Abstract
The aim of the current study was to investigate the regulatory effect of sericin on the hepatic insulin-phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) signaling pathway in a type 2 diabetes rat model. Male Sprague Dawley rats were randomly divided into four groups: Control group, diabetic model group, high-dose sericin group and low-dose sericin group, with 12 rats in each group. Fasting blood glucose was detected by the glucose oxidase method, and hepatic glycogen was determined by periodic acid-Schiff staining. The morphology of the liver was observed by hematoxylin and eosin staining. Immunohistochemical staining, western blotting and reverse transcription-quantitative polymerase chain reaction were used to determine the protein and mRNA expression levels of insulin receptor (IR), IR substrate-1 (IRS-1), PI3K and AKT. Compared with the control group, the blood glucose of the diabetic model group was significantly increased (P<0.05). The glycogen content and the expression levels of IR, IRS-1, PI3K and AKT in the diabetic model group were significantly lower (P<0.05), and the liver morphological structure of the diabetic model group exhibited obvious pathological changes compared with the control group. Compared with the diabetic model group, the blood glucose of the high- and low-dose sericin groups was significantly reduced, while the glycogen content and the expression levels of IR, IRS-1, PI3K and AKT in the sericin treatment groups were significantly increased (P<0.05). Additionally, the liver pathological changes of high-dose and low-dose sericin groups were markedly reduced. Sericin may enhance the signaling transduction effect of insulin by upregulating the expression levels of key factors (IR, IRS-1, PI3K and AKT) in the liver insulin-PI3K/AKT signaling pathway, thus promoting glucose transport and liver glycogen synthesis, and further reducing blood glucose.
Keywords: sericin, type 2 diabetes mellitus, liver, insulin, phosphoinositide 3-kinase/protein kinase B signaling pathway
Introduction
Type 2 diabetes is primarily characterized by insulin resistance, and one of the important causes of insulin resistance is insulin signal transduction disorder (1,2). Insulin is the only hormone in the body that is able to lower blood glucose level. It first binds to the insulin receptor (IR) on the cell membrane and then activates the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) or Ras/Raf/mitogen-activated protein kinase signaling pathway (3). The PI3K/AKT signaling pathway is the primary pathway of insulin signaling transduction, through which insulin regulates glucose uptake, glycogen synthesis and degradation (4).
In the liver, insulin binds to the α subunit of IR on liver cells, and then activates IR substrate (IRS). IRS then binds to p85, the regulatory subunit of PI3K, and activates p110, the catalytic subunit of PI3K. The activated PI3K produces the second messengers Phosphatidylinositol (3,4)-trisphosphate [PtdIns(3,4)P2] and PtdIns(3,4,5)P3, which promote the activation of AKT (5). Activated AKT regulates the process of carbohydrate metabolism in hepatocytes via the following pathways: First, activated AKT promotes the transfer of glucose transporter 4 to cell membranes, which facilitates the transportation of extracellular glucose into the cells (6). Second, activated AKT mediates glycogen synthesis through glycogen synthase kinase 3 (GSK3) (7). Third, activated AKT inhibits hepatocyte gluconeogenesis through peroxisome proliferator-activated receptor-γ coactivator-α (8). Therefore, the insulin-PI3K/AKT signaling pathway serves an important function in the regulation of carbohydrate metabolism in liver.
At present, chemical drugs, including metformin, are primarily used for the clinical treatment of diabetes (9). Blood glucose level is significantly reduced by these drugs, but insulin resistance is not effectively improved. In addition, long-term use of these drugs can cause a variety of serious side effects, leading to liver and kidney damage (10). Therefore, it is necessary to develop natural drugs with reliable hypoglycemic effects and less toxic side effects.
Sericin is a water-soluble protein of the silkworm cocoon, which is composed of 18 different amino acids (11). The content of serine, aspartic acid, threonine and other polar amino acids (which are rich in hydroxyl, amino and carboxyl groups) in sericin is >70%, therefore sericin possesses good water solubility (12). Sericin has been used in wound healing, tissue regeneration, drug delivery and biomedicine (11,13). Studies have indicated that the addition of sericin to the daily diet may reduce the intestinal intake of cholesterol, triglycerides and other lipids, as well as improve the body's tolerance to glucose (14,15). Previous studies by our group indicated that sericin could effectively reduce blood glucose level in a streptozotocin-induced type 2 diabetic rat model (16,17). However, the hypoglycemic mechanism of sericin remains unclear.
In the current study, a type 2 diabetes rat model was established by administration with high-fat and high-glucose diet combined with streptozotocin intraperitoneal injection. The expression levels of IR, IRS-1, PI3K and AKT in the rat liver were analyzed to investigate whether sericin exerts a hypoglycemic effect via the insulin-PI3K/AKT signaling pathway. The current study may provide an experimental basis for the use of sericin in the treatment of diabetes in the clinic.
