Abstract
In the animal kingdom, behavioral variation among individuals has often been reported. However, stable among‐individual differences along a behavioral continuum—reflective of personality variation—have only recently become a key target of research. While a vast body of descriptive literature exists on animal personality, hypothesis‐driven quantitative studies are largely deficient. One of the main constraints to advance the field is the lack of suitable model organisms. Here, we explore whether N. furzeri could be a valuable model to bridge descriptive and hypothesis‐driven research to further unravel the causes, function and evolution of animal personality. As a first step toward this end, we perform a common garden laboratory experiment to examine if behavioral variation in the turquoise killifish Nothobranchius furzeri reflects personality divergence. Furthermore, we explore if multiple behavioral traits are correlated. We deliver “proof of principle” of personality variation among N. furzeri individuals in multiple behavioral traits. Because of the vast body of available genomic and physiological information, the well‐characterized ecological background and an exceptionally short life cycle, N. furzeri is an excellent model organism to further elucidate the causes and implications of behavioral variation in an eco‐evolutionary context.
Keywords: animal personality, behavioral ecology, behavioral variation, Nothobranchius, repeatability
1. INTRODUCTION
Behavioral variation among individuals has been reported in a wide range of animals. Traditionally, such variation has been explained as the result of random stochastic variation (Briffa & Weiss, 2010; Tran & Gerlai, 2013) and behavior was assumed to be indefinitely plastic in response to environmental cues (Sih, Bell, & Chadwick, 2004). However, the finding that behavioral variation could be adaptive and is often consistent, with stable individual differences along a behavioral continuum, caused a shift in that classic interpretation and fueled the introduction of the term “animal personality” (Briffa & Weiss, 2010; Sih et al., 2004). Animal personality has since been demonstrated in an array of animals, ranging from mammals (Réale, Gallant, Leblanc, & Festa‐Bianchet, 2000), to birds (Drent, van Oers, & van Noordwijk, 2002), reptiles (Le Galliard, Paquet, Cisel, & Montes‐Poloni, 2013), amphibians (Wilson & Krause, 2012), fish (Adriaenssens & Johnsson, 2008), and invertebrates (Biro, Adriaenssens, & Sampson, 2014). There is growing awareness that animal personality should be considered to fully understand the ecology and evolutionary biology of species and can have major implications for ecosystem functioning (Mittelbach, Ballew, & Kjelvik, 2014; Roche, Careau, & Binning, 2016). Personality has been shown to influence an array of ecological and evolutionary aspects, including distribution patterns, feeding niche, population growth and persistence, migration, dispersal, species interactions, social evolution, and adaptive potential (Briffa & Weiss, 2010; Mittelbach et al., 2014; Wolf & Weissing, 2012). For instance, Ioannou, Payne, and Krause (2008) illustrated that bolder three‐spined stickleback (Gasterosteus aculeatus) preyed more heavily upon Chironomus prey, whereas Chapman et al. (2011) showed that bolder individuals of roach (Rutilus rutilus) had a higher tendency to migrate. The study of individual behavioral responses is, therefore, not only interesting from a fundamental perspective, but is also important for, for example, nature conservation, harvest and resource management as outlined in more detail by Mittelbach et al. (2014).
While a growing body of literature reports on animal personality in a variety of species, the ecological underpinnings that determine personality and its consequences remain poorly understood (Adriaenssens & Johnsson, 2017; Mittelbach et al., 2014). In addition, underlying proximate mechanisms (e.g. genetic, physiology) should be identified (Briffa, Sneddon, & Wilson, 2015; Oswald, Singer, & Robison, 2013) and animal personality should be studied across the ontogeny of organisms. This is a crucial step toward understanding the function, evolution, and mechanism of animal personality (Stamps & Groothuis, 2010). To tackle these goals, studies of individual behavioral variation in natural populations of well‐characterized species along with laboratory and mesocosm studies have been promoted (Killen et al., 2016; Mittelbach et al., 2014).
A number of fish model organisms are used in various fields of biological research (Polačik, Blažek, & Reichard, 2016). Best studied species include zebrafish (Danio rerio), medaka (Oryzias latipes), fathead minnow (Pimephales promelas), and stickleback (Gasterosteus aculeatus). One constraint with these models is their relatively slow maturation and long lifespan, hampering whole life or multigenerational studies (Harel et al., 2015). Annual killifish combine the perks of traditional fish models with the short generation time of invertebrate model species (Polačik et al., 2016). Still, in order to be used as models in personality research, the existence of consistent among‐individual variation in behavior needs to be demonstrated. Species of the African genus Nothobranchius inhabit temporary freshwater pools and are adapted to the seasonal desiccation of their habitat by completing their life cycle in typically 3–4 weeks (Cellerino, Valenzano, & Reichard, 2015; Polačik et al., 2016). Moreover, annual fish produce drought‐resistant, dormant eggs that form an egg bank in the sediment and hatch when the pool is inundated again (Reichard, Polačik, & Sedláček, 2009).
The African annual killifish Nothobranchius furzeri (Turquoise killifish) has the shortest lifespan of any vertebrate in captivity. The species typically matures after three weeks (both in laboratory and in field conditions) and survives for only 5–6 months post‐hatching (Blažek, Polačik, & Reichard, 2013; Terzibasi et al., 2008), with even shorter lifespans recorded for inbred (homozygous) lines (Polačik et al., 2016; Wang, Promislow, & Kaeberlein, 2015). The “fastest” fish in our breeding facility even reached maturity in a little under two weeks (E. Thoré, Personal observation). In addition to a very short lifespan and generation time, N. furzeri is relatively easy to culture (Polačik et al., 2016). Moreover, individuals typically have a high reproductive output and are naturally bold in behavior (Cellerino et al., 2015; Polačik et al., 2016). Dormant eggs can easily be stored and allow for a synchronized hatching for experiments (Philippe et al., 2017; Polačik et al., 2016).
Nothobranchius furzeri is a relatively novel, yet already widely used, model organism that is rapidly gaining popularity in many fields of biological research, including ecology (Grégoir et al., 2017; Grégoir et al., 2017; Pinceel et al., 2015; Reichard, Polačik, Blažek, & Vrtílek, 2014), evolutionary biology (Blažek et al., 2016), ecotoxicology (Philippe et al., 2017), gerontology (Reichwald et al., 2015), genome‐wide gene expression studies and quantitative genetics (Cellerino et al., 2015; Valenzano et al., 2015). This surge of interest has, for instance, resulted in the construction of a whole brain atlas (D'angelo, 2013), age‐related histopathological analyses, an annotated genome (Reichwald et al., 2015; Valenzano et al., 2015) and transcriptome (Di Cicco, Terzibasi Tozzini, Rossi, & Cellerino, 2011), transgenesis and the generation of transgenic lines (Hartmann & Englert, 2012; Valenzano, Sharp, & Brunet, 2011).
