Abstract
Background
The potential of ascorbic acid and two botanical decoctions, green tea and cat's claw, to limit cell death in response to oxidants were evaluated in vitro.
Methods
Cultured human gastric epithelial cells (AGS) or murine small intestinal epithelial cells (IEC-18) were exposed to oxidants – DPPH (3 μM), H2O2 (50 μM), peroxynitrite (300 μM) – followed by incubation for 24 hours, with antioxidants (10 μg/ml) administered as a 1 hour pretreatment. Cell number (MTT assay) and death via apoptosis or necrosis (ELISA, LDH release) was determined. The direct interactions between antioxidants and DPPH (100 μM) or H2O2 (50 μM) were evaluated by spectroscopy.
Results
The decoctions did not interact with H2O2, but quenched DPPH although less effectively than vitamin C. In contrast, vitamin C was significantly less effective in protecting human gastric epithelial cells (AGS) from apoptosis induced by DPPH, peroxynitrite and H2O2 (P < 0.001). Green tea and cat's claw were equally protective against peroxynitrite and H2O2, but green tea was more effective than cat's claw in reducing DPPH-induced apoptosis (P < 0.01). Necrotic cell death was marginally evident at these low concentrations of peroxynitrite and H2O2, and was attenuated both by cat's claw and green tea (P < 0.01). In IEC-18 cells, all antioxidants were equally effective as anti-apoptotic agents.
Conclusions
These results indicate that dietary antioxidants can limit epithelial cell death in response to oxidant stress. In the case of green tea and cat's claw, the cytoprotective response exceed their inherent ability to interact with the injurious oxidant, suggestive of actions on intracellular pathways regulating cell death.
Introduction
Epithelial apoptosis in the gastro-intestinal tract is normally restricted to superficial cells but in pathological states of inflammation or infection, apoptotic cell death can be far more expansive. Under these conditions apoptosis may result from the production of cytokines [1], cell activation [2-4], infective agents [5] and adverse responses to pharmaceuticals [6,7]. Depending on the agonist or eliciting milieu, apoptotic cell death is accompanied by the activation of various cell death pathways including caspases, ceramide, altered gene expression, mitochondrial dysfunction and consumption of ATP e.g., with DNA repair, that result in histone-associated DNA fragmentation [8,9]. Many of these cell death pathways are also associated with the secondary production of oxidants [10] which appears to contribute to the magnitude of cell death. For example, over-expression of antioxidants like intracellular SOD reduces cell death [11], or alternatively, depletion of antioxidant reserves exacerbates cell death to a variety of stimuli [12].
Despite the knowledge that apoptosis is increased in gut inflammation, either from idiopathic causes like inflammatory bowel disease [8,9] or associated with infections like H. pylori[13], little has been done to examine the effects of antioxidants on epithelial cell death. We have previously demonstrated an excessive degree of apoptosis in patients with gastritis induced by H. pylori, and that the degree of apoptosis could be reduced by dietary supplementation with vitamin C, irrespective of whether the infection was cleared or not [13]. This observation was suggestive that dietary antioxidants could limit the gut pathology by limiting cell death mechanisms. We have also demonstrated that mesalamine (5-aminosalicylic acid), used as a frontline treatment for inflammatory bowel disease, could prevent apoptotic epithelial cell death in response to peroxynitrite [14]. Peroxynitrite is a potent oxidant; formed from two free radicals – nitric oxide and superoxide – that catalyzes the nitration of tyrosine residues. The resultant nitrotyrosine is used as a marker of peroxynitrite or at least nitration reactions in gastritis [13], inflammatory bowel disease [15,16] as well as general inflammation [17]. While we have demonstrated that peroxynitrite-induced apoptosis of macrophages and colon epithelial cells is reduced by ascorbic acid [18], it is not known if other dietary antioxidants exert similar benefits or if they are present in epithelial cells from the upper gastrointestinal tract. However, flavonoids and related phenolic compounds have been described to be more effective than vitamin C in limiting DNA damage induced by nitrating/nitrosating species in cell free systems [19].
Altered intracellular redox balance with depletion of antioxidant reserves is associated with the activation of transcription factors like NF-κB, and genes (TNFα, IL-1β) associated with apoptosis, proliferation and inflammation [3,4,8,9]. Indeed, dietary antioxidants like resveratrol [20], green tea [21], cucumin [22] and cat's claw [23,24] have been shown to inhibit gene expression associated with inflammation and states of immune activation. From this one could postulate that the benefits of diets rich in antioxidants could be in part due to a reduction in signals leading to apoptosis and cell activation. This possibility was addressed in this study by examining the ability of three antioxidants, vitamin C and two decoctions commonly used in Asia and South America (green tea and cat's claw) to limit epithelial cell apoptosis in response to a variety of oxidative stresses. The latter two forms of antioxidants were chosen because the antioxidant and anti-inflammatory phytochemical constituents (polyphenols, flavanols) are distinct from ascorbate but yet there is a growing appreciation for their role in managing disorders associated with oxidative stress.
Methods
The South American ethnomedicine, Cat's claw (Vinicol™), Uncaria guianensis, was obtained as a freeze dried concentrate from Rainforest Phytoceuticals, LLC, Delmar, NY. This concentrate is prepared in accord with ethnomedical traditions, where a decoction is made from the bark, and the solutes concentrated by freeze drying the decoction. The subspecies Uncaria guianensis was used over Uncaria tomentosa, as it is a more potent anti-inflammatory agent and has negligible amounts of alkaloids. A green tea extract was prepared from commercial sources by a decoction method. Briefly, dry leaves (2.5 g) were added to 100 ml of boiling water and steeped for 15 min. The infusion was cooled down to room temperature, decanted and total solids separated by filtration with a Whatman N° 4 filter paper. The filtrate was then freeze-dried using a Freezemobile 6 concentrator (Virtis, Gardiner, NY). The light brown solid extract was then used for all subsequent studies. For spectrophotometric analysis and cell culture studies the extract was filtered at 20 μm and 0.2 μm, respectively. Vitamin C (ascorbic acid) was purchased from Sigma Chemical Company, St. Louis, Missouri, USA.
