Abstract
Lactation is the most energetically demanding stage of reproduction in female mammals. Increased energetic allocation toward current reproduction may result in fitness costs, although the mechanisms underlying these trade‐offs are not well understood. Trade‐offs during lactation may include reduced energetic allocation to cellular maintenance, immune response, and survival and may be influenced by resource limitation. As the smallest marine mammal, sea otters (Enhydra lutris) have the highest mass‐specific metabolic rate necessitating substantial energetic requirements for survival. To provide the increased energy needed for lactation, female sea otters significantly increase foraging effort, especially during late‐lactation. Caloric insufficiency during lactation is reflected in the high numbers of maternal deaths due to End‐Lactation Syndrome in the California subpopulation. We investigated the effects of lactation and resource limitation on maternal stress responses, metabolic regulation, immune function, and antioxidant capacity in two subspecies of wild sea otters (northern: E. l. nereis and southern: E. l. kenyoni) within the California, Washington, and Alaska subpopulations. Lactation and resource limitation were associated with reduced glucocorticoid responses to acute capture stress. Corticosterone release was lower in lactating otters. Cortisol release was lower under resource limitation and suppression during lactation was only evident under resource limitation. Lactation and resource limitation were associated with alterations in thyroid hormones. Immune responses and total antioxidant capacity were not reduced by lactation or resource limitation. Southern sea otters exhibited higher concentrations of antioxidants, immunoglobulins, and thyroid hormones than northern sea otters. These data provide evidence for allocation trade‐offs during reproduction and in response to nutrient limitation but suggest self‐maintenance of immune function and antioxidant defenses despite energetic constraints. Income‐breeding strategists may be especially vulnerable to the consequences of stress and modulation of thyroid function when food resources are insufficient to support successful reproduction and may come at a cost to survival, and thereby influence population trends.
Keywords: lactation, resource limitation, sea otter, trade‐offs
1. INTRODUCTION
Lactation is the most energetically expensive portion of the mammalian reproductive cycle (Hanwell & Peaker, 1977). Mean daily caloric intake can be 66–200% greater, and peak energy expenditure may be 2.5–5 times higher in lactating females compared with nonlactating conspecifics (Gittleman & Thompson, 1988). During lactation, behavioral changes often coincide with increased energetic demands. For example, females may significantly increase duration and frequency of foraging events in order to balance increased energetic costs (Clutton‐Brock, Iason, Albon, & Guinness, 1982; Stebbins, 1977). In addition to increasing energy intake, reproducing females are able to catabolize body tissue as a nutrient source (Millar, 1977; Randolph, Randolph, Mattingly, & Foster, 1977).
Species are frequently classified along a continuum of reproductive strategies ranging from capital (rely mostly on stored energy during reproduction) to income breeders (rely mostly on acquired energy during reproduction), depending on the importance of stored body reserves in relation to reproductive effort (Jönsson, 1997; Stearns, 1992). Yet even for income breeders, for which body reserves provide only a small proportion of reproductive expenditure, reserves may still be important to supplement physiological limits at the periods of peak energy investment (Tyler, 1987). Foraging success in relation to reproduction may be a key determinant of the magnitude of reproductive effort (Clutton‐Brock et al., 1982; Crocker, Williams, Costa, & Le Boeuf, 2001).
For many species, the high energetic demands required for reproduction necessitate various life‐history trade‐offs. For example, increased energetic allocation toward a current reproductive event may come at a cost to future survival or reproduction (Stearns, 1992; Williams, 1966). These trade‐offs may involve ecological (e.g., increased risk of predation due to more time spent foraging) and physiological processes (e.g., competing cellular functions) (Speakman, 2008; Zera & Harshman, 2001). Trade‐offs during lactation may drive reductions in energy allocation to cellular maintenance and somatic function, and thereby reduce future reproduction and survival (Zera & Harshman, 2001). Direct costs arise from the energetic demands of the reproductive event, such as dramatic increases in nutrients for milk production (Hanwell & Peaker, 1977; Speakman, 2008). Indirect costs can be compensatory, where the female makes optional allocation trade‐offs in other components of its physiology such as altering thermoregulation (Trayhurn, 1989) and immunosuppression (Peck, Costa, Crocker, & Goldbogen, 2015) to increase the energy available for parental investment. Indirect costs can also be obligatory; for example, reproductive events may decrease maternal bone mass (Miller & Bowman, 2004) and increase oxidative stress (Sharick, Vazquez‐Medina, Ortiz, & Crocker, 2015; Wiersma, Selman, Speakman, & Verhulst, 2004). In all of the above examples, reductions in the environmental availability of food resources (henceforth, resource limitation) will tend to exacerbate trade‐offs between competing life‐history processes (Boggs & Ross, 1993; Cotter, Simpson, Raubenheimer, & Wilson, 2011; Martin, 1987).
Fluctuations in environmental and state conditions have the potential to affect homeostasis of wild animals. Energy demands of reproduction may illicit a stress response by activating the hypothalamic–pituitary–adrenal (HPA) axis to release glucocorticoid (GC) hormones (e.g., corticosterone and cortisol). Upon activation of the HPA axis, most species release predominant quantities of a single glucocorticoid—either cortisol or corticosterone, and smaller quantities of the other (Palme, Rettenbacher, Touma, El‐Bahr, & Möstl, 2005). Within mammals, cortisol is the predominant GC in humans, ruminants, and carnivores (Palme et al., 2005), but many mammals also release biologically significant amounts of corticosterone (Koren et al., 2012). GCs have expansive physiological consequences on metabolism, reproduction, and immune function (Ensminger, Somo, Houser, & Crocker, 2014; Maule, Schreck, & Kaattari, 1987; Tilbrook, Turner, & Clarke, 2000). GCs are known to be immunosuppressive (Råberg, Grahn, Hasselquist, & Svensson, 1998; Sheldon & Verhulst, 1996) and have been hypothesized as a proximate mediator of allocation trade‐offs between reproduction and immune function. During periods when GC levels are increased to support energy mobilization for reproduction, there may be a reduced ability to respond to additional stressors. Under conditions of chronic stress, an additional acute stressor can elicit the HPA axis to respond in either of two ways: facilitation or acclimation (Dickens & Romero, 2013; Figueiredo, Bodie, Tauchi, Dolgas, & Herman, 2003; Pitman, Ottenweller, & Natelson, 1988; Romero, 2004). The literature describing downregulation of GC activity (i.e., gene regulation, receptor availability and resistance, hypocortisolism) in response to an acute stressor under conditions of chronic stress are robust in the biomedical field (Heim, Ehlert, & Hellhammer, 2000; McEwen, 2000; Miller, Chen, & Zhou, 2007; Juster , McEwen, & Lupien, 2010). Fewer studies have assessed the effects of chronic stress on HPA sensitivity in wild animals, especially mammals, some of which show that under chronic stress (e.g., population decline, density, and predation pressure) some species respond to acute stressors by facilitation (Boonstra, Hik, Singleton, & Tinnikov, 1998; Clinchy, Zanette, Boonstra, Wingfield, & Smith, 2004; Boonstra, 2013; Dantzer et al., 2013). Whereas others exhibit decreased basal GC levels, and/or less GC is released in response to an acute stressor (Harlow, Lindzey, Sickle, & Gern, 1992; Krause, Dorsa, & Wingfield, 2014; Rich & Romero, 2005; Wingfield & Mukai, 2009). Therefore, chronic stress may not always be identifiable by upregulated GC concentrations. The intricacies of the stress response in wildlife do not allow for consistent predications of response outcomes, rather a best approach to identifying chronic stress is to measure multiple features of GC regulation and changes in those responses (Dickens & Romero, 2013).
Glucocorticoids interact with thyroid hormones (thyroxine, T4; and triiodothyronine, T3), which are regulators of metabolic pathways. In response to a stressor, such as nutrient limitation, GCs tend to increase to mobilize energy stores (McMahon, Gerich, & Rizza, 1988; Sapolsky, Romero, & Munck, 2000) and thyroid hormones tend to decrease to reduce energy expenditure (Oppenheimer et al., 1987). GCs reduce levels of circulating thyroid hormone by suppressing release of thyroid‐stimulating hormone and inhibiting enzymatic deiodination of T4 into T3, the more physiologically active form (Oppenheimer et al., 1987). Additionally, upregulated GCs increase concentrations of reverse T3 (rT3), an inactive form of the hormone which functions as a thyroid receptor blocker (Charmandari, Tsigos, & Chrousos, 2005). Reductions in thyroid hormones may directly decrease maternal energy expenditure but at the expense of thermoregulation and activity.
Oxidative stress may be an obligatory cost of reproduction, and when present, restricts energy allocation to other important life stages (Costantini, 2008; Dowling & Simmons, 2009). Although oxidative stress may regulate life‐history trade‐offs, its effects on different tissues during reproduction are still poorly understood (Speakman et al., 2015). Oxidative stress occurs when reactive oxygen species (ROS) production overwhelms the body's antioxidant defense systems (Monaghan, Metcalfe, & Torres, 2009). Life stages with increased energy investment associated with reproduction, such as lactation, may lead to overproduction of ROS (Alonso‐Alvarez et al., 2004; Balaban, Nemoto, & Finkel, 2005), which may result in the depletion of antioxidant defenses and progress to oxidative damage and inflammation, especially under conditions of nutrient limitation (Mårtensson, 1986; Sharick et al., 2015). Conversely, increased foraging during lactation may enhance availability of exogenous antioxidants, specifically if the food sources contain high levels of antioxidants that can act efficiently in vivo, helping mitigate the enhanced oxidative stress associated with high rates of energy expenditure during lactation (Beaulieu & Schaefer, 2013; Schinella, Marín, de Alaniz, de Buschiazzo, & Tournier, 1999).
Sea otters (Enhydra lutris) (Figure 1) provide an excellent study system for examining life‐history trade‐offs and physiological responses to the energetic demands of lactation, particularly in the context of resource limitation. Female sea otters give birth to a single pup at approximately 1‐year intervals—annual birth rates vary from 0.84 to 1.07 and appear to be relatively invariant across populations (Bodkin, Mulcahy, & Lensink, 1993; Monson, Estes, Bodkin, & Siniff, 2000; Riedman, Estes, Staedler, Giles, & Carlson, 1994; Tinker et al., 2017). The consistency of birth rates, even during periods of reduced food abundance, is thought to represent a form of bet‐hedging that is adaptive given temporally unpredictable prey resources (Monson, Estes, et al., 2000): females initiate pregnancy irrespective of their current condition in order to capitalize on possible future increases in prey abundance occurring during the ~6 month gestation period and before parturition. Only females provide pup care, and the average pup dependency period is 6 months (Monson, Estes, et al., 2000; Riedman et al., 1994). The elevated energetic costs associated with lactation necessitate significant increases in maternal foraging activity, especially during the latter half of the lactation period (Esslinger, Bodkin, Breton, Burns, & Monson, 2014; Staedler, 2011; Thometz, Staedler, et al., 2016). Necropsies of pregnant females found that they appear to maximize fat deposition during gestation (Chinn et al., 2016), suggesting that they may accrue some reserves prior to parturition. However, once sexually mature, female sea otters are perpetually alternating between pregnancy and lactation, with little allowance for energetic recovery, and therefore provide an excellent example of an “income” strategist.