In this study, we explore and discuss how N. furzeri could be a valuable model organism to move from descriptive studies to quantitative personality research to further unravel the causes, function, and evolution of animal personality. Despite the magnitude of recent advances in a range of biological disciplines that continue to promote N. furzeri as a promising model organism, individual behavioral variation in N. furzeri remains to be examined. Consequently, it remains unknown whether behavioral differences among N. furzeri individuals reflect personality variation or are due to random stochastic variation. We study a range of behavioral measures in N. furzeri individuals to determine whether variation is reflective of stable individual differences or rather represents random stochastic variation as a first and necessary step for quantitative personality research. In addition, we examine if and how different behavioral traits are correlated with each other. As personality variation has already been confirmed in a range of fish species (Budaev & Brown, 2011), we expect stable individual differences in behavioral expression and correlated behaviors also to be present in N. furzeri.
2. METHODS
2.1. General setup and fish maintenance
A total of 20 N. furzeri fish (9 females, 11 males) were reared from egg to adulthood while quantifying a range of behavioral measures under common garden rearing conditions in the laboratory. Fish originate from the natural population MZCS‐414 (central Mozambique) and had been laboratory‐reared for three generations under optimal common garden conditions prior to the start of the experiment in accordance with the protocols as specified by Polačik et al. (2016). Fish were hatched (synchronized) by submerging eggs and peat in reconstituted water (type II RO water with added Instant Ocean salt mix; 8.3 pH, 600 μS/cm conductivity) at a temperature of 14°C, based on the protocol outlined by Polačik et al. (2016). Two days post‐hatching, fish larvae were transferred to housing tanks at a density of 20 larvae per 4‐L water. At an age of 2 weeks post‐hatching, fish were transferred to 10‐L aquaria in groups of 10. Three weeks post‐hatching and for the remainder of the experiment, fish were housed individually in 9‐L tanks for individual monitoring. Fish were placed in a housing compartment (one compartment per tank, approx. 12 cm L × 19 cm W × 16 cm H) to habituate them to the tank setup for behavioral testing (see below). Each compartment was provided with an air‐driven filter to ensure good water quality. The sides of the tanks were covered with opaque plastic partitions to prevent confounding social contact between individuals. If social contact was allowed, neighboring dominant males could have interacted more with each other and have higher energetic needs than would neighboring females or submissive males. Water was renewed every 2 days from the moment of hatching until 3 weeks post‐hatching. Afterward, water was renewed on a weekly basis. Fish larvae were fed an ad libitum quantity of Artemia franciscana nauplii (Ocean Nutrition, Essen, Belgium) twice a day until 3 weeks post‐hatching and frozen Chironomus larvae (Ocean Nutrition, Essen, Belgium), supplemented with Artemia nauplii, from an age of 3 weeks onward. On observation days, fish were fed only once a day to avoid interference with the observations. At an age of 6 weeks, a small amount of the solvent DMSO (dimethyl sulfoxide) was added to the water of the housing tanks as part of a concurrent experiment studying the effects of antidepressant exposure on behavioral expression in killifish. The applied solvent concentration was, however, negligible (0.00001 vol%) and identical for all individuals. Still, to account for any potential differences before/after DMSO addition, we included it as a random factor in our statistical analyses.
2.2. Behavioral setup
At an age of one month post‐hatching, individual fish were subjected to four different behavioral tests, each of which was repeated once per week for a total of five consecutive trials per test. For each trial, each fish was placed individually in an experimental arena and allowed to acclimate for 5 min prior to the start of the observation trial. After each behavioral trial, individuals were transferred back to their housing tanks. Behavioral tests included (a) an emergence test; (b) an open field test. To also include behavior with direct ecological relevance, a habitat choice test (c) and a life skills test (d) were included. For practical reasons and to minimize behavioral changes associated with timing of the day (Tran & Gerlai, 2013), each sampling burst per behavioral trial lasted for a maximum of 3.5 hr. Fish were divided in two observation cohorts for practical reasons, following the scheme presented in Table 1. In order to motivate fish to explore the arena and to prevent disinterest in the applied food, fish were abstained from food for 24 hr before the emergence test and life skills test.
Table 1.
Weekly scheme of sampling bursts
| Moment of the week | Cohort | Behavioral test |
|---|---|---|
| Tuesday | ||
| Morning | 2 | Habitat choice test |
| Afternoon | 1 | Emergence test |
| Wednesday | ||
| Morning | 1 | Habitat choice test |
| Afternoon | 2 | Emergence test |
| Thursday | ||
| Morning | 2 | Open field test |
| Afternoon | 1 | Life skills test |
| Friday | ||
| Morning | 1 | Open field test |
| Afternoon | 2 | Life skills test |
Every sampling burst lasted for a maximum of 3.5 hr. Fish were divided in two cohorts to improve the logistic feasibility of the experiment. Every Monday, medium of the housing tanks was renewed and there were no behavioral tests.
As body size can be an important covariate of behavioral expression (Polverino, Bierbach, Killen, Uusi‐Heikkilä, & Arlinghaus, 2016), it was determined on the day before the first behavioral observations by briefly transferring each individual to a petri dish with a small amount of water and taking size‐calibrated photographs (mean ± SD = 23.94 ± 1.70 mm). Measurements were performed using the open source image processing software ImageJ 1.50i (Schneider, Rasband, & Eliceiri, 2012).
2.3. Emergence test
Exploration tendency was studied by means of an emergence test. Individuals were transferred to an experimental arena (Figure 1a), similar to their housing tank (i.e. a smaller compartment, separated from a “novel” larger compartment). After the acclimatization period, a doorway was opened to allow the individual to leave the small compartment and explore the larger one. Latency time to enter the newly available compartment was recorded during the next 45 min. Fish that did not enter the compartment during this time were assigned the maximum score of 45 min.
Figure 1.
