Abstract
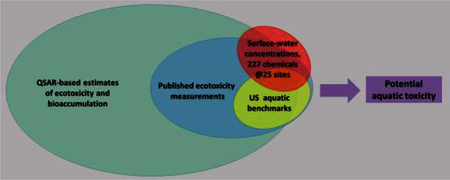
We describe screening level estimates of potential aquatic toxicity posed by 227 chemical analytes that were measured in 25 ambient water samples collected as part of a joint USGS/USEPA drinking water plant study. Measured concentrations were compared to biological effect concentration (EC) estimates, including USEPA aquatic life criteria, effective plasma concentrations of pharmaceuticals, published toxicity data summarized in the USEPA ECOTOX database, and chemical structure-based predictions. Potential dietary exposures were estimated using a generic 3-tiered food web accumulation scenario. For many analytes, few or no measured effect data were found, and for some analytes, reporting limits exceeded EC estimates, limiting the scope of conclusions. Results suggest occasional occurrence above ECs for copper, aluminum, strontium, lead, uranium, and nitrate. Sparse effect data for manganese, antimony, and vanadium suggest that these analytes may occur above ECs, but additional effect data would be desirable to corroborate EC estimates. These conclusions were not affected by bioaccumulation estimates. No organic analyte concentrations were found to exceed EC estimates, but ten analytes had concentrations in excess of 1/10th of their respective EC: triclocarban, norverapamil, progesterone, atrazine, metolachlor, triclosan, para-nonylphenol, ibuprofen, venlafaxine, and amitriptyline, suggesting more detailed characterization of these analytes.
Keywords: Aquatic, Contaminant, Hazard
Graphical Abstract
1. Introduction
Between 2010 and 2012, the US Geological Survey (USGS) and the US Environmental Protection Agency (USEPA) conducted a study of aquatic contaminants during which they collected one-time grab samples of source and finished drinking water from 25 drinking water plants across the US (see companion paper Glassmeyer et al., 2017-in this issue, for an overview). In each water sample, > 200 chemical analytes were measured, including traditional toxicants, such as metals, pesticides, nutrients, and solvents, as well as ‘contaminants of emerging concern’, such as pharmaceuticals, hormones, and perfluorinated compounds. In order to provide some toxicological context to this large collection of measurements, we compare the observed concentrations with readily available estimates of aquatic toxicity (effect concentrations, or ECs), and express results as the ratio of observed concentration to effect concentration (termed here the hazard quotient, or HQ) for aquatic life potentially chronically exposed to ambient water containing concentrations of analyte similar to the concentrations found in our source water samples. Readers should note that higher HQs indicate higher hazard, which is opposite to the implication of higher margins of exposure (MOE: defined as the ratio of toxic concentration divided by the exposure concentration) metric typically used in human risk assessment. A companion paper (Benson et al., 2017-in this issue) reports MOEs for potential human exposures (via drinking water) to analyte concentrations like those we measured in finished drinking water.
A significant challenge to estimating HQs is the sparseness (or absence) of directly measured data available for estimating potential ecological effect concentrations (ECs). Official benchmark concentrations, based on a relatively large body of carefully considered toxicity data, are available for a few analytes. These benchmarks typically incorporate safety factors to account for various uncertainties, and are intended to represent safe reference concentrations below which no adverse biological effects are expected. HQs below one constructed with these values can be considered safe when evaluating single-chemical exposures. By contrast, values above one do not necessarily mean that a hazard exists, since a safety margin is typically built into the benchmark EC. For most analytes, such benchmarks have not been established, but often some published toxicity data are available for comparison to occurrence levels. These values typically reflect toxicity testing results in a single species, and are reported in a variety of forms (depending on the choice of the authors), including lowest observable effect concentrations (LOECs), and effect concentrations spanning a range of effect intensities from around 1% to 100% of the maximal effect. While HQs above one constructed with a LOEC or ECx (concentration at which x% of the maximal effect is observed; e.g. EC50 or EC10) imply some hazard for at least some species, HQ values less than one are harder to interpret as they may or may not be capable of eliciting some biological effects.
References to most published aquatic toxicity data can be found in the ECOTOX database (USEPA, 2013a). In addition, for pharmaceuticals, ECs might be crudely approximated using pharmacological parameters consistently made publically available during the regulatory approval process. For steroidal estrogens, ECs (NOECs and LOECs) have been proposed (Caldwell et al., 2012). For analytes which have few or no directly measured concentration response data, toxicity can be roughly estimated using quantitative structure–activity relationships. In addition to consideration of direct exposure in the water column, food webexposure to bioaccumulative analytes (which can result in much higher exposure rates than direct water exposure) can be estimated based on hydrophobicity of analytes, along with estimates of food web structure.
Here, we compare analyte concentrations in 25 source water samples to a variety of EC estimates, including official government benchmarks, pharmacodynamic parameters, published concentration–response data, and estimates from structure-based toxicity prediction algorithms. Conservative default values are substituted for missing information throughout, in an attempt to reduce the likelihood that concerns about observed concentrations of environmentally important analytes will be prematurely discounted due to limitations in EC data. For most of our analytes, we anticipate that insufficient effect information exists for definitive hazard estimation. Nevertheless, evaluation of the limited data available serves to provide some ecotoxicological context to measured concentrations of the analytes, and may suggest more detailed investigation of some of the 227 evaluated analytes, particularly when measured concentrations exceed ECs.
2. Materials and methods
2.1. Measured occurrence concentrations
Measured concentrations (details of which are provided in companion publications) were excluded from analysis if they were less than three times field or lab blank concentrations, analyte recovery was outside the range of 50% to 150%, or they otherwise failed quality controls. Measured concentrations are listed in the Supporting Information. Mean, median and maximum HQs were calculated by substituting the reporting limit (RL) for concentrations below the RL, whenever the RL was less than the EC estimate. When the RL was greater than the EC estimate, only measurements in excess of the estimate were used for calculating mean, median, and maximum HQs. Water samples were filtered (0.45 µm) in the laboratory prior to analysis, so concentrations may not reflect solid or colloidal material suspended in the water column.