Materials and methods
Animals, grouping and sampling
A total of 48 specific-pathogen-free 6-week-old male Sprague-Dawley rats (purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd., Beijing, China) were used in the current study. The rats were housed with a natural light cycle at 18–26°C and relative humidity of 40–70%. They were randomly divided into four groups: Control group, diabetic model group, high-dose sericin group and low-dose sericin group, with 12 rats in each group. The type 2 diabetes rat model was established as follows: by high-fat and high-glucose diet combined with streptozotocin (35 mg/kg, twice) intraperitoneal injection. The rats were fed with high-fat and high-glucose diets as previously described (18) for 4 weeks and streptozotocin (35 mg/kg) was administered twice in succession by intraperitoneal injection. After 72 h, blood glucose was measured and rats with a fasting blood glucose of 11.1 mmol/l were deemed to have diabetes (17,19). Model rats were fed with high-fat and high-glucose diet for another 3 weeks and the diet was changed to normal diet when they were fed with sericin. The control group was fed with normal diet throughout the experiment. For the high- and low-dose sericin groups, the rats were continuously administered with 2.4 and 1.8 g/kg/day sericin for 35 days, respectively, while the control rats were administered with the same volume of saline for 35 days. All animal experiments were approved by the Ethics Committee of Chengde Medical University (Chengde, China) and conducted according to the ethical guidelines of Chengde Medical University.
The rats in each group were fasted for 12 h following treatment and anesthetized by intraperitoneal injection of 10% chloral hydrate (300 mg/kg body weight) (20–22). Then, the thoracic cavity was opened and venous blood samples were collected from the right atrium of the rats. During blood sample collection, rats were in deep anesthesia and no signs of peritonitis were observed. Then, the rats were sacrificed by decapitation and the left hepatic lobe was collected following opening of the abdominal cavity. The blood was centrifuged at 800 × g for 20 min at 4°C, and the serum was isolated. The liver tissues were kept in liquid nitrogen until further analysis.
Determination of glucose level
The blood glucose level of rats in each group was measured using a glucose oxidase method (23) on a Beckman Coulter AU5800 Clinical Chemistry Analyzer (Beckman Coulter, Inc., Brea, CA, USA).
Hematoxylin and eosin (H&E) staining
The liver morphology of each group was observed under an Olympus BH-2 light microscope (Olympus Corporation, Tokyo, Japan) following H&E staining. Briefly, the liver tissues were fixed with Bouins fixative [a mixture of picric acid saturated liquid (1.22%), formaldehyde and glacial acetic acid at a ratio of 15:5:1] for 24 h at room temperature and embedded in paraffin, and continuously sliced into 5-µm-thick sections. At room temperature, the sections were stained with hematoxylin for 7 min, differentiated with 1% HCl in 70% alcohol for 5 sec, stained with eosin for 1 min and dehydrated in a serial ethanol solution of increasing concentrations. Then, the sections were deparaffinized with xylene and sealed.
Periodic acid-Schiff staining
The hepatic glycogen content was determined by periodic acid-Schiff staining. The liver tissues were fixed as above described and embedded in paraffin, and continuously sliced into 5-µm-thick sections. The sections were oxidized with periodic acid for 10 min and stained with Schiff reagent for 15 min at room temperature. Cell nuclei were re-stained with hematoxylin for 5 min at room temperature. Then, sections were deparaffinized with xylene and sealed. Cells with red or magenta particles in the cytoplasm were defined as positive for glycogen. Tissue sections digested with amylase were used as negative controls.
For the quantification of glycogen content, six rat livers were randomly selected from each group, three sections were selected from each rat liver, and three views were observed in each section. The liver lobules with intact tissue structure were selected for observation using an light microscope (BH-2; Olympus Corporation, Tokyo, Japan; magnification, ×200). Image-Pro Plus 6.0 image analysis software (Media Cybernetics, Inc., Rockville, MD, USA) was used to calculate the integral optical density of glycogen in hepatocyte cytoplasm, and the mean value was determined as the glycogen content.
Immunohistochemical staining
Protein expression of IR, IRS-1, PI3K and AKT in the liver was analyzed. Briefly, the sections were dewaxed and incubated in 3% H2O2-methanol at 37°C for 30 min. Following antigen retrieval by microwaving, the sections were blocked with 10% goat serum (OriGene Technologies, Inc., Beijing, China) at 37°C for 30 min. The sections were then incubated with primary antibodies against IR (1:100; cat. no. ab131238), IRS-1 (1:100; cat. no. ab131487; both Abcam, Cambridge, MA USA), PI3K (1:100; cat. no. 611398; BD Biosciences, San Jose, CA, USA) and AKT (1:200; cat. no. ab179463; Abcam) at 4°C overnight. Next, the sections were incubated with goat anti-rabbit IgG secondary antibodies, which was provided by the rabbit streptavidin-biotin assay system (cat. no. SP-9001; Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China) according to the manufacturer's protocol, and then counterstained with DAB for 5–8 min at room temperature. Cell nuclei were re-stained with hematoxylin for 10 min at room temperature. PBS was used to substitute the primary antibody as the negative control. Cells with brownish yellow and/or brown particles were defined as positive staining.
For quantification, six rat livers were randomly selected from each group, three sections were selected from each rat liver, and three views were observed in each section. The liver lobules with intact tissue structure were selected for observation by an Olympus BH-2 microscope (magnification, ×200). Image-Pro Plus 6.0 image analysis software was used to calculate the integral optical density of each protein, and the mean value was determined as the corresponding protein expression level.
