to analysis (Supporting Information for Rationale and Implications). Nonsignificant interactions of air and soil temperature thus indicate that relative effects of soil temperature, that is, the percent change from 4 to 9°C, are independent of air temperature. Dependent variables that were ratios (e.g., the fraction of plant 14C that was allocated to roots) were analyzed untransformed because the calculation of the ratio already standardized for the total amount of label. Our experimental design did not allow for tests of air temperature effects on absolute amounts of 14C. The reason is that microcosms exposed to low and high air temperatures were labelled separately. The amount of 14C released in each run therefore largely determined total uptake so that differences in assimilation rates between 4 and 9°C air temperature could not manifest (Discussion in Supporting Information). However, the analysis of the proportional distribution of 14C among ecosystem compartments is unaffected by this caveat because it does not depend on label amounts. This limitation also does not apply to tests of soil temperature effects because the microcosms exposed to low and high soil temperature were exposed to the same atmospheric 14C concentrations, during the same labelling event.
3. RESULTS
We first analyzed effects of the temperature treatments in an overall model with both species. Despite some commonalities, specific response patterns differed among species to an extent that made interpretation and presentation difficult. Also, the experiment was not randomized at the level of species (L. alpina was labelled first, followed by P. mugo). We therefore present results from separate analyses. The main statistical results are summarized in Table 1.
Table 1.
Statistical tests for effects of air temperature (Air), soil temperature (Soil), and their interaction (Air × Soil) in microcosms with either Leucanthemopsis alpina or Pinus mugo
| Fraction | L. alpina | P. mugo | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Air | Soil | Air × Soil | Air | Soil | Air × Soil | |||||||
| df, ddf | F‐value | df, ddf | F‐value | df, ddf | F‐value | df, ddf | F‐value | df, ddf | F‐value | df, ddf | F‐value | |
| Plant biomass | ||||||||||||
| Total | 1, 1 | 6.84 n.s. | 1, 10 | 7.67 * | 1, 10 | 1.04 n.s. | 1, 3 | 0.07 n.s. | 1, 14 | 0.82 n.s. | 1, 14 | 0.87 n.s. |
| Shoots | 1, 1 | 4.13 n.s. | 1, 10 | 3.44 (*) | 1, 10 | 0.22 n.s. | 1, 3 | 0.00 n.s. | 1, 14 | 0.17 n.s. | 1, 14 | 1.49 n.s. |
| Roots | 1, 1 | 0.20 n.s. | 1, 10 | 6.60 * | 1, 10 | 1.14 n.s. | 1, 3 | 0.64 n.s. | 1, 14 | 2.37 n.s. | 1, 14 | 0.08 n.s. |
| Plant 14C | ||||||||||||
| Total | 1, 1 | 2.15 n.s. | 1, 10 | 4.62 (*) | 1, 10 | 0.31 n.s. | 1, 3 | 2.02 n.s. | 1, 14 | 0.77 n.s. | 1, 14 | 0.39 n.s. |
| Shoots | 1, 1 | 1.66 n.s. | 1, 10 | 3.22 n.s. | 1, 10 | 0.32 n.s. | 1, 3 | 7.88 (*) | 1, 14 | 1.67 n.s. | 1, 14 | 5.62 * |
| Roots | 1, 1 | 0.05 n.s. | 1, 10 | 15.05 ** | 1, 10 | 0.42 n.s. | 1, 3 | 43.6 ** | 1, 14 | 0.00 n.s. | 1, 14 | 2.10 n.s. |
| Root fraction | 1, 1 | 0.03 n.s. | 1, 10 | 4.62 (*) | 1, 10 | 0.31 n.s. | 1, 3 | 61.3 ** | 1, 14 | 2.86 n.s. | 1, 14 | 20.2 *** |
| Soil 14C | ||||||||||||
| Total (excl. roots) | 1, 1 | 8.62 n.s. | 1, 10 | 8.64 * | 1, 10 | 0.06 n.s. | 1, 3 | 31.3 * | 1, 14 | 0.18 n.s. | 1, 14 | 1.35 n.s. |
| Soil microbial biomass | – | – | – | 1, 1 | 0.38 n.s. | 1, 8 | 0.00 n.s. | 1, 8 | 0.22 n.s. | |||
| Soil depth dependency of temperature effects | ||||||||||||
| Root biomass | 1, 2 | 5.10 n.s. | 1, 10 | 5.04 * | 1, 10 | 0.05 n.s. | 1, 6 | 0.00 n.s. | 1, 14 | 5.27 * | 1, 14 | 6.89 * |
| Root 14 C | 1, 2 | 0.21 n.s. | 1, 10 | 88.42 *** | 1, 10 | 0.10 n.s. | 1, 6 | 1.87 n.s. | 1, 14 | 0.05 n.s. | 1, 14 | 3.67 (*) |
| Soil 14C | 1, 2 | 2.17 n.s. | 1, 10 | 6.43 * | 1, 10 | 0.11 n.s. | 1, 6 | 0.07 n.s. | 1, 14 | 0.02 n.s. | 1, 14 | 12.58 ** |
Results are shown for the analysis of plant biomass (total = shoots + roots, shoots, and roots), the amount of 14C recovered in these plant fractions plus the root fraction of 14C (14C in roots relative to total plant 14C), and the amount of 14C recovered in soils (total, and soil microbial biomass for P. mugo). For roots and soil 14C, tests for the temperature‐dependency of their depth‐distribution are provided (soil layers: 0–5 cm, 5–10 cm, and 10–15 cm). A significant soil temperature × depth interaction indicates a shift in depth distribution with soil temperature, for example, downwards with increasing temperature. All data were log‐transformed. F‐values are given with nominator and denominator degrees of freedom. ***p < 0.001, **p < 0.01, *p < 0.05, (*) p < 0.1, n.s. p > 0.1). Methods and Results for details.
3.1. L. alpina microcosms
3.1.1. Plant biomass
Air temperature did not affect total plant biomass, that is, the sum of shoot and root mass. However, plant biomass was 20% lower at 4°C soil temperature than at 9°C (p < 0.05, Figure 2). A similar effect was found for root mass (p < 0.05).
Figure 2.