Figure 1.

Sea otter (Enhydra lutris) female with small pup. Sea otters are the smallest marine mammal spanning the nearshore intertidal habitats of the North Pacific
Sea otter pups suckle throughout the dependency period, but also begin to receive solid prey after 4 weeks, and by the end of lactation may consume up to a third of the prey captured by their mothers (Riedman et al., 1994). Respirometry studies indicate that a mother's daily energy consumption increases 85–110% over nonlactating periods (Thometz, Kendall, Richter, & Williams, 2016; Thometz, Tinker, Staedler, Mayer, & Williams, 2014). Despite increasing their foraging effort to as much as 60% of each 24‐h period (Esslinger et al., 2014; Thometz, Staedler, et al., 2016), females in resource‐limited environments are often emaciated by the end of lactation. The extreme energetic demands elicited by lactation and pup‐rearing, in addition to exogenous stressors such as resource limitation (Tinker, Doak, & Estes, 2008; Tinker et al., 2006), can increase risk of metabolic collapse and mortality, a phenomenon known as “End‐Lactation Syndrome” (ELS) (Chinn et al., 2016).
Our objective was to investigate the physiological dimensions of the life‐history trade‐offs made by sea otters, in order to understand how an extremely energy‐limited mammal is able to adjust to the increased energetic demands of lactation. Sea otters are well suited for this analysis because their history of overexploitation and subsequent population recovery has created a natural experiment where populations exist with widely varying levels of resource availability. Sea otters were hunted nearly to extinction during the maritime fur trade in the 18th and 19th centuries, which left a fraction of the original population scattered throughout their range in 12 remnant populations (Kenyon, 1969). Following protection in 1911 and with the assistance of translocations into areas of SE Alaska, British Columbia, and Washington in the 1970's (Jameson, Kenyon, Johnson, & Wight, 1982), these remnant populations began to grow and reoccupy habitats that had not had otters in some cases for over 100 years. Sea otters are a keystone species having strong direct and indirect impacts on the nearshore food webs of which they are a part (Breen, Carson, Foster, & Stewart, 1982; Duggins, Simenstad, & Estes, 1989; Estes & Duggins, 1995; Estes & Palmisano, 1974). As a result, the invertebrate prey populations in unoccupied habitats increased dramatically with standing stocks often orders of magnitude greater than in areas with long‐established populations (Estes & Duggins, 1995; Estes & Palmisano, 1974). Currently, areas of unoccupied habitat still exist with adjacent sea otter populations living with abundant prey as they expand their range (Bodkin & Udevitz, 1999; Estes, 1990). In contrast, other populations expanded and recovered to pre‐exploitation (i.e., equilibrium) densities as early as the 1950s where food became an important limiting resource (Coletti, Bodkin, Monson, Ballachey, & Dean, 2016; Estes, 1990; Kenyon, 1969; Monson, Estes, et al., 2000). Most recently, a top‐down‐driven decline in the western Aleutians sea otter population caused by killer whale predation has again created low‐density sea otter populations existing with nonlimiting prey resources (Estes, Tinker, Williams, & Doak, 1998; Williams, Estes, Doak, & Springer, 2004). This spatio/temporal pattern of populations existing with various levels of food resources has been used to understand demographic factors controlling sea otter population dynamics in the past (Monson, Estes, et al., 2000). In addition, it has allowed researchers the ability to develop tools to assess population status relative to food resources including assessments of sea otter body size and condition, direct measures of energy recovery rates and time‐activity budgets, and dietary diversity (Bodkin, Monson, & Esslinger, 2007; Monson & Bowen, 2015; Newsome et al., 2015; Tinker, Bentall, & Estes, 2008).
Here, we explore how lactation affects maternal immune function, energy regulation, and antioxidant capacity across several subpopulations of wild sea otters existing at varying levels of resource availability. We compared a suite of blood analytes between lactating and nonlactating sea otters and subspecies, and compared populations for evidence of effects of resource limitation. We predicted changes in GC response to the acute stress of capture in females that were already experiencing chronic stress due to lactation. We predicted allocation trade‐offs due to energetic demands of lactation to suppress immune function and metabolism, and decreased antioxidant availability to combat oxidative damage.
2. MATERIALS AND METHODS
2.1. Sample collection
Wild, reproductively active adult female sea otters were captured along the California, USA, coastline from 1999 to 2016, Alaska, USA, from 2006 to 2011 and Washington, USA, in 2011 with floating tangle nets, dip nets or by rebreather divers using modified Wilson traps (Ames, Hardy, & Wendell, 1986). The sample (n = 278) included 137 individuals from southern subspecies in California (E. l. nereis, subsequently referred to as SSO) and 141 from the northern subspecies in Alaska and Washington (E. l. kenyoni, subsequently referred to as NSO) (Figure 2). Of these, 112 females were captured with a dependent pup (lactating) and 166 were reproductively mature, nonlactating females. Lactating females were kept with their pups until anesthetized, and pups were returned to their mother prior to the female coming out of anesthesia and subsequently released together to avoid separation. Captured sea otters were held for variable times in wooden crates before anesthetization and sample collection. Otters were anesthetized via an intramuscular injection using a combination of 0.22–0.33 mg/kg fentanyl citrate and 0.07–0.11 mg/kg midazolam hydrochloride (Monson, McCormick, & Ballachey, 2001; Tinker et al., 2006). Morphometrics, nutritional state, and reproductive status were recorded, and blood was collected at a single event. A premolar tooth was collected for aging via analysis of cementum annuli (Bodkin et al., 1993; Matson's Lab, Milltown, MT, USA). When a cementum age was not available, age was determined by all physical characteristics including condition of teeth and coloration of pelage. Precise capture times were recorded for sea otters caught with Wilson traps and dip nets, and preanesthesia hold times were calculated. Precise capture times with tangle nets were not known but hold times for these sea otters were calculated from time of removal from net (which also represents a stressful event). Hold times were only recorded and available for a subset of the captures (n = 186).
Figure 2.
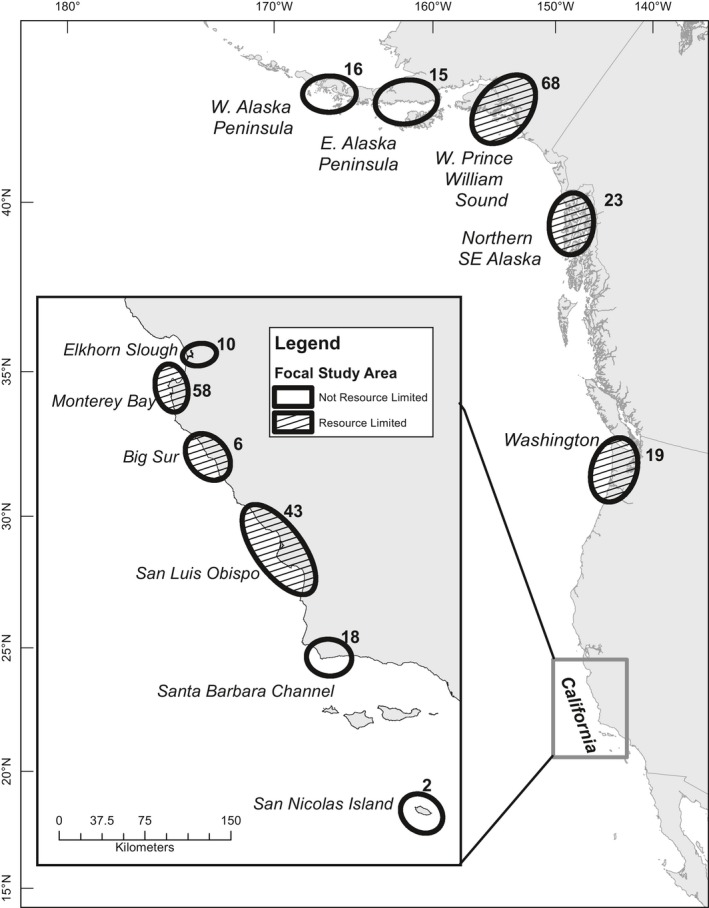
All subpopulations of sea otters sampled from California, Washington, and Alaska, USA. Circles with hatched fill were considered resource limited at the time of sampling. Number of otters sampled in each are listed above/to the right of each circle. Resource limitation status was assessed based on population density, recent trends, diet diversity, and biomass intake rates while foraging: for details, refer to Newsome et al. (2015)
Following sedation, whole blood was obtained from the jugular vein with 21 or 19 g butterfly winged blood collection sets into vacutainers and centrifuged (5–15 min at 2500–3700 RPM) to separate serum or plasma, then frozen at −80°C until analysis.
Subpopulations sampled were classified as resource‐limited or resource‐abundant, based on patterns of covariation in key metrics known to be responsive to resource abundance: population density and trends; diet diversity and prey recovery rates from foraging observations; relative body condition; and time‐activity budgets (Monson & Bowen, 2015; Newsome et al., 2015; Tinker, Doak, et al., 2008, 2017) (Figure 2; for additional details see Appendix Table S1).
2.2. Sample analysis
All analytes were measured in duplicate using commercially available assay platforms according to manufacturer's specifications. Cortisol and thyroid (total T3, total T4, and rT3) concentrations were measured using hormone specific I125 radioimmunoassays (MP Biological, Santa Ana, CA, USA, and ALPCO, Salem, NH, USA, respectively). Total antioxidant capacity (TAC) and corticosterone concentrations were measured using fluorometric and colorimetric enzyme‐immunoassays, respectively (Cayman Chemicals, Ann Arbor, MI, USA). Adaptive immune function was measured in two classes of total immunoglobulins, IgG, and IgM, using antibody‐sensitized microsphere microagglutination assays (Thermo Fisher Scientific, Waltham, MA, USA).