Cell culture
AGS or IEC-18 cells were obtained from the American Type Culture Collection (Rockville, MD). The cells were grown in DMEM (high glucose), 10% FCS and supplemented with 25 mM HEPES, pH 7.4; 4 mM L-glutamine; 40 μg/ml penicillin; 90 μg/ml streptomycin and 1.2 g/L NaHCO3. Cell cultures were maintained in a humidified 5% CO2 incubator at 37°C. Harvested cells were plated in 96-well microplates (5 × 104 cells/well) and allowed to grow to confluence over 24 h before use. Baseline effects on cell proliferation by the antioxidants i.e., without oxidant co-administration, was followed for 72 hours.
In the experiments evaluating antioxidants/oxidant interactions on cell number (MTT assay) the antioxidants were added to the culture media 1 hour before the oxidant being evaluated, with incubation for an additional 24 hours. At the conclusion of this incubation period either cell number was determined by the MTT assay.
The cytoprotection activity of antioxidants, cat's claw, green tea and vitamin C, was investigated against the toxicity of the oxidants (DPPH, H2O2, and peroxynitrite) using acridine orange staining as a means of confirming the absence or presence of apoptosis by morphological criteria. Cells, AGS or IEC-18, were seeded in 96-well plates (1.5 × 104 cells/well) and treated in a similar manner as described above for the DNA fragmentation ELISA. Briefly, cell suspensions (100 μl) were mixed with acridine orange (5 μg/ml) and from this mixture an aliquot of 25 μl was dropped on to a microscope slide. Cells were visualized for nuclear fragmentation under blue-green fluorescence using a phase-contrast inverted microscope DMIRB (Leica Mikroscopie und, Germany). Induction of apoptosis with the oxidants was confirmed qualitatively, as well as reduction in the extent of the apoptotic response with antioxidants. Quantification of these events was achieved with the Cell Death ELISA (DNA fragmentation assay).
DPPH radical scavenging
The DPPH radical scavenging capacity as previously reported [24] was modified as follows. The aqueous extract of freeze-dried cat's claw (Uncaria tomentosa or Uncaria guianensis), vitamin C or green tea was standardized to give a stock solution (25 mg/ml) and filtered at 20 μm using a Whatman paper N° 4. Aliquots (25 μl) were placed in a cuvette and an ethanolic solution of DPPH (100 μM) was added to a final volume of 1 ml. The decrease in absorbance at 515 nm was determined continuously with data capturing at 30 sec intervals with a spectrophotometer UV-1601 PC (Shimadzu Corporation, Japan). The degree of DPPH radical scavenging activity of the antioxidants was calculated as percentage of inhibition (% inhibition) by the following expression: % inhibition = [(Acontrol - Asample) / Acontrol] * 100 where A control is the absorbance at time = 0, and Asample is the absorbance of the sample at time = 5 min. An EC50 value was determined as the concentration that elicited a half-maximal response.
Hydrogen peroxide scavenging
Hydrogen peroxide levels were monitored spectrophotometrically at 240 nm in a manner similar to what is described above for DPPH. Reductions in H2O2 concentration from an initial 50 μM were calculated from the reduction in the absorbance signal after subtracting any inherent signal associated with the antioxidant.
Quenching of peroxynitrite was not evaluated as it can only be effectively studied at a severe alkaline pH, given the lability of peroxynitrite at neutral pH. Peroxynitrite was synthesized as previously described [14]. Briefly, solutions of (a) 0.7 M NaNO2, 0.7 M H2O2 and (b) 0.6 M HCl, were pumped using a syringe infusion pump (Harvard Apparatus, South Natick, MA) at 25 ml/min, into a Y-junction and mixed in a 2 mm-diameter by 0.5 cm silica tube. The mixture was collected in a beaker containing a 1.5 M KOH solution. To eliminate the excess of H2O2, the peroxynitrite solution was filtered in a column containing MnO2 (4 g). The prepared solution contained 40 mM peroxynitrite, as determined by absorbance at 302 nm. A fresh solution of peroxynitrite (5 mM) was prepared in 5 mM KOH for each experiment and filtered at 0.2 μm.
Cell death photometric enzyme linked immuno-sorbent assay (ELISA)
To investigate the protection cytotoxic effects of oxidants (DPPH, H2O2, and ONOO-), cells (AGS and IEC-18) were seeded in 96-well plates (1.5 × 104 cells/well). Cells were treated with DPPH (3 μM), H2O2 (50 μM), peroxynitrite (300 μM) and/or supplemented with 10 μg/ml of each antioxidant (cat's claw, green tea, ascorbic acid), and incubated for 6 h. Apoptosis was quantified by a cellular DNA fragmentation ELISA. Cell death was characterized by measuring DNA fragments released into the medium (early necrosis and late apoptosis), and apoptosis (DNA fragments in the cytoplasm). The assay is based on the principle of incubating cells with the non-radioactive thymidine analogue BrdU, which is added to the cells an media at the time of seeding and incorporated into the genomic DNA. BrdU-labeled DNA fragments are released from the cells into the cytoplasm during apoptosis or into the cell culture medium during cell-mediated cytotoxicity. The DNA fragments were detected immunologically by the ELISA technique using an anti-DNA-antibody coated microplate to capture the DNA fragments, and an anti-BrdU-antibody peroxidase conjugate to detect the BrDU contained in the captured DNA fragments. The degree of apoptosis (cytosolic DNA fragments) and early necrosis (DNA released into the medium) was quantified following the manufacturer's recommendations (Roche Molecular Biochemicals, Nutley, NJ).
Cell membrane integrity (Necrosis)
The degree of cellular necrosis (cell membrane integrity) in AGS and IEC-18 cells was determined by measuring the level of lactate dehydrogenase (LDH) released into culture media. LDH release was quantified using a commercially available colorimetric kit (Sigma, St. Louis, MO). Cells (AGS or IEC-18) were seeded into 96-well plates (1.5 × 104 cells/well) and allowed to adhere for 24 hours. Cells were then exposed to oxidants at the previously stated concentrations for an additional 24 hours. In experiments evaluating antioxidant/oxidant interactions, antioxidants were added to the culture media 1 hour prior to oxidants. Following a 24 hour incubation, culture plates were centrifuged at 250 × g for 4 minutes and a 100 μL aliquot transferred to a clean 96-well plate. The amount of LDH released into the culture media was quantified according to the protocol outlined by the manufacturer. Data were expressed as the percent of LDH released compared to control cells.
Statistical analysis
Results were analyzed by ANOVA with post hoc analysis, where appropriate, by the Dunnett's test, using a commercial statistical software package (Instat®). Data is expressed as the mean ± SEM with a minimum of three experiments performed for each variable.