Western blot analysis
Total protein was extracted by RIPA Tissue/Cell Lysates (Beijing Solarbio & Technology Co., Ltd., Beijing, China) from 100 mg liver tissues, and the protein concentration was determined using a BCA protein kit (Kangwei Shiji Biotechnology Co., Ltd., Beijing, China). Proteins (108 µg/lane) were separated by 10% SDS-PAGE and transferred on to a PVDF membrane. Following blocking with 5% skim milk overnight at 4°C, the membrane was incubated with primary antibodies [IR, IRS-1, AKT (all 1:1,000), PI3K (1:500) and β-actin (1:1,000; cat no. AF7018; Affinity Biosciences, Cincinnati, OH, USA)] at room temperature for 2 h. Then, the membrane was incubated with goat anti-rabbit IgG (1:5,000; cat. no. 074-1506) or goat anti-mouse IgG (1:5,000; cat. no. 074-1806; both KPL, Inc., Gaithersberg, MD, USA) for a further 1.5 h at room temperature. The membrane was developed with Super ECL Plus ultra-sensitive luminescent liquid (Applygen Technologies, Inc., Beijing, China). The protein bands were analyzed with Quantity One v4.6.2 software (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The density of each target band was calculated relative to the β-actin band to determine relative expression levels.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from 100 mg liver tissues with a TaKaRa MiniBEST Universal RNA Extraction kit (cat. no. 9767) and reverse transcribed into cDNA using a PrimeScript™ RT Master mix (cat. no. RR036A; both Takara Biotechnology Co., Ltd., Dalian, China). The reverse transcription protocol was as follows: 37°C for 15 min and 85°C for 5 sec. The sample was put on ice and the obtained cDNA was stored at −20°C. Then, the mRNA expression of IR, IRS-1, PI3K, AKT and GAPDH was determined by qPCR with a SYBR Premix Ex Taq™ II kit (cat. no. RR820A; Takara Biotechnology Co., Ltd.). The primer sequences are listed in Table I. The PCR procedure was as follows: Pre-denaturation at 98°C for 1 min, followed by 40 cycles of denaturation at 98°C for 7 sec, annealing and polymerization at 60°C for 30 sec, then final polymerization at 60°C for 5 min. A relative standard curve method (24) was used to quantify the mRNA and the relative mRNA expression level of each target gene was determined relative to the corresponding GAPDH.
Table I.
Primer sequences used for quantitative polymerase chain reaction analysis.
| Gene | Primer sequence (5′-3′) |
|---|---|
| IR | F: TCATGGATGGAGGCTATCTGGA |
| R: TCCTTGAGCAGGTTGACGATTTC | |
| IRS-1 | F: AAGCACCTATGCCAGCATCAAC |
| R: GAGGATTGCTGAGGTCATTTAGGTC | |
| PI3K | F: CCAGAAGAAGGGACAGTGGTATG |
| R: TCGTAGCCAATCAGGGAGGT | |
| AKT | F: ATGGACTTCCGGTCAGGTTCA |
| R: GCCCTTGCCCAGTAGCTTCA | |
| GAPDH | F: GGCACAGTCAAGGCTGAGAATG |
| R: ATGGTGGTGAAGACGCCAGTA |
IR, insulin receptor; IRS-1, IR substrate-1; PI3K, phosphoinositide 3-kinase; AKT, protein kinase B.
Statistical analysis
Statistical analysis was performed with the statistical software SPSS 20.0 (IBM Corp., Armonk, NY, USA). All data are expressed as mean ± standard deviation. One-way analysis of variance was used to compare multiple groups, followed by Tukey's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Morphological changes of liver following sericin treatment
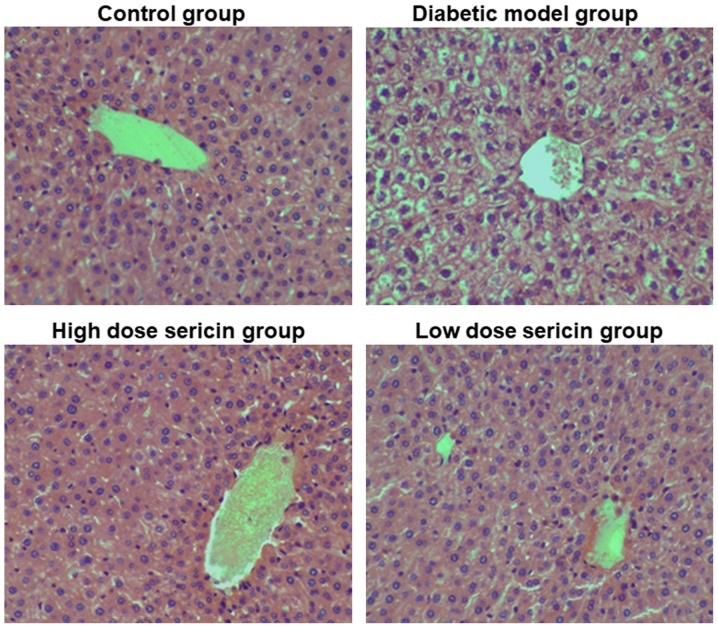
To observe the effect of sericin on the liver morphology of type 2 diabetic rats, H&E staining was performed. In the control group, the structure of the hepatic lobules was clear and the hepatocytes were arranged in a cord-like manner around the central vein (Fig. 1). The cell nuclei were large and round, located in the center of cells, and the cytoplasm was stained uniformly. The liver sinus was clear. In the diabetic model group, the hepatocytes were basically arranged in a cord-like manner around the central vein, but the liver cells were swollen, the volume increased, and obvious vacuolar structure appeared in the cytoplasm. A number of the liver cells exhibited soluble necrosis, and the hepatic sinus exhibited stenosis or atresia. Compared with the diabetic model group, the pathological changes of the rat liver in the high- and low-dose sericin groups were markedly reduced (Fig. 1). Rat liver lobular structures in these two sericin treatment groups were clear, and the hepatocytes were arranged in a cord-like manner around the central vein, with large round nuclei in the center of the cells. A small number of hepatocytes exhibited vacuolar structure in the cytoplasm, and the liver sinus was clear. This indicates that sericin may improve the liver morphological structure of type 2 diabetic rats.