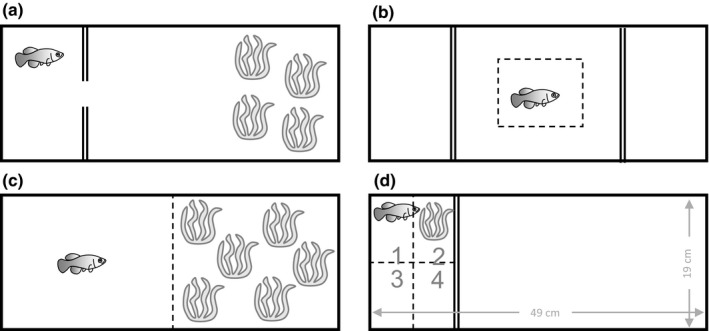
Schematic representation of the different test arenas used (top view). All tanks are LxWxH 49 × 19 × 16 cm and hold 9 L of water, except for the open field arena which only holds water to a height of 2 cm (approx. 1.9 L of water). (a) Experimental setup for the emergence test. The start compartment resembles the housing conditions. A doorway (diameter 20 mm) allows individuals to explore the novel, larger part of the tank which holds artificial plants as shelter in the furthest half of the compartment. (b) Open field experimental setup. (c) Experimental setup for the habitat choice test. The tank is equally divided in an open part and a part provided with artificial plants as shelter. The dotted line represents a virtual barrier. (d) Experimental setup for the life skills test, used to characterize feeding and antipredator behavior. The experimental compartment was virtually divided in four equally sized zones (delineated by the dotted lines). Zone 2 holds an artificial plant as shelter, whereas both feeding stimulus and simulated avian attack were applied in zone 3
2.4. Open field test
An open field test was performed to explore basic locomotor activity parameters and the propensity to take risks (boldness). Individuals were transferred to an open field arena (Figure 1b) and spontaneous activity was recorded for 20 min. The tank was virtually divided in a central (50% of length and width of open field) and peripheral area with activity in the centrum zone being considered as more risk‐prone than activity in the peripheral zone (Ansai, Hosokawa, Maegawa, & Kinoshita, 2016). Water level in this setup was lowered to a height of 2 cm to allow activity in the horizontal plane only.
2.5. Habitat choice test
The test arena to explore habitat choice was divided in two equal parts: a part with artificial plants for shelter and an open, barren part (Figure 1c). Fish were introduced individually to the centrum of the open part and habitat preference (expressed as the proportion of time spent in the open zone compared to the duration of the test) was recorded for 30 min after 5 min of acclimatization.
2.6. Life skills test
Feeding and antipredator behaviors were explored by means of a life skills test (Figure 1d). The experimental arena was virtually divided in four equal‐sized zones. After an acclimatization period, the test was initiated as soon as fish entered either zone 1 or 4, after which food (Chironomus larvae) was gently added in zone 3. The latency time to feed was assessed. At the onset of feeding, an avian predator attack was simulated by means of a suspended, weighted 15‐ml falcon tube (beak shaped, opaque) that was dropped and allowed to touch the water surface in zone 3 (see for instance Bell & Sih, 2007 and Hedgespeth, Nilsson, & Berglund, 2016 for a similar setup). Subsequently, the time until a fish that froze or fled in response to the simulated attack resumed movement and the time that was needed to resume feeding were recorded. Again, a maximum time of 45 min was allowed.
All behavioral measures were recorded (top view) using Logitech C920 HD Pro webcams and were manually analyzed afterward (observer‐blind), except for open field data which were analyzed using EthoVision XT Version 9.0 video‐tracking software (Noldus Information Technologies Inc; http://www.noldus.com).
2.7. Animal welfare note
This study was approved by the ethical committee of KU Leuven (file number: P160/2016). All performed procedures are conform the legal requirements for animal research in Belgium. The condition and health of every individual was checked multiple times a day by two researchers separately (ESJT and LS). In addition, water parameters were measured in each individual tank on a daily basis to keep track of water quality (pH: mean ± SD = 8.20 ± 0.41; conductivity: mean ± SD = 679.57 ± 22.64 μS/cm; temperature: mean ± SD = 24.55 ± 0.89°C). Animals were housed under optimal conditions and the handmade air‐driven filter provided shelter in all tanks. Disturbance and handling was kept to a minimum. Fish were part of a concurrent experiment studying the effects of antidepressant exposure on behavioral expression in killifish (only control fish were used for analysis in the current study). This approach enabled us to reduce the use of laboratory animals, by assessing multiple research objectives per experiment and avoiding the subsequent use of additional animals consistent with the “three Rs” guiding principles for more ethical use of laboratory animals (Fenwick, Griffin, & Gauthier, 2009).
2.8. Statistical analysis
All statistical analyses were conducted in R 3.3.1 (R Development Core Team, 2016) at a significance level of 0.05. Model assumptions were verified graphically for all analyses. Linear mixed models with Gaussian error distribution were fitted for all behavioral response variables (lme4 package; Bates et al., 2017) with sex, body size, and trial number (referring to the repeated measures, to account for behavioral changes over time) as fixed factors, including interaction between sex and body size and between sex and trial number. Fish identity was added to the model as random factor. To also account for variation explained by potential differences between cohorts and before/after DMSO treatment (0.00001 vol%), these factors were added to the models as random factors. Significance of fixed effects was tested using Wald chi‐square tests (car package; Fox et al., 2017). To determine if individual behavioral variation is reflective of personality variation, repeatability measures were calculated using the rptR package (Stoffel, Nakagawa, & Schielzeth, 2018) as the between‐individual variance over the sum of between‐individual and residual variance (Nakagawa & Schielzeth, 2010). Statistical significance of the repeatability measures was tested by likelihood‐ratio tests (comparing the model with and without the fish identity random effect structure) in the rptR package. Correlations between behavioral traits were assessed by averaging individual scores and calculating the Spearman correlation coefficient per pair of significantly repeatable behavioral traits.
2.9. Behavioral response variables
Latency time (in seconds) to enter the novel environment was assessed in the emergence test and was log‐transformed to meet model assumptions. Due to a low resolution in the data (36% did not emerge within the given 45 min and were assigned the maximum value), model assumption of homoscedasticity could not completely be met. Therefore, these results should be interpreted with some caution.
Total distance moved (cm) in the open field test was assessed as a measure of activity. As measures of boldness, the number of times the fish entered the centrum zone (log + 1 transformed) and the cumulative time (log + 1 transformed, in seconds) spent in the centrum zone were assessed.
Habitat choice (habitat choice test) was expressed as the proportion of time spent in the open zone compared to the total duration of the test (time spent in the open zone plus time spent in the plant zone).
Behavioral response variables for the life skills test included latency time (in seconds) to feed before and after the simulated predator attack and the time until movement (in seconds) after the attack for fish that froze or fled in response to the simulated attack. Latency time to feed before the predator attack was double log‐transformed to meet the model assumptions while latency time to resume feeding and time till movement after the predator attack were log‐transformed.