Results
Cell proliferation and antioxidants
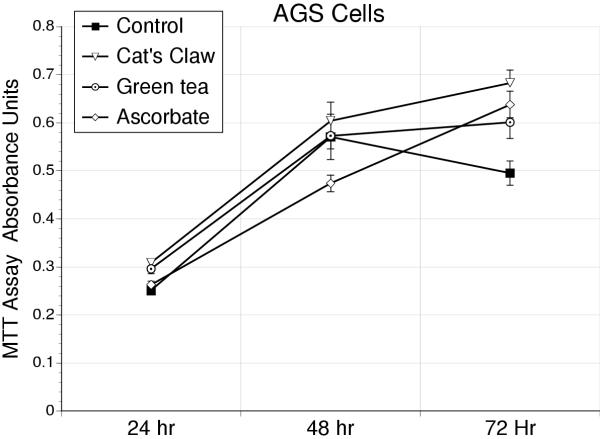
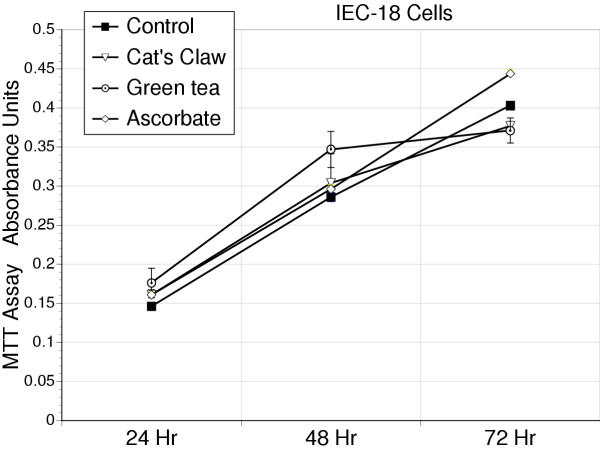
At the concentrations studied, addition of antioxidants to either AGS (Figure 1) or IEC-18 cells (Figure 2) failed to affect cell proliferation over 72 hours.
Figure 1.
Proliferation curves for AGS cells, based on the MTT assay, for cell respiration over a 72 hour period in response to the antioxidants – cat's claw, green tea, or vitamin C. These antioxidants had no detrimental effect on cell proliferation at the concentrations used in this study, 10 μg/ml.
Figure 2.
Proliferation curves for IEC-18 cells, based on the MTT assay, for cell respiration over a 72 hour period in response to the antioxidants – cat's claw, green tea, or vitamin C. These antioxidants had no detrimental effect on cell proliferation at the concentrations used in this study, 10 μg/ml.
Cell death in response to DPPH
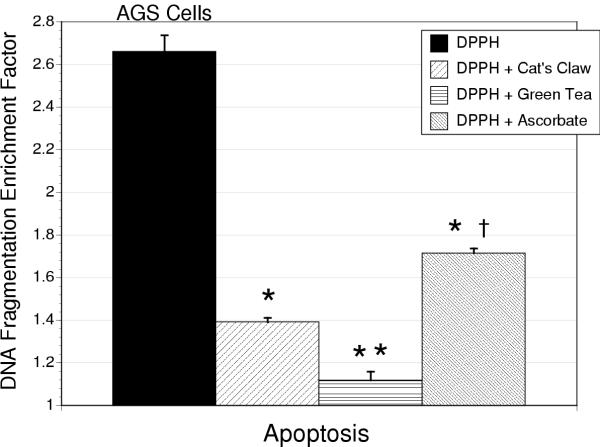
In response to the addition of DPPH (3 μM) to the culture media there was a marked induction in apoptosis as determined by ELISA and acridine orange assessment. This response was attenuated by the antioxidants in the following order of potency green tea > cat's claw > ascorbic acid for AGS cells (Figure 3). Similar reductions in apoptosis were observed in IEC-18 cells, although in contrast to AGS cells differences between antioxidants in their cytoprotective actions were not significant (Table 1).
Figure 3.
Apoptosis, measured as by histone-associated cytosolic DNA fragmentation, in AGS cells treated with DPPH alone (3 μM) or in combination with cat's claw, green tea extract or ascorbic acid (10 μg/ml). The * designates a significant difference from DPPH alone (P < 0.01), ** a significant difference between green tea and cat's claw (P < 0.05) and † significant difference between ascorbic acid and either green tea or cat's claw treated groups (P < 0.1).
Table 1.
Apoptosis Induction by Oxidants in IEC-18 cells and the Anti-apoptotic Effects of Dietary Antioxidants.
| Treatment | Agonist | Agonist + Cat's claw | Agonist + Green Tea | Agonist + Vitamin C |
| H2O2 | 269 ± 10† | 178 ± 7* | 161 ± 7* | 184 ± 12* |
| Peroxynitrite | 248 ± 5† | 146 ± 6* | 143 ± 5* | 141 ± 6* |
| DPPH | 249 ± 6† | 173 ± 7* | 159 ± 4* | 177 ± 4* |
Results are displayed as a percentage of control values (Mean ± SEM), N = 6 in each group, † P < 0.001 vs. control, *P < 0.001 vs. Agonist.
In both AGS and IEC-18 cells, DPPH (3 μM) produced a 20–30% reduction in cell viability (Table 2, 3 and 4). This reduction in cell viability was unaffected by antioxidants with the exception of green tea, which protected against this loss in cell number (P < 0.05). Using media release of BrdU-labeled DNA fragments as an index of early stage necrosis, it was noted that DPPH (3 μM) did not raise rates of necrosis above that evident in untreated control AGS cells (Table 4). Antioxidants did not change this response with the exception of cat's claw, which reduced this assay of early stage necrosis (P < 0.01) when compared to either control (untreated) values or the cells treated with DPPH.
Table 2.
Changes in AGS Cell Viability (MTT Assay) in Response to Oxidants and the Effects of Dietary Antioxidants
| Treatment | Agonist | Agonist + Cat's claw | Agonist + Green tea | Agonist + Vitamin C |
| H2O2 | 91.2 ± 3.6 | 104.0 ± 4.1 | 105 ± 3.1 | 89.8 ± 2.7 |
| Peroxynitrite | 86.8 ± 3.7 | 93.3 ± 3.1 | 101.0 ± 3.2 | 91.3 ± 2.7 |
| DPPH | 70.4 ± 1.3 | 74.2 ± 2.5 | 79.7 ± 1.8* | 69.5 ± 1.4 |
Viability was determined by the MTT assay and results are expressed as a percentage of control values (Mean ± SEM), N = 6 in each group. The * indicates a significant improvement in cell viability when compared to agonist alone (P < 0.05).