Figure 1.
Analysis of liver morphology. Changes in liver morphology in the control group, diabetic model group, high-dose sericin group and low-dose sericin group were observed with hematoxylin and eosin staining. Representative images are presented. Magnification, ×200.
Glycogen content in liver following sericin treatment
To determine the effect of sericin on the glycogen content in type 2 diabetic rat livers, periodic acid-Schiff staining was performed. Hepatic glycogen positive expression was observed in the liver sections of all groups, indicated by red and purple particles in the cytoplasm. As indicated in Fig. 2A, the number of positive cells in the control group was high, and the staining was dark purple. In the diabetic model group, there were fewer positively stained cells, and the staining was a lighter reddish color. In the high-dose sericin group, there was a higher number of positively stained cells compared with the low-dose sericin group, and the staining was darker. As indicated in Fig. 2B, compared with the control group, the glycogen content in the rat liver of the diabetic model group was significantly decreased (P<0.05). Compared with the diabetic model group, the glycogen content in the liver of the high- and low-dose sericin groups was significantly increased (P<0.01). Furthermore, the glycogen content in the rat liver of the high-dose sericin group was significantly higher compared with the low-dose sericin group (P<0.01). This indicates that sericin may significantly increase the liver glycogen content of type 2 diabetic rats.
Figure 2.
Analysis of liver glycogen content and blood glucose level. (A) Liver glycogen content was evaluated by periodic acid-Schiff staining. Representative images are presented. Magnification, ×200. (B) Quantification of liver glycogen content. (C) Blood glucose level in each group. *P<0.05 vs. control group; #P<0.05 vs. diabetic model group; &P<0.05 vs. low-dose sericin group.
Blood glucose levels following sericin treatment
To determine the effect of sericin on the blood glucose level of the type 2 diabetic rats, the rat blood glucose level was measured. As indicated in Fig. 2C, the blood glucose level in the diabetic model group was significantly increased compared with the control group (P<0.05). Compared with the diabetic model group, the blood glucose levels of the rats in the high- and low-dose sericin groups were significantly decreased (P<0.01). However, there was no significant difference in the blood glucose levels between the high- and how-dose sericin groups. This indicates that sericin may significantly decrease the blood glucose levels of type 2 diabetic rats.
Expression of associated factors in the PI3K/AKT signaling pathway
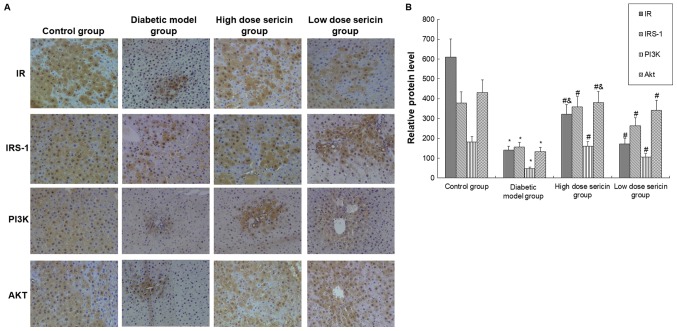
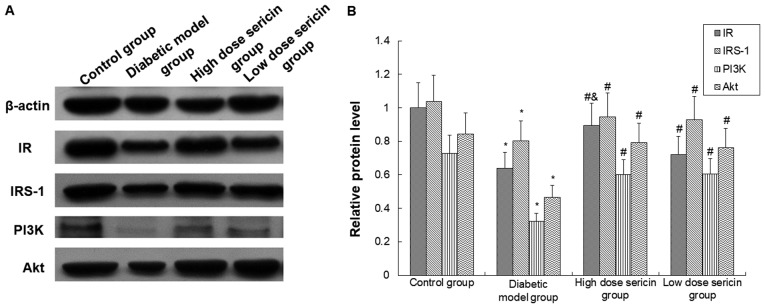
The protein levels of IR, IRS-1, PI3K, and AKT were detected with immunohistochemical staining and western blot analysis. Compared with the control group, the protein expression levels of IR, IRS-1, PI3K and AKT were significantly decreased in the diabetic model group (P<0.05; Figs. 3 and 4). Compared with the diabetic model group, the expression levels of these proteins in the liver of the high- and low-dose sericin groups were significantly increased (P<0.05; Figs. 3 and 4). Furthermore, the expression levels of AKT (Fig. 3) and IR (Figs. 3 and 4) in the high-dose sericin group were significantly higher compared with the low-dose sericin group (P<0.05).