Samples were run “neat” (undiluted) except for corticosterone (diluted 1:15, with EIA buffer), IgM and IgG (diluted 1:100 000 or 1:2400, with dilution buffer) and TAC (diluted 1:10, with EIA buffer) because sample values were off the standard curve. All assay platforms were validated for use in sea otters. Serially diluted samples yielded curves that were parallel to the standard curves and between 89% and 106% of added standards were recovered from samples. The mean coefficient of variation was ≤5% for all analytes.
2.3. Statistical analysis
Data analyses were performed with JMP 13 Pro (SAS Institute, Cary, NC, USA). General linear models (GLM) were used to assess the effects of subspecies, maternal age, lactation status, resource limitation, and the interaction of lactation status with resource limitation on analyte concentrations. To examine associations between the markers, we used a GLM ANCOVA with an interaction term for lactation status and the independent variable. To address the ability to mount an acute stress response, sea otters with known time prior to anesthesia (duration between capture time or first observation in tangle net, until anesthesia) were plotted against GC values. If hold times exceeded 60 min, those individuals were excluded from analysis to account for clearance of GC from the initial stress response. While the half‐life of endogenous GCs in sea otters is unknown, cortisol half‐life in juvenile mink was typical for mammals (60 min; Sangild & Elnif, 1996). All individuals that were not anesthetized during sampling (n = 12) were removed from this analysis. ANCOVA was used to compare the slope of these relationships between lactating/nonlactating and resource‐limited/nonresource‐limited otters in a model that also included maternal age. Model residuals of GLMs were visually assessed for approximate normality, and residual plots were assessed for homoscedascity. For all tests, results were considered significant when p < 0.05.
3. RESULTS
3.1. Glucocorticoid response to capture
The timing of the acute stress response to the capture event was assessed in all sea otters with known time between capture and anesthesia of ≤60 min (n = 85). Cortisol concentration increased with hold time (F 1,79 = 8.80, p = 0.004). This effect was similar for both lactating and nonlactating females (ANCOVA, F 1,79 = 0.01, p = 0.91) and was not influenced by resource limitation (F 1,79 = 0.76, p = 0.39). Corticosterone levels did not increase with hold time (F 1,79 = 0.01, p = 0.91). Across all samples, cortisol was the dominant GC released but was strongly correlated with corticosterone (Figure 5a). The ratio of cortisol:corticosterone averaged 11.8 ± 7.4 (SD). This ratio falls within the range reported as typical (10–100) for terrestrial mammals (Koren et al., 2012). Because independent effects were detected as potential drivers of the magnitude of GC release, we also evaluated the cortisol:corticosterone ratio as a response variable.
Figure 5.
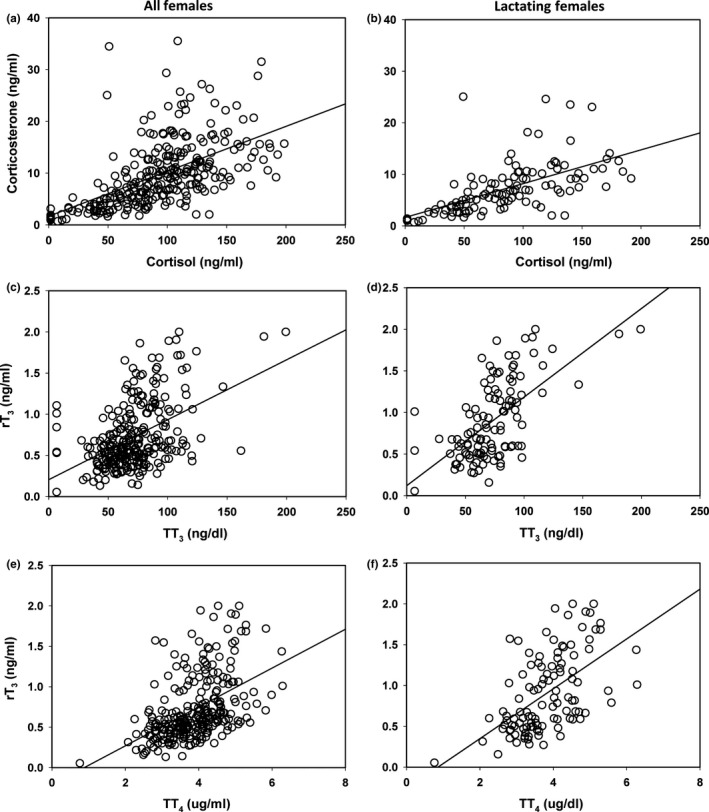
Interrelationships between measured analytes. (a) Relationship between cortisol and corticosterone of all female sea otters, y = 1.67 + 0.09x, r 2 = 0.33, F 1,276 = 133.73, p < 0.0001. (b) Relationship between cortisol and corticosterone for lactating females, y = 1.64 + 0.07x, r 2 = 0.35, F 1,110 = 59.31, p < 0.0001. (c) Relationship between T3 and rT 3, y = 0.20 + 0.007x, r 2 = 0.22, F 1,272 = 76.10, p < 0.0001. (d) Relationship between T3 and rT 3 in lactating females, y = 0.12 + 0.01x, r 2 = 0.38, F 1,110 = 68.32, p < 0.0001. (e) Relationship between T4 and rT3, y = −0.21 + 0.24x, r 2 = 0.24, F 1,264 = 82.91, p < 0.0001. (f) Relationship between T4 and rT 3 in lactating females, y = −0.26 + 0.31x, r 2 = 0.31, F 1,108 = 48.15, p < 0.0001
GC concentrations after the capture stress response showed differences with lactation status and resource limitation, but not by subspecies. Nonlactating females had higher corticosterone concentrations than lactating females (Tables 1, 2; Figure 3a). Resource limitation was associated with a reduced magnitude of GC release for both GCs (Tables 1, 2; Figure 3b). There was a significant interaction of lactation status and resource limitation for cortisol release in response to capture (Table 2; Figure 4). Cortisol release was lower in resource‐limited sea otters and lactating females from resource‐abundant subpopulations exhibited a stronger acute stress response. Corticosterone release increased significantly with maternal age (Table 2). There were no differences between subspecies in the magnitude of GC release in response to capture (p > 0.05). The cortisol:corticosterone ratio was strongly impacted by lactation status (F 1,256 = 11.88, p < 0.001), having a value of 10.3 ± 0.4 (SE) for lactating females and 14.0 ± 0.9 (SE) for nonlactating otters, but was not altered by any other explanatory factor (p > 0.05). For all sea otters, corticosterone increased with cortisol concentrations (Figure 5a). The slope of this association was greater in nonlactating sea otters (ANCOVA, F 1,274 = 4.2, p = 0.04; Figure 5b).
Table 1.
Mean ± SE of analytes
| NSO | SSO | Nonlactating | Lactating | Resource‐abundant | Resource‐limited | |
|---|---|---|---|---|---|---|
| TAC (mM) | 0.56 ± 0.03* | 1.09 ± 0.03* | 0.80 ± 0.02 | 0.79 ± 0.04 | 0.87 ± 0.04 | 0.77 ± 0.02 |
| IgM (mg/ml) | 0.22 ± 0.02* | 0.60 ± 0.02* | 0.37 ± 0.02 | 0.43 ± 0.03 | 0.39 ± 0.03 | 0.30 ± 0.01 |
| IgG (mg/ml) | 17.10 ± 1.00* | 23.79 ± 1.06* | 18.35 ± 0.88 | 22.75 ± 1.38 | 17.73 ± 1.47ᵟ | 20.77 ± 0.72ᵟ |
| Cortisol (ng/ml) | 94.97 ± 4.21 | 94.58 ± 4.36 | 99.04 ± 3.61 | 87.68 ± 5.83 | 111.43 ± 6.16ᵟ | 89.74 ± 2.98ᵟ |
| Corticosterone (ng/ml) | 10.11 ± 0.61 | 9.51 ± 0.63 | 11.30 ± 0.52# | 7.39 ± 0.84# | 11.70 ± 0.89ᵟ | 9.27 ± 0.43ᵟ |
| TT4 (ug/dl) | 3.59 ± 0.08* | 4.19 ± 0.08* | 3.92 ± 0.06 | 3.75 ± 0.11 | 3.75 ± 0.11 | 3.89 ± 0.05 |
| TT3 (ng/dl) | 60.96 ± 2.28* | 82.63 ± 2.40* | 70.26 ± 2.00 | 71.65 ± 3.16 | 78.25 ± 3.36ᵟ | 68.57 ± 1.62ᵟ |
| rT3 (ng/ml) | 0.52 ± 0.03* | 0.90 ± 0.03* | 0.60 ± 0.02# | 0.86 ± 0.04# | 0.75 ± 0.04ᵟ | 0.68 ± 0.02ᵟ |
TAC: total antioxidant capacity; TT4: total T4; TT3: total T3; rT3: reverse T3; NSO: northern sea otter; SSO: southern sea otter. Different superscripts denote significant differences between subspecies, lactation, or resource limitation status from a general linear model.
Table 2.
Results from general linear model examining effects of subspecies, and lactation and resource‐limited status on serum or plasma analytes in sea otters
| TAC | IgM | IgG | Cortisol | Corticost | TT4 | TT3 | rT3 | |
|---|---|---|---|---|---|---|---|---|
| Den df | 245 | 246 | 247 | 256 | 256 | 244 | 252 | 256 |
| Subspecies | ||||||||
| F | 221.90 | 289.62 | 31.52 | 0.16 | 1.76 | 44.01 | 58.63 | 138.44 |
| P |
<0.0001
S > N |
<0.0001
S > N |
<0.0001
S > N |
0.69 | 0.19 |
<0.0001
S > N |
<0.0001
S > N |
<0.0001
S > N |
| Lactating | ||||||||
| F | 0.43 | 2.07 | 3.42 | 0.002 | 12.52 | 1.52 | 2.08 | 56.83 |
| P | 0.51 | 0.15 | 0.07 | 0.96 |
0.0005
NL > L |
0.22 | 0.15 |
<0.0001
L > NL |
| Resource‐limited | ||||||||
| F | 1.72 | 0.43 | 4.57 | 15.91 | 5.23 | 2.09 | 8.68 | 9.13 |
| P | 0.19 | 0.51 |
0.03
RL > RA |
<0.0001
RA > RL |
0.02
RA > RL |
0.15 |
0.004
RA > RL |
0.003
RA > RL |
| Maternal age | ||||||||
| F | 5.10 | 1.18 | 2.08 | 0.75 | 11.31 | 1.94 | 0.66 | 8.58 |
| P |
0.02
INCR |
0.28 | 0.15 | 0.39 |
0.0009
INCR |
0.17 | 0.42 |
0.004
DECR |
| RL × Lact | ||||||||
| F | 0.52 | 1.81 | 1.45 | 5.12 | 0.62 | 0.07 | 0.80 | 2.44 |
| P | 0.47 | 0.18 | 0.23 | 0.02 | 0.43 | 0.80 | 0.37 | 0.12 |
S: southern sea otter; N: northern sea otter; NL: nonlactating; L: lactating; RL: resource‐limited; RA: resource‐abundant; INCR: concentration increased with age; DECR: concentration decreased with age; TAC: total antioxidant capacity; TT4: total T4; TT3: total T3; rT3: reverse T3. Inequalities denote directions of significant differences.