Table 3.
Changes in IEC-18 Cell Viability (MTT Assay) in Response to Oxidants and the Effects of Dietary Antioxidants
| Treatment | Agonist | Agonist + Cat's claw | Agonist + Green tea | Agonist + Vitamin C |
| H2O2 | 102.3 ± 2.3 | 105.8 ± 2.3 | 123.1 ± 3.7 | 99.9 ± 2.4 |
| Peroxynitrite | 76.9 ± 1.9 | 79.7 ± 2.5 | 85.6 ± 2.2 | 90.5 ± 2.2 |
| DPPH | 70.4 ± 1.3 | 74.2 ± 2.5 | 79.7 ± 1.8* | 69.5 ± 1.4 |
Viability was determined by the MTT assay and results are expressed as a percentage of control values (Mean ± SEM), N = 6 in each group. The * indicates a significant improvement in cell viability when compared to agonist alone (P < 0.05).
Table 4.
Changes in Membrane Integrity (LDH Release) in IEC-18 and AGS Cells in Response to Oxidants.
| Treatment | IEC-18 Cells | AGS Cells |
| H2O2 | -12.0 ± 0.8 | -8.2 ± 1.6 |
| Peroxynitrite | -3.7 ± 1.5 | 1.4 ± 2.0 |
| DPPH | 33.1 ± 2.7* | 8.0 ± 3.6 |
Results are presented as a percent change from control values, Mean ± SEM, n = 4 per group. The increase in LDH release evident in DPPH treated IEC-18 cells (*P < 0.05 vs. control) was significantly reduced by cat's claw (11.6 ± 6.7%) or green tea (11.3 ± 3.9%), P < 0.05 compared to DPPH treatment alone, but not by vitamin C (29.3 ± 6.0%).
LDH release into the media was used as an index of the integrity of cell membranes, or necrosis, in response to the oxidant burden. Neither peroxynitrite or H2O2 at the concentrations chosen affected LDH levels in either AGS or IEC-18 cells, indicating that cell death by necrosis was minimal. DPPH had no effect on media LDH levels in AGS cells but significantly raised levels in IEC-18 cells (Table 4). This response was attenuated by cat's claw or green tea (P < 0.05) but not ascorbic acid (Table 4).
Cell death in response to hydrogen peroxide
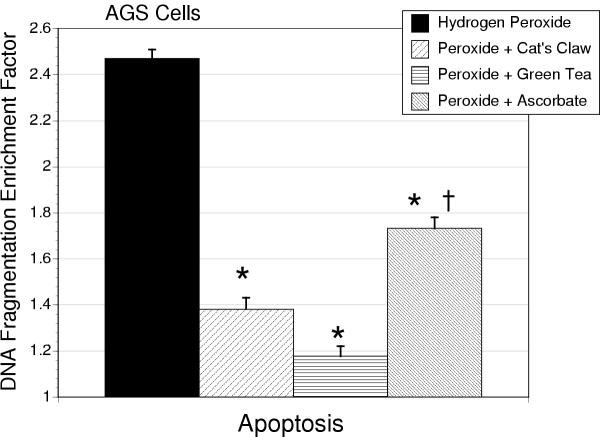
Hydrogen peroxide (50 μM) elicited a marked increase in apoptosis in AGS (Figure 4) and IEC-18 cells (Table 1), confirmed by acridine orange morphological assessment. Ascorbic acid treatment was less effective than either green tea or cat's claw in attenuating hydrogen peroxide-induced apoptosis in AGS cells (P < 0.01). In a manner similar to that seen with DPPH in IEC-18 cells, all antioxidants displayed comparable anti-apoptotic actions against H2O2 in IEC-18 cells (Table 1).
Figure 4.
Apoptosis, measured as by histone-associated cytosolic DNA fragmentation, in AGS cells treated with hydrogen peroxide (50 μM) alone or in combination with cat's claw, green tea extract or ascorbic acid (10 μg/ml). The * indicates a significant reduction in apoptosis compared to hydrogen peroxide alone (P < 0.01), and † designates a significant difference between ascorbic acid and either cat's claw or green tea extract (P < 0.01).
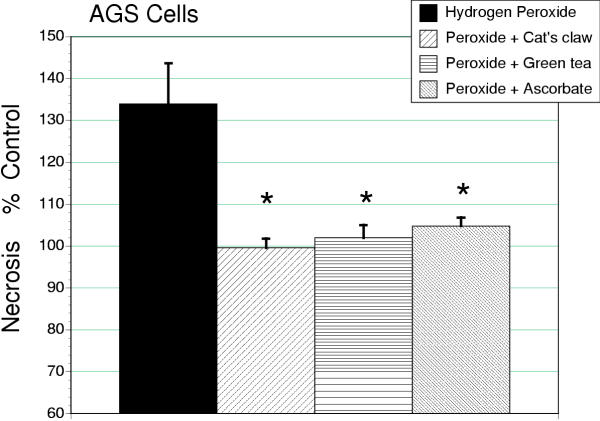
Using the release of BrdU-labeled DNA assay for early stage necrosis, it was noted that hydrogen peroxide (50 μM) increased necrosis in AGS cells by approximately 34% (Figure 5). The induction or necrosis by H2O2 in AGS cells was prevented by cat's claw, green tea and ascorbic acid (P < 0.01). In contrast, the MTT assay did not reveal changes in cell number in response to hydrogen peroxide in either AGS or IEC-18 cells (Tables 2 and 3).
Figure 5.
Early stage necrosis in AGS cells treated with hydrogen peroxide (50 μM) expressed as a percentage of untreated controls. All antioxidants reduced cell death to control values (*P < 0.01).