Figure 3.
Analysis of IR, IRS-1, PI3K and AKT protein expression by immunohistochemical staining. (A) IR, IRS-1, PI3K and AKT protein expression in the rat livers of each group was detected by immunohistochemical staining (magnification, ×200). (B) Quantification of immunohistochemical staining in each group. *P<0.05 vs. control group; #P<0.05 vs. diabetic model group; &P<0.05 vs. low-dose sericin group. IR, insulin receptor; IRS-1, IR substrate-1; PI3K, phosphoinositide 3-kinase; AKT, protein kinase B.
Figure 4.
Analysis of IR, IRS-1, PI3K and AKT protein expression levels by western blotting. (A) Expression of IR, IRS-1, PI3K and AKT proteins in the rat livers of each group was detected by western blot analysis. (B) Quantification of protein expression in each group. *P<0.05 vs. control group; #P<0.05 vs. diabetic model group; &P<0.05 vs. low-dose sericin group. IR, insulin receptor; IRS-1, IR substrate-1; PI3K, phosphoinositide 3-kinase; AKT, protein kinase B.
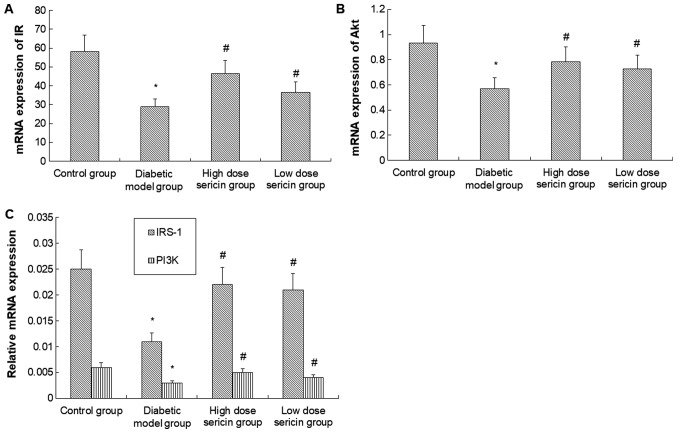
To further verify these results, RT-qPCR was conducted to detect the mRNA levels of IR, IRS-1, PI3K and AKT. As indicated in Fig. 5, the mRNA levels of IR, IRS-1, PI3K and AKT in the diabetic model group were significantly lower compared with the control group (P<0.05). However, following sericin treatment in the high- and how-dose sericin groups, mRNA expression was significantly higher compared with the diabetic model group (P<0.05). No significant difference in mRNA levels of these genes was identified between the high- and low-dose sericin groups.
Figure 5.
Analysis of IR, IRS-1, PI3K and AKT mRNA expression levels. The expression of (A) IR, (B) AKT and (C) IRS-1 and PI3K mRNA in the rat livers of each group was detected by reverse transcription-quantitative polymerase chain reaction. *P<0.05 vs. control group; #P<0.05 vs. diabetic model group. IR, insulin receptor; IRS-1, IR substrate-1; PI3K, phosphoinositide 3-kinase; AKT, protein kinase B.
In summary, these results indicate that sericin may promote the insulin/PI3K/AKT signaling pathway in type 2 diabetic rat liver by upregulating IR, IRS-1, PI3K and AKT expression, and thus may reduce blood glucose levels.
Discussion
Type 2 diabetes is a chronic metabolic disease characterized by insulin resistance. On a global scale, the incidence of type 2 diabetes is increasing year by year, with faster growth rates in developing countries (25,26). The expected number of patients with type 2 diabetes in 2030 is 552 million (27). At present, diabetes has become the third most prevalent non-communicable disease that threatens human life and health after cardiovascular disease and cancer (28). Therefore, it is of great importance to explore the pathogenesis of type 2 diabetes and search for effective and economical treatments.
Insulin resistance is the initiating factor for the development of type 2 diabetes. Researchers have identified that the PI3K/AKT signaling pathway is closely associated with insulin resistance-associated diseases, including diabetes and obesity (29,30). Furthermore, the PI3K/AKT signaling pathway is one of the key pathways by which insulin regulates blood glucose balance. Reduced expression of PI3K regulatory subunit p85, as well as functional defects of PI3K regulatory subunit p85 and catalytic subunit p110, may lead to glucose and lipid metabolism disorder (31,32) which further indicates the important role of this pathway in regulating glucose and lipid metabolism.
Hepatic insulin resistance also serves a key function in the pathophysiology of diabetes. It has been reported that feeding with high-fat diet and alcohol simultaneously could induce rat liver insulin resistance through inhibition of mRNA and protein expression levels of IRS-1 and PI3K (33). Rats with non-alcoholic liver disease also present typical symptoms of fatty liver, insulin resistance and glucose metabolism disorder, and silinin, a hepatoprotective agent, may attenuate hepatic steatosis and insulin resistance caused by non-alcoholic fatty liver via the IRS-1/PI3K/AKT pathway (34). The results of the current study also suggest that there may be associations among the hepatic insulin PI3K/AKT signaling pathway, hepatic insulin resistance and diabetes mellitus.