3. RESULTS
All behavioral measures were repeatable. Latency to enter a novel environment in the emergence test was repeatable with R = 0.334 (p < 0.001). In the open field test, the total distance moved, the number of times the fish entered the centrum and the cumulative duration spent in the centrum were repeatable with R = 0.457 (p < 0.001), R = 0.266 (p < 0.001), and R = 0.259 (p = 0.002), respectively. Habitat choice was repeatable with R = 0.178 (p = 0.016). In the life skills test, latency time to feed before attack, latency time to resume feeding and time till movement after attack were repeatable with R = 0.174 (p = 0.012), R = 0.108 (p = 0.011), and R = 0.32 (p = 0.005), respectively. Mean value, standard deviation and minimum and maximum value for all behavioral response variables are presented in Table 2. Results of the mixed models are presented in Table 3. Correlation coefficients per pair of behavioral traits are presented in Figure 2.
Table 2.
Mean value, standard deviation, and minimum and maximum value for all behavioral response variables, separated per sex. Latency time to initiate and resume feeding are expressed in seconds, as is latency time to resume movement after attack, latency time to enter novel environment, and cumulative time spent in centrum zone. Habitat choice was calculated as the total amount of time spent in the open zone (in seconds) over the total amount of time spent in the open and plant zone (i.e. duration of the test) and ranges between 0 (higher preference for plant zone) and 1 (higher preference for open zone). Total distance moved is expressed in centimeter
| Behavioral response | Mean value | Standard deviation | Min. value | Max. value | ||||
|---|---|---|---|---|---|---|---|---|
| Males | Females | Males | Females | Males | Females | Males | Females | |
| Latency time to feed before attack (s) | 24.462 | 109.744 | 43.261 | 299.682 | 2 | 3 | 200 | 1,597 |
| Latency time to resume feeding (s) | 155.173 | 277.878 | 308.888 | 486.678 | 3 | 5 | 1,679 | 2,296 |
| Time till movement after attack (s) | 17.526 | 14.353 | 24.669 | 17.029 | 1 | 2 | 123 | 80 |
| Latency time to enter novel environment (s) | 1404.077 | 1265.86 | 1083.338 | 993.898 | 36 | 42 | 2,700 | 2,700 |
| Habitat choice | 0.443 | 0.395 | 0.277 | 0.214 | 0 | 0.035 | 0.996 | 0.936 |
| Total distance moved (cm) | 2226.952 | 2119.377 | 910.573 | 891.153 | 661.78 | 679.55 | 3,892.33 | 4,306.76 |
| Number of times entered in centrum | 7.577 | 5.690 | 6.458 | 4.841 | 0 | 0 | 26 | 19 |
| Cumulative duration in centrum (s) | 38.005 | 25.371 | 44.046 | 21.787 | 0 | 0 | 197.21 | 95.9 |
Table 3.
Results of the mixed models per behavioural measure
| Behavioral response | Sex | Trial | Sex × Trial | Body size | Sex × Body size |
|---|---|---|---|---|---|
| Emergence test | |||||
| Latency time to enter novel environment | χ2 = 0.361 | χ2 = 2.103 | χ2 = 0.834 | χ2 = 0.439 | χ2 = 0.690 |
| p = 0.548 | p = 0.147 | p = 0.361 | p = 0.508 | p = 0.406 | |
| Open field test | |||||
| Total distance moved | χ2 < 0.001 | χ2 = 9.152 | χ2 = 1.712 | χ2 = 0.108 | χ2 = 3.187 |
| p = 0.992 | p = 0.002 | p = 0.191 | p = 0.743 | p = 0.074 | |
| Number of times the fish entered centrum | χ2 = 1.287 | χ2 = 0.047 | χ2 = 1.748 | χ2 = 0.472 | χ2 = 3.250 |
| p = 0.257 | p = 0.829 | p = 0.186 | p = 0.492 | p = 0.071 | |
| Cumulative duration in centrum | χ2 = 1.586 | χ2 = 1.214 | χ2 = 1.370 | χ2 = 2.130 | χ2 = 2.896 |
| p = 0.208 | p = 0.271 | p = 0.242 | p = 0.145 | p = 0.089 | |
| Habitat choice test | |||||
| Habitat choice | χ2 < 0.001 | χ2 = 1.651 | χ2 = 0.615 | χ2 = 0.535 | χ2 = 0.030 |
| p = 0.985 | p = 0.199 | p = 0.433 | p = 0.465 | p = 0.863 | |
| Life skills test | |||||
| Latency time to feed before attack | χ2 = 1.305 | χ2 = 0.623 | χ2 = 3.291 | χ2 = 0.047 | χ2 = 0.047 |
| p = 0.253 | p = 0.430 | p = 0.070 | p = 0.829 | p = 0.829 | |
| Latency time to resume feeding | χ2 = 1.028 | χ2 = 1.755 | χ2 = 2.123 | χ2 = 0.011 | χ2 = 0.363 |
| p = 0.311 | p = 0.185 | p = 0.145 | p = 0.918 | p = 0.547 | |
| Time till movement after attack | χ2 = 3.107 | χ2 = 0.165 | χ2 = 9.451 | χ2 = 2.145 | χ2 = 0.154 |
| p = 0.078 | p = 0.684 | p = 0.002 | p = 0.143 | p = 0.695 | |
p‐values <0.05 are shown in bold.
Figure 2.