Cell death in response to Peroxynitrite
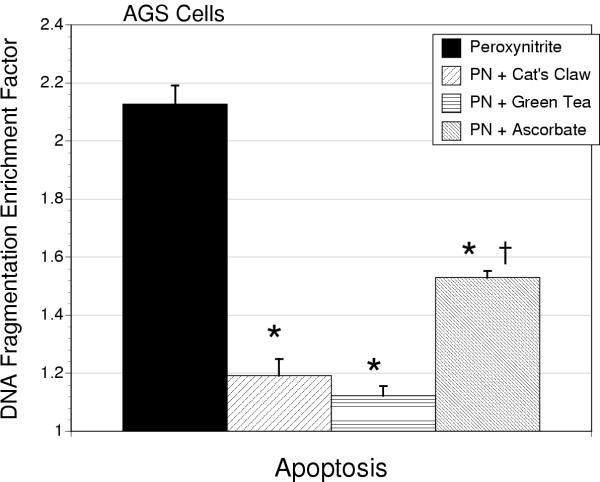
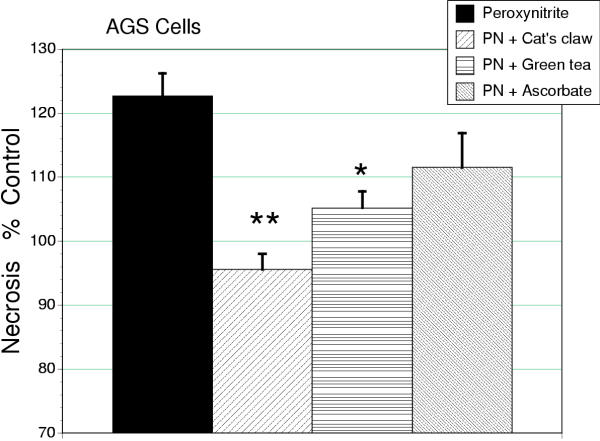
Peroxynitrite (300 μM) exposure reduced cell number (MTT assay) that was not significantly altered by antioxidant therapy in either AGS or IEC-18 cell types (Tables 2 and 3). Apoptosis was induced in both AGS and IEC-18 cells by peroxynitrite (P < 0.001), quantified by ELISA and confirmed morphologically by acridine orange. In AGS cells, ascorbic acid caused a reduction in the apoptotic response to peroxynitrite (P < 0.01). However, both cat's claw or green tea treatment were more effective than ascorbate in reducing the apoptotic response to peroxynitrite (P < 0.05, Figure 6). Values for apoptosis for green tea or cat's claw, but not ascorbate, were lowered to a level that was indistinguishable from control, untreated cells. Using the media release of BrdU-labeled DNA as an index of early necrotic cell death, it was observed that peroxynitrite treatment induced necrosis in AGS cells (P < 0.001). This effect was reduced by either cat's claw (P < 0.001) or green tea (P < 0.01) but not ascorbic acid (Figure 7). In contrast, in IEC-18 cells all three antioxidants produced similar degrees of cytoprotection, limiting peroxynitrite-induced apoptosis (Table 1).
Figure 6.
Apoptosis, measured as by histone-associated cytosolic DNA fragmentation, in AGS cells treated with peroxynitrite (300 μM) alone or in combination with cat's claw, green tea extract or ascorbic acid (10 μg/ml). All antioxidants reduced the extent of apoptosis (*P < 0.01). However, ascorbic acid was significantly less effective than either cat's claw or green tea extract (P < 0.01).
Figure 7.
Early stage necrosis in AGS cells treated with peroxynitrite (300 μM) expressed as a percentage of untreated controls. Both cat's claw (**P < 0.01) and green tea extract (*P0.05) were effective in preventing this form of cell death. Ascorbic acid treatment was not associated with a significant reduction in cell death.
DPPH radical and Hydrogen Peroxide quenching
The DPPH radical was quenched by the antioxidants as indicated by an acceleration of the decay of the absorbance signal (515 nm). Ascorbic acid was significantly more potent than green tea or cat's claw (P < 0.05), with green tea being more effective than cat's claw (P < 0.05). The EC50 activities for these antioxidants against DPPH are summarized in Table 5. This rank order of potency contrasts the rank order of potency in protecting epithelial cells form DPPH-induced apoptosis.
Table 5.
Quenching of the DPPH Radical and H2O2 by Dietary Antioxidants
| Treatment | Cat's claw | Green Tea | Vitamin C |
| H2O2 | ND | ND | 4.6 |
| DPPH | 22.7 | 15.7† | 5.2* |
Results are expressed as the half-maximal concentration (EC50, μg/ml) required to quench either H 2O2 or DPPH radicals, determined by inhibition of absorbance at 240 and 515 nm, respectively. *P < 0.05 vs. other treatments † P < 0.05 vs. Cat's claw ND Not detected
The hydrogen peroxide signal, determined by spectroscopy at 240 nm, was quenched by ascorbic acid but not by the decoctions, green tea or cat's claw. Results are summarized in Table 5.
Discussion
From this study it is clear that concentrations of oxidants that elicit minimal to minor degrees of epithelial cell necrosis can promote significant apoptotic cell death. This result is not specific to a particular oxidant, as a comparable response was observed with the three distinct oxidants – peroxynitrite, hydrogen peroxide, and the free radical DPPH. In each case, these oxidants caused significant apoptosis and cell death which was dramatically reduced by the addition of dietary antioxidants. With the exception of vitamin C, the potency of these antioxidants as cytoprotective (anti-apoptotic) agents, appears to exceed their inherent ability to quench the specific oxidant stimulus. In other words, at concentrations of green tea or cat's claw that had minimal quenching effects on the oxidant signal itself, a significant benefit to cellular systems was seen. This may reflect the combined contributions of intracellular antioxidants with the exogenous antioxidant. However, if that were the sole explanation, the rank order of potency would not change when switching from cell free to cellular systems. Ascorbate would remain more effective, rather than being the weakest of the antioxidants studied to limit apoptosis (in AGS cells). It appears that other factors influence the anti-apoptotic activity of antioxidants.
We chose to evaluate cat's claw and green tea for several reasons. Beyond being extracts and hence they are made up of a collection of phytochemicals, in contrast to vitamin C, these "teas" are used for health maintenance in various cultures. Recently, both green tea and cat's claw have been reported to reduce the expression of inflammatory genes; responses that are linked to apoptosis and proliferation [23-27]. Indeed, cat's claw has been described as a potent inhibitor of the transcription factor NF-κB activation, preventing the expression of TNFα and inducible nitric oxide synthase [23,24]. These immune/inflammatory products are associated with gastritis and epithelial cell death [5-7,13] and hence cat's claw may have utility in limiting gut inflammation [23]. Indeed, this matches its ethnomedical use in South America where it is used to treat gastritis and other forms of chronic inflammation like arthritis [28]. Green tea, and its constituent polyphenols, has been associated with reduced expression of inflammatory genes and signal transduction processes leading to cell death [21,25-27].