IR is an important mediator of the insulin PI3K/AKT signaling pathway, and its downregulation or mutation may lead to reduced insulin sensitivity and insulin resistance (35,36). Consistent with this, the current study identified that IR protein and mRNA levels in the type 2 diabetic model rat liver were significantly reduced. Furthermore, IRS-1, PI3K and AKT exhibited the same trend as IR. A decrease in AKT level could decrease the release and transport of glucose transporter in the vesicles (37). A decrease in AKT could also interfere with GSK3-mediated hepatic glycogen synthesis, leading to a significant decrease in hepatic glycogen content in diabetic model rats. AKT also reduces the inhibition of gluconeogenesis (7). These three effects of AKT may finally lead to a significant increase in blood glucose levels and insulin resistance in the diabetic rats.
Currently, oral hypoglycemic drugs are primarily used for the treatment of diabetes. Although various types of hypoglycemic drugs may effectively control blood sugar and delay the progression of the disease and complications, the majority of drugs exert side effects to varying degrees, including ineffective improvement of insulin resistance or islet cell protection, and drug resistance (38). Therefore, in recent years, researchers have turned their attention to natural substances that exert hypoglycemic effects, so as to obtain a drug that has good absorption and efficacy without obvious side effects. The ‘Compendium of Materia Medica’ records that cocoons may have effects on diabetes (39). Sericin used in the current study is a natural protein in silkworm cocoons, which is coated on the outside of silk fibroin, accounting for approximately 25% of the cocoon. Sericin is usually discarded when reeling. However, cocoons soaked in boiling water have long been used to control blood glucose in Chinese folk medicine. Modern research indicates that sericin is a water-soluble protein consisting of 18 amino acids. It is biocompatible, biodegradable and non-toxic to humans, and has no obvious side effects (40). Our previous studies also identified that sericin could effectively reduce blood glucose level (16,17). However, it is not yet clear whether the PI3K/AKT signaling pathway is involved in the effect of sericin on blood glucose.
The current study observed the effect of sericin on associated factors of the PI3K/AKT signaling pathway, including IR, IRS-1, PI3K and AKT, in the liver of type 2 diabetic rats, and investigated whether sericin could reduce blood glucose and improve insulin resistance. Following administration of sericin in diabetic model rats, the expression levels of IR, IRS-1, PI3K and AKT in the model rat liver significantly increased, the blood glucose level significantly reduced and the hepatic glycogen content significantly increased.
In conclusion, the current findings demonstrate that sericin is able to increase the expression of IR, IRS-1, PI3K and AKT in the liver of diabetic rats at the gene and protein level. Sericin may regulate the PI3K/AKT signaling pathway in diabetes to enhance the transduction effect of insulin signal and promote the synthesis of hepatic glycogen. This may be the mechanism by which sericin is able to lower blood glucose and improve insulin resistance.
Acknowledgements
Not applicable.
Funding
The current study was supported by the National Natural Science Foundation of China (grant no. 81441133) and the Natural Science Foundation of Hebei Province (grant no. H2013406096).
Availability of data and materials
All data generated or analyzed in the present study are included in this published article.
Authors' contributions
CS performed all experiments. DL fed the animals, collected the samples and participated in the blood glucose test. SY participated in the immunohistochemistry experiments. LC helped with the hematoxylin and eosin staining and glycogen staining. EX participated in the polymerase chain reaction analysis. ZC designed and directed the study.
Ethics approval and consent to participate
All animal experiments were approved by the Ethics Committee of Chengde Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Chattopadhyay T, Singh RR, Gupta S, Surolia A. Bone morphogenetic protein-7 (BMP-7) augments insulin sensitivity in mice with type II diabetes mellitus by potentiating PI3K/AKT pathway. Biofactors. 2017;43:195–209. doi: 10.1002/biof.1334. [DOI] [PubMed] [Google Scholar]