Figure 3.
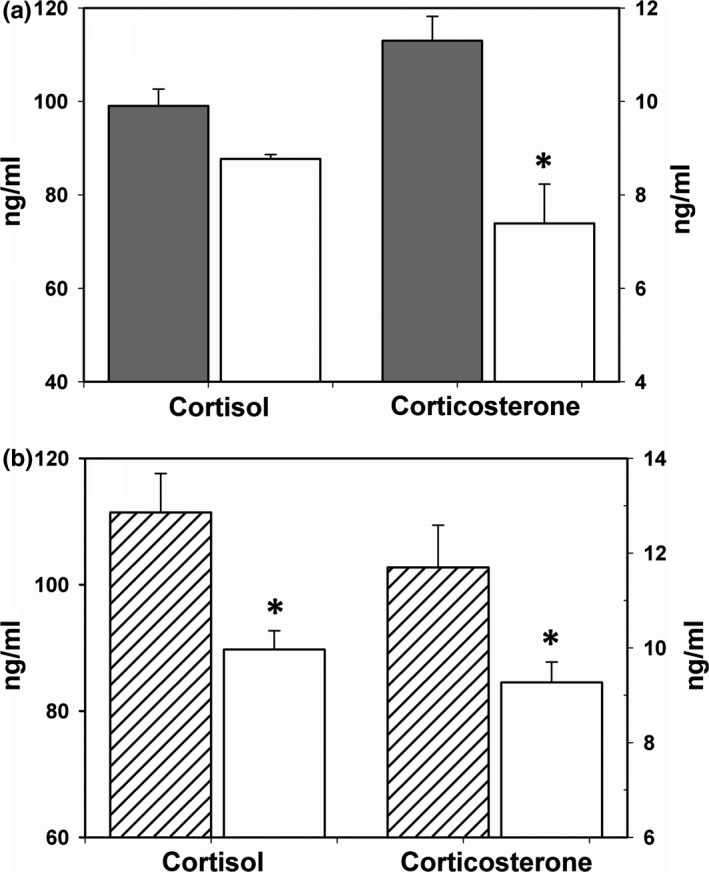
Alterations in the endocrine response from capture were evident during lactation and resource limitation. (a) Filled bars represent nonlactating females, open bars represent lactating females. (b) Filled bars represent pooled resource‐abundant females; open bars represent pool resource‐limited females. “*” denotes significance in least square means between lactation and nonlactating or resource‐abundant and resource‐limited sea otters from the general linear model
Figure 4.
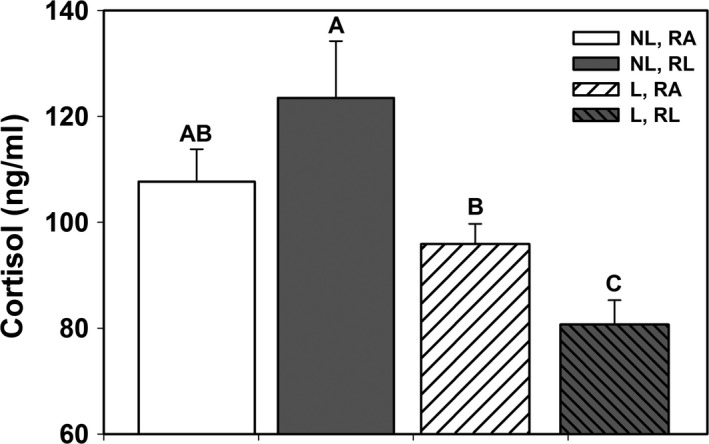
Suppression of cortisol release after capture was most apparent in lactating sea otters, and lowest under conditions of resource limitation. Bar shading denotes lactation status (lactating, L; nonlactating, NL) and resource limitation status (resource abundant, RA; resource limited, RL). Different letters denote differences in least square means from the GLM. Suppression of cortisol release during lactation was evident in resource‐limited otters
3.2. Thyroid hormones
There was no relationship between time to sampling and the serum concentration of any thyroid hormones (T4, T3, rT3; p > 0.05). T4 and T3, and rT3, all showed significant covariation with each other. T3 and rT3 showed a positive relationship (Figure 5c), and the slope of association was greater in lactating females (ANCOVA, F 1,270 = 23.15, p < 0.0001, Figure 5d). T3 and rT3 also increased with an increase in T4 (r 2 = 0.19, F 1,264 = 62.49, p < 0.0001; Figure 5e, respectively). The slope of association between rT3 and T4 was greater in lactating females (ANCOVA, F 1,262 = 4.27, p = 0.04, Figure 5f).
There was a strong impact of subspecies on all three thyroid hormones (Tables 1, 2) with SSO having greater concentrations (T4: 17% higher, T3: 36% higher, rT3: 73% higher). There was no impact of lactation status on T3 or T4 (p > 0.05) but mean rT3 concentration was 48% greater in lactating otters (Tables 1, 2). Resource limitation impacted both T3 and rT3 concentrations (Tables 1, 2). In resource‐limited sea otters, mean T3 and rT3 concentrations declined 12% and 9%, respectively. There were no significant interactions between lactation status and resource limitation for any thyroid hormone (p > 0.05). Maternal age did not influence T4 or T3 concentrations (p > 0.05). In contrast, rT3 concentrations decreased with maternal age (Table 2).
3.3. Immune markers
The two immune markers were weakly associated across samples. (r 2 = 0.19, F 1,266 = 62.27, p < 0.0001). Mean concentrations of immune markers, IgM and IgG, were 172% and 39% higher, respectively, in SSO than NSO (Table 1). There were no differences in immune markers between lactating and nonlactating otters (p > 0.05; Tables 1, 2). Resource‐limited otters had higher IgG concentrations than resource‐abundant otters (Tables 1, 2). There was no evident interaction between lactation status and resource limitation for either immune marker (Table 2). Similarly, there was no influence of maternal age on either immune marker (Table 2).
3.4. Total antioxidant capacity
There was a strong difference in the TAC of the subspecies, with SSO exhibiting a mean TAC that was 89% greater than NSO (Tables 1, 2). There was no evident impact of lactation status, resource limitation, or their interaction on TAC (Tables 1, 2). There was a significant increase in TAC with maternal age (Tables 1, 2).
3.5. Associations between markers
We examined relationships among analytes of different categories (e.g., GCs vs. thyroid hormones) to determine possible covariation or suggested downstream effects on another marker. We included lactation status and an interaction term with lactation status in the model to assess whether the slope of the regression was similar between groups. T4 and T3 increased with cortisol (F 1,262 = 14.83, p < 0.0001; F 1,270 = 13.69, p = 0.0003, respectively), and the slope of this association was similar among lactating and nonlactating otters. Both immune markers covaried with thyroid hormones. IgM and IgG increased with T4 (F 1,262 = 28.38, p < 0.0001; F 1,262 = 8.84, p = 0.003, respectively), T3 (F 1,264 = 46.78, p < 0.0001; F 1,265 = 7.41; p = 0.007, respectively) and rT3 (Figure 6a, F 1,264 = 63.75, p < 0.0001; Figure 6b, F 1,265 = 20.80, p < 0.0001, respectively). These associations were not affected by lactation status except for the effect of T3 on IgG which was greater in lactating otters (ANCOVA, F 1,265 = 18.50, p < 0.0001).
Figure 6.
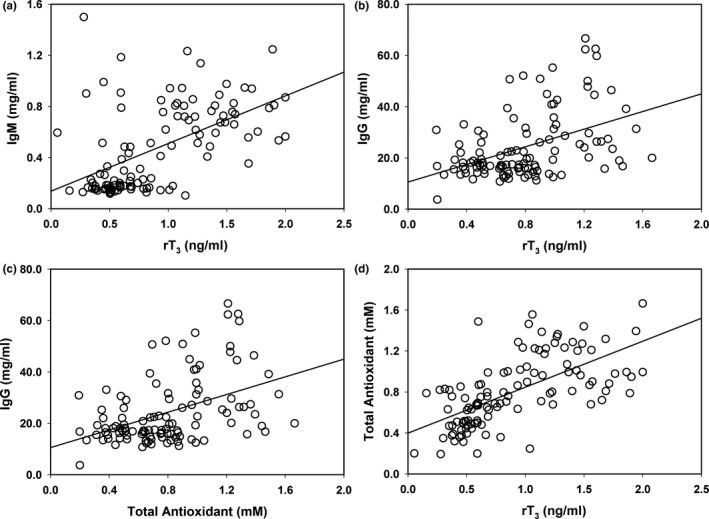
Interrelationships between measured analytes for lactating females. (a) Relationship between rT 3 and IgM, y = 0.14 + 0.37x, r 2 = 0.29, F 1,109 = 44.63, p < 0.0001. (b) Relationship between rT 3 and IgG, y = 13.66 + 11.77x, r 2 = 0.18, F 1,110 = 24.64, p < 0.0001. (c) Relationship between total antioxidant capacity and IgG, y = 10.57 + 17.21x, r 2 = 0.20, F 1,109 = 26.44, p < 0.0001. (d) Relationship between rT 3 and total antioxidant capacity, y = 0.40 + 0.45x, r 2 = 0.41, F 1,109 = 74.48, P < 0.0001
Increased TAC was associated with increased immunoglobulins and thyroid hormones. Both immunoglobulins showed a positive association with TAC (IgM: F 1,262 = 101.13, p < 0.0001; IgG: F 1,263 = 31.14, p < 0.0001). The slope of the increase in IgG with TAC was greater in lactating otters (ANCOVA, F 1,263 = 14.39, p = 0.0002; Figure 6c). TAC increased with T4 (F 1,260 = 38.58, p < 0.0001), and the slope of this relationship was greater in lactating otters (ANCOVA, F 1,260 = 6.30, p = 0.01). TAC also increased with T3 (F 1,263 = 72.34, p < 0.0001) and rT3 (Figure 6d) but no effects of lactation on the slopes of these associations were evident (ANCOVA, p > 0.05).
4. DISCUSSION
4.1. Glucocorticoids
We measured the release of GCs in response to an acute capture stress. The wide individual variability in this response likely reflected both individual differences (e.g., personalities) but also the several factors that showed impacts on the magnitude of cortisol release. Cortisol was the predominant GC released, but sea otters also released biologically significant and varying quantities of corticosterone in response to acute stress.