We focused on epithelial cells of the upper gastrointestinal tract because the sites of highest epithelial exposure to these dietary antioxidants would be the stomach and small intestine following ingestion. While it is likely that these antioxidants are absorbed and exert actions from the vascular space systemically (hence applications in arthritis), knowledge of their pharmacokinetics is limited. Given that gastritis, whether it is induced by infection with H. pylori or toxicity responses to NSAIDs, is associated with NF-κB activation, TNFα expression and apoptosis in epithelial cells [5-7], strategies to limit these responses may have significant therapeutic potential. This is particularly true if the therapeutic modalities are available to a wide section of the population for little expense. However, the doses of antioxidants used in this study are above that noted with diet or simple tea consumption and are more reflective of therapeutic intervention based on dietary supplementation.
Gastritis associated with persistent H. pylori infection is associated with an increased incidence of gastric cancer. Whether dietary antioxidant supplementation retards this expression has been an appealing hypothesis. Several lines of evidence support this contention. Correa et al [29] have clearly shown in a large trial conducted over six years that dietary antioxidant supplementation alone (vitamin C or beta-carotene), without antimicrobial therapy, can limit the progress of gastric cancer and actually may promote regression of precancerous states. Additionally, it appears that the population sub-groups at greatest risk for gastric cancer have a limited ability to secrete ascorbic acid from the plasma through the mucosal and into the gastric lumen [30]. In a related study, supplementation with vitamin C reduces the degree of apoptosis and nitrotyrosine immunohistochemistry [13], a marker of peroxynitrite formation [15]. NF-κB is activated in gastric epithelia with H. pylori infection, and clearance of this infection reduced NF-κB levels as well as expression of inducible nitric oxide [5,13], thereby reducing the mucosal burden of nitric oxide and possibly peroxynitrite. While clearance of the infection is curative, much of the world's population cannot afford the therapy, and further clearance of the infection with antimicrobial therapy is not guaranteed. Thus, treating the host response to the infection with common, readily available antioxidants may afford some degree of protection. In contrast, a recent study in Japan indicated that the consumption of green tea was not associated with either a reduction or an increase in gastric cancer rates [31]. This raises concern as to the ability of polyphenols derived from green tea to afford protection from mutagenic substances or limit the progression of established cancer. However, the discrepancy is more likely a reflection that higher doses of polyphenols are required to achieve benefit than that which is achieved with simple ingestion of tea. This is evident when one evaluates the concentrations required to achieve anticancer, cytoprotective actions in vitro [19,21,25-27]. More recently in the Apcmin mouse model of familial adenomatous polyposis, a condition with a massive induction of polyps in the gastrointestinal tract, green tea extracts reduced polyp load and potentiated the effects of sulindac, a cyclo-oxygenase inhibitor used to treat gastrointestinal cancer [32]. This further affirms the potential of this strategy in managing disorders characterized by epithelial cell death and transformation.
The gastric mucosal responses to non-steroidal anti-inflammatory drugs also involve the activation of NF-κB, generation of cytotoxic cytokines like TNFα and epithelial apoptosis [6,7]. Administration of low concentrations of nitric oxide by an ester linkage reduces this effect, suggesting that nitric oxide was offering benefit by quenching other radicals involved in cell activation. Under these circumstances, could co-administration of dietary antioxidants also limit the toxicity of the pharmaceutical? If so, this approach could offer significant cost savings over the use of newer cyclo-oxygenase 2 (COX2) inhibitors. In a recent paper by Laine [33], it was calculated that although COX2 inhibitors reduced the incidence of gastrointestinal damage when compared to non-selective COX inhibitors, these benefits were achieved at a significant increase in health care costs (approximately $560 per patient per year). In contrast, we have reported that cat's claw prevents the significant enteropathy associated with sustained use of indomethacin, a non-selective COX inhibitor [23], and this combination could alleviate the gastrointestinal damage without the high costs of COX2 inhibitors.
In the present study, oxidant stress was induced by signals that are largely hydrophilic. In other words apoptosis was not provoked with lipid peroxides. As the chosen antioxidants were hydrophilic, a significant interaction was anticipated. However, these antioxidants may be less effective against a lipid peroxide generating system, and under those conditions an antioxidant like vitamin E may be more effective. However, lipid hydroperoxide-induced apoptosis is accompanied by an alteration in the intracellular redox balance leading to a depletion of intracellular thiols and subsequent activation of caspases and PARP cleavage [33], responses that would be attenuated by hydrophilic antioxidants.
Peroxynitrite is a potent oxidant that can induce lipid peroxidation and as such may provide important insight. However, endogenous production of peroxynitrite remains controversial. Nitration of tyrosine residues is used as an index of peroxynitrite formation but it is not specific [13]. However, the purpose of using peroxynitrite in this study was to address the effects of a potent oxidant and not to evaluate the role of peroxynitrite in pathological states. Indeed some oxidants may be environmental and not produced endogenously but yet antioxidant supplementation may provide therapeutic utility.
In the present study, AGS cells (human gastric epithelial cells) were used. AGS are a transformed cell line, consequently, one could argue that dietary antioxidants could prevent the elimination of these transformed cells by the mucosal immune response. Indeed that is a concern that has been raised following the failure of prospective antioxidant trials in smokers to limit the incidence/progression of lung cancer [35] and the recent epidemiological analysis of gastric cancer incidence and green tea consumption [32]. However, other evidence has indicated that the antioxidants, vitamin E or N-acetylcysteine, enhanced the ability of the chemotherapeutic agent 5-fluorouracil to induce apoptosis in colon cancer cells [36]. These in vitro observations are supported by the Apcmin animal model [32] and sustained clinical trial in patients with H. pylori gastritis [30] suggesting that dietary antioxidants do not exacerbate precancerous states but may indeed promote regression. In addition, cells of a lymphoid origin where NF-κB is constitutively activated, inhibition of NF-κB confers complete protection to hydrogen peroxide and pervanadate, but rendered HeLa cells more susceptible to apoptosis induced by TNFα [37].