- 2.Chaudhury A, Duvoor C, Reddy Dendi VS, Kraleti S, Chada A, Ravilla R, Marco A, Shekhawat NS, Montales MT, Kuriakose K, et al. Clinical review of antidiabetic drugs: Implications for type 2 diabetes mellitus management. Front Endocrinol (Lausanne) 2017;8:6. doi: 10.3389/fendo.2017.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horita S, Nakamura M, Suzuki M, Satoh N, Suzuki A, Seki G. Selective insulin resistance in the kidney. Biomed Res Int. 2016;2016:5825170. doi: 10.1155/2016/5825170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao YF, Zhang MN, Wang TX, Wu TC, Ai RD, Zhang ZS. Hypoglycemic effect of D-chiro-inositol in type 2 diabetes mellitus rats through the PI3K/Akt signaling pathway. Mol Cell Endocrinol. 2016;433:26–34. doi: 10.1016/j.mce.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Hou XL, Wang WQ, Shi CY, Tong Q, Fang JG. Research progress in pharmacological effects of dihydromyricelin. Chinese Traditional and Herbal Drugs. 2015;46:603–609. [Google Scholar]
- 6.Gandhi GR, Jothi G, Antony PJ, Balakrishna K, Paulraj MG, Ignacimuthu S, Stalin A, Al-Dhabi NA. Gallic acid attenuates high-fat diet fed-streptozotocin-induced insulin resistance via partial agonism of PPARgamma in experimental type 2 diabetic rats and enhances glucose uptake through translocation and activation of GLUT4 in PI3K/p-Akt signaling pathway. Eur J Pharmacol. 2014;745:201–216. doi: 10.1016/j.ejphar.2014.10.044. [DOI] [PubMed] [Google Scholar]
- 7.Liu TY, Shi CX, Gao R, Sun HJ, Xiong XQ, Ding L, Chen Q, Li YH, Wang JJ, Kang YM, Zhu GQ. Irisin inhibits hepatic gluconeogenesis and increases glycogen synthesis via the PI3K/Akt pathway in type 2 diabetic mice and hepatocytes. Clin Sci (Lond) 2015;129:839–850. doi: 10.1042/CS20150009. [DOI] [PubMed] [Google Scholar]
- 8.Chang S. Progress in studying the relationship between PDK/Akt signal access and insulin resistance. Zhong Yi Yao Dao Bao. 2008;14:113–116. [Google Scholar]
- 9.Ahrén B, Masmiquel L, Kumar H, Sargin M, Karsbøl JD, Jacobsen SH, Chow F. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): A 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017;5:341–354. doi: 10.1016/S2213-8587(17)30092-X. [DOI] [PubMed] [Google Scholar]
- 10.Type 2 diabetes and metformin. First choice for monotherapy: Weak evidence of efficacy but well-known and acceptable adverse effects. Prescrire Int. 2014;23:269–272. [PubMed] [Google Scholar]
- 11.Chen H, Zhu LJ, Min SJ, Hu GL. Structure, property and utilization of silk sericin. Journal of Functional Polymers. 2001;14:344–347. [Google Scholar]
- 12.Takasu Y, Yamada H, Tamura T, Sezutsu H, Mita K, Tsubouchi K. Identification and characterization of a novel sericin gene expressed in the anterior middle silk gland of the silkworm Bombyx mori. Insect Biochem Mol Biol. 2007;37:1234–1240. doi: 10.1016/j.ibmb.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Aramwit P, Siritientong T, Srichana T. Potential applications of silk sericin, a natural protein from textile industry by-products. Waste Manag Res. 2012;30:217–224. doi: 10.1177/0734242X11404733. [DOI] [PubMed] [Google Scholar]
- 14.Okazaki Y, Kakehi S, Xu Y, Tsujimoto K, Sasaki M, Ogawa H, Kato N. Consumption of sericin reduces serum lipids, ameliorates glucose tolerance and elevates serum adiponectin in rats fed a high-fat diet. Biosci Biotechnol Biochem. 2010;74:1534–1538. doi: 10.1271/bbb.100065. [DOI] [PubMed] [Google Scholar]
- 15.Limpeanchob N, Trisat K, Duangjai A, Tiyaboonchai W, Pongcharoen S, Sutheerawattananonda M. Sericin reduces serum cholesterol in rats and cholesterol uptake into Caco-2 cells. J Agric Food Chem. 2010;58:12519–12522. doi: 10.1021/jf103157w. [DOI] [PubMed] [Google Scholar]
- 16.Chen Z, He Y, Song C, Dong Z, Su Z, Xue J. Sericin can reduce hippocampal neuronal apoptosis by activating the Akt signal transduction pathway in a rat model of diabetes mellitus. Neural Regen Res. 2012;7:197–201. doi: 10.3969/j.issn.1673-5374.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song CJ, Yang ZJ, Tang QF, Chen ZH. Effects of sericin on the testicular growth hormone/insulin-like growth factor-1 axis in a rat model of type 2 diabetes. Int J Clin Exp Med. 2015;8:10411–10419. [PMC free article] [PubMed] [Google Scholar]
- 18.Hao WJ, Li JY, Jiang H, Li YH, Shen Z, Hou JJ, Xiong J, Li XR. Advantages of purified high-fat and high-glucose diet. Wei Sheng Yan Jiu. 2017;46:143–147. [Google Scholar]
- 19.Li N, Liu Q, Li XJ, Bai XH, Liu YY, Jin ZY, Jing YX, Yan ZY, Chen JX. Establishment and evaluation of a rat model of type 2 diabetes associated with depression. Zhongguo Ying Yong Sheng Li Xue Za. 2015;31:23–26. (In Chinese) [PubMed] [Google Scholar]
- 20.Santos EL, Dias BH, Andrade AC, Pascoal AM, Vasconcelos Filho FE, Medeiros Fd, Guimarães SB. Effects of acupuncture and electroacupuncture on estradiol-induced inflammation and oxidative stress in health rodents. Acta Cir Bras. 2013;28:582–588. doi: 10.1590/S0102-86502013000800005. [DOI] [PubMed] [Google Scholar]