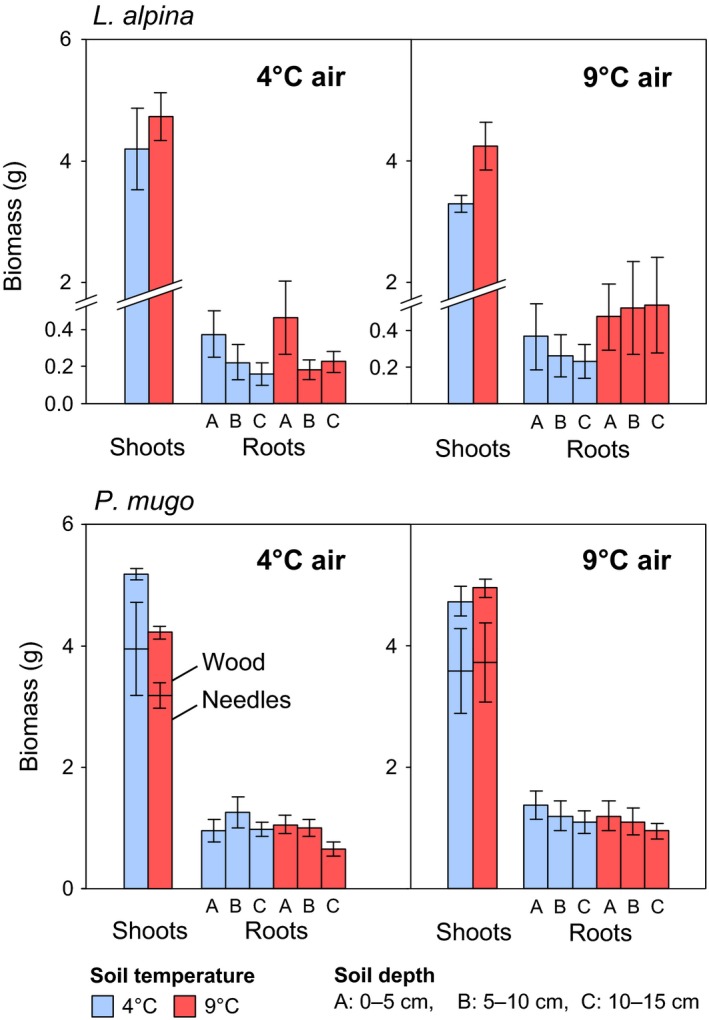
Effects of air and soil temperature on shoot and root biomass of 7‐month old L. alpina and 4‐year‐old P. mugo saplings. Error bars are standard errors (n = 8 for L. alpina, n = 12 for P. mugo)
3.1.2. Distribution of 14C among plant and soil fractions
Total 14C recovered in the microcosms (plant plus soil material) averaged 40% of the activity released. Independent of air and soil temperature, 94% of the microcosm 14C was in plant biomass (6% in soil). Root 14C did not depend on air temperature but increased with soil temperature (p < 0.01, Table 2). A similar trend (p < 0.1) was found for the fraction of plant 14C in roots.
Table 2.
Distribution of 14C recovered in microcosms at final harvest. Data show percentages of totals per labelling run (means ± SE)
| Fraction | Temperature treatments | Treatment averages | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 4°C Air | 9°C Air | Air | Soil | |||||||
| 4°C Soil | 9°C Soil | 4°C Soil | 9°C Soil | 4°C | 9°C | Δ% | 4°C | 9°C | Δ% | |
| L. alpina | ||||||||||
| Shoots | 40.8 ± 7.8 | 47.9 ± 3.5 | 34.5 ± 4.4 | 53.0 ± 8.7 | 44.3 ± 0.1 | 43.8 ± 1.6 | −1 | 37.6 ± 4.3 | 50.4 ± 4.5 | +34 |
| Roots | 1.63 ± 0.32 | 4.24 ± 0.65 | 2.05 ± 0.81 | 3.69 ± 1.38 | 2.93 ± 0.05 | 2.87 ± 1.61 | −2 | 1.84 ± 0.41 | 3.97 ± 0.71 | +115 |
| 0–5 cm | 1.11 ± 0.14 | 1.91 ± 0.33 | 1.43 ± 0.59 | 1.06 ± 0.32 | 1.51 ± 0.21 | 1.24 ± 0.57 | −18 | 1.27 ± 0.29 | 1.48 ± 0.27 | +17 |
| 5–10 cm | 0.30 ± 0.10 | 0.98 ± 0.21 | 0.49 ± 0.22 | 1.48 ± 0.65 | 0.64 ± 0.11 | 0.98 ± 0.65 | +54 | 0.39 ± 0.12 | 1.23 ± 0.33 | +212 |
| 10–15 cm | 0.22 ± 0.08 | 1.35 ± 0.40 | 0.13 ± 0.04 | 1.16 ± 0.48 | 0.79 ± 0.15 | 0.65 ± 0.39 | −18 | 0.18 ± 0.04 | 1.26 ± 0.29 | +612 |
| Soil | 1.86 ± 0.31 | 3.66 ± 0.70 | 2.45 ± 0.48 | 4.25 ± 0.69 | 2.76 ± 0.09 | 3.35 ± 0.05 | +21 | 2.15 ± 0.29 | 3.96 ± 0.47 | +84 |
| 0–5 cm | 0.95 ± 0.19 | 1.35 ± 0.16 | 0.98 ± 0.23 | 1.15 ± 0.31 | 1.15 ± 0.03 | 1.07 ± 0.05 | −7 | 0.96 ± 0.14 | 1.25 ± 0.16 | +30 |
| 5–10 cm | 0.39 ± 0.11 | 1.06 ± 0.33 | 0.72 ± 0.15 | 1.37 ± 0.23 | 0.72 ± 0.02 | 1.05 ± 0.14 | +45 | 0.55 ± 0.11 | 1.22 ± 0.20 | +119 |
| 10–15 cm | 0.52 ± 0.13 | 1.26 ± 0.24 | 0.75 ± 0.20 | 1.72 ± 0.22 | 0.89 ± 0.13 | 1.24 ± 0.03 | +39 | 0.64 ± 0.12 | 1.49 ± 0.18 | +134 |
| P. mugo | ||||||||||
| Shoots | 45.7 ± 8.0 | 33.2 ± 5.2 | 33.5 ± 5.6 | 36.4 ± 6.2 | 44.4 ± 8.8 | 38.9 ± 7.7 | −12 | 39.6 ± 5.0 | 34.8 ± 3.9 | −12 |
| Roots | 19.26 ± 4.79 | 22.23 ± 3.71 | 31.00 ± 7.93 | 23.11 ± 3.92 | 23.54 ± 5.38 | 31.11 ± 7.99 | +32 | 25.13 ± 4.76 | 22.67 ± 2.58 | −10 |
| 0–5 cm | 5.81 ± 0.95 | 9.94 ± 1.90 | 15.99 ± 5.05 | 10.67 ± 2.17 | 8.78 ± 1.66 | 15.81 ± 4.99 | +80 | 10.90 ± 2.89 | 10.30 ± 1.38 | −6 |
| 5–10 cm | 9.17 ± 3.47 | 7.49 ± 1.55 | 9.42 ± 2.08 | 7.00 ± 0.87 | 9.92 ± 3.15 | 9.08 ± 1.85 | −8 | 9.29 ± 1.93 | 7.25 ± 0.85 | −22 |
| 10–15 cm | 4.28 ± 0.70 | 4.80 ± 0.48 | 5.59 ± 1.42 | 5.44 ± 1.21 | 4.84 ± 0.64 | 6.22 ± 1.25 | +28 | 4.93 ± 0.78 | 5.12 ± 0.63 | +4 |
| Soil | 6.86 ± 0.91 | 6.10 ± 0.97 | 4.59 ± 0.67 | 4.74 ± 0.42 | 7.03 ± 1.29 | 4.99 ± 0.60 | −29 | 5.73 ± 0.64 | 5.42 ± 0.55 | −5 |
| 0–5 cm | 3.04 ± 0.90 | 3.77 ± 1.15 | 2.04 ± 0.36 | 1.92 ± 0.40 | 3.90 ± 1.43 | 2.01 ± 0.31 | −48 | 2.54 ± 0.49 | 2.85 ± 0.64 | +12 |