Conclusion
Dietary antioxidants confer significant protection to gut epithelial cells from pro-apoptotic oxidant stress. The phytochemical mixtures found in the teas, cat's claw and green tea, appear to be more effective than vitamin C in some cell lines and at concentrations that suggest that they may be acting at levels distinct from the mere scavenging of the oxidant signal. Diet supplementation with these or related antioxidants may prove valuable in limiting the pathophysiology of numerous disorders associated with gut inflammation.
Competing interests
There are no competing interests for BK Reuter or F. Angeles. Both MJS Miller and M Sandoval have financial interests in Rainforest Phytoceuticals, LLC who supplied the cat's claw used in this study.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgement
This work was supported by a Public Health Service grant P01CA28842 from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, USA
Contributor Information
Mark JS Miller, Email: millermj@mail.amc.edu.
Fausto M Angeles, Email: fmangelesf@usa.com.
Brian K Reuter, Email: ok_go@hotmail.com.
Paul Bobrowski, Email: bobrowski@amazonmedicines.com.
Manuel Sandoval, Email: sandovm@mail.amc.edu.
References
- Neurath MF, Becker C, Barbulescu K. Role of NF-kappa B in immune and inflammatory responses in the gut. Gut. 1998;43:856–860. doi: 10.1136/gut.43.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourd'heuil D, Morise Z, Conner EM, Kurose J, Grisham MB. Oxidant-regulation of gene expression in the chronically inflamed intestine. Keio J Med. 1997;46:10–15. doi: 10.2302/kjm.46.10. [DOI] [PubMed] [Google Scholar]
- Winyard PG, Blake DR. Antioxidants, redox-regulated transcription factors. Adv in Pharmacol. 1997;18:403–421. doi: 10.1016/s1054-3589(08)60993-x. [DOI] [PubMed] [Google Scholar]
- Marks-Honczalik J, Chu SC, Moss J. Cytokine-mediated transcriptional induction of human inducible nitric oxide synthase gene requires both activator protein-1 and nuclear factor κB binding sites. J Biol Chem. 1998;273:22201–22208. doi: 10.1074/jbc.273.35.22201. [DOI] [PubMed] [Google Scholar]
- Zhang X-J, Ruiz B, Correa P, Miller MJS. Cellular disassociation of NF-κB and iNOS in Helicobacter pylori infection. Free Radical Biol Med. 2000;29:730–735. doi: 10.1016/S0891-5849(00)00375-0. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Antonelli E, Santucci L, Morelli O, Miglietti M, Federici B, Mannucci R, del Soldato P, Morelli A. Gastrointestinal safety of nitric oxide-derived aspirin is related to inhibition of ICE-like cysteine proteases in rats. Gastroenterology. 1999;116:1089–1106. doi: 10.1016/s0016-5085(99)70012-0. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Santucci L, Federici B, Antonelli E, Distrutti E, Morelli O, di Renzo G, Coata G, Cirino G, del Soldato P, Morelli A. Nitric oxide-releasing NSAIDs inhibit interleukin-1 β converting enzyme-like cystein proteases and protect endothelial cells from apoptosis induced by TNFα. Aliment Pharmacol Ther. 1999;13:421–435. doi: 10.1046/j.1365-2036.1999.00442.x. [DOI] [PubMed] [Google Scholar]
- Jobin C, Sartour RB. The IκB/NF-κB system: a key determinant of mucosal inflammation and protection. Am J Physiol. 2000;278:C451–C462. doi: 10.1152/ajpcell.2000.278.3.C451. [DOI] [PubMed] [Google Scholar]
- Schmid RM, Adler G. NF-κB/Rel/IκB: Implications in gastrointestinal diseases. Gastroenterology. 2000;118:1208–1228. doi: 10.1016/s0016-5085(00)70374-x. [DOI] [PubMed] [Google Scholar]
- Bohler T, Waiser J, Hepburn H, Gaedeke J, Lehmann C, Hambach P, Budde K, Neumayer HH. TNF-alpha and IL-1 alpha induce apoptosis in subconfluent rat mesangial cells. Evidence for the involvement of hydrogen peroxide and lipid peroxidation as second messengers. Cytokine. 2000;12:986–991. doi: 10.1006/cyto.1999.0633. [DOI] [PubMed] [Google Scholar]
- Kahlos K, Soini Y, Paako P, Saily M, Linnainmaa K, Kinnula VL. Proliferation, apoptosis, and manganese superoxide dismutase in malignant mesothelioma. Int J Cancer. 2000;88:37–43. doi: 10.1002/1097-0215(20001001)88:1<37::AID-IJC6>3.3.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Li J, Huang CY, Zheng RL, Cui KR, Li JF. Hydrogen peroxide induces apoptosis in human hepatoma cells and alters cell redox status. Cell Biol Int. 2000;24:9–23. doi: 10.1006/cbir.1999.0438. [DOI] [PubMed] [Google Scholar]
- Mannick EE, Bravo LE, Zarama G, Realpe JL, Zhang X-J, Ruiz B, Fontham ETH, Mera R, Miller MJS, Correa P. Inducible nitric oxide synthase, nitrotyrosine and apoptosis in Helicobacter pylori gastritis: Effects of antibiotics and antioxidants. Cancer Research. 1996;56:3238–3243. [PubMed] [Google Scholar]
- Sandoval M, Liu X, Clark DA, Miller MJS. Peroxynitrite induced-apoptosis in human epithelial cells is attenuated by mesalamine. Gastroenterology. 1997;113:1480–1488. doi: 10.1053/gast.1997.v113.pm9352850. [DOI] [PubMed] [Google Scholar]
- Miller MJS, Thomson JH, Zhang X-J, Sadowska-Krowicka H, Kakais JL, Munshi UK, Sandoval M, Rossi JE, Eloby-Childress S, Beckman JS, Ye YZ, Roddi CP, Manning PT, Currie MG, Clark DA. Role of inducible nitric oxide synthase expression and peroxynitrite formation in guinea pig ileitis. Gastroenterology. 1995;109:1475–1483. doi: 10.1016/0016-5085(95)90633-9. [DOI] [PubMed] [Google Scholar]