- 21.Silachev DN, Usatikova EA, Pevzner IB, Zorova LD, Babenko VA, Gulyaev MV, Pirogov YA, Plotnikov EY, Zorov DB. Effect of anesthetics on efficiency of remote ischemic preconditioning. Biochemistry. Biokhimiia (Mosc) 2017;82:1006–1016. doi: 10.1134/S0006297917090036. [DOI] [PubMed] [Google Scholar]
- 22.Vollmar B, Janata J, Yamauchi JI, Menger MD. Attenuation of microvascular reperfusion injury in rat pancreas transplantation by L-arginine. Transplantation. 1999;67:950–955. doi: 10.1097/00007890-199904150-00004. [DOI] [PubMed] [Google Scholar]
- 23.Kura RR, Kilari EK, Shaik M. Influence of aprepitant on the pharmacodynamics and pharmacokinetics of gliclazide in rats and rabbits. PeerJ. 2018;6:e4798. doi: 10.7717/peerj.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang NN, Liu F, Xu X, Zhang SW, Nie R, Yin DD, Liu GQ, Wang GJ. Establishment and preliminary application of fluorescence quantitative RT-PCR detection method for tambus virus. Zhong Guo Shou Yi Ke Xue. 2015;45:15–19. [Google Scholar]
- 25.Chahardah-Cherik SM, Gheibizadeh MP, Jahani SP, Cheraghian BP. The relationship between health literacy and health promoting behaviors in patients with type 2 diabetes. Int J Commun Based Nurs Midwifery. 2018;6:65–75. [PMC free article] [PubMed] [Google Scholar]
- 26.Papier K, D'Este C, Bain C, Banwell C, Seubsman S, Sleigh A, Jordan S. Consumption of sugar-sweetened beverages and type 2 diabetes incidence in Thai adults: Results from an 8-year prospective study. Nutr Diabetes. 2017;7:e283. doi: 10.1038/nutd.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 28.Gautam S, Banerjee M. The macrophage Ox-LDL receptor, CD36 and its association with type II diabetes mellitus. Mol Genet Metab. 2011;102:389–398. doi: 10.1016/j.ymgme.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 29.Hu X, Wang S, Xu J, Wang DB, Chen Y, Yang GZ. Triterpenoid saponins from Stauntonia chinensis ameliorate insulin resistance via the AMP-activated protein kinase and IR/IRS-1/PI3K/Akt pathways in insulin-resistant HepG2 cells. Int J Mol Sci. 2014;15:10446–10458. doi: 10.3390/ijms150610446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu J, Chen LN, Ma X. PI3K/Akt signaling pathway and insulin resistance related diseases. Guo Wai Yi Xue (Yi Xue Di Li Fen Ce) 2012;33:127–131. (In Chinese) [Google Scholar]
- 31.Winnay JN, Dirice E, Liew CW, Kulkarni RN, Kahn CR. p85α deficiency protects β-cells from endoplasmic reticulum stress-induced apoptosis. Proc Natl Acad Sci USA. 2014;111:1192–1197. doi: 10.1073/pnas.1322564111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson VL, Jiang YP, Dickman KG, Ballou LM, Lin RZ. Adipose tissue insulin resistance due to loss of PI3K p110alpha leads to decreased energy expenditure and obesity. Am J Physiol Endocrinol Metab. 2014;306:E1205–E1216. doi: 10.1152/ajpendo.00625.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qu W, Hao L, Chen Y, Zhou S. Effect of chronic ethanol intake on insulin receptor, insulin receptor subsrate-1 and phosphoinositide 3-kinase mRNA expression in skeletal muscle of rats. Wei Sheng Yan Jiu. 2007;36:172–175. (In Chinese) [PubMed] [Google Scholar]
- 34.Zhang Y, Hai J, Cao M, Zhang Y, Pei S, Wang J, Zhang Q. Silibinin ameliorates steatosis and insulin resistance during non-alcoholic fatty liver disease development partly through targeting IRS-1/PI3K/Akt pathway. Int Immunopharmacol. 2013;17:714–720. doi: 10.1016/j.intimp.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 35.Wu QP, Xiao C, Yang XB, Zhang JM. Hypoglycemic effects of components extracted from edible and medicinal fungi and their mechanisms of action. Acta Edulis Fungi. 2009;16:80–86. [Google Scholar]
- 36.Tao MX, Wang F, Liu J, Cheng GY, Jin BQ. Hypoglycemic Effect of Pleurotus citrinopileats Polysaccharide. Food Sci. 2009;30:227–230. [Google Scholar]
- 37.Kuai M, Li Y, Sun X, Ma Z, Lin C, Jing Y, Lu Y, Chen Q, Wu X, Kong X, Bian H. A novel formula Sang-Tong-Jian improves glycometabolism and ameliorates insulin resistance by activating PI3K/AKT pathway in type 2 diabetic KKAy mice. Biomed Pharmacother. 2016;84:1585–1594. doi: 10.1016/j.biopha.2016.10.101. [DOI] [PubMed] [Google Scholar]
- 38.Bodmer M, Meier C, Krähenbühl S, Jick SS, Meier CR. Metformin, sulfonylureas, or other antidiabetes drugs and the risk of lactic acidosis or hypoglycemia: A nested case-control analysis. Diabetes Care. 2008;31:2086–2091. doi: 10.2337/dc08-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li SZ. Compendium of Materia Medica. Beijing: People's Health Publishing House; 1985. pp. 1–1052. [Google Scholar]
- 40.Zhang HP, Zhu LJ, Hu H. Studies on the properties of L-asparaginase immobilized on sericin protein powder. Bulletin of Sericulture. 2003;34:16–19. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed in the present study are included in this published article.