| 5–10 cm | 2.32 ± 0.40 | 1.15 ± 0.13 | 1.80 ± 0.46 | 1.65 ± 0.39 | 1.83 ± 0.24 | 2.02 ± 0.53 | +10 | 2.06 ± 0.30 | 1.40 ± 0.21 | −32 |
| 10–15 cm | 1.51 ± 0.15 | 1.17 ± 0.26 | 0.76 ± 0.13 | 1.17 ± 0.05 | 1.30 ± 0.19 | 0.96 ± 0.11 | −26 | 1.13 ± 0.15 | 1.17 ± 0.13 | +3 |
| Soil microbes | 4.30 ± 1.51 | 3.86 ± 1.36 | 2.98 ± 1.05 | 2.66 ± 0.84 | 4.93 ± 1.55 | 2.93 ± 1.32 | −41 | 3.64 ± 0.90 | 3.26 ± 0.78 | −10 |
For roots and soil organic matter, data are given as totals and separately for the three depth layers (0–5, 5–10, and 10–15 cm). A labelling run includes a single air temperature but two replicates for each soil temperature. The sum of shoots, roots, and soil is 100 (%) for each air temperature. The standard errors provided are based on the statistical replicates, which are pairs of microcosms with equal soil temperature except for air temperature averages where replicates are labelling runs. Methods and Supporting Information for details.
3.1.3. Vertical distribution of 14C in roots and soil
Root biomass decreased with soil depth (F 1,10 = 11.3, p < 0.01, Figure 2). This decrease was independent of air temperature but stronger in cold soils (p < 0.05 for depth × soil temperature). Root 14C also decreased with depth (Table 2, F 1,10 = 148.4, p < 0.001), independent of air temperature. However, the root 14C decrease with depth was only pronounced at 4°C soil temperature with only a small gradient at 9°C (p < 0.001, Table 1, Table 2).
Soil 14C, that is, net rhizodeposition, approximately followed the distribution of root 14C (Table 2) and showed similar temperature effects (air temperature: n.s.; soil temperature × depth: p < 0.05). No soil temperature × depth interactions were found for 14C recorded in autoradiographies (Figure 3).
Figure 3.
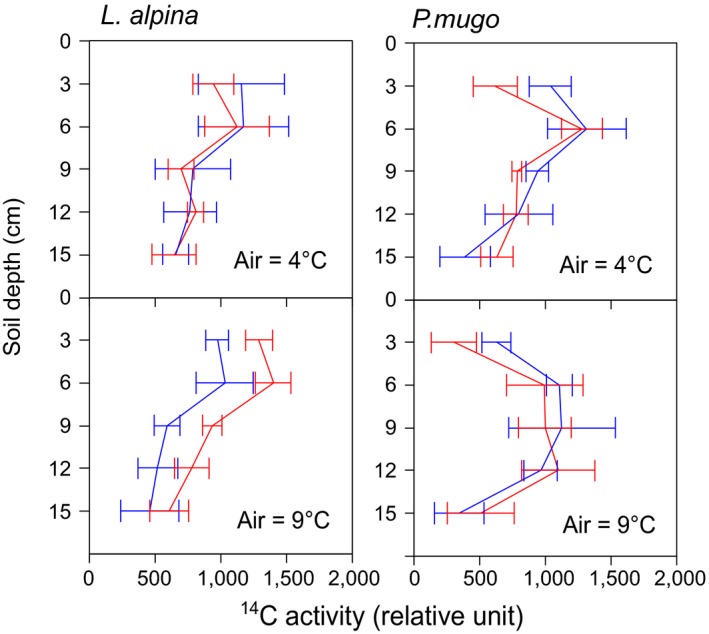
14C distribution over the soil profile, determined by autoradiography (Supporting Information Figure S5 for image examples); data were averaged to 3 cm depth layers. Error bars are standard errors (n = 6 for L. alpina, n = 8 for P. mugo)
3.1.4. Soil respiration
Soil CO2 efflux increased with soil temperature (Figure 4, p < 0.001), and this effect tended to be larger at low than at high air temperature (p < 0.05, for soil × air temperature). Soil 14CO2 efflux also increased with soil temperature (p < 0.01), independent of air temperature. Soil 14CO2 efflux decreased rapidly over time (Figure 4). We quantified temporal decay rates of 14CO2 evolution by fitting a first‐order exponential decay curve; decay rate constants did not vary with air or soil temperature (mean of k = 0.76 d−1).
Figure 4.
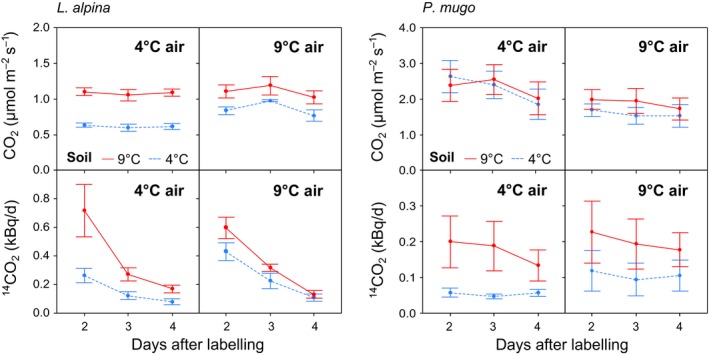
Air and soil temperature effects on soil CO 2 and 14 CO 2 efflux in microcosms planted with L. alpina and P. mugo. Soil respiration was trapped over 24 hr intervals. Data for the first 24 hr after pulse‐labelling (day 1) are not available because respired CO 2 could not be collected without contamination during labelling. Error bars are standard errors (n = 8 for L. alpina, n = 16 for P. mugo)
3.2. P. mugo microcosms
3.2.1. Plant biomass
Neither air temperature nor soil temperature affected total plant biomass, shoot biomass, or root biomass (Figure 2).