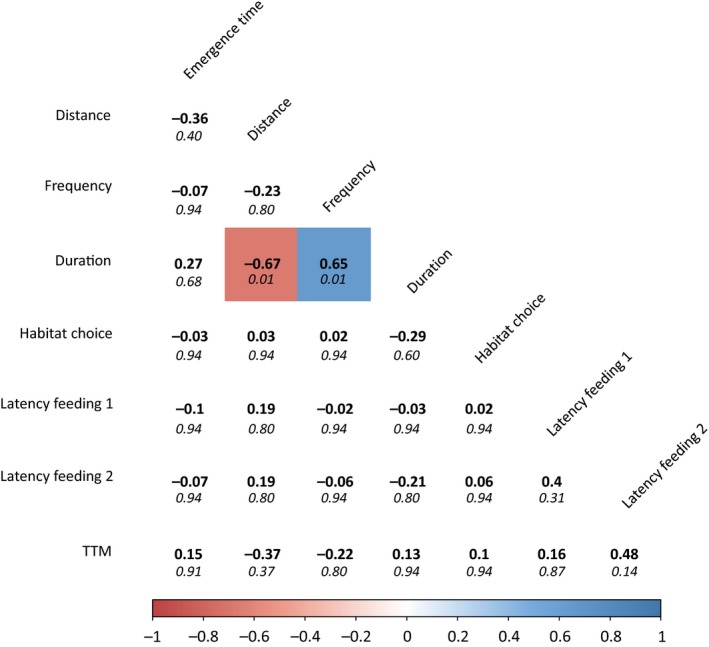
Spearman rank correlation coefficients (in bold) per pair of behavioral traits. p‐values (false discovery rate controlled) are shown in italics. Significant correlation coefficients are depicted in color (red for negative correlation, blue for positive correlation). Emergence time: latency time to enter novel environment (emergence test), Distance: total distance moved (open field test), Frequency: number of times the fish entered centrum (open field test), Duration: cumulative duration in centrum (open field test), Habitat choice: habitat preference (habitat choice test), Latency feeding 1: latency time to feed before attack (life skills test), Latency feeding 2: latency time to resume feeding (life skills test), TTM: time till movement after attack (life skills test)
4. DISCUSSION
Individual behavioral variation is a common phenomenon in animals. Scientists now increasingly recognize the importance of decomposing such variation in among‐ and within‐individual variation and argue that consistent individual differences along a behavioral continuum exist (Briffa & Weiss, 2010; Roche et al., 2016; Sih et al., 2004). Here, we investigated if behavioral variation in the upcoming vertebrate model organism N. furzeri is reflective of stable individual differences or rather represents random stochastic variation. Overall, our results support consistent inter‐individual differences in multiple behavioral responses, suggestive of personality variation. All behavioral measures that reflect locomotor activity and the propensity to take risks were found to be repeatable. This suggests that consistent behavioral differences exist among N. furzeri individuals.
Locomotor activity and movement resumption after a simulated predator attack of N. furzeri individuals differed between the trials. This variation should most likely be interpreted as the result of behavioral plasticity or age‐related behavioral changes. However, our results show that relative differences in both locomotor activity and movement resumption after a simulated predator attack between individuals were maintained across trials. This implies the existence of consistent variation in behavior among individuals. Since behavioral responses and body size were not correlated in the studied fish we suggest that neurophysiological rather than biophysical differences underlie these observations, which is consistent with findings on zebrafish (Danio rerio) (Tran & Gerlai, 2013).
In personality research, behavioral traits are often found to be dependent of each other (Class & Brommer, 2015). Such behavioral correlations are however not unanimously supported in the literature (Garamszegi & Herczeg, 2012). Likewise, in this study we found only limited support for behavioral correlations. Behavioral correlations across different test setups could not be demonstrated. However, in the open field test, total distance traveled (as a measure of activity) was negatively correlated with the time spent in the centrum zone of the open field (as a measure of boldness). This result suggests that active individuals prefer the more risk‐averse peripheral zone, whereas less active individuals spend more time in the risk‐prone central zone in comparison to active individuals. As expected, time spent in the centrum zone was positively correlated with the number of times the fish entered the centrum. The absence of any further behavioral correlations should be subject to future research. We do remark however that the current study is not optimally designed to explore behavioral correlations, as such studies typically require larger sample sizes (Garamszegi & Herczeg, 2012).
The historical lack of interest in between‐individual behavioral variation has been used as justification to launch the field of personality research (Beekman & Jordan, 2017). However, this notion recently received criticism. It can be argued that individual behavioral variation was already the implicit corner‐stone of behavioral ecology—a discipline that explains animal behavior from a functional and evolutionary perspective (Beekman & Jordan, 2017; Owens, 2006). Moreover, the bulk of animal personality literature is descriptive rather than hypothesis‐driven and often lacks an evolutionary context and insight into the mechanistic underpinnings of behavioral variation (Beekman & Jordan, 2017; Roche et al., 2016; Sih, 2013). In light of the task ahead, the use of model organisms could be indispensable to bridge descriptive and hypothesis‐driven research, as suggested by Owens (2006). Behavioral ecologists typically study behavior of wild organisms rather than that of model organisms (Monaghan, 2014; Owens, 2006). Owens (2006) reports that <2% of the studies published in three leading journals in behavioral ecology between 2001 and 2005 made use of traditional model organisms. Likewise, Monaghan (2014) reports that in 2007–2014 less than 0.5% of the papers published in Behavioral Ecology made use of traditional model organisms. This is partly attributed to a general lack of knowledge on the natural history of traditional model organisms which has, in turn, been largely ascribed to an unfortunate division between biomedical sciences and eco‐evolutionary research (Alfred & Baldwin, 2015; Owens, 2006; Parichy, 2015). Consequently, behavioral ecologists miss out on a large amount of biochemical and physiological data that is available for traditional model organisms and fail to effectively link individual behavioral variation to the genetic and physiological mechanisms that underpin this variation (Beekman & Jordan, 2017; Owens, 2006). Although (field) studies on natural populations are of crucial importance, model organisms are under‐used in behavioral ecology. This has, in our opinion, contributed to the predominantly descriptive nature of animal personality research. The use of model organisms in combination with natural populations could extend the scope from descriptive studies to targeted quantitative research designs. In addition, it would also solve the lack of replication across laboratories that is common in behavioral ecology and help to validate findings, adding to the scrutiny of the field (Owens, 2006).
Nothobranchius furzeri is already a commonly used model organism in a range of biomedical research fields, in ecology and in evolutionary biology (Polačik et al., 2016). We argue that N. furzeri has great potential to further add to the advancement of ethology and behavioral ecology. Whereas personality variation has been reported in a range of traditional model organisms such as zebrafish (Tran & Gerlai, 2013), stickleback (Jolles, Taylor, & Manica, 2016), and rainbow trout (Elias, Thrower, & Nichols, 2018), this is the first study to report on personality variation in N. furzeri.
The main advantage of N. furzeri is its extremely short maturation time (3 weeks) and lifespan (typically <6 months). This is much shorter than that of traditional model organisms such as zebrafish with a typical maturation time of 2 months and lifespan of up to 5 years (Lawrence et al., 2012). In addition, the life history of the species is well characterized across ecological gradients (e.g. aridity, predation) and ongoing ecological and evolutionary research continuously adds to this knowledge (Blažek et al., 2016; Watters, 2009). This facilitates field studies to further elucidate the ecological causes and consequences of behavioral variation (Mittelbach et al., 2014). In complement with this ecological information, a whole brain atlas (D'angelo, 2013) along with an annotated genome (Reichwald et al., 2015; Valenzano et al., 2015) and transcriptome (Di Cicco et al., 2011) allow for an in‐depth investigation of neurophysiological and genetic underpinnings of behavioral variation. Moreover, successful transgenesis and the generation of transgenic lines (Hartmann & Englert, 2012) allows for the construction of specific lines. If such lines are characterized by different behavioral profiles, the molecular mechanisms underlying behavioral variation and the ecological and evolutionary implications of personality variation could be studied under well‐controlled experimental conditions (Tran & Gerlai, 2013).