2.2. Cross-referencing salts and derivatives
For each analyte, associated CAS registry numbers were identified by keyword searching chemical structure databases, including the National Library of Medicine ChemIdPlus database (http://chem.sis.nlm.nih.gov/chemidplus/), the USEPA ACToR (http://actor.epa.gov) database, and the Royal Society of Chemistry ChemSpider database (http://www.chemspider.org). Because analytical methods employed in this project were not stereospecific, CAS numbers of stereoisomers were identified from these chemical structure databases by searching lists of structurally related molecules. Because toxicity experiments may have been conducted with salts of ionizable analytes, rather than neutral forms, common salt forms were also identified. CAS numbers of enantiomers and salts were used to associate analytes with toxicity data. For analytes and associated variants, Simplified Molecular-Input Line-Entry System (SMILES; Weininger, 1988) structural descriptors were downloaded from the ChemIdPlus database. SMILES descriptors were used as input for prediction of physiochemical properties (see Section 2.6) and toxicity (see Section 2.7). Cross-references are listed in the Supplement.
2.3. Selection of ECs
In order to accommodate the varying availability and quality of data available for estimating ECs of different chemicals, we used a tiered scheme for selecting ECs, in the hopes of providing more reliable and interpretable HQs, while casting a net as broad as possible for applicable data. First priority was given to the use of benchmark concentrations (see Section 2.4), because these values are based on carefully curated and analyzed data and represent safe concentrations below which adverse biological effects are not expected. No benchmarks were available for pharmaceuticals, so minimally effective therapeutic concentrations were used to estimate ECs (see Section 2.5), since these are uniformly available, reflect the chemicals’ primary known modes of action, and are often considerably lower (and therefore more protective) than ECs from traditional toxicity testing. For other analytes without benchmarks, we used the lowest published toxicity values (see Section 2.6) to construct HQs. For the remaining chemicals, we used structure–activity relationships to estimate ECs (see Section 2.8).
2.4. Benchmark concentrations
USEPA Office of Water National (OW) Recommended Water Quality Criteria (WQC) for aquatic life were downloaded on January 22, 2014 from: <http://water.epa.gov/scitech/swguidance/standards/criteria/current/index.cfm>.
USEPA Office of Pesticide Programs (OPP) Aquatic Life Benchmarks were downloaded on January 22, 2014 from: <http://www.epa.gov/oppefed1/ecorisk_ders/aquatic_life_benchmark.htm>.
The Aquatic Life Benchmarks were subsequently moved to: <https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/aquatic-life-benchmarks-pesticide-registration>.
Freshwater criterion continuous concentrations (CCC) for the most sensitive biological taxa were used in downstream analysis.
In accordance with WQC guidelines, site-specific CCC for lead and chromium were defined by regression-based WQC formulas that account for effects of water hardness on toxicity. We calculated hardness (which was not measured directly) from measured calcium and magnesium concentrations by accounting for relative molecular weights of the respective carbonates:
Site-specific chromium CCCs (μg/L) were then calculated as
CrCCC = exp(0.819*log(hardness) + 0.6848)*0.86*1000
where ‘log(x)’ is the natural log of x, and ‘exp(x)’ is the base of the natural log raised to the power of x. Site-specific CCCs (μg/L) for lead were calculated as
PbCCC = exp(1.273*log(hardness)−4.705)*(1.46203−log(hardness)*0.145712)*1000
WQC recommends site-specific CCCs for copper calculated using the Biotic Ligand Model (BLM; Di Toro et al., 2001; Windows Interface, Version 2.2.1; Hydroqual Inc., 2007). Not all of the thirteen required BLM parameters were available in all cases: pH and chloride were not measured at particular locations, while dissolved organic carbon (DOC), sulfate, and alkalinity were not measured in our study. Two sets of ECs were calculated: one based on more protective default values (pH = 4.9; DOC = 0.05 mg/L; SO4 = 0.096 mg/L; Cl = 0.32 mg/L; alkalinity = 1.99 mg CaCO3 eq/L) selected from the input value limits for the BLM software, representing the lower CCC limits of what was possible given the available data; and one based on less protective default values selected from input limits (pH = 9.2; DOC = 29.65 mg/L; SO4 = 278.4 mg/L; Cl = 0.32 (279.72 mg/L; alkalinity = 360 mg CaCO3eq/L) representing the upper CCC limits of possibility given available data. For several sites, copper CCCs can change by > 1000 depending on the choice of defaults.
Benchmarks and criteria are listed in the Supporting Information.
2.5. Pharmaceutical effect concentrations
Pharmaceutical ECs were estimated as the maximum freely dissolved plasma concentrations expected in human patients after a minimum therapeutic dose. For antibiotics, ECs were estimated as the lowest minimum inhibitory concentration (MIC: concentrations that inhibit growth of the tested organism under standard conditions) for the most sensitive microbe. The plasma concentrations after dosing, fraction of drug bound to plasma proteins, MICs, and breakpoint concentrations (concentrations defining clinical resistance) were adapted from Kostich et al., 2014, and from prescribing information provided by drug manufacturers. Lowest MICs for sulfamethizole and sulfadimethoxine were from Williams (1978). Pharmaceutical ECs are listed in the Supporting Information.
2.6. Published toxicity values
Published experimental toxicity data for each analyte, and for derivatives associated with an analyte (see Section 2.2. Cross-referencing salts and derivatives), were retrieved from the USEPA ECOTOX database (http://www.epa.gov/ecotox) on September 16, 2013. Only toxicity values directly translatable into water or food-based exposure that reflected the endpoints of growth, survival, or reproduction were included. In particular, data involving parenteral administration, cellular assays, enzymatic assays, and biochemical endpoints were excluded. Only LOECs, ECx, and LCx (concentration at which x% of the maximum possible effect/lethality is observed) where a statistically significant difference from controls was shown were used for HQ construction. NOECs were not used, since the absence of a detectable effect in one species provides no suggestion of lack of effects in other species, while using a LOEC, ECx and LCx strongly suggests that at least one species displays effects at the given concentration. For each chemical, the lowest available EC was used. For chemicals with HQ (based on data from ECOTOX) estimated to be > 0.01, ECOTOX ECs were checked for accuracy against the original literature, except in a few cases where reliable translations of the articles were not available. Uncorrected and corrected ECOTOX ECs are listed in the Supporting Information.
2.7. Physical–chemical properties
Measured physiochemical properties, including water solubility, melting point, and octanol–water partitioning coefficients were collected from ChemIdPlus, ACToR, or the USEPA EPI Suite (USEPA, 2013b) Physical Properties Database (Boethling et al., 2004), and used for subsequent calculations whenever available. When measured values were not available, they were estimated using the USEPA EPI Suite software package collection (USEPA, 2013b). Melting points were estimated using the MPBPWIN package, and octanol–water partitioning coefficients were estimated using the KOWWIN package (Meylan and Howard, 2005). Water solubilities were estimated using the WATERNT (Meylan and Howard, 2005) and WSKOWWIN (Meylan et al., 1996) packages. Properties were only predicted for organic chemicals without metal atoms, in order to restrict predictions to EPI Suite’s recommended domain of applicability. Physical–chemical properties are listed in the Supporting Information.
2.8. Structure–toxicity prediction
Toxicity was estimated with the USEPA ECOSAR software tool (USEPA, 2012; http://www.epa.gov/oppt/newchems/tools/21ecosar.htm), for analytes and associated derivatives within the domain of applicability of the program (organic molecules of moderate molecular weight, not containing metal atoms). When available, experimentally determined properties (Kow, melting point, water solubility, and molecular weight) were used to parameterize this software, otherwise estimated values were used (see Section 2.6, physiochemical properties). Predicted toxicity values are listed in the Supporting Information.
2.9. Food web exposure
Bioconcentration factors (BCFs—the ratio of whole body concentration in organisms exposed to water to the concentration in water) as well as bioaccumulation factors (BAFs—the ratio of concentration in organisms achieved due to food web accumulation plus water exposure, divided by the concentration in the water) of each analyte, and for each related chemical associated with that analyte (see Section 2.2), were estimated using a partitioning equation (Arnot and Gobas, 2003). A generic three-tiered food web scenario (Arnot and Gobas, 2003) was used to estimate trophic transfers, with biodegredation rates assumed to be negligible. This should result in conservative BAF estimates for high-level predators. The BAF/BCF ratio for each chemical was used to estimate food level exposure as a function of water column concentration. Toxicity values for water exposure were extrapolated to include food web exposure by dividing the toxicity values by the BAF/BCF ratio. This assumes that toxicity is proportional to the body burden of a chemical. BAF estimates are listed in the Supporting Information.
2.10. Computations
Data were retrieved and formatted using Strawberry Perl version 5.8.2, MSWin32-× 64-multi-thread build (http://strawberryperl.com/). Data analysis was performed using R version 3.02, x86_64-w64-mingw32/× 64 build ( R Core Team, 2013). A p-value cutoff of 0.05 was used for determining significance.
3. Results and discussion
3.1. Source water concentrations
Sixteen sampled sites used river water as their primary water supply, while two used lakes, four used reservoirs, and three used ground water as their primary water source. Each plant was sampled once, which means the typical ranges of concentrations at the collection locations are unknown and we could miss episodic pulses of analytes associated with (for example) agricultural activities, which are typically applied seasonally during a relatively narrow time window.
Across the 227 analytes, RLs ranged between 0.03 ng/L (for perfluorobutanesulfonic acid = PFBS) and 300,000 ng/L (for potassium). Nine hundred and ninety-five measurements exceeded RLs (termed detections). Eighty-nine analytes were detected at least once, and 138 analytes were never detected. Eight analytes were always detected (barium, calcium, magnesium, potassium, silicon, sodium, strontium, and sulfur). The most commonly detected organic compounds were perfluorinated compounds (PFBS, perfluoro-n-heptanoic acid = PFHpA, perfluorohexanoic acid = PFHxA, and perfluoro-n-nonanoic acid = PFNA, each detected 24 times). Frequent detection of perfluorinated compounds may reflect low RLs for these compounds (all < 0.1 ng/L). Consistent with this hypothesis, the median concentrations of these analytes were lower than the reporting limits of > 75% of the 227 analytes. Logistic regression using the entire analyte set suggests the association between detections (expressed as a proportion of measurements) and RL is statistically significant, but the strength of this association appears small, suggesting that the pattern of detects across the dataset is not primarily reflecting heterogeneity in RLs for different analytes.
3.2. Benchmark concentrations
OW WQC CCC exist for 15 analytes, from which we adopted freshwater CCC for the most sensitive biological taxa for HQ estimation. Summary statistics for copper, lead, and chromium CCCs across the 25 sample sites are listed in Table 1. Readers should keep in mind these screening-level estimates are based on parameters (e.g. hardness or pH) determined from a single sample per site, whereas definitive hazard estimates require that the parameters be estimated from a series of measurements across time in order to account for temporal variations.
Table 1.
Summary statistics for USEPA Office of Water Criteria Continuous Concentrations (CCC).
| Pb CCC | Cr CCC | Cu CCC Lo | Cu CCC Hi | |
|---|---|---|---|---|
| Mean | 3.8 | 96 | 0.55 | 42 |
| Std Dev | 3.2 | 64 | 0.59 | 88 |
| Min | 0.29 | 15 | 0.019 | 1.3 |
| Max | 13 | 279 | 2.3 | 451 |
Location-specific CCCs in micrograms per liter, for lead (Pb), chromium (Cr) and copper (Cu) calculated for the 25 sampled sites. CCCs for Pb and Cr were calculated based on water hardness, and the CCC for Cu were calculated using biotic ligand model (BLM)-based software. Not all BLM parameters were measured. Substituting the range of allowable values for missing values in the BLM would result in CCC between CCC Lo and CCC Hi.
RLs exceeded CCC for three analytes: chlorpyrifos (concentration 2.9 × higher than CCC), diazinon (2.4 ×), and cadmium (1.2 ×). These analytes were never detected. HQs for the remaining 12 analytes are listed in Table 2. HQs greater than one were observed for copper in 22 out of 25 samples when using the lower CCC estimate, while only three samples had HQs greater than one when using the higher CCC estimate. HQs greater than one were also seen for aluminum (N = 13), iron (N = 2), and lead (N = 1). The analytical methods we employed were not designed to remove all colloidal material or particulates smaller than 0.45 μm, therefore some of the material we measured during elemental analysis may reflect undissolved material with limited bioavailability. Iron and aluminum are widespread in the earth’s crust, but concentrations in ambient water largely depend on redox potential (for iron) and pH (Hem, 1985). We did not have enough information to determine what portion of the measured iron or aluminum concentrations was derived from human activity.
Table 2.
Hazard quotients (HQs) calculated using USEPA Office of Water National Recommended Water Quality Criteria Chronic Concentrations (CCCs).
| CAS RN | Analyte | RL | N | EC | N Exceed | Avg HQ | Med HQ | Max HQ |
|---|---|---|---|---|---|---|---|---|
| 7440-50-8 | Copper | 1000 | 25 | Table 1 | 3–22* | 0.52– 75* |
0.21–6.2* | 3.1–589* |
| 7429-90-5 | Aluminum | 4000 | 25 | 87,000 | 13 | 1.5 | 1.1 | 11 |
| 7439-89-6 | Iron | 1000 | 25 | 1E6 | 2 | 0.33 | 0.19 | 1.7 |
| 7439-92-1 | Lead | 65 | 22 | Table 1 | 1 | 0.23 | 0.16 | 1.7 |
| 7440-47-3 | Chromium | 1000 | 25 | Table 1 | 0 | 0.041 | 0.011 | 0.56 |
| 7782-49-2 | Selenium | 1000 | 22 | 5000 | 0 | 0.21 | 0.20 | 0.31 |
| 16,887-00-6 | Chloride | NA | 23 | 2.3E08 | 0 | 0.096 | 0.077 | 0.23 |
| 7440-66-6 | Zinc | 500 | 25 | 120,000 | 0 | 0.033 | 0.028 | 0.19 |
| 7440-38-2 | Arsenic | 4000 | 25 | 150,000 | 0 | 0.057 | 0.032 | 0.17 |
| 84,852-15-3 | Para- nonylphenol |
1000 | 21 | 6600 | 0 | 0.15 | 0.15 | 0.15 |
| 7664-41-7 | Ammonia (NH3) |
12,159 | 25 | 1,900,000† | 0 | 0.034 | 0.026 | 0.15 |
| 7440-02-0 | Nickel | 1000 | 25 | 52,000 | 0 | 0.024 | 0.019 | 0.042 |
Concentrations in ng/L. Method is the analytical method. RL is the reporting limit. N is the number of measurements passing quality control. EC is the effect concentration, which is set to the CCC in this table. NExceed is the number of measurements above the EC. Avg HQ is the ratio of the average (across N samples) concentration to the EC. Med HQ is the ratio of median (of N samples) concentration to the EC. Max HQ is the ratio of the maximum measured concentration to the EC.
Pairs of values listed for copper reflect use of Cu CCC Lo vs. Cu CCC Hi (see Table 1).
CCC for pH 7 at 20 °C.
OPP Aquatic Life Benchmarks exist for 9 analytes. RLs are greater than benchmark concentrations for chlorpyrifos (3 × higher) and diazinon (3.5 ×). Neither analyte was ever detected, but given the RLs, HQs up to about three cannot be ruled out with the analytical methods used. HQs for the remaining seven analytes are listed in Table 3. Only copper was observed to exceed the corresponding OPP benchmark (N = 20 samples with measurements greater than the benchmark, out of 25 samples).
Table 3.
HQs calculated using USEPA Office of Pesticide Programs Aquatic Life Benchmarks.
| CAS RN | Analyte | RL | N | EC | N Exceed | Avg HQ | Med HQ | Max HQ |
|---|---|---|---|---|---|---|---|---|
| 7440-50-8 | Copper | 1000 | 25 | 1110 | 20 | 7.6 | 1.8 | 48 |
| 1912-24-9 | Atrazine | 0.25 | 23 | 1000 | 0 | 0.07 | 0.011 | 0.87 |
| 51,218-45-2 | Metolachlor | 49 | 25 | 1000 | 0 | 0.056 | 0.049 | 0.13 |
| 314-40-9 | Bromacil | 370 | 23 | 6800 | 0 | 0.054 | 0.054 | 0.054 |
| 57,837-19-1 | Metalaxyl | 270 | 25 | 100,000 | 0 | 0.003 | 0.003 | 0.003 |
| 1610-18-0 | Prometon | 63 | 25 | 98,000 | 0 | 0.0006 | 0.0006 | 0.0006 |
| 51-03-6 | Piperonylbutoxide | 3.2 | 25 | 30,000 | 0 | 0.0001 | 0.0001 | 0.0001 |
See Table 2 for key.
3.3. Hormones and pharmaceuticals
Aquatic life predicted no-effect concentrations (PNECs) have been estimated for the steroidal estrogens estrone (E1), estradiol (E2), estriol (E3), and ethinyl estradiol (EE2) as 6, 2, 60, and 0.1 ng/L, respectively (Caldwell et al., 2012). RLs for E1, E2, E3, and EE2 in this study were 0.092, 0.1, 0.092, and 0.43 ng/L, respectively. Only E1 was ever detected in our samples, with a maximum concentration of 0.29 ng/L. HQs appear to be well below one for E1, E2, and E3. Although EE2 was not detected, the EE2 RL is above its PNEC. Therefore, an EE2 HQ up to about four cannot be ruled out with the analytical method employed.
Ninety-two of our analytes were pharmaceuticals. Little is known about the ecotoxicology of most pharmaceuticals, especially the relative sensitivity of different species to each pharmaceutical. Therapeutic plasma concentrations of pharmaceuticals have been suggested (Länge and Dietrich, 2002, Huggett et al., 2003, Kostich et al., 2014) as a conservative, although crude estimator of concentrations of pharmaceuticals ECs. Although this approach has not yet been accepted by the regulatory community for performing formal risk assessments, available evidence has corroborated using this approach for screening-level EC estimation in aquatic life (Kostich et al., 2014). We adjusted therapeutic plasma concentrations to account for bioaccumulation estimates, resulting in more conservative EC estimates. RLs were greater than predicted ECs for: tamoxifen (RL 9240 × greater), raloxifene (6806 ×), atorvastatin (128 ×), desmethyl sertraline (9.99 ×), simvastatin (4.9 ×), duloxetine (3.85 ×), sertraline (2.77 ×), and ezetimibe (2.54 ×). For all the detected pharmaceutical analytes, HQs calculated using the adjusted maximum therapeutic plasma concentrations are shown in Table 4. None of the HQs are greater than one, although three (norverapamil, progesterone, and amitriptyline) exceed 0.1, and an additional four (testosterone, venlafaxine, verapamil, and diphenhydramine) exceed 0.01, which suggests there is a wide margin of safety for most pharmaceuticals examined.
Table 4.
HQs calculated using pharmaceutical therapeutic plasma concentrations, adjusted for estimate of bioaccumulation potential.
| CAS RN | Analytes | RL | N | EC | N Exceed | Mean HQ | Median HQ | Max HQ |
|---|---|---|---|---|---|---|---|---|
| 67,018-85-3 | Norverapamil | 8.5 | 2 | 112 | 0 | 0.26 | 0.26 | 0.42 |
| 57-83-0 | Progesterone | 0.13 | 24 | 0.43 | 0 | 0.30 | 0.30 | 0.35 |
| 50-48-6 | Amitriptyline | 4 | 25 | 109 | 0 | 0.040 | 0.037 | 0.11 |
| 58-22-0 | Testosterone | 0.1 | 24 | 2.64 | 0 | 0.039 | 0.038 | 0.057 |
| 93,413-69-5 | Venlafaxine | 0.3 | 24 | 18,572 | 0 | 0.0014 | < 0.0001 | 0.028 |
| 52-53-9 | Verapamil | 7.8 | 24 | 2094 | 0 | 0.0045 | 0.0037 | 0.022 |
| 58-73-1 | Diphenhydramine | 6.9 | 25 | 584 | 0 | 0.012 | 0.012 | 0.018 |
| 137,862-53-4 | Valsartan | 7.2 | 22 | 14,419 | 0 | 0.0008 | 0.0005 | 0.0055 |
| 83,799-24-0 | Fexofenadine | 25 | 25 | 33,432 | 0 | 0.0010 | 0.0007 | 0.0049 |
| 54-31-9 | Furosemide | 14 | 25 | 9548 | 0 | 0.0015 | 0.0015 | 0.0018 |
| 125-29-1 | Hydrocodone | 4.1 | 25 | 5049 | 0 | 0.0008 | 0.0008 | 0.0016 |
| 58-93-5 | Hydrochlorthiazide | 10 | 25 | 41,825 | 0 | 0.0004 | 0.0002 | 0.0016 |
| 93,413-62-8 | Desvenlafaxine | 15 | 25 | 39,673 | 0 | 0.0004 | 0.0004 | 0.0015 |
| 85,100-17-0 | Diltiazem- desmethyl |
4.4 | 15 | 11,730 | 0 | 0.0004 | 0.0004 | 0.0005 |
| 42,399-41-7 | Diltiazem | 6.8 | 21 | 38,115 | 0 | 0.0002 | 0.0002 | 0.0004 |
| 27,203-92-5 | Tramadol | 8.7 | 23 | 89,002 | 0 | 0.0001 | 0.0001 | 0.0003 |
| 51,384-51-1 | Metoprolol | 4.7 | 17 | 131,208 | 0 | 0.0001 | < 0.0001 | 0.0003 |
| 29,122-68-7 | Atenolol | 23 | 23 | 578,318 | 0 | < 0.0001 | < 0.0001 | 0.0001 |
| 298-46-4 | Carbamazepine | 7.1 | 25 | 275,437 | 0 | < 0.0001 | < 0.0001 | 0.0001 |
See Table 2 for key.
Five antibiotic analytes were measured in the study: erythromycin, sulfadimethoxine, sulfamethizole, sulfamethoxazole, and trimethoprim. Since antibiotics are designed to be selectively toxic to certain prokaryotes, we calculated an HQ for each antibiotic by dividing the lowest MIC we found for any microbe by the sample concentration. Antibiotics were measured using methods with reporting limits at least three orders of magnitude lower than the corresponding MIC. Nevertheless, only sulfamethoxazole and trimethoprim were ever detected, with HQs reaching 0.001 and 0.012 respectively.
3.4. ECOSAR measured toxicity
ECOSAR publishes a database of curated toxicity values from the literature that were used to train the ECOSAR QSAR toxicity prediction algorithm, including toxicity values for 32 of our analytes. We adjusted ECs from this database to account for bioaccumulation estimates. Three analytes have RLs higher than adjusted ECs: chlorpyrifos (90 × higher), diazinon (3.4 ×), and 4-tert-octylphenol (7.5 ×). None of these compounds was ever detected. For the six analytes that were detected, HQs are listed in Table 5. All HQs are below one, with the highest HQs associated with 3,4,4′-trichloro carbanilide (also known as triclocarban; maximum HQ = 0.72), metolachlor (0.25), and atrazine (0.036).
Table 5.
HQs calculated using ECOSAR experimental toxicity values, adjusted for estimated bioaccumulation potential.
| CAS RN | Analyte | RL | N | EC | NExceed | Mean HQ |
Median HQ |
Max HQ |
|---|---|---|---|---|---|---|---|---|
| 101-20-2 | 3,4,4-Trichloro carbanalide |
1 | 15 | 4.0 | 0 | 0.32 | 0.27 | 0.72 |
| 51,218-45-2 | Metolachlor | 49 | 25 | 522 | 0 | 0.11 | 0.094 | 0.25 |
| 1912-24-9 | Atrazine | 22 | 25 | 8936 | 0 | 0.0052 | 0.0025 | 0.036 |
| 78-51-3 | Tri(2-butoxyethyl) phosphate |
410 | 17 | 4,756,755 | 0 | < 0.0001 | < 0.0001 | < 0.0001 |
| 80-05-7 | Bisphenol-A | NA | 1 | 658,953 | 0 | < 0.0001 | < 0.0001 | < 0.0001 |
| 134-62-3 | DEET | 72 | 22 | 67,296,485 | 0 | < 0.0001 | < 0.0001 | < 0.0001 |
See Table 2 for key.
3.5. ECOTOX measured toxicity
The ECOTOX database contained ecotoxicity values from the literature for 147 of our analytes. The ECs we used represent a variety of endpoints, as well as a range of effect levels from EC1 to EC100. This variability reflects choices made by the original authors of the studies abstracted in the ECOTOX database. For each analyte, we selected the lowest EC related to growth, survival, and reproduction, adjusting them to account for bioaccumulation potential, in order to provide more protective EC estimates. Results for compounds with HQs > 0.01 are listed in Table 6. Complete results are listed in the Supporting Information. Maximum HQs based on lowest relevant toxicity values in the ECOTOX database exceed one for twelve analytes, which are described here.
Table 6.
Raw HQs calculated using ECOTOX ECs, listed for analytes where maximum HQ > 0.01, adjusted for estimated bioaccumulation potential.
| AnalyteCas | Analyte | RL | N | EC | NExceed | Mean HQ |
Median HQ |
Max HQ |
|---|---|---|---|---|---|---|---|---|
| 7723-14-0 | Phosphorus | 5000 | 24 | 400 | 20 | 469 | 316 | 1403 |
| 7704-34-9 | Sulfur | 3000 | 25 | 160,000 | 25 | 142 | 83 | 517 |
| 7439-96-5 | Manganese | 1000 | 25 | 4365 | 22 | 33 | 8.2 | 343 |
| 7440-36-0 | Antimony | 3000 | 25 | 100 | 3 | 47 | 49 | 59 |
| 7440-24-6 | Strontium | 1000 | 25 | 32,000 | 22 | 8.1 | 5.5 | 32 |
| 14,265-44-2 | Phosphate (PO4) | 25,000 | 24 | 94,970 | 12 | 1.7 | 1.0 | 5.9 |
| 7440-62-2 | Vanadium | 1000 | 25 | 1210 | 7 | 1.4 | 0.83 | 4.8 |
| 3380-34-5 | Triclosan | 0.68 | 16 | 1 | 2 | 0.99 | 0.68 | 3.5 |
| 7440-61-1 | Uranium | 50 | 22 | 2700 | 4 | 0.46 | 0.044 | 3.3 |
| 7440-70-2 | Calcium | 10,000 | 25 | 52,000,000 | 6 | 0.73 | 0.75 | 2.5 |
| 7439-95-4 | Magnesium | 5000 | 25 | 27,400,000 | 2 | 0.45 | 0.39 | 1.6 |
| 14,797-55-8 | Nitrate (NO3) | 88,536 | 24 | 22,142,860 | 1 | 0.22 | 0.15 | 1.0 |
| 14,866-68-3 | Chlorate (ClO3) | 10,000 | 16 | 80,000 | 0 | 0.19 | 0.13 | 0.88 |
| 101-20-2 | 3,4,4-Trichloro carbanalide |
1.1 | 15 | 5 | 0 | 0.28 | 0.22 | 0.58 |
| 7440-39-3 | Barium | 1000 | 25 | 200,000 | 0 | 0.25 | 0.25 | 0.57 |
| 15,687-27-1 | Ibuprofen | 6.9 | 25 | 31 | 0 | 0.25 | 0.22 | 0.57 |
| 16,984-48-8 | Flouride | NA | 23 | 1,221,649 | 0 | 0.18 | 0.16 | 0.46 |
| 15,541-45-4 | Bromate (BrO3) | 2300 | 22 | 30,000,000 | 0 | 0.020 | < 0.0001 | 0.44 |
| 14,797-65-0 | Nitrite (NO2) | 32,845 | 24 | 460,000 | 0 | 0.12 | 0.071 | 0.40 |
| 7439-93-2 | Lithium | 5000 | 25 | 167,880 | 0 | 0.078 | 0.033 | 0.27 |
| 10,035-10-6 | Bromide | 5000 | 24 | 1,022,270 | 0 | 0.065 | 0.049 | 0.25 |
| 14,808-79-8 | Sulfate (SO4) | NA | 23 | 1,000,000,000 | 23 | 0.039 | 0.024 | 0.23 |
| 7440-31-5 | Tin | 1000 | 25 | 80,000 | 0 | 0.041 | 0.013 | 0.22 |
| 93,413-69-5 | Venlafaxine | 6.2 | 25 | 183 | 0 | 0.041 | 0.034 | 0.14 |
| 51,384-51-1 | Metoprolol | 4.7 | 17 | 457 | 0 | 0.021 | 0.010 | 0.083 |
| 298-46-4 | Carbamazepine | 7.1 | 25 | 453 | 0 | 0.022 | 0.016 | 0.079 |
| 723-46-6 | Sulfamethoxazole | 6.5 | 25 | 2065 | 0 | 0.013 | 0.0031 | 0.078 |
| 1763-23-1 | PFOS | 0.13 | 25 | 1199 | 0 | 0.0036 | 0.0012 | 0.040 |
| 80-05-7 | BPA | NA | 1 | 1318 | 0 | 0.022 | 0.022 | 0.022 |
| 335-67-1 | PFOA | 0.56 | 25 | 6138 | 0 | 0.0017 | 0.00056 | 0.018 |
See Table 2 for key. Please see discussion of highest HQs in Section 3.5, for important caveats.
Phosphorus (median sample concentration 99,000 ng/L; maximum 561,000 ng/L) had the highest maximum HQ (1400), based on a LOEC for reduced hatching of Pimephales promelas eggs at 400 ng/L phosphorus (Bentley et al., 1978). Fletcher et al. (1970) report a brook trout larval lethality LOEC of 500 ng/L; Maddock and Taylor (1976) report an LC50 in cod of 14,400 ng/L (corresponding to a maximum HQ of 39). However, these results are for elemental phosphorus toxicity, while the phosphorus we measured with our destructive analytical methods (our elemental analysis dissociates analyte molecules into individual atoms prior to element detection, so information about the chemical structure containing the element is lost) more likely represents much more common, and far less toxic, phosphorus-containing substances such as phosphate ions (Hem, 1985).
Sulfur (median 13,330,000 ng/L; max 82,720,000 ng/L) had a very high maximum HQ (517), which was based on an LC50 for elemental sulfur reported for Tetrahymena pyriformis of 160,000 ng/L (Toth and Tomasovicova, 1979). The next highest EC for elemental sulfur is more than one hundred-fold lower: Nishiuchi and Asano (1979) report an LC50 for mayfliesof 40,000,000 ng/L (corresponding maximum HQ is 2.068). The Pesticide Ecotoxicity Database (USEPA, 2013c) reports a sulfur LC50 in trout of 100,000,000 ng/L (maximum HQ 0.83) and bluegill of 180,000,000 ng/L (maximum HQ 0.46). However, most sulfur in surface or ground water is expected to be in the form of naturally occurring sulfate (Hem, 1985), rather than elemental sulfur, but the different forms cannot be distinguished using the destructive analytical method employed in this study. The lowest ECs for sulfate listed in ECOTOX are about 1 g/L, which translates into an HQ of about 0.25 if all the sulfur was in the form of sulfate.
Manganese (median 35,800 ng/L; max 1,497,000 ng/L) was associated with a very high maximum HQ (343), based on an EC50 for increased time to pupation and emergence of mosquito larvae at 4365 ng/L (Suzuki, 1959). The next highest effect concentration is more than one hundred fold higher: Vedamanikam and Shazilli (2008) report an LC50 of 1,658,914 ng/L in midge larvae, which corresponds to a much lower, but still relatively high, maximum HQ of about 0.9. This broad distribution of toxicity values suggests the need for additional toxicological investigation, particularly in arthropods.
Although antimony (median value was a non-detect [< 3000 ng/L]; max 5900 ng/L) was only detected three times, all detects were well above (maximum HQ 59) reported ECs (100 ng/L for LC50 in shrimp—Amiard, 1976). However, the next lowest EC (10,100 ng/L LC50 for Daphnia magna—Kimball, 1978), corresponds to an HQ of 0.58. This suggests that antimony may occasionally occur at levels near or above potential ECs for arthropods, and invites further characterization of the sensitivity distribution in arthropods and closely related taxa.
Strontium (median 176,800 ng/L; max 1,014,000 ng/L) had a maximum HQ of 32, based on a reported lower confidence bound for an LC10 for trout embryos and larvae of 32,000 ng/L (Black et al., 1980). The point estimate LC10 was 49,000 ng/L, still well below the median strontium concentration of 176,800 ng/L. Birge et al. (1981) also report a lower 95% bound for the LC50 in trout as 100,000 ng/L (point estimate 200,000 ng/L), which is also near the median strontium concentration. Therefore, it appears likely that strontium concentrations at or above ECs in a variety of fish may be common.
Phosphate (median 99,000 ng/L; max 561,000 ng/L) had a maximum HQ of 5.9 based on an LOEC of 94,970 ng/L for negative effects on growth in unidentified Microcystis species (Saxton et al., 2011) grown in mixed cultures. However, effects were small and inconsistent, perhaps reflecting nutrient effects of phosphate in promoting growth of competing species in phosphate-limited water. The next highest ECs (LC50 in Gambusia affinis—Wallen et al., 1957) corresponded to an HQ below 0.001.
Vanadium (median value was a non-detect [< 1000 ng/L], max 5800 ng/L) had a maximum HQ of 4.8 based on a reported LC50 for P. promelas of 1210 ng/L (Kimball, 1978). However, LC50s reported for other fish are substantially higher, with Birge et al. (1981) giving a lower bound estimate of the LC50 in trout of 70,000 ng/L (corresponding to a maximum HQ of 0.08), and Knudtson (1979) reporting a lower bound LC50 estimate in Poecilia reticulata of 240,000 ng/L, and in Carassius auratus of 1,730,000 ng/L. This suggests additional concentration response studies, particularly in fish.
Triclosan had a maximum HQ of about 3.5, based on a LOEC for inhibition of biomass growth of unspecified Chlamydomonas species of 15 ng/L, which was then adjusted for bioaccumulation potential to 1 ng/L. All the un-adjusted triclosan ECs below 600 ng/L were measured in plants or cyanobacteria. Since these are all primary producers, adjustment for bioaccumulation may not be justified for triclosan when trying to estimate minimum ECs. If the bioaccumulation adjustment is not made, the maximum HQ for triclosan is about 0.23, suggesting some of our samples contained triclosan concentrations slightly below effect levels for some plants and cyanobacteria.
Uranium (median 120 ng/L; max 8920 ng/L) had a maximum HQ of about 3.3, which was based on a mortality LOEC of 2700 ng/L in Ceriodaphnia dubia (Pickett et al., 1993). Barata et al. (1998) report a lower bound LC50 estimate in D. magna of 4120 ng/L, corresponding to a maximum HQ of 2.17. LC50 in Hyalella azteca corresponding to a maximum HQ 0.52 were reported by Borgmann et al. (2005), and a mortality LOEC in Moinodaphnia macleayicorresponding to a maximum HQ of 0.36 have been reported by Hyne et al. (1993). These observations suggest occurrence levels similar to some arthropod ECs may occur at some sites.
Calcium (median 38,880,000 ng/L; max 128,800,000 ng/L) had a maximum HQ of 2.5, based on an LC50 reported for D. magna of 52,000,000 ng/L (Biesinger and Christensen, 1972). Magnesium (median 10,640,000 ng/L; max 44,570,000 ng/L) had a maximum HQ of 1.63 based on a lower bound estimate of the LC50 in Daphnia hyalina of 27,400,000 ng/L (Baudouin and Scoppa, 1974). Ca++ and Mg++ are typically the two predominant cations in river water, and concentrations similar to those we observed in our samples can occur naturally (Hem, 1985). It is not clear how much of the calcium and magnesium we measured is accounted for by human activity.
Nitrate (median 3,421,916 ng/L; max 22,550,119 ng/L) had a maximum HQ of about one, based on a LOEC for asymmetry in frog limb development at 22,143,000 ng/L (Earl and Whiteman, 2009), statistically significant effects on frog larval activity (Hatch and Blaustein, 2000) at 20,000,000 ng/L, and an estimated tolerance limit of 23,471,000 ng/L for marine polychaetes (Reish, 1970). These data suggest that nitrate may occasionally reach levels comparable to potential ECs in a variety of taxa.
3.6. Structure-based toxicity predictions
In order to provide some basis for evaluation of chemicals with little or no directly measured toxicity data, we resorted to estimating ECs based on chemical structure-based predictive software, even though chemical structure-based predictions of toxicity are not currently very reliable (Reuschenbach et al., 2008). We generated structure-based toxicity predictions for the 188 non-metallic analytes, then adjusted the results to account for estimated bioaccumulation potential.
HQs calculated using the adjusted values for detected analytes are listed in the Supporting Information. HQs are greater than one in at least one sample for six analytes: cholesterol(maximum HQ of 51 million), hexahydrohexamethyl cyclopentabenzopyran (HHCB: maximum HQ = 165), perfluorododecanoic acid (PFDoDA: maximum HQ = 48), perfluoro-n-undecanoic acid (PFUnDA: maximum HQ = 24), tri(2-butoxyethyl) phosphate (maximum HQ = 14), and perfluorodecanoic acid (PFDA: maximum HQ = 8.8). The very high HQ for cholesterol highlights the potentially error-prone nature of this approach, particularly for hydrophobic analytes. Even without the adjustment for bioaccumulation, ECOSAR predicts toxicity of cholesterol concentrations well below 1 ng/L, and bioaccumulation adjustment reduces EC estimates another three orders of magnitude. HQs for HHCB and the perfluorinated compounds drop below 1 without bioaccumulation adjustment; however, tri(2-butoxyethyl) phosphate has an HQ of 5.7 even without adjustment. Only two studies looking at this analyte were found in ECOTOX, but the lowest toxicity value reported (based on only two species examined) would suggest an HQ of 0.0001. The ECOSAR results suggest more detailed toxicological characterization of this analyte.
3.7. Reflections on data limitations
Relatively good evidence of occasional occurrence above ECs was found for copper, aluminum, strontium, lead, uranium, and nitrate. Sparse effect data for manganese, antimony, and vanadium suggest these analytes may occur above ECs, but additional effect data would be desirable to corroborate EC estimates. These conclusions were not affected by bioaccumulation estimates. No organic analyte concentrations were found to exceed EC estimates, but ten analytes had concentrations in excess of 1/10th of their respective EC: triclocarban, norverapamil, progesterone, atrazine, metolachlor, triclosan, para-nonylphenol, ibuprofen, venlafaxine, and amitriptyline, suggesting more detailed characterization of these analytes.
Data on the nationwide distribution of most of our analytes is sparse, and time profiles of analyte concentrations are particularly rare. The present study only provides one-time grab samples from each of 25 sampling locations that are not particularly representative of any definable general class of sites. The relevance of our HQ estimates to environmental protection depend on the assumption that there are many ambient water sites with chemical concentration profiles similar to the sites we sampled, which we expect is true. In addition, almost all of the ECs we used were based on experiments employing sustained exposures to (nominally) constant concentrations of toxicant, while the temporal profile of our analytes could not be determined using our sampling scheme. We can proceed despite this uncertainty if we accept the simplifying assumption that actual concentrations at the sampling site are relatively constant (which is probably not true). In fact, virtually no experimental exposure profile is likely to exactly match any real-world aquatic exposure profile—simplifying assumptions are almost always required to combine occurrence data with ECs.
It is harder to accommodate the shortfall of evidence from which to estimate ECs for many of our analytes. No assumptions or surrogates provide a convincing solution. In species for which toxicological studies for a given analyte have been carried out, typically only a small subset of developmental stages and toxicological endpoints have been tested, and usually only acute exposures have been examined. Multi-generational studies in a variety of species sufficient for reliable estimation of species sensitivity distributions have been conducted for only a tiny proportion of our analytes. Furthermore, some analyte classes such as pharmaceuticals may affect traditional endpoints (e.g. mortality) via mechanisms such as predator avoidance that are less likely to be detected using traditional toxicological assays. These limitations suggest that most of the ECs listed in this study should be considered very preliminary. The variations in data shortcomings for different analytes also make assignment of safety factors to systematically account for data gaps very challenging. For example, it is not clear what factor to apply to estimates based on destructive elemental analysis, where exact identity of the analyte is unknown; or what factors would make, e.g. HQs based on LC50s in larvae comparable with HQs based on EC10s for effects on adult growth. In addition, the lack of EC data for many of our analytes, incomparability of available EC types for different analytes (different species, endpoints, durations of exposure, etc.), almost complete lack of directly measured mixture toxicity data for pairs (let alone higher-order mixtures) of the analytes considered here, and lack of a reliable theoretical basis for estimating mixture effects prevented us from attempting an analysis of potential mixture effects.
Despite these limitations, the pattern of analyte occurrence found in our study and the limited toxicological data assembled has identified analytes whose ambient water concentrations appear to frequently exceed well established ECs. This type of observation would suggest the importance of further characterizing the nationwide aquatic occurrence of those analytes and their pathways into the environment. If analytes were frequently detected and there is sparse evidence suggesting that ECs are near or below occurrence concentrations, additional toxicological data to firm up ECs may be more immediately useful than occurrence studies. The limited available effect data may suggest which species to employ in toxicological tests, the durations of exposure to be considered, and the endpoints to be scored. Frequently detected analytes for which no experimental EC data are available may be good candidates for toxicological follow-up based on results from SAR-based estimates of bioaccumulation potential or toxicity. Finally, for analytes which have very little or no effect data and which cannot be discriminated based on predictive toxicological algorithms, high frequency of occurrence may suggest the need for additional experimental toxicological evaluation.
Supplementary Material
Acknowledgments
Acknowledgments
The authors would like to thank all participating drinking water treatment plants for their involvement in the project and for their assistance in collecting the samples.
Appendix A.
Supplementary data
Supporting information is presented as a Microsoft Excel spreadsheet containing the following sheets:
Concentrations: Analyte source water concentrations, tabulated by analyte and drinking water plant.
Cross reference: Cross reference to salts, enantiomers, and other derivatives of analytes.
Physical chemical: Physical–chemical properties of parents, salts, enantiomers, and other derivatives.
Effect concentrations: ECOTOX (uncorrected) and ECOSAR toxicity; EPA benchmarks and CCC.
Epa Cr and Pb: Hardness adjusted CCC for chromium and lead Epa Cu: BLM-based CCC for copper.
Pharmaceuticals: Pharmaceutical effect concentrations. ECOSAR EXP: HQ results using ECOSAR curated experimental ECs not adjusted for bioaccumulation
ECOSAR EXP BAF: HQ results using ECOSAR curated experimental ECs adjusted for bioaccumulation
ECOTOX: ECs and HQs based on uncorrected ECOTOX estimates not adjusted for bioaccumulation.
ECOTOX BAF: ECs and HQs based on uncorrected ECOTOX estimates adjusted for bioaccumulation.
ECOTOX CORRECTED: ECs and HQs based on ECOTOX estimates checked against original literature.
ECOSAR EST HQ: HQs based on ECOSAR predicted ECs not adjusted for bioaccumulation
ECOSAR EST BAF: HQs based on ECOSAR predicted ECs adjusted for bioaccumulation.
Footnotes
Disclaimers
The research described in this article has been funded in part by the U.S. Environmental Protection Agency through Interagency Agreement DW14922330 to the U.S. Geological Survey, and through programmatic support of the USGS’ Toxic Substances HydrologyProgram and the USEPA’s Office of Research and Development, Office of Water, Office of Chemical Safety and Pollution Prevention, and Region 8. Information Collection Rule approval for the Phase II Questionnaire was granted under EPA ICR No. 2346.01, OMB Control No. 2080–0078. This document has been reviewed in accordance with USEPA and USGS policy and approved for publication. Approval does not signify that the contents reflect the views of the USEPA and mention of trade names or commercial products does not constitute endorsement or recommendation for use by USEPA. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
References
- Amiard JC, 1976. Experimental study of acute toxicity of salts of cobalt, antimony, strontium