A previous investigation in sea otters found that corticosterone was most responsive to capture stress (Larson, Monson, Ballachey, Jameson, & Wasser, 2009), and that levels of this GC may be affected by genetic diversity (Larson, Ralls, & Ernest, 2015). In the current study, corticosterone levels after capture were significantly decreased in lactating sea otters suggesting reduced HPA responsiveness to the acute capture stress. Although it might be expected that lactation status would be reflected by higher levels of baseline GCs, the prolonged energetic stress of lactation may have blunted the ability to mount an additional stress response to handling for corticosterone (Harlow et al., 1992; Krause et al., 2014; Rich & Romero, 2005), resulting in acclimation of the stress response (Juster, McEwen, & Lupien, 2010; McEwen, 2000; Wingfield & Mukai, 2009). The reduced corticosterone release in lactating females and the lack of a similar effect for cortisol resulted in increases in the cortisol: corticosterone ratios during lactation. Cortisol release was lower in resource‐limited otters, suggesting impacts of nutritional stress on HPA sensitivity. Resource limitation also interacted with the effect of lactation on cortisol release, with cortisol only showing lactation effects under conditions of resource limitation (Figure 4). Under lactation stress, females from subpopulations with abundant nutritional resources mounted a more pronounced acute stress response. Together, these results suggest a complex semi‐independent release of these two GCs with each one responding differently to varying types and levels of stressors while being modulated by individual genetic makeup and background environmental and physiological stress levels. Though limited, data from mammals suggest that GCs can vary independently showing seasonal variation, circadian patterns, and responses to acute stress (Vera, Antenucci, & Zenuto, 2011). Cortisol binding globulin (CBG) generally shows greater specificity for the dominant glucocorticoid (Westphal, 1986); thus, the two GCs may have different representation in the “free” bioavailable glucocorticoid pool and may have independent signaling and functional roles (Koren et al., 2012) including partitioning of physiological and behavioral effects.
4.2. Thyroid hormones
Thyroid hormones regulate metabolic pathways and metabolism. Decreases in maintenance metabolism may serve to increase the proportion of assimilated energy available for milk synthesis but reduce metabolic heat production to thermoregulation. Thyroid hormones were substantially higher in SSO than in NSO. This difference, assessed in a model that controlled for lactation status and nutritional limitation, suggests potential phenotypic differences among the two subspecies in energy requirements. This may reflect differences in habitat and resource availability during the divergence of the subspecies, or simply reflect differences created through bottlenecking and founder effects resulting from historic over exploitation of sea otters in the 18th and 19th centuries (Larson, Jameson, Enier, Fleming, & Bentzen, 2002; Larson, Jameson, Etnier, Jones, & Hall, 2012). Also, although our southern and northern samples had comparable numbers of nonresource‐limited individuals (SSO = 30, NSO = 31), it is worth noting that our sample of resource‐limited SSO otters (n = 107) came from subpopulations that had been at or near carrying capacity for much longer than most of the northern subpopulations we sampled. For example, our resource‐limited subpopulations in central California were among the first areas to recover from the fur trade in the early 1900s (Kenyon, 1969) and have been at or near carrying capacity for many decades (Tinker et al., 2017). Conversely, sea otter populations in both Washington and SE Alaska were established by re‐introduction in 1969 and 1970 (Jameson et al., 1982; Kenyon, 1969), and thus, our resource‐limited samples from these regions come from subpopulations that have only recently reached carrying capacity. Similarly, sea otters in western Prince William Sound are believed to have been at carrying capacity for several decades, although this population was depleted by the Exxon Valdez Oil Spill in 1989, and only recently has recovered to the point that resources are once again the primary limiting factor (Ballachey et al., 2014; Monson, Doak, Ballachey, & Bodkin, 2011; Monson, Doak, Ballachey, Johnson, & Bodkin, 2000).
Lactation had no effect on T4 or T3, suggesting that downregulation of maintenance metabolism is not accomplished by suppressing thyroid availability. However, rT3, which should compete with T3 for receptor sites, increased by 86% in lactating females, which should downregulate metabolism. Similarly, T3 decreased in resource‐limited otters, suggesting downregulation of metabolism under stronger nutritional constraints. Sea otters under resource limitation stress may face intense prey competition, increased foraging effort (number of dives, dive length, and foraging bout duration), and still experience a negative energy balance (Thometz, Staedler, et al., 2016; Tinker et al., 2017). It may be adaptive for individuals under nutrient restriction to decrease thyroid levels to decrease metabolic rate, and thus energy requirements. More difficult to explain is the small decrease in rT3 in resource‐limited sea otters, although the difference is small (9%) and may reflect lower overall rates of deiodination under nutritional stress.
T4, T3, and rT3 all showed strong, positive associations with each other. T4 may act as a reservoir for downstream processes that may require conversion to T3 (for promotion of thermogenesis or muscle catabolism) or conversion to rT3 (for suppression of metabolic rate). T4 and T3 both weakly increased with cortisol after handling. If higher magnitude cortisol responses to handling are associated with lower baseline levels of stress, this pattern may reflect the reduced suppression of thyroid by baseline GCs (Ferguson & Peterson, 1992).
T4, T3, and rT3 showed positive associations with TAC, IgM, and IgG that were strongest in lactating females. This suite of associations suggests a role for thyroid in regulating energy mobilization for allocation toward immune and oxidative defense. Biomedical studies have shown strong links between variation in thyroid and tissue antioxidant responses (Das & Chainy, 2001; Venditti, Balestrieri, Di Meo, & De Leo, 1997). Similarly, thyroid variation has strong impacts on humoral immune responses (Fabris, 1973), mounting an immune response creates oxidative stress (Costantini & Møller, 2009), and animals may mitigate oxidative stress and enhance their response to immune challenges with increased dietary antioxidants (Catoni, Peters, & Schaefer, 2008; Park, Chyun, Kim, Line, & Chew, 2010). Together these associations may reflect a critical role for thyroid in regulating metabolism and allocation toward systemic defenses under the strong nutrient constraints of lactation.
4.3. Immune markers
Differences between subspecies were the strongest drivers of variation in circulating immunoglobulins. Immunoglobulin concentrations, especially IgM, were greater in SSO. Previous studies have shown dramatically higher exposure of SSO to some pathogens (e.g., Leptospira spp., T. gondii) (Hanni et al., 2003). Coastal urbanization and freshwater run‐off may result in increased pathogen exposure in SSO (Miller et al., 2002). Together, these findings suggest higher rates of pathogen exposure in SSO. Our findings suggest that immune responses to these pathogens are an important component of sea otter survival and are not downregulated in response to lactation. IgG concentrations were higher in resource‐limited subpopulations. This is consistent with earlier work that suggests that density‐dependent changes in prey choice and habitat use may increase sea otter pathogen exposure in the resource‐limited California coastal system (Johnson et al., 2009). The current study suggests self‐maintenance by mothers and that immune responses are not reduced under constraints of nutritional stress due to lactation or resource limitation.
4.4. Total antioxidant capacity
Contrary to our prediction, there were no differences in TAC due to lactation status. Females appear to balance any potential increases in ROS production induced by elevated energy expenditure or breath‐hold diving during lactation (Vázquez‐Medina et al., 2011) and the associated stressors correlated with pup care, which may be elevated under conditions of resource limitation. Females may compensate for increased ROS by endogenously producing more antioxidants or increasing antioxidant concentration by consuming a more diverse diet or switch to prey with higher antioxidant concentrations, as reflected in the maintenance of TAC despite the strong potential for increased oxidative stress. TAC was almost twofold higher in SSO. This difference may reflect a physiological response to increased ROS production due to increased breath‐hold diving for foraging differences between habitats. Alternatively, increased TAC may reflect increased consumption of antioxidants from prey. Mothers are required to spend significantly more time foraging to meet the energy demands of lactation and to supplement a growing pup (Esslinger et al., 2014; Thometz, Staedler, et al., 2016). In addition, dietary diversity tends to increase under conditions of resource limitation (Newsome et al., 2015). This may enhance the supply of exogenous antioxidants available through alternate prey types and reduce the need for antioxidant enzyme synthesis. Measurement of antioxidant enzyme concentrations is necessary to differentiate between these two possibilities. Similarly, measures of oxidative damage are necessary to know whether this increased antioxidant capacity is sufficient to prevent oxidative stress. In either case, our data do not suggest loss of antioxidant capacity or enhanced oxidative stress during reproduction in sea otters. Maternal age had a positive effect on antioxidant capacity. This may reflect enhanced diving or foraging efficiency with larger body size or increased experience, which may reduce the potential for oxidative damage in older females.
Our data suggest that lactation has significant effects on physiological functions in female sea otters. Evidence of blunted stress responses and downregulated thyroid function in lactating sea otters suggest fitness costs to current reproduction, especially in populations experiencing resource limitation. These alterations may lessen the ability of lactating females to respond to additional stressors such as injury or infection and may increase risk of ELS, a significant cause of mortality in California, attributed to >50% of the deaths of stranded, reproductively mature females examined between 2005 and 2012 (Chinn et al., 2016). Resource limitation may require more pronounced metabolic alterations during lactation to balance reproductive demands with those for survival, further exacerbating ELS. In top‐predator species that functionally modulate species composition within their environment and where prey resources are often a significant limiting factor, physiological trade‐offs during lactation may have downstream effects on parental care and life‐history decisions ultimately manifest in population‐level consequences (i.e., a high proportion of prime‐age reproductively active females succumb to ELS), which likely mediate predator–prey dynamics and affect food‐web stability. These population‐level and ecosystem effects are even more likely for income‐breeding strategists, where reproductive effort is directly linked to energy acquisition during lactation.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
All authors conceived the ideas and designed methodology. SMC, MTT, DHM, and MMS collected the data. SMC conducted laboratory analyses. SMC and DEC analyzed the data. SMC led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
DATA ACCESSIBILITY
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.mk59gk0.
Supporting information
ACKNOWLEDGMENTS
We thank the researchers, technicians, and volunteers of the US Geological Survey, US Fish and Wildlife Service, The Monterey Bay Aquarium, California Department of Fish and Wildlife, Alaska Department of Fish and Game, The Marine Mammal Center, and the University of California Santa Cruz, who sampled sea otters. Additional support for sample collection was provided by California Coastal Conservancy and Bureau of Ocean Energy Management. Thank you to F. Batac, E. Dodd, K. Kloecker, M. Young, and Dr. M. Murray for their help with collecting, processing, and locating archived samples. This research was supported by grants from the Office of Naval Research (#N000141410393, to DEC), California State University's Council on Ocean Affairs, Science & Technology, the Sea Otter Foundation & Trust, and the American Cetacean Society – Monterey Bay (to SMC). Any use of trade names is for descriptive purposes only and does not represent endorsement by the U.S. Government.