3.2.2. Distribution of 14C among plant and soil fractions
Total 14C recovery in the microcosms (plant plus soil material) averaged 46% of the activity originally released. This fraction was independent of air and soil temperature. Independent of air and soil temperature, 91% of microcosm 14C were recovered in plant biomass (9% in soil). A number of statistically significant effects of temperature occurred for plant 14C fractions (Table 1). These were largely driven by a root 14C increase with air temperature that only occurred when soils were at 4°C and manifested in significant main effects of air temperature and interactions between air and soil temperature on root 14C and the root fraction of plant 14C (Table 2). The fraction of microcosm 14C recovered in soil decreased with air temperature (p < 0.05) but was not affected by soil temperature. Microbial biomass accounted for 62% of total soil 14C (Table 2) with no effects of air and soil temperature.
3.2.3. Vertical distribution of 14C in roots and soil
Root biomass slightly decreased with soil depth (p < 0.05, Figure 2), independent of air and soil temperature. Root 14C decreased steeply with soil depth in all air and soil temperature combinations (p < 0.001), but this effect was less regular when both air and soil were at 4°C and 14C amounts were highest in the middle soil layer. Soil 14C approximately followed the distribution of root 14C (Table 2); similar to root 14C, soil 14C decreased least when both air and soil were cold; this manifested in a significant depth × air temperature × soil temperature interaction (p < 0.01).
The autoradiographies of belowground sections (Figure 3) revealed that 14C depth distribution depended on both air (F 4,46 = 2.81, p < 0.05 for depth × air temperature) and soil temperatures (F 4,46 = 4.67, p < 0.01 for depth × soil temperature). Mean 14C allocation depth increased with soil (but not with air) temperature (F 1,7 = 13.4, p < 0.01) from 5.9 to 6.9 cm.
3.2.4. Soil respiration
Soil CO2 efflux was slightly higher in warm soils (Figure 4), but independent of air temperature. Soil 14CO2 efflux increased with soil temperature (p < 0.01), but remained unaffected by air temperature. The rate of decrease in soil 14CO2 efflux over time (Figure 4) was independent of air and soil temperature, although there was a statically nonsignificant trend toward a higher decay rate constants in warmer soils (k = 0.009 ± 0.061 in cold and 0.091 ± 0.056 in warm soils).
4. DISCUSSION
Do air or soil temperature control C allocation in cold environments? We independently manipulated air and soil temperature in experimental microcosms of a nonwoody forb (Leucanthemopsis alpina) and of a tree (Pinus mugo) that both occur naturally at the alpine treeline. While some of the responses that we observed where species‐specific, the general pattern that emerged was that C allocation was more strongly affected by belowground than by aboveground temperature. Our findings therefore suggest that the ultimate mechanisms that control C allocation within the plant, and also the subsequent turnover of rhizodeposits in the soil, are primarily located below ground. Our experiment further demonstrates that these processes are sensitive to temperature changes in the range we studied (approx. 2–10°C). At the alpine treeline, such temperatures are frequently reached in the shoulder season but also around night‐time during peak growing season or during cold spells, indicating that these effects are ecologically relevant.
Low soil temperatures restricted C cycling, in particular belowground. One of the clearest manifestations of this limitation was that the release of 14CO2 strongly increased with soil temperature, in microcosms with both species. Given that plant aboveground and belowground physiological processes are strongly linked, the ultimate physiological drivers of this temperature dependency cannot unambiguously be identified. However, there is compelling evidence that root metabolism is temperature‐dependent (Ericsson, Rytter, & Vapaavuori, 1996; Iivonen, Rikala, Ryyppo, & Vapaavuori, 1999; Pregitzer, King, Burton, & Brown, 2000). In particular, root growth virtually ceases when soil temperatures drop below approximately 6°C (Alvarez‐Uria & Körner, 2007; Schenker et al., 2014; Vapaavuori, Rikala, & Ryyppo, 1992). This mechanism easily explains the temperature effects that we observed. Plant growth, and therefore also root growth, obviously also depend on photosynthesis. However, leaf temperatures are remarkably decoupled from air temperatures (Helliker & Richter, 2008). In hot environments, transpiration substantially reduces leaf temperatures. Conversely, the heating of leaves by absorbed solar radiation is more important in cold environments (Körner, 2003). We did not measure leaf temperatures in our study. Also, our experimental setup did not allow quantifying air temperature effects on the net assimilation of labelled CO2 because net uptake was largely determined by the amount of label released (Methods for details). Nevertheless, air temperatures were well above the freezing point and radiation from overhead lamps was strong, so that we consider it unlikely that air temperature limited CO2 assimilation. However, net assimilation may be reduced indirectly when soil temperatures are low because a low root activity will reduce belowground assimilate consumption (Domisch, Finer, & Lehto, 2001; Hoch & Körner, 2009; Hoch et al., 2002; Kontunen‐Soppela, Lankila, Lähdesmäki, & Laine, 2002).
Another mechanism by which plant growth may be limited at low soil temperature is reduced nutrient supply from organic matter mineralization (Dieleman et al., 2012; Dormann & Woodin, 2002; Melillo, Steudler, Aber, & Newkirk, 2002). Soil microbial activity generally drops at lower temperatures, but how closely the supply of available N to plants tracks the effects of temperature on decomposition is less clear, because net N mineralization depends on microbial turnover through both mineralization and immobilization processes. In our study, exposure of microcosms to the different temperature treatments was short and we therefore think that it is unlikely that soil warming caused a substantial increase in N availability. Furthermore, a general pattern found in plants is that they respond to a shortage of mineral nutrients by increasing root growth relatively to shoot growth. In our study, however, we observed a reduced 14C allocation belowground which suggests that belowground plant activity was limited by factors other than nutrients when soils were cold. This reasoning is compatible with the notion of a temperature‐limitation on tissue growth as was postulated by Körner (1998).
In a soil warming experiment at the alpine treeline, we recently have shown that the temperature sensitivity of rhizosphere respiration is higher than that of total soil respiration between 5 and 10°C soil temperature, but smaller between 10 and 15°C (Ferrari, Hagedorn, & Niklaus, 2016). The pattern we have found between 10 and 15°C is in line with many other studies that also have shown a higher temperature dependency of total soil respiration compared to root and rhizosphere respiration (Hagedorn et al., 2010; Hartley, Heinemeyer, Evans, & Ineson, 2007; Streit et al., 2014; Vogel, Bronson, Gower, & Schuur, 2014; Wang et al., 2014). These seemingly conflicting patterns can be reconciled by assuming that soil microbial activity is generally more temperature sensitive than root activity, except in the temperature range around 4–8°C where root growth ceases relatively abruptly and the pattern therefore is reversed.
In natural ecosystems, soil temperature follows a depth gradient. An interesting consequence of a strong temperature limitations of assimilate investment into roots in cold soil is that soil temperature changes will lead to a change in the depth in which organic matter is deposited. The specific patterns are complicated to predict because soil temperature gradients often reverse during diurnal and seasonal cycles. Soil temperature at depth is relatively well buffered and integrates heat budgets (convective, conductive, and radiative fluxes) over longer time scales. Closer to the soil surface, temperatures are higher at daytime and early in the growing season, whereas the opposite occurs at night and at the end of the season. In our experiment, soil temperatures were relative homogenous over the entire soil profile, varying only over the upper 2–3 cm of soil when air and soil temperatures differed. A direct effect of the air treatment on soil temperatures can therefore be excluded. The root and soil 14C we found in combusted samples and in autoradiographies indicate that C is deposited at larger depth when soils are warmer. This is likely driven by stronger C sink activity in warmer soils, while reduced C sink‐strength in cold soil reduced the downwards transport of recent assimilates.
Many responses to the temperature treatments differed between L. alpina and P. mugo. While woody and nonwoody vegetation clearly differ in functional traits, the two species also were labelled at different times in the growing season and were in different ontogenetic stages. It therefore is not possible to attribute the different effects we found to growth form only (forb vs. tree). A generalization would require a setup with a larger number of species, and ideally multiple labeling events throughout the season and with plants of different age. Nevertheless, our findings suggest that species‐specific responses may contribute to the conflicting observations of temperatures sensitivities of autotrophic and heterotrophic soil respiration (Janssens & Pilegaard, 2003; Schindlbacher, Zechmeister‐Boltenstern, & Jandl, 2009).
Our study addressed short‐term effects of air and soil temperature of C allocation and the processing of rhizodeposits. Extrapolating these to growth and longer time scales is difficult. Some of the applied label will have been in nonstructural fractions used as carbohydrate stores. Whether these would eventually have been allocated to growth in the same organ is unclear. Over longer time scales, effects also could be modified, for example by acclimation. At the population and community level, responses might change further because other genotypes or species are favored. At the ecosystem level, feedback mechanisms including carbon and nutrient dynamics will modify the initial effects we addressed. For example, many studies have found that warming effects on soil respiration decreased with time (Carey et al., 2016; Luo, Wan, Hui, & Wallace, 2001; Romero‐Olivares, Allison, & Treseder, 2017). The mechanisms that have been put forward as explanation include the acclimation of root metabolism (Atkin, Edwards, & Loveys, 2000; Burton, Melillo, & Frey, 2008), the exhaustion of labile soil organic matter pools that fuel microbial respiration (Caprez, Niklaus, & Körner, 2012; Eliasson et al., 2005), and a thermal adaption of soil microbial communities (Bradford et al., 2008; Heinemeyer, Ineson, Ostle, & Fitter, 2006).
In summary, our study suggests that soil temperature is a more important controller of C allocation in cold ecosystems than air temperature. Most of the patterns that we found were compatible with the idea that root metabolism is strongly inhibited below a critical temperature between 4 and 9°C. Given the increasing frequency of extreme meteorological events, which are often associated with a decoupling of above‐ and belowground temperatures, the understanding of both short‐term and long‐term temperature responses appears important for predictions of ecosystem responses to warming. Our results thus emphasize that air and soil temperature variation must be considered separately when assessing ecosystem responses to global change, both in field warming experiments and in numeric models used to simulate plant and ecosystem performance in a future climate.
AUTHOR CONTRIBUTIONS
AF set up the experiment and carried out all laboratory and data analyses, with assistance from PAN. AF wrote the manuscript, with contributions from FH and PAN. FH and PAN conceived the study and wrote the grant that funded the project.
DATA ACCESSIBILITY
All data shown in this article (plant and microbial biomass, 14C distribution, soil and air temperatures) are deposited under Dryad https://doi.org/10.5061/dryad.mk1vd47.
Supporting information
ACKNOWLEDGMENTS
We thank Marcel Freund and Reto Maier for help with the construction of the incubation chamber. Alicia Argüello, René Husi and Saeed Karbin helped with analytical procedures. Patrick Schleppi provided the lyophiliser to freeze‐dry the soil cores. This study was funded by COST‐SBF grant to FH and PAN and additional funding by WSL Birmensdorf and the University of Zurich. PAN acknowledges support from the University of Zurich Priority Program on Global Change and Biodiversity.