Whereas studying the development of animal personality across ontogeny is pivotal to our understanding of the proximal causation, function (adaptive value), and evolution of personality, this remains largely understudied. Understanding the development of animal personality typically requires time‐consuming research as behavioral changes across the lifespan of individuals need to be monitored (Stamps & Groothuis, 2010). In this regard, the short lifespan of N. furzeri offers a major advantage over other vertebrate species. Moreover, its fast life cycle allows for time‐ and cost‐efficient examination of environmental underpinnings of behavioral variation across ontogeny—including experiential factors—and multigenerational setups.
While in the current study we identified stable individual differences in behavioral expression, thereby adding necessary fundamental information to the descriptive animal personality literature, we also provide a potential stepping stone to quantitative research designs by introducing the model organism N. furzeri. The inclusion of N. furzeri in behavioral sciences answers the call for an increased diversity in studied organisms (Monaghan, 2014). Its well‐characterized biomedical and ecological background in combination with its short life cycle make it an excellent model organism to further elucidate the causes and implications of behavioral variation in an eco‐evolutionary context.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
The study was designed by ESJT and TP and performed by ESJT and LS. Data was analyzed by ESJT. The manuscript was written by ESJT and reviewed by TP, LS, CP, and LB. All authors gave final approval for publication.
DATA ACCESSIBILITY
Data is accessible at the FigShare repository (https://doi.org/10.6084/m9.figshare.6741185.v1).
ACKNOWLEDGMENTS
We are grateful to Dr. Martin Reichard and coworkers for providing the parental fish for this project. We furthermore thank the reviewers for their constructive comments on the manuscript.
Thoré ESJ, Steenaerts L, Philippe C, Grégoir A, Brendonck L, Pinceel T. Individual behavioral variation reflects personality divergence in the upcoming model organism Nothobranchius furzeri . Ecol Evol. 2018;8:8448–8457. 10.1002/ece3.4356
Funding Information
This work was supported by Fonds Wetenschappelijk Onderzoek—Vlaanderen (SB151323 and 12F0716N to ESJT and TP, respectively) and by the Excellence Center ‘Eco and socio‐evolutionary dynamics' (PF/10/007 to CP).
REFERENCES
- Adriaenssens, B. , & Johnsson, J. I. (2011). Shy trout grow faster: Exploring links between personality and fitness‐related traits in the wild. Behavioral Ecology, 22, 135–143. 10.1093/beheco/arq185 [DOI] [Google Scholar]
- Adriaenssens, B. , & Johnsson, J. I. (2013). Natural selection, plasticity and the emergence of a behavioural syndrome in the wild. Ecology Letters, 16, 47–55. 10.1111/ele.12011 [DOI] [PubMed] [Google Scholar]
- Alfred, J. , & Baldwin, I. T. (2015). New opportunities at the wild frontier. eLife, 4, e06956 10.7554/eLife.06956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansai, S. , Hosokawa, H. , Maegawa, S. , & Kinoshita, M. (2016). Chronic fluoxetine treatment induces anxiolytic responses and altered social behaviors in medaka, Oryzias latipes . Behavioural Brain Research, 303, 126–136. 10.1016/j.bbr.2016.01.050 [DOI] [PubMed] [Google Scholar]
- Bates, D. , Maechler, M. , Bolker, B. , Walker, S. , Christensen, R. H. B. , Singmann, H. , … Green, P. (2017). lme4: Linear mixed‐effects models using Eigen and S4. R package version 1.1‐14. Retrieved from http://CRAN.R-project.org/package=lme4
- Beekman, M. , & Jordan, L. A. (2017). Does the field of animal personality provide any new insights for behavioral ecology? Behavioral Ecology, 28, 617–623. https://doi.org/0.1093/beheco/arx022 [Google Scholar]
- Bell, A. M. , & Sih, A. (2007). Exposure to predation generates personality in threespined sticklebacks (Gasterosteus aculeatus). Ecology Letters, 10, 828–834. 10.1111/j.1461-0248.2007.01081.x [DOI] [PubMed] [Google Scholar]
- Biro, P. A. , Adriaenssens, B. , & Sampson, P. (2014). Individual and sex‐specific differences in intrinsic growth rate covary with consistent individual differences in behaviour. Journal of Animal Ecology, 83, 1186–1195. 10.1111/1365-2656.12210 [DOI] [PubMed] [Google Scholar]
- Blažek, R. , Polačik, M. , Kačer, P. , Cellerino, A. , Řežucha, R. , Methling, C. , … Reichard, M. (2016). Repeated intraspecific divergence in life span and aging of African annual fishes along an aridity gradient. Evolution, 71, 386–402. 10.1111/evo.13127 [DOI] [PubMed] [Google Scholar]
- Blažek, R. , Polačik, M. , & Reichard, M. (2013). Rapid growth, early maturation and short generation time in African annual fishes. EvoDevo, 4, 24 10.1186/2041-9139-4-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briffa, M. , Sneddon, L. U. , & Wilson, A. J. (2015). Animal personality as a cause and consequence of contest behaviour. Biology Letters, 11, 20141007 10.1098/rsbl.2014.1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briffa, M. , & Weiss, A. (2010). Animal personality. Current Biology, 20, 912–914. 10.1016/j.cub.2010.09.019 [DOI] [PubMed] [Google Scholar]
- Budaev, S. , & Brown, C. (2011). Personality traits and behaviour In Brown C., Laland K. N., & Krause J. (Eds.), Fish cognition and behaviour (pp. 135–165). Oxford, UK: Wiley‐Blackwell. [Google Scholar]
- Cellerino, A. , Valenzano, D. R. , & Reichard, M. (2015). From the bush to the bench: The annual Nothobranchius fishes as a new model system in biology. Biological Reviews Cambridge Philosophical Society, 91, 511–533. 10.1111/brv.12183 [DOI] [PubMed] [Google Scholar]
- Chapman, B. B. , Hulthén, K. , Blomqvist, D. R. , Hansson, L. , Nilsson, J. , Brodersen, J. , … Brönmark, C. (2011). To boldly go: Individual differences in boldness influence migratory tendency. Ecology Letters, 14, 871–876. 10.1111/j.1461-0248.2011.01648.x [DOI] [PubMed] [Google Scholar]