Chinn SM, Monson DH, Tinker MT, Staedler MM, Crocker DE. Lactation and resource limitation affect stress responses, thyroid hormones, immune function, and antioxidant capacity of sea otters (Enhydra lutris). Ecol Evol. 2018;8:8433–8447. 10.1002/ece3.4280
REFERENCES
- Alonso‐Alvarez, C. , Bertrand, S. , Devevey, G. , Prost, J. , Faivre, B. , Sorci, G. , & Loeschcke, V. (2004). Increased susceptibility to oxidative stress as a proximate cost of reproduction. Ecology Letters, 7, 363–368. 10.1111/j.1461-0248.2004.00594.x [DOI] [Google Scholar]
- Ames, J. A. , Hardy, R. A. , & Wendell, F. E. (1986) A simulated translocation of sea otters, Enhydra lutris, with a review of capture, transport and holding techniques.
- Balaban, R. S. , Nemoto, S. , & Finkel, T. (2005). Mitochondria, oxidants, and aging. Cell, 120, 483–495. 10.1016/j.cell.2005.02.001 [DOI] [PubMed] [Google Scholar]
- Ballachey, B. E. , Monson, D. H. , Esslinger, G. G. , Kloecker, K. , Bodkin, J. , Bowen, L. , & Miles, A. K. (2014). 2013 update on sea otter studies to assess recovery from the 1989 Exxon Valdez oil spill, Prince William Sound, Alaska: U.S. Geological Survey Open‐File Report 2014‐1030, 40 p., 10.3133/ofr20141030 [DOI]
- Beaulieu, M. C. A. B. , & Schaefer, H. M. (2013). Rethinking the role of dietary antioxidants through the lens of self‐medication. Animal Behaviour, 86, 17–24. 10.1016/j.anbehav.2013.05.022 [DOI] [Google Scholar]
- Bodkin, J. L. , Monson, D. H. , & Esslinger, G. G. (2007). Activity budgets derived from time‐depth recorders in a diving mammal. Journal of Wildlife Management, 71, 2034–2044. 10.2193/2006-258 [DOI] [Google Scholar]
- Bodkin, J. L. , Mulcahy, D. , & Lensink, C. J. (1993). Age‐specific reproduction in female sea otters (Enhydra lutris) from south‐central Alaska: Analysis of reproductive tracts. Canadian Journal of Zoology, 71, 1811–1815. 10.1139/z93-258 [DOI] [Google Scholar]
- Bodkin, J. L. , & Udevitz, M. S. (1999). An aerial survey method to estimate sea otter abundance (pp. 13–26). Balekema, Rotterdam, Netherlands: Marine Mammal Survey and Assessment Methods. [Google Scholar]
- Boggs, C. L. , & Ross, C. L. (1993). The effect of adult food limitation on life history traits in Speyeria mormonia (Lepidoptera: Nymphalidae). Ecology, 74, 433–441. 10.2307/1939305 [DOI] [Google Scholar]
- Boonstra, R. , Hik, D. , Singleton, G. R. , & Tinnikov, A. (1998). The impact of predator‐induced stress on the snowshoe hare cycle. Ecological Monographs, 68, 371 10.1890/0012-9615(1998)068[0371:TIOPIS]2.0.CO;2 [DOI] [Google Scholar]
- Boonstra, R. , (2013). Reality as the leading cause of stress: rethinking the impact of chronic stress in nature. Functional Ecology, 27, 11–23 10.1111/1365-2435.12008 [DOI] [Google Scholar]
- Breen, P. , Carson, T. , Foster, J. , & Stewart, E. (1982). Changes in subtidal community structure associated with British Columbia sea otter transplants. Marine Ecology Progress Series, 7, 13–20. 10.3354/meps007013 [DOI] [Google Scholar]
- Catoni, C. , Peters, A. , & Schaefer, H. M. (2008). Life history trade‐offs are influenced by the diversity, availability and interactions of dietary antioxidants. Animal Behaviour, 76, 1107–1119. 10.1016/j.anbehav.2008.05.027 [DOI] [Google Scholar]
- Charmandari, E. , Tsigos, C. , & Chrousos, G. (2005). Endocrinology of the stress response 1. Annual Review of Physiology, 67, 259–284. 10.1146/annurev.physiol.67.040403.120816 [DOI] [PubMed] [Google Scholar]
- Chinn, S. M. , Miller, M. A. , Tinker, M. T. , Staedler, M. M. , Batac, F. I. , Dodd, E. M. , & Henkel, L. A. (2016). The high cost of motherhood: End‐lactation syndrome in southern sea otters (Enhydra lutris nereis) on the Central California Coast, USA. Journal of Wildlife Diseases, 52, 307–318. 10.7589/2015-06-158 [DOI] [PubMed] [Google Scholar]
- Clinchy, M. , Zanette, L. , Boonstra, R. , Wingfield, J. C. , & Smith, J. N. M. (2004). Balancing food and predator pressure induces chronic stress in songbirds. Proceedings of the Royal Society B: Biological Sciences, 271, 2473–2479. 10.1098/rspb.2004.2913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton‐Brock, T. , Iason, G. , Albon, S. , & Guinness, F. (1982). Effects of lactation on feeding behaviour and habitat use in wild red deer hinds. Journal of Zoology, 198, 227–236. [Google Scholar]
- Coletti, H. A. , Bodkin, J. L. , Monson, D. H. , Ballachey, B. E. , & Dean, T. A. (2016). Detecting and inferring cause of change in an Alaska nearshore marine ecosystem. Ecosphere, 7, e01489 10.1002/ecs2.1489 [DOI] [Google Scholar]
- Costantini, D. (2008). Oxidative stress in ecology and evolution: Lessons from avian studies. Ecology Letters, 11, 1238–1251. 10.1111/j.1461-0248.2008.01246.x [DOI] [PubMed] [Google Scholar]
- Costantini, D. , & Møller, A. P. (2009). Does immune response cause oxidative stress in birds? A meta‐analysis. Comparative Biochemistry and Physiology Part A Molecular Integrative Physiology, 153, 339–344. 10.1016/j.cbpa.2009.03.010 [DOI] [PubMed] [Google Scholar]
- Cotter, S. C. , Simpson, S. J. , Raubenheimer, D. , & Wilson, K. (2011). Macronutrient balance mediates trade‐offs between immune function and life history traits. Functional Ecology, 25, 186–198. 10.1111/j.1365-2435.2010.01766.x [DOI] [Google Scholar]
- Crocker, D. E. , Williams, J. D. , Costa, D. P. , & Le Boeuf, B. J. (2001). Maternal traits and reproductive effort in northern elephant seals. Ecology, 82, 3541–3555. 10.1890/0012-9658(2001)082[3541:MTAREI]2.0.CO;2 [DOI] [Google Scholar]
- Dantzer, B. , Newman, A. E. M. , Boonstra, R. , Palme, R. , Boutin, S. , Humphries, M. M. , & Mcadam, A. G. (2013). Density triggers maternal hormones that increase adaptive offspring growth in a wild mammal. Science, 340, 1215–1217. 10.1126/science.1235765 [DOI] [PubMed] [Google Scholar]
- Das, K. , & Chainy, G. (2001). Modulation of rat liver mitochondrial antioxidant defence system by thyroid hormone. Biochimica et Biophysica Acta (BBA)‐Molecular Basis of Disease, 1537, 1–13. 10.1016/S0925-4439(01)00048-5 [DOI] [PubMed] [Google Scholar]
- Dickens, M. J. , & Romero, L. M. (2013). A consensus endocrine profile for chronically stressed wild animals does not exist. General and Comparative Endocrinology, 191, 177–189. 10.1016/j.ygcen.2013.06.014 [DOI] [PubMed] [Google Scholar]
- Dowling, D. K. , & Simmons, L. W. (2009). Reactive oxygen species as universal constraints in life‐history evolution. Proceedings of the Royal Society of London B: Biological Sciences, 276, 1737–1745. 10.1098/rspb.2008.1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggins, D. O. , Simenstad, C. A. , & Estes, J. A. (1989). Magnification of secondary production by kelp detritus in coastal marine ecosystems. Science, 245, 170–173. 10.1126/science.245.4914.170 [DOI] [PubMed] [Google Scholar]
- Ensminger, D. C. , Somo, D. A. , Houser, D. S. , & Crocker, D. E. (2014). Metabolic responses to adrenocorticotropic hormone (ACTH) vary with life‐history stage in adult male northern elephant seals. General and Comparative Endocrinology, 204, 150–157. 10.1016/j.ygcen.2014.04.024 [DOI] [PubMed] [Google Scholar]
- Esslinger, G. G. , Bodkin, J. L. , Breton, A. R. , Burns, J. M. , & Monson, D. H. (2014). Temporal patterns in the foraging behavior of sea otters in Alaska. Journal of Wildlife Management, 78, 689–700. 10.1002/jwmg.701 [DOI] [Google Scholar]
- Estes, J. A. (1990). Growth and equilibrium in sea otter populations. Journal of Animal Ecology, 59, 385 10.2307/4870 [DOI] [Google Scholar]
- Estes, J. A. , & Duggins, D. O. (1995). Sea otters and kelp forests in Alaska: generality and variation in a community ecological paradigm. Ecological Monographs, 65, 75–100. 10.2307/2937159 [DOI] [Google Scholar]
- Estes, J. A. , & Palmisano, J. F. (1974). Sea otters: Their role in structuring nearshore communities. Science, 185, 1058–1060. 10.1126/science.185.4156.1058 [DOI] [PubMed] [Google Scholar]
- Estes, J. A. , Tinker, M. T. , Williams, T. M. , & Doak, D. F. (1998). Killer whale predation on sea otters linking oceanic and nearshore ecosystems. Science, 282, 473–476. 10.1126/science.282.5388.473 [DOI] [PubMed] [Google Scholar]
- Fabris, N. (1973). Immunodepression in thyroid‐deprived animals. Clinical and Experimental Immunology, 15, 601. [PMC free article] [PubMed] [Google Scholar]
- Ferguson, D. C. , & Peterson, M. E. (1992). Serum free and total iodothyronine concentrations in dogs with hyperadrenocorticism. American Journal of Veterinary Research, 53, 1636–1640. [PubMed] [Google Scholar]
- Figueiredo, H. F. , Bodie, B. L. , Tauchi, M. , Dolgas, C. M. , & Herman, J. P. (2003). Stress integration after acute and chronic predator stress: differential activation of central stress circuitry and sensitization of the hypothalamo‐pituitary‐adrenocortical axis. Endocrinology, 144, 5249–5258. 10.1210/en.2003-0713 [DOI] [PubMed] [Google Scholar]
- Gittleman, J. L. , & Thompson, S. D. (1988). Energy allocation in mammalian reproduction. American Zoologist, 28, 863–875. 10.1093/icb/28.3.863 [DOI] [Google Scholar]