- Singer II, Kawka DW, Scott S, Weidner JR, Mumford RA, Riehl TE, Stenson WF. Expression of inducible nitric oxide synthase and nitrotyrosine in colonic epithelia in inflammatory bowel disease. Gastroenterology. 1996;111:871–885. doi: 10.1016/s0016-5085(96)70055-0. [DOI] [PubMed] [Google Scholar]
- Kaur H, Halliwell B. Evidence of nitric-oxide oxidative damage in chronic inflammation. Nitrotyrosine in serum and synovial fluid from rheumatoid patients. Febs Lett. 1994;350:9–12. doi: 10.1016/0014-5793(94)00722-5. [DOI] [PubMed] [Google Scholar]
- Sandoval M, Zhang X-J, Liu X, Mannick EE, Clark DA, Miller MJS. Peroxynitrite-induced apoptosis in T84 and RAW 264-7 cells: attenuation by L-ascorbic acid. Free Rad Biology Medicine. 1997;22:495. doi: 10.1016/s0891-5849(96)00374-7. [DOI] [PubMed] [Google Scholar]
- Oldreive C, Zhao K, Paganga G, Halliwel B, Rice-Evans C. Inhibition of nitrous acid-dependent tyrosine nitration and DNA base deamination by flavonoids and other phenolic compounds. Chem Res Toxicol. 1998;11:1574–1579. doi: 10.1021/tx980163p. [DOI] [PubMed] [Google Scholar]
- Manna SK, Mukhopadhyay A, Aggarwal BB. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-κB, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J Immunol. 2000;164:6509–6519. doi: 10.4049/jimmunol.164.12.6509. [DOI] [PubMed] [Google Scholar]
- Ahmad N, Gupta S, Mukhtar H. Green tea polyphenol epigallocatechin-3-gallate differentially modulates nuclear factor kappa B in cancer cells versus normal cells. Arch Biochem Biophys. 2000;376:338–346. doi: 10.1006/abbi.2000.1742. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Zhang Y, Quehenberger P, Luther T, Haase M, Muller M, Mackman N, Ziegler R, Nawroth PP. The dietary pigment curcumin reduces endothelial tissue factor gene expression by inhibiting binding of AP-1 to the DNA and activation of NF-kappa B. Thromb Haemost. 1997;77:772–782. [PubMed] [Google Scholar]
- Sandoval-Chacon M, Thompson JH, Liu X, Mannick EE, Sadowska-Krowicka H, Charbonnet R, Clark DA, Miller MJS. Anti-inflammatory actions of cat's claw: the role of NF-κB. Alimentary Pharmacol Ther. 1998;12:1279–1289. doi: 10.1046/j.1365-2036.1998.00424.x. [DOI] [PubMed] [Google Scholar]
- Sandoval M, Charbonnet RM, Okuhama NN, Roberts J, Krenova Z, Trentacosti AM, Miller MJS. Cat's claw inhibits TNFα production and scavenges free radicals: Role in cytoprotection. Free Radical Biol Med. 2000;29:71–78. doi: 10.1016/S0891-5849(00)00327-0. [DOI] [PubMed] [Google Scholar]
- Chen C, Yu R, Owuor ED, Kong AN. Activation of antioxidant-response element (ARE), mitogen-activated protein kinases (MAPKs) and caspases by major green tea polyphenol components during cell survival and death. Arch Pharm Res. 2000;23:605–612. doi: 10.1007/BF02975249. [DOI] [PubMed] [Google Scholar]
- Fu YC, Jin XP, Wei SM. The effects on cell growth of tea polyphenols acting as a strong anti-peroxidant and an inhibitor of apoptosis in primary cultured rat skin cells. Biomed Environ Sci. 2000;13:170–170. [PubMed] [Google Scholar]
- Jung YD, Kim MS, Shin BA, Chay KO, Ahn BW, Liu W, Bucana CD, Gallick GE, Elllis LM. EGCG, a major component of green tea, inhibits tumour growth by inhibiting VEGF induction in human colon carcinoma cells. Br J Cancer. 2001;84:844–850. doi: 10.1054/bjoc.2000.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. Cat's Claw. Healing vine of Peru. Seattle, USA Sylvan Press. 1995.
- Correa P, Fontham ET, Bravo JC, Bravo LE, Ruiz B, Zarama G, Realpe JL, Malcom GT, Li D, Johnson WD, Mera R. Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti-Helicobacter pylori therapy. J Natl Cancer Inst. 2000;92:1881–1888. doi: 10.1093/jnci/92.23.1881. [DOI] [PubMed] [Google Scholar]
- Ruiz B, Rood JC, Fontham ET, Malcolm G, Hunter FM, Sobhan M, et al. Vitamin C concentration in gastric juice before and after anti-Helicobacter pylori treatment. Am J Gastroenterol. 1994;22:65–72. [PubMed] [Google Scholar]
- Tsubono Y, Nishino Y, Komatsu S, Hsieh CC, Kanemura S, Tsuji I, Nakatsuka H, Fukao A, Satoh H, Hisamichi S. Green tea and the risk of gastric cancer in Japan. N Engl J Med. 2001;344:632–636. doi: 10.1056/NEJM200103013440903. [DOI] [PubMed] [Google Scholar]
- Suganamu M, Ohkura Y, Okabe S, Fujiki H. Combination cancer chemoprevention with green tea extract and sulindac shown in intestinal tumor formation in Min mice. J Cancer Res Clin Oncol. 2001;127:69–72. doi: 10.1007/s004320000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine L. Approaches to non-steroidal anti-inflammatory drug use in the high-risk patient. Gastroenterology. 2001;120:594–606. doi: 10.1053/gast.2001.21907. [DOI] [PubMed] [Google Scholar]
- Wang TG, Gotoh Y, Jennings MH, Rhoads CA, AW TY. Lipid peroxide-induced apoptosis in human colonic CaCo-2 cells is associated with an early loss of cellular redox balance. FASEB J. 2000;11:1567–1576. doi: 10.1096/fj.14.11.1567. [DOI] [PubMed] [Google Scholar]
- The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- Adeyemo D, Imtiaz F, Toffa S, Lowdell M, Wickremasinghe RG, Winslet M. Antioxidant enhance the susceptibility of colon carcinoma cells to 5-fluorouracil by augmenting the induction of the bax protein. Cancer Lett. 2001;164:77–84. doi: 10.1016/S0304-3835(00)00720-5. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt B, Kaltschmidt C, Hofman TG, Hehner SP, Droge W, Schmitz ML. The pro- or anti-apoptotic function of NF-kappa B is determined by the nature of the stimulus. Eur J Biochem. 2000;267:3828–3835. doi: 10.1046/j.1432-1327.2000.01421.x. [DOI] [PubMed] [Google Scholar]