Ferrari A, Hagedorn F, Niklaus PA. Disentangling effects of air and soil temperature on C allocation in cold environments: A 14C pulse‐labelling study with two plant species. Ecol Evol. 2018;8:7778–7789. 10.1002/ece3.4215
REFERENCES
- Alef, K. , & Nannipieri, P. (1995). Methods in applied soil microbiology and biochemistry. The Journal of Applied Ecology, 33, 178. [Google Scholar]
- Alvarez‐Uria, P. , & Körner, C. (2007). Low temperature limits of root growth in deciduous and evergreen temperate tree species. Functional Ecology, 21, 211–218. 10.1111/j.1365-2435.2007.01231.x [DOI] [Google Scholar]
- Atkin, O. K. , Edwards, E. J. , & Loveys, B. R. (2000). Response of root respiration to changes in temperature and its relevance to global warming. New Phytologist, 147, 141–154. 10.1046/j.1469-8137.2000.00683.x [DOI] [Google Scholar]
- Berry, J. , & Bjorkman, O. (1980). Photosynthetic response and adaptation to temperature in higher plants. Annual Review of Plant Physiology, 31, 491–543. 10.1146/annurev.pp.31.060180.002423 [DOI] [Google Scholar]
- Bradford, M. A. , Davies, C. A. , Frey, S. D. , Maddox, T. R. , Melillo, J. M. , Mohan, J. E. , … Wallenstein, M. D. (2008). Thermal adaptation of soil microbial respiration to elevated temperature. Ecology Letters, 11, 1316–1327. 10.1111/j.1461-0248.2008.01251.x [DOI] [PubMed] [Google Scholar]
- Burton, A. J. , Melillo, J. M. , & Frey, S. D. (2008). Adjustment of forest ecosystem root respiration as temperature warms. Journal of Integrative Plant Biology, 50, 1467–1483. 10.1111/j.1744-7909.2008.00750.x [DOI] [PubMed] [Google Scholar]
- Caprez, R. , Niklaus, P. A. , & Körner, C. (2012). Forest soil respiration reflects plant productivity across a temperature gradient in the Alps. Oecologia, 170, 1143–1154. 10.1007/s00442-012-2371-3 [DOI] [PubMed] [Google Scholar]
- Carey, J. C. , Tang, J. , Templer, P. H. , Kroeger, K. D. , Crowther, T. W. , Burton, A. J. , … Tietema, A. (2016). Temperature response of soil respiration largely unaltered with experimental warming. Proceedings of the National Academy of Sciences, 113, 13797–13802. 10.1073/pnas.1605365113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin, F. I. , McFarland, J. , McGuire, A. , Euskirchen, E. , Ruess, R. , & Kielland, K. (2009). The changing global carbon cycle: Linking plant‐soil carbon dynamics to global consequences. Journal of Ecology, 97, 840–850. [Google Scholar]
- Crowther, T. W. , Todd‐Brown, K. E. O. , Rowe, C. W. , Wieder, W. R. , Carey, J. C. , Machmuller, M. B. , … Bradford, M. A. (2016). Quantifying global soil carbon losses in response to warming. Nature, 540, 104–108. 10.1038/nature20150 [DOI] [PubMed] [Google Scholar]
- Davidson, E. A. , & Janssens, I. A. (2006). Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature, 440, 165–173. 10.1038/nature04514 [DOI] [PubMed] [Google Scholar]
- Dawes, M. A. , Philipson, C. D. , Fonti, P. , & Bebi, P. (2015). Soil warming and CO2 enrichment induce biomass shifts in alpine tree line vegetation. Global Change Biology, 21, 1–17. [DOI] [PubMed] [Google Scholar]
- Dieleman, W. I. J. , Vicca, S. , Dijkstra, F. A. , Hagedorn, F. , Hovenden, M. J. , Larsen, K. S. , … Janssens, I. A. (2012). Simple additive effects are rare: A quantitative review of plant biomass and soil process responses to combined manipulations of CO2 and temperature. Global Change Biology, 18, 2681–2693. 10.1111/j.1365-2486.2012.02745.x [DOI] [PubMed] [Google Scholar]
- Domisch, T. , Finer, L. , & Lehto, T. (2001). Effects of soil temperature on biomass and carbohydrate allocation in Scots pine (Pinus sylvestris) seedlings at the beginning of the growing season. Tree Physiology, 21, 465–472. 10.1093/treephys/21.7.465 [DOI] [PubMed] [Google Scholar]
- Dormann, C. , & Woodin, S. (2002). Climate change in the Arctic: Using plant functional types in a meta‐analysis of field experiments. Functional Ecology, 16, 4–17. 10.1046/j.0269-8463.2001.00596.x [DOI] [Google Scholar]
- Eliasson, P. E. , McMurtrie, R. E. , Pepper, D. A. , Stromgren, M. , Linder, S. , & Agren, G. I. (2005). The response of heterotrophic CO2 flux to soil warming. Global Change Biology, 11, 167–181. 10.1111/j.1365-2486.2004.00878.x [DOI] [Google Scholar]
- Ericsson, T. , Rytter, L. , & Vapaavuori, E. (1996). Physiology of carbon allocation in trees. Biomass and Bioenergy, 11, 115–127. 10.1016/0961-9534(96)00032-3 [DOI] [Google Scholar]
- Ferrari, A. , Hagedorn, F. , & Niklaus, P. A. (2016). Experimental soil warming and cooling alters the partitioning of recent assimilates: Evidence from a 14C‐labelling study at the alpine treeline. Oecologia, 181, 25–37. 10.1007/s00442-015-3427-y [DOI] [PubMed] [Google Scholar]
- Grace, J. (2002). Impacts of climate change on the tree line. Annals of Botany, 90, 537–544. 10.1093/aob/mcf222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn, F. , Bruderhofer, N. , Ferrari, A. , & Niklaus, P. A. (2015). Tracking litter‐derived dissolved organic matter along a soil chronosequence using 14C imaging: Biodegradation, physico‐chemical retention or preferential flow? Soil Biology and Biochemistry, 88, 333–343. 10.1016/j.soilbio.2015.06.014 [DOI] [Google Scholar]
- Hagedorn, F. , Martin, M. , Rixen, C. , Rusch, S. , Bebi, P. , Zurcher, A. , … Hättenschwiler, S. (2010). Short‐term responses of ecosystem carbon fluxes to experimental soil warming at the Swiss alpine treeline. Biogeochemistry, 97, 7–19. 10.1007/s10533-009-9297-9 [DOI] [Google Scholar]
- Hartley, I. P. , Heinemeyer, A. , Evans, S. P. , & Ineson, P. (2007). The effect of soil warming on bulk soil vs. rhizosphere respiration. Global Change Biology, 13, 2654–2667. 10.1111/j.1365-2486.2007.01454.x [DOI] [Google Scholar]
- Heinemeyer, A. , Ineson, P. , Ostle, N. , & Fitter, A. H. (2006). Respiration of the external mycelium in the arbuscular mycorrhizal symbiosis shows strong dependence on recent photosynthates and acclimation to temperature. New Phytologist, 171, 159–170. 10.1111/j.1469-8137.2006.01730.x [DOI] [PubMed] [Google Scholar]