- Hanni, K. D. , Mazet, J. A. , Gulland, F. M. , Estes, J. , Staedler, M. , Murray, M. J. , … Jessup, D. A. (2003). Clinical pathology and assessment of pathogen exposure in southern and Alaskan sea otters. Journal of Wildlife Diseases, 39, 837–850. 10.7589/0090-3558-39.4.837 [DOI] [PubMed] [Google Scholar]
- Hanwell, A. , & Peaker, M. (1977). Physiological effects of lactation on the mother. Symposia of the Zoological Society of London, 41, 297–312. [Google Scholar]
- Harlow, H. J. , Lindzey, F. G. , Sickle, W. D. V. , & Gern, W. A. (1992). Stress response of cougars to nonlethal pursuit by hunters. Canadian Journal of Zoology, 70, 136–139. 10.1139/z92-020 [DOI] [Google Scholar]
- Heim, C. , Ehlert, U. , & Hellhammer, D. H. (2000). The potential role of hypocortisolism in the pathophysiology of stress‐related bodily disorders. Psychoneuroendocrinology, 25, 1–35. 10.1016/S0306-4530(99)00035-9 [DOI] [PubMed] [Google Scholar]
- Jameson, R. J. , Kenyon, K. W. , Johnson, A. M. , & Wight, H. M. (1982). History and status of translocated sea otter populations in North America. Wildlife Society Bulletin, 10, 100–107. [Google Scholar]
- Johnson, C. K. , Tinker, M. T. , Estes, J. A. , Conrad, P. A. , Staedler, M. , Miller, M. A. , … Mazet, J. A. (2009). Prey choice and habitat use drive sea otter pathogen exposure in a resource‐limited coastal system. Proceedings of the National Academy of Sciences, 106, 2242–2247. 10.1073/pnas.0806449106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jönsson, K. I. (1997). Capital and income breeding as alternative tactics of resource use in reproduction. Oikos, 78, 57–66. 10.2307/3545800 [DOI] [Google Scholar]
- Juster, R.‐P. , Mcewen, B. S. , & Lupien, S. J. (2010). Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience & Biobehavioral Reviews, 35, 2–16. 10.1016/j.neubiorev.2009.10.002 [DOI] [PubMed] [Google Scholar]
- Kenyon, K. W. (1969). The sea otter in the eastern Pacific Ocean. North American Fauna, 68, 1–352. 10.3996/nafa.68.0001 [DOI] [Google Scholar]
- Koren, L. , Whiteside, D. , Fahlman, Å. , Ruckstuhl, K. , Kutz, S. , Checkley, S. , … Wynne‐Edwards, K. (2012). Cortisol and corticosterone independence in cortisol‐dominant wildlife. General Comparative Endocrinology, 177, 113–119. 10.1016/j.ygcen.2012.02.020 [DOI] [PubMed] [Google Scholar]
- Krause, J. S. , Dorsa, D. , & Wingfield, J. C. (2014). Changes in plasma concentrations of progesterone, dehydroepiandrosterone and corticosterone in response to acute stress of capture, handling and restraint in two subspecies of white‐crowned sparrows. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 177, 35–40. 10.1016/j.cbpa.2014.07.019 [DOI] [PubMed] [Google Scholar]
- Larson, S. , Jameson, R. , Enier, M. , Fleming, M. , & Bentzen, P. (2002). Loss of genetic diversity in sea otters (Enhydra lutris) associated with the fur trade of the 18th and 19th centuries. Molecular Ecology, 11, 1899–1903. 10.1046/j.1365-294X.2002.01599.x [DOI] [PubMed] [Google Scholar]
- Larson, S. , Jameson, R. , Etnier, M. , Jones, T. , & Hall, R. (2012). Genetic diversity and population parameters of sea otters, Enhydra lutris, before Fur Trade Extirpation from 1741‐1911. PLoS ONE, 7, e32205 10.1371/journal.pone.0032205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson, S. , Monson, D. , Ballachey, B. , Jameson, R. , & Wasser, S. K. (2009). Stress‐related hormones and genetic diversity in sea otters (Enhydra lutris). Marine Mammal Science, 25, 351–372. 10.1111/j.1748-7692.2008.00260.x [DOI] [Google Scholar]
- Larson, S. , Ralls, K. , & Ernest, H. (2015) Sea otter conservation genetics In Larson S. E., Bodkin J. L., VanBlaricom G. R. (Eds.), Sea otter conservation (pp. 97–120). Amsterdam, Netherlands: Elsevier Inc. 10.1016/B978-0-12-801402-8.00005-6 [DOI] [Google Scholar]
- Mårtensson, J. (1986). The effect of fasting on leukocyte and plasma glutathione and sulfur amino acid concentrations. Metabolism, 35, 118–121. 10.1016/0026-0495(86)90110-1 [DOI] [PubMed] [Google Scholar]
- Martin, T. E. (1987). Food as a limit on breeding birds: A life‐history perspective. Annual Review of Ecology and Systematics, 18, 453–487. 10.1146/annurev.es.18.110187.002321 [DOI] [Google Scholar]
- Maule, A. G. , Schreck, C. B. , & Kaattari, S. L. (1987). Changes in the immune system of coho salmon (Oncorhynchus kisutch) during the parr‐to‐smolt transformation and after implantation of cortisol. Canadian Journal of Fisheries and Aquatic Sciences, 44, 161–166. 10.1139/f87-021 [DOI] [Google Scholar]
- McEwen, B. S. (2000). The neurobiology of stress: From serendipity to clinical relevance Published on the World Wide Web on 22 November 2000. Brain Research, 886, 172–189. 10.1016/S0006-8993(00)02950-4 [DOI] [PubMed] [Google Scholar]
- McMahon, M. , Gerich, J. , & Rizza, R. (1988). Effects of glucocorticoids on carbohydrate metabolism. Diabetes/Metabolism Reviews, 4, 17–30. 10.1002/(ISSN)1099-0895 [DOI] [PubMed] [Google Scholar]
- Millar, J. S. (1977). Adaptive features of mammalian reproduction. Evolution, 31, 370–386. 10.1111/j.1558-5646.1977.tb01019.x [DOI] [PubMed] [Google Scholar]
- Miller, S. C. , & Bowman, B. M. (2004). Rapid improvements in cortical bone dynamics and structure after lactation in established breeder rats. Anatomical Record Part A: Discoveries in Molecular, Cellular, and Evolutionary Biology, 276, 143–149. 10.1002/(ISSN)1097-0185 [DOI] [PubMed] [Google Scholar]
- Miller, G. E. , Chen, E. , & Zhou, E. S. (2007). If it goes up, must it come down? Chronic stress and the hypothalamic‐pituitary‐adrenocortical axis in humans. Psychological Bulletin, 133, 25–45. 10.1037/0033-2909.133.1.25 [DOI] [PubMed] [Google Scholar]
- Miller, M. , Gardner, I. , Kreuder, C. , Paradies, D. , Worcester, K. , Jessup, D. , … Packham, A. (2002). Coastal freshwater runoff is a risk factor for Toxoplasma gondii infection of southern sea otters (Enhydra lutris nereis). International Journal for Parasitology, 32, 997–1006. 10.1016/S0020-7519(02)00069-3 [DOI] [PubMed] [Google Scholar]
- Monaghan, P. , Metcalfe, N. B. , & Torres, R. (2009). Oxidative stress as a mediator of life history trade‐offs: Mechanisms, measurements and interpretation. Ecology Letters, 12, 75–92. 10.1111/j.1461-0248.2008.01258.x [DOI] [PubMed] [Google Scholar]
- Monson, D. H. , & Bowen, L. (2015) Evaluating the status of individuals and populations: Advantages of multiple approaches and time scales In Larson S. E. Bodkin J. L., VanBlaricom G. R. (Eds.), Sea otter conservation (pp. 121–158). Amsterdam, Netherlands: Elsevier Inc. 10.1016/B978-0-12-801402-8.00006-8 [DOI] [Google Scholar]
- Monson, D. H. , Doak, D. F. , Ballachey, B. E. , & Bodkin, J. L. (2011). Could residual oil from the Exxon Valdez spill create a long‐term population “sink” for sea otters in Alaska? Ecological Applications, 21, 2917–2932. 10.1890/11-0152.1 [DOI] [Google Scholar]
- Monson, D. H. , Doak, D. F. , Ballachey, B. E. , Johnson, A. , & Bodkin, J. L. (2000). Long‐term impacts of the Exxon Valdez oil spill on sea otters, assessed through age‐dependent mortality patterns. Proceedings of the National Academy of Sciences, 97, 6562–6567. 10.1073/pnas.120163397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson, D. H. , Estes, J. A. , Bodkin, J. L. , & Siniff, D. B. (2000). Life history plasticity and population regulation in sea otters. Oikos, 90, 457–468. 10.1034/j.1600-0706.2000.900304.x [DOI] [Google Scholar]
- Monson, D. H. , McCormick, C. , & Ballachey, B. E. (2001). Chemical anesthesia of northern sea otters (Enhydra lutris): Results of past field studies. Journal of Zoo and Wildlife Medicine, 32, 181–189. [DOI] [PubMed] [Google Scholar]
- Newsome, S. D. , Tinker, M. T. , Gill, V. A. , Hoyt, Z. N. , Doroff, A. , Nichol, L. , & Bodkin, J. L. (2015). The interaction of intraspecific competition and habitat on individual diet specialization: A near range‐wide examination of sea otters. Oecologia, 178, 45–59. 10.1007/s00442-015-3223-8 [DOI] [PubMed] [Google Scholar]
- Oppenheimer, J. , Schwartz, H. , Mariash, C. , Kinlaw, W. , Wong, N. , & Freake, H. (1987). Advances in our understanding of thyroid hormone action at the cellular level. Endocrine Reviews, 8, 288–308. 10.1210/edrv-8-3-288 [DOI] [PubMed] [Google Scholar]
- Palme, R. , Rettenbacher, S. , Touma, C. , El‐Bahr, S. , & Möstl, E. (2005). Stress hormones in mammals and birds: Comparative aspects regarding metabolism, excretion, and noninvasive measurement in fecal samples. Annals of the New York Academy of Sciences, 1040, 162–171. 10.1196/annals.1327.021 [DOI] [PubMed] [Google Scholar]
- Park, J. S. , Chyun, J. H. , Kim, Y. K. , Line, L. L. , & Chew, B. P. (2010). Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutrition & Metabolism, 7(18), 1–10. 10.1186/1743-7075-7-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck, H. E. , Costa, D. P. , Crocker, D. E. , & Goldbogen, J. (2015). Body reserves influence allocation to immune responses in capital breeding female northern elephant seals. Functional Ecology, 30, 389–397. [Google Scholar]