- Class, B. , & Brommer, J. E. (2015). A strong genetic correlation underlying a behavioural syndrome disappears during development because of genotype‐age interactions. Proceedings of the Royal Society B: Biological Sciences, 282(1809), 20142777 10.1098/rspb.2014.2777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'angelo, L. (2013). Brain atlas of an emerging Teleostean model: Nothobranchius furzeri . Anatomical Record (Hoboken), 296, 681–691. 10.1002/ar.22668 [DOI] [PubMed] [Google Scholar]
- Di Cicco, E. , Terzibasi Tozzini, E. , Rossi, G. , & Cellerino, A. (2011). The short‐lived annual fish Nothobranchius furzeri shows a typical teleost aging process reinforced by high incidence of age‐dependent neoplasias. Experimental Gerontology, 46, 249–256. 10.1016/j.exger.2010.10.011 [DOI] [PubMed] [Google Scholar]
- Drent, P. J. , van Oers, K. , & van Noordwijk, A. J. (2002). Realized heritability of personalities in the great tit (Parus major). Proceedings of the Royal Society B: Biological Sciences, 270, 45–51. 10.1098/rspb.2002.2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias, A. , Thrower, F. , & Nichols, K. M. (2018). Rainbow trout personality: Individual behavioural variation in juvenile Oncorhynchus mykiss . Behaviour, 155, 205–230. 10.1163/1568539X-00003483 [DOI] [Google Scholar]
- Fenwick, N. , Griffin, G. , & Gauthier, C. (2009). The welfare of animals used in science: How the “Three Rs” ethic guides improvements. The Canadian Veterinary Journal, 50, 523–530. [PMC free article] [PubMed] [Google Scholar]
- Fox, J. , Weisberg, S. , Adler, D. , Bates, D. , Baud‐Bovy, G. , Ellison, S. , … Murdoch, D. (2017). car: Companion to applied regression. R package version 2.1‐6. Retrieved from https://CRAN.R-project.org/package=car
- Garamszegi, L. Z. , & Herczeg, G. M. G. (2012). A meta‐analysis of correlated behaviours with implications for behavioural syndromes: Mean effect size, publication bias, phylogenetic effects and the role of mediator variables. Evolutionary Ecology, 26, 1213–1235. 10.1007/s10682-012-9589-8 [DOI] [Google Scholar]
- Grégoir, A. F. , Philippe, C. , Pinceel, T. , Reniers, J. , Thoré, E. S. J. , Vanschoenwinkel, B. , & Brendonck, L. (2017. a). Life stage dependent responses to desiccation risk in the annual killifish Nothobranchius wattersi . Journal of Fish Biology, 91, 880–895. 10.1111/jfb.13385 [DOI] [PubMed] [Google Scholar]
- Grégoir, A. F. , Thoré, E. S. J. , Philippe, C. , Pinceel, T. , Brendonck, L. , & Vanschoenwinkel, B. (2017. b). Squeezing out the last egg—annual fish increase reproductive efforts in response to a predation threat. Ecology and Evolution, 00, 1–9. 10.1002/ece3.3422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel, I. , Benayoun, B. A. , Machado, B. , Singh, P. P. , Hu, C. , Pech, M. F. , … Brunet, A. (2015). A platform for rapid exploration of aging and diseases in a naturally short‐lived vertebrate. Cell, 160, 1013–1026. 10.1016/j.cell.2015.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann, N. , & Englert, C. (2012). A microinjection protocol for the generation of transgenic killifish (species: Nothobranchius furzeri). Developmental Dynamics, 241, 1133–1141. 10.1002/dvdy.23789 [DOI] [PubMed] [Google Scholar]
- Hedgespeth, M. L. , Nilsson, P. A. , & Berglund, O. (2016). Assessing potential vulnerability and response of fish to simulated avian predation after exposure to psychotropic pharmaceuticals. Toxics, 4, 9 10.3390/toxics4020009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannou, C. C. , Payne, M. , & Krause, J. (2008). Ecological consequences of the bold‐shy continuum: The effect of predator boldness on prey risk. Oecologia, 157, 177–182. 10.1007/s00442-008-1058-2 [DOI] [PubMed] [Google Scholar]
- Jolles, J. W. , Taylor, B. A. , & Manica, A. (2016). Recent social conditions affect boldness repeatability in individual sticklebacks. Animal Behaviour, 112, 139–145. 10.1016/j.anbehav.2015.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killen, S. S. , Adriaenssens, B. , Marras, S. , Claireaux, G. , & Cooke, S. J. (2016). Context dependency of trait repeatability and its relevance for management and conservation of fish populations. Conservation Physiology, 4, cow007 10.1093/conphys/cow007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence, C. , Adatto, I. , Best, J. , James, A. , & Maloney, K. (2012). Generation time of zebrafish (Danio rerio) and medakas (Orysias latipes) housed in the same aquaculture facility. Lab Animal, 41, 158–165. 10.1038/laban0612-158 [DOI] [PubMed] [Google Scholar]
- Le Galliard, J. , Paquet, M. , Cisel, M. , & Montes‐Poloni, L. (2013). Personality and the pace‐of‐life syndrome: Variation and selection on exploration, metabolism and locomotor performances. Functional Ecology, 27, 136–144. 10.1111/1365-2435.12017 [DOI] [Google Scholar]
- Mittelbach, G. G. , Ballew, N. G. , & Kjelvik, M. K. (2014). Fish behavioural types and their ecological consequences. Canadian Journal of Fisheries and Aquatic Sciences, 71, 927–944. 10.1139/cjfas-2013-0558 [DOI] [Google Scholar]
- Monaghan, P. (2014). Behavioral ecology and the successful integration of function and mechanism. Behavioral Ecology, 25, 1019–1021. 10.1093/beheco/aru082 [DOI] [Google Scholar]
- Nakagawa, S. , & Schielzeth, H. (2010). Repeatability for Gaussian and non‐Gaussian data: A practical guide for biologists. Biological Reviews of the Cambridge Philosophical Society, 85, 935–956. 10.1111/j.1469-185X.2010.00141.x [DOI] [PubMed] [Google Scholar]
- Oswald, M. E. , Singer, M. , & Robison, B. D. (2013). The quantitative genetic architecture of the bold‐shy continuum in zebrafish, Danio rerio . PLoS ONE, 8, e68828 10.1371/journal.pone.0068828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens, I. P. F. (2006). Where is behavioural ecology going? Trends in Ecology and Evolution, 21, 356–361. 10.1016/j.tree.2006.03.014 [DOI] [PubMed] [Google Scholar]