- Helliker, B. R. , & Richter, S. L. (2008). Subtropical to boreal convergence of tree‐leaf temperatures. Nature, 454, 511–514. 10.1038/nature07031 [DOI] [PubMed] [Google Scholar]
- Hobbie, S. , & Chapin, F. (1998). The response of tundra plant biomass, aboveground production, nitrogen, and CO2 flux to experimental warming. Ecology, 79, 1526–1544. [Google Scholar]
- Hoch, G. (2013). Reciprocal root‐shoot cooling and soil fertilization effects on the seasonal growth of two treeline conifer species. Plant Ecology & Diversity, 6, 21–30. 10.1080/17550874.2011.643324 [DOI] [Google Scholar]
- Hoch, G. , & Körner, C. (2009). Growth and carbon relations of tree line forming conifers at constant vs. variable low temperatures. Journal of Ecology, 97, 57–66. 10.1111/j.1365-2745.2008.01447.x [DOI] [Google Scholar]
- Hoch, G. , Popp, M. , & Körner, C. (2002). Altitudinal increase of mobile carbon pools in Pinus cembra suggests sink limitation of growth at the Swiss treeline. Oikos, 98, 361–374. 10.1034/j.1600-0706.2002.980301.x [DOI] [Google Scholar]
- Hollister, R. , & Webber, P. (2006). Soil thaw and temperature response to air warming varies by plant community: Results from an open‐top chamber experiment in northern Alaska. Arctic, Antarctic, and Alpine Research., 38, 206–215. 10.1657/1523-0430(2006)38[206:STATRT]2.0.CO;2 [DOI] [Google Scholar]
- Hudson, J. M. G. , Henry, G. H. R. , & Cornwell, W. K. (2011). Taller and larger: Shifts in Arctic tundra leaf traits after 16 years of experimental warming. Global Change Biology, 17, 1013–1021. 10.1111/j.1365-2486.2010.02294.x [DOI] [Google Scholar]
- Iglesias, D. , Lliso, I. , Tadeo, F. , & Talon, M. (2002). Regulation of photosynthesis through source: Sink imbalance in citrus is mediated by carbohydrate content in leaves. Physiologia Plantarum, 116, 563–572. 10.1034/j.1399-3054.2002.1160416.x [DOI] [Google Scholar]
- Iivonen, S. , Rikala, R. , Ryyppo, A. , & Vapaavuori, E. (1999). Responses of Scots pine (Pinus sylvestris) seedlings grown in different nutrient regimes to changing root zone temperature in spring. Tree Physiology, 19, 951–958. 10.1093/treephys/19.14.951 [DOI] [PubMed] [Google Scholar]
- IPCC (2013). Summary for policymakers In Stocker T. F., Qin D., Plattner G.‐K., Tignor M., Allen S. K., Boschung J., Nauels A., Xia Y., Bex V. & Midgle P. M. (Eds.), Climate change 2013: The physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK & New York, NY, USA: Cambridge University Press. [Google Scholar]
- Janssens, I. A. , & Pilegaard, K. (2003). Large seasonal changes in Q10 of soil respiration in a beech forest. Global Change Biology, 9, 911–918. 10.1046/j.1365-2486.2003.00636.x [DOI] [Google Scholar]
- Jungqvist, G. , Oni, S. K. , Teutschbein, C. , & Futter, M. N. (2014). Effect of climate change on soil temperature in Swedish boreal forests. PLoS ONE, 9, e93957 10.1371/journal.pone.0093957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball, B. A. , Conley, M. M. , Wang, S. , Lin, X. , Luo, C. , Morgan, J. , & Smith, D. (2008). Infrared heater arrays for warming ecosystem field plots. Global Change Biology, 14, 309–320. [Google Scholar]
- Kirschbaum, M. U. F. (1995). The temperature dependence of soil organic matter decomposition, and the effect of global warming on soil organic C storage. Soil Biology and Biochemistry, 27, 753–760. 10.1016/0038-0717(94)00242-S [DOI] [Google Scholar]
- Kirschbaum, M. (2000). Will changes in soil organic carbon act as a positive or negative feedback on global warming? Biogeochemistry, 48, 21–51. 10.1023/A:1006238902976 [DOI] [Google Scholar]
- Kontunen‐Soppela, S. , Lankila, J. , Lähdesmäki, P. , & Laine, K. (2002). Response of protein and carbohydrate metabolism of Scots pine seedlings to low temperature. Journal of Plant Physiology, 159, 175–180. 10.1078/0176-1617-00538 [DOI] [Google Scholar]
- Körner, C. (1998). A re‐assessment of high elevation treeline positions and their explanation. Oecologia, 115, 445–459. [DOI] [PubMed] [Google Scholar]
- Körner, C. (2003). Alpine plant life: Functional plant ecology of high mountain ecosystems, 2nd ed. Heidelberg, DE: Springer; 10.1007/978-3-642-18970-8 [DOI] [Google Scholar]
- Luo, Y. , Wan, S. , Hui, D. , & Wallace, L. L. (2001). Acclimatization of soil respiration to warming in a tall grass prairie. Nature, 413, 622–625. 10.1038/35098065 [DOI] [PubMed] [Google Scholar]
- Luo, C. , Xu, G. , Chao, Z. , Wang, S. , Lin, X. , Hu, Y. , … Kimball, B. (2010). Effect of warming and grazing on litter mass loss and temperature sensitivity of litter and dung mass loss on the Tibetan plateau. Global Change Biology, 16, 1606–1617. 10.1111/j.1365-2486.2009.02026.x [DOI] [Google Scholar]
- Maurer, G. E. , & Bowling, D. R. (2014). Seasonal snowpack characteristics influence soil temperature and water content at multiple scales in interior western U.S. mountain ecosystems. Water Resources Research, 50, 5216–5234. 10.1002/2013WR014452 [DOI] [Google Scholar]
- Medlyn, B. E. , Dreyer, E. , Ellsworth, D. , Forstreuter, M. , Harley, P. C. , Kirschbaum, M. U. F. , … Loustau, D. (2002). Temperature response of parameters of a biochemically based model of photosynthesis. II. A review of experimental data. Plant, Cell and Environment, 25, 1167–1179. 10.1046/j.1365-3040.2002.00891.x [DOI] [Google Scholar]
- Melillo, J. , Steudler, P. , Aber, J. , & Newkirk, K. (2002). Soil warming and carbon‐cycle feedbacks to the climate system. Science, 298, 2173–2176. 10.1126/science.1074153 [DOI] [PubMed] [Google Scholar]
- Natali, S. M. , Schuur, E. A. G. , & Rubin, R. L. (2012). Increased plant productivity in Alaskan tundra as a result of experimental warming of soil and permafrost. Journal of Ecology, 100, 488–498. 10.1111/j.1365-2745.2011.01925.x [DOI] [Google Scholar]
- Ochsner, T. E. , Horton, R. , & Ren, T. (2001). A new perspective on soil thermal properties. Soil Science Society of America Journal, 65, 1641 10.2136/sssaj2001.1641 [DOI] [Google Scholar]
- Pammenter, N. W. , Loreto, F. , & Sharkey, T. D. (1993). End product feedback effects on photosynthetic electron transport. Photosynthesis Research, 35, 5–14. 10.1007/BF02185407 [DOI] [PubMed] [Google Scholar]
- Peterjohn, W. , Melillo, J. , & Steudler, P. (1994). Responses of trace gas fluxes and N availability to experimentally elevated soil temperatures. Ecological Applications, 4, 617–625. 10.2307/1941962 [DOI] [Google Scholar]
- Pregitzer, K. S. , King, J. S. , Burton, A. J. , & Brown, S. E. (2000). Responses of tree fine roots to temperature. New Phytologist, 147, 105–115. 10.1046/j.1469-8137.2000.00689.x [DOI] [Google Scholar]
- Pumpanen, J. , Heinonsalo, J. , Rasilo, T. , Villemot, J. , & Ilvesniemi, H. (2012). The effects of soil and air temperature on CO2 exchange and net biomass accumulation in Norway spruce, Scots pine and silver birch seedlings. Tree Physiology, 32, 724–736. 10.1093/treephys/tps007 [DOI] [PubMed] [Google Scholar]
- Repo, T. , Leinonen, I. , Ryyppö, A. , & Finér, L. (2004). The effect of soil temperature on the bud phenology, chlorophyll fluorescence, carbohydrate content and cold hardiness of Norway spruce seedlings. Physiologia Plantarum, 121, 93–100. 10.1111/j.0031-9317.2004.00307.x [DOI] [PubMed] [Google Scholar]
- Rime, T. , & Niklaus, P. A. (2017). Spatio‐temporal dynamics of soil CH4 uptake after application of N fertilizer with and without the nitrification inhibitor 3,4‐ dimethylpyrazole phosphate (DMPP). Soil Biology and Biochemistry, 104, 218–225. 10.1016/j.soilbio.2016.11.001 [DOI] [Google Scholar]
- Romero‐Olivares, A. L. , Allison, S. D. , & Treseder, K. K. (2017). Soil microbes and their response to experimental warming over time: A meta‐analysis of field studies. Soil Biology and Biochemistry, 107, 32–40. 10.1016/j.soilbio.2016.12.026 [DOI] [Google Scholar]
- Rustad, L. , Campbell, J. , Marion, G. , & Norby, R. (2001). A meta‐analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia, 126, 543–562. 10.1007/s004420000544 [DOI] [PubMed] [Google Scholar]
- Rustad, L. E. , & Fernandez, I. J. (1998). Experimental soil warming effects on CO2 and CH4 flux from a low elevation spruce‐fir forest soil in Maine, USA. Global Change Biology, 4, 597–605. 10.1046/j.1365-2486.1998.00169.x [DOI] [Google Scholar]
- Savitch, L. , Gray, G. , & Huner, N. (1997). Feedback‐limited photosynthesis and regulation of sucrose‐starch accumulation during cold acclimation and low‐temperature stress in a spring and winter wheat. Planta, 201, 18–26. 10.1007/BF01258676 [DOI] [Google Scholar]
- Schenker, G. , Lenz, A. , Körner, C. , & Hoch, G. (2014). Physiological minimum temperatures for root growth in seven common European broad‐leaved tree species. Tree Physiology, 34, 302–313. 10.1093/treephys/tpu003 [DOI] [PubMed] [Google Scholar]
- Schindlbacher, A. , Zechmeister‐Boltenstern, S. , & Jandl, R. (2009). Carbon losses due to soil warming: Do autotrophic and heterotrophic soil respiration respond equally? Global Change Biology, 15, 901–913. 10.1111/j.1365-2486.2008.01757.x [DOI] [Google Scholar]
- Sistla, S. A. , Moore, J. C. , Simpson, R. T. , Gough, L. , Shaver, G. R. , & Schimel, J. P. (2013). Long‐term warming restructures Arctic tundra without changing net soil carbon storage. Nature, 497, 615–618. 10.1038/nature12129 [DOI] [PubMed] [Google Scholar]
- Stiehl‐Braun, P. A. , Powlson, D. S. , Poulton, P. R. , & Niklaus, P. A. (2011). Effects of N fertilizers and liming on the micro‐scale distribution of soil methane assimilation in the long‐term Park Grass experiment at Rothamsted. Soil Biology and Biochemistry, 43, 1034–1041. 10.1016/j.soilbio.2011.01.020 [DOI] [Google Scholar]
- Streit, K. , Hagedorn, F. , Hiltbrunner, D. , Portmann, M. , Saurer, M. , Buchmann, N. , … Siegwolf, R. T. W. (2014). Soil warming alters microbial substrate use in alpine soils. Global Change Biology, 20, 1327–1338. 10.1111/gcb.12396 [DOI] [PubMed] [Google Scholar]
- Turnbull, M. , Murthy, R. , & Griffin, K. (2002). The relative impacts of daytime and night‐time warming on photosynthetic capacity in Populus deltoides. Plant, Cell & Environment, 25, 1729–1737. 10.1046/j.1365-3040.2002.00947.x [DOI] [Google Scholar]
- Vance, E. , Brookes, P. , & Jenkinson, D. (1987). An extraction method for measuring soil microbial biomass C. Soil Biology and Biochemistry, 19, 703–707. 10.1016/0038-0717(87)90052-6 [DOI] [Google Scholar]
- Vapaavuori, E. M. , Rikala, R. , & Ryyppo, A. (1992). Effects of root temperature on growth and photosynthesis in conifer seedlings during shoot elongation. Tree Physiology, 10, 217–230. 10.1093/treephys/10.3.217 [DOI] [PubMed] [Google Scholar]
- Vogel, J. G. , Bronson, D. , Gower, S. T. , & Schuur, E. A. G. (2014). The response of root and microbial respiration to the experimental warming of a boreal black spruce forest. Canadian Journal of Forest Research, 44, 986–993. 10.1139/cjfr-2014-0056 [DOI] [Google Scholar]
- Wang, X. , Liu, L. , Piao, S. , Janssens, I. A. , Tang, J. , Liu, W. , … Xu, S. (2014). Soil respiration under climate warming: Differential response of heterotrophic and autotrophic respiration. Global Change Biology, 20, 3229–3237. 10.1111/gcb.12620 [DOI] [PubMed] [Google Scholar]
- Yamori, W. , Hikosaka, K. , & Way, D. A. (2014). Temperature response of photosynthesis in C3, C4, and CAM plants: Temperature acclimation and temperature adaptation. Photosynthesis Research, 119, 101–117. 10.1007/s11120-013-9874-6 [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Chen, W. , Smith, S. L. , Riseborough, D. W. , & Cihlar, J. (2005). Soil temperature in Canada during the twentieth century: Complex responses to atmospheric climate change. Journal of Geophysical Research D: Atmospheres, 110, 1–15. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data shown in this article (plant and microbial biomass, 14C distribution, soil and air temperatures) are deposited under Dryad https://doi.org/10.5061/dryad.mk1vd47.