- Pitman, D. L. , Ottenweller, J. E. , & Natelson, B. H. (1988). Plasma corticosterone levels during repeated presentation of two intensities of restraint stress: Chronic stress and habituation. Physiology & Behavior, 43, 47–55. 10.1016/0031-9384(88)90097-2 [DOI] [PubMed] [Google Scholar]
- Råberg, L. , Grahn, M. , Hasselquist, D. , & Svensson, E. (1998). On the adaptive significance of stress‐induced immunosuppression. Proceedings of the Royal Society of London B: Biological Sciences, 265, 1637–1641. 10.1098/rspb.1998.0482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph, P. A. , Randolph, J. , Mattingly, K. , & Foster, M. M. (1977). Energy costs of reproduction in the cotton rat, Sigmodon hispidus . Ecology, 58, 31–45. 10.2307/1935106 [DOI] [Google Scholar]
- Rich, E. L. , & Romero, L. M. (2005). Exposure to chronic stress downregulates corticosterone responses to acute stressors. American Journal of Physiology‐Regulatory, Integrative and Comparative Physiology, 288, R1628–R1636. 10.1152/ajpregu.00484.2004 [DOI] [PubMed] [Google Scholar]
- Riedman, M. L. , Estes, J. A. , Staedler, M. M. , Giles, A. A. , & Carlson, D. R. (1994). Breeding patterns and reproductive success of California sea otters. The Journal of Wildlife Management, 58, 391–399. 10.2307/3809308 [DOI] [Google Scholar]
- Romero, L. (2004). Physiological stress in ecology: Lessons from biomedical research. Trends in Ecology & Evolution, 19, 249–255. 10.1016/j.tree.2004.03.008 [DOI] [PubMed] [Google Scholar]
- Sangild, P. T. , & Elnif, J. (1996). Intestinal hydrolytic activity in young mink (Mustela vison) develops slowly postnatally and exhibits late sensitivity to glucocorticoids. Journal of Nutrition, 126, 2061–2068. 10.1093/jn/126.9.2061 [DOI] [PubMed] [Google Scholar]
- Sapolsky, R. M. , Romero, L. M. , & Munck, A. U. (2000). How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions 1. Endocrine Reviews, 21, 55–89. [DOI] [PubMed] [Google Scholar]
- Schinella, G. R. , Marín, M. C. , de Alaniz, M. J. T. , de Buschiazzo, P. M. , & Tournier, H. A. (1999). Antioxidant defence system and lipid peroxidation in lactating rats: Effect of dietary vitamin E during gestation and lactation. Nutrition Research, 19, 795–803. 10.1016/S0271-5317(99)00041-X [DOI] [Google Scholar]
- Sharick, J. T. , Vazquez‐Medina, J. P. , Ortiz, R. M. , & Crocker, D. E. (2015). Oxidative stress is a potential cost of breeding in male and female northern elephant seals. Functional Ecology, 29, 367–376. 10.1111/1365-2435.12330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon, B. C. , & Verhulst, S. (1996). Ecological immunology: Costly parasite defences and trade‐offs in evolutionary ecology. Trends in Ecology & Evolution, 11, 317–321. 10.1016/0169-5347(96)10039-2 [DOI] [PubMed] [Google Scholar]
- Speakman, J. R. (2008). The physiological costs of reproduction in small mammals. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 363, 375–398. 10.1098/rstb.2007.2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman, J. R. , Blount, J. D. , Bronikowski, A. M. , Buffenstein, R. , Isaksson, C. , Kirkwood, T. B. L. , … Selman, C. (2015). Oxidative stress and life histories: Unresolved issues and current needs. Ecology and Evolution, 5, 5745–5757. 10.1002/ece3.1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staedler, M. M. (2011). Maternal care and provisioning in the southern sea otter (Enhydra lutris nereis): Reproductive consequences of diet specialization in an apex predator. Santa Cruz, CA: University of California. [Google Scholar]
- Stearns, S. C. (1992). The evolution of life histories. Oxford, UK: Oxford University Press. [Google Scholar]
- Stebbins, L. L. (1977). Energy requirements during reproduction of Peromyscus maniculatus . Canadian Journal of Zoology, 55, 1701–1704. 10.1139/z77-220 [DOI] [Google Scholar]
- Thometz, N. M. , Kendall, T. L. , Richter, B. P. , & Williams, T. M. (2016). The high cost of reproduction in sea otters necessitates unique physiological adaptations. Journal of Experimental Biology, 219, 2260–2264. 10.1242/jeb.138891 [DOI] [PubMed] [Google Scholar]
- Thometz, N. , Staedler, M. , Tomoleoni, J. , Bodkin, J. , Bentall, G. , & Tinker, M. (2016). Trade‐offs between energy maximization and parental care in a central place forager, the sea otter. Behavioral Ecology, 27, 1552–1566. 10.1093/beheco/arw089 [DOI] [Google Scholar]
- Thometz, N. M. , Tinker, M. T. , Staedler, M. M. , Mayer, K. A. , & Williams, T. M. (2014). Energetic demands of immature sea otters from birth to weaning: Implications for maternal costs, reproductive behavior and population‐level trends. Journal of Experimental Biology, 217, 2053–2061. 10.1242/jeb.099739 [DOI] [PubMed] [Google Scholar]
- Tilbrook, A. , Turner, A. , & Clarke, I. (2000). Effects of stress on reproduction in non‐rodent mammals: The role of glucocorticoids and sex differences. Reviews of Reproduction, 5, 105–113. 10.1530/ror.0.0050105 [DOI] [PubMed] [Google Scholar]
- Tinker, M. T. , Bentall, G. , & Estes, J. A. (2008). Food limitation leads to behavioral diversification and dietary specialization in sea otters. Proceedings of the National Academy of Sciences of the United States of America, 105, 560–565. 10.1073/pnas.0709263105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinker, M. T. , Doak, D. F. , & Estes, J. A. (2008). Using demography and movement behavior to predict range expansion of the southern sea otter. Ecological Applications, 18, 1781–1794. 10.1890/07-0735.1 [DOI] [PubMed] [Google Scholar]
- Tinker, M. T. , Doak, D. F. , Estes, J. A. , Hatfield, B. B. , Staedler, M. M. , & Bodkin, J. L. (2006). Incorporating diverse data and realistic complexity into demographic estimation procedures for sea otters. Ecological Applications, 16, 2293–2312. 10.1890/1051-0761(2006)016[2293:IDDARC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Tinker, M. T. , Tomoleoni, J. , LaRoche, N. , Bowen, L. , Miles, A. K. , Murray, M. , Staedler, M. , and Randell, Z. (2017). Southern sea otter range expansion and habitat use in the Santa Barbara Channel, California: U.S. Geological Survey Open‐File Report 2017–1001 (OCS Study BOEM 2017‐002), 76 p., 10.3133/ofr20171001 [DOI] [Google Scholar]
- Trayhurn, P. (1989). Brown Adipose Tissue and the Regulation of Energy Balance: Studies on Thermogenesis in Lactation. Hormones, Thermogenesis, and Obesity: Proceedings of the Eighteenth Steenbock Symposium Held June 12th Through June 16th, 1988, at the University of Wisconsin–Madison, USA, pp. 149 Elsevier Publishing Company. [Google Scholar]
- Tyler, N. (1987). Body composition and energy balance of pregnant and non‐pregnant Svalbard reindeer during winter. Symposia of the Zoological Society of London, 57, 203–229. [Google Scholar]
- Vázquez‐Medina, J. P. , Zenteno‐Savín, T. , Tift, M. S. , Forman, H. J. , Crocker, D. E. , & Ortiz, R. M. (2011). Apnea stimulates the adaptive response to oxidative stress in elephant seal pups. Journal of Experimental Biology, 214, 4193–4200. 10.1242/jeb.063644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venditti, P. , Balestrieri, M. , Di Meo, S. , & De Leo, T. (1997). Effect of thyroid state on lipid peroxidation, antioxidant defences, and susceptibility to oxidative stress in rat tissues. Journal of Endocrinology, 155, 151–157. 10.1677/joe.0.1550151 [DOI] [PubMed] [Google Scholar]
- Vera, F. , Antenucci, C. D. , & Zenuto, R. R. (2011). Cortisol and corticosterone exhibit different seasonal variation and responses to acute stress and captivity in tuco‐tucos (Ctenomys talarum). General Comparative Endocrinology, 170, 550–557. 10.1016/j.ygcen.2010.11.012 [DOI] [PubMed] [Google Scholar]
- Westphal, U. (1986). Steroid‐protein interactions II (monographs on endocrinology), vol. 27 New York, NY: Springer‐Verlag; 10.1007/978-3-642-82486-9 [DOI] [PubMed] [Google Scholar]
- Wiersma, P. , Selman, C. , Speakman, J. R. , & Verhulst, S. (2004). Birds sacrifice oxidative protection for reproduction. Proceedings of the Royal Society of London B: Biological Sciences, 271, S360–S363. 10.1098/rsbl.2004.0171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, G. C. (1966). Natural selection, the costs of reproduction, and a refinement of Lack's principle. American Naturalist, 100, 687–690. 10.1086/282461 [DOI] [Google Scholar]
- Williams, T. M. , Estes, J. A. , Doak, D. F. , & Springer, A. M. (2004). Killer appetites: Assessing the role of predators in ecological communities. Ecology, 85(12), 3373–3384. 10.1890/03-0696 [DOI] [Google Scholar]
- Wingfield, J. C. , & Mukai, M. (2009). Endocrine disruption in the context of life cycles: Perception and transduction of environmental cues. General and Comparative Endocrinology, 163, 92–96. 10.1016/j.ygcen.2009.04.030 [DOI] [PubMed] [Google Scholar]
- Zera, A. J. , & Harshman, L. G. (2001). The physiology of life history trade‐offs in animals. Annual review of Ecology and Systematics, 32, 95–126. 10.1146/annurev.ecolsys.32.081501.114006 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.mk59gk0.