- Parichy, D. M. (2015). Advancing biology through a deeper understanding of zebrafish ecology and evolution. eLife, 4, e05635 10.7554/eLife.05635.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe, C. , Grégoir, A. F. , Janssens, L. , Pinceel, T. , De Boeck, G. , & Brendonck, L. (2017). Acute and chronic sensitivity to copper of a promising ecotoxicological model species, the annual killifish Nothobranchius furzeri . Ecotoxicology and Environmental Safety, 144, 26–35. 10.1016/j.ecoenv.2017.05.047 [DOI] [PubMed] [Google Scholar]
- Pinceel, T. , Vanschoenwinkel, B. , Deckers, P. , Grégoir, A. , Ver Eecke, T. , & Brendonck, L. (2015). Early and late developmental arrest as complementary embryonic risk spreading strategies in African killifish. Biological Journal of the Linnean Society, 114, 941–948. [Google Scholar]
- Polačik, M. , Blažek, R. , & Reichard, M. (2016). Laboratory breeding of the short‐lived annual killifish Nothobranchius furzeri . Nature Protocols, 11, 1396–1413. 10.1038/nprot.2016.080 [DOI] [PubMed] [Google Scholar]
- Polverino, G. , Bierbach, D. , Killen, S. S. , Uusi‐Heikkilä, S. , & Arlinghaus, R. (2016). Body length rather than routine metabolic rate and body condition correlates with activity and risk‐taking in juvenile zebrafish Danio rerio . Journal of Fish Biology, 89, 2251–2267. 10.1111/jfb.13100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team . (2008). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; ISBN 3‐900051‐07‐0, http://www.R-project.org [Google Scholar]
- Réale, D. , Gallant, B. Y. , Leblanc, M. , & Festa‐Bianchet, M. (2000). Consistency of temperament in bighorn ewes and correlates with behaviour and life history. Animal Behaviour, 60, 589–597. 10.1006/anbe.2000.1530 [DOI] [PubMed] [Google Scholar]
- Reichard, M. , Polačik, M. , Blažek, R. , & Vrtílek, M. (2014). Female bias in the adult sex ratio of African annual fishes: Interspecific differences, seasonal trends and environmental predictors. Evolutionary Ecology, 28, 1105–1120. 10.1007/s10682-014-9732-9 [DOI] [Google Scholar]
- Reichard, M. , Polačik, M. , & Sedláček, O. (2009). Distribution, colour polymorphism and habitat use of the African killifish Nothobranchius furzeri, the vertebrate with the shortest life span. Journal of Fish Biology, 74, 198–212. 10.1111/j.1095-8649.2008.02129.x [DOI] [PubMed] [Google Scholar]
- Reichwald, K. , Petzold, A. , Koch, P. , Downie, B. R. , Hartmann, N. , Pietsch, S. , … Platzer, M. (2015). Insights into sex chromosome evolution and aging from the genome of a short‐lived fish. Cell, 163, 1527–1538. 10.1016/j.cell.2015.10.071 [DOI] [PubMed] [Google Scholar]
- Roche, D. G. , Careau, V. , & Binning, S. A. (2016). Demystifying animal ‘personality’ (or not): Why individual variation matters to experimental biologists. Journal of Experimental Biology, 219, 3832–3843. 10.1242/jeb.146712 [DOI] [PubMed] [Google Scholar]
- Schneider, C. A. , Rasband, W. S. , & Eliceiri, K. W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nature Methods, 9, 671–675. 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sih, A. (2013). Frontiers on the interface between behavioral syndromes and social behavioral ecology In Carere C., & Maestripieri D. (Eds.), Animal personalities: Behavior, physiology, and evolution (pp. 221–251). Chicago, IL: Chicago University Press. [Google Scholar]
- Sih, A. , Bell, A. , & Chadwick, J. (2004). Behavioral syndromes: An ecological and evolutionary overview. Trends in Ecology and Evolution, 19, 372–378. 10.1016/j.tree.2004.04.009 [DOI] [PubMed] [Google Scholar]
- Stamps, J. , & Groothuis, T. G. G. (2010). The development of animal personality: Relevance, concepts and perspectives. Biological Reviews of the Cambridge Philosophical Society, 85, 301–325. 10.1111/j.1469-185X.2009.00103.x [DOI] [PubMed] [Google Scholar]
- Stoffel, M. , Nakagawa, S. , & Schielzeth, H. (2018). rptR: Repeatability estimation for Gaussian and non‐Gaussian data. R package version 0.9.21. Retrieved from https://CRAN.R-project.org/package=rptR
- Terzibasi, E. , Valenzano, D. R. , Benedetti, M. , Roncaglia, P. , Domenici, L. , & Cellerino, A. (2008). Large differences in aging phenotype between strains of the short‐lived annual fish Nothobranchius furzeri . PLoS ONE, 3, e3866 10.1371/journal.pone.0003866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran, S. , & Gerlai, R. (2013). Individual differences in activity levels in zebrafish (Danio rerio). Behavioural Brain Research, 257, 224–229. 10.1016/j.bbr.2013.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzano, D. R. , Benayoun, B. A. , Singh, P. P. , Zhang, E. , Etter, P. D. , Hu, C. K. , … Brunet, A. (2015). The African Turquoise killifish genome provides insights into evolution and genetic architecture of lifespan. Cell, 163, 1539–1554. 10.1016/j.cell.2015.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzano, D. R. , Sharp, S. , & Brunet, A. (2011). Transposon‐mediated transgenesis in the short‐lived African killifish Nothobranchius furzeri, a vertebrate model for aging. G3 (Bethesda), 7, 531–538. 10.1534/g3.111.001271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, A. M. , Promislow, D. E. L. , & Kaeberlein, M. (2015). Fertile waters for aging research. Cell, 160, 814–815. 10.1016/j.cell.2015.02.026 [DOI] [PubMed] [Google Scholar]
- Watters, B. R. (2009). The ecology and distribution of Nothobranchius fishes. Journal of the American Killifish Association, 42, 37–76. [Google Scholar]
- Wilson, A. D. M. , & Krause, J. (2012). Personality and metamorphosis: Is behavioural variation consistent across ontogenetic niche shifts? Behavioral Ecology, 23, 1316–1323. 10.1093/beheco/ars123 [DOI] [Google Scholar]
- Wolf, M. , & Weissing, F. J. (2012). Animal personalities: Consequences for ecology and evolution. Trends in Ecology and Evolution, 27, 452–461. 10.1016/j.tree.2012.05.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is accessible at the FigShare repository (https://doi.org/10.6084/m9.figshare.6741185.v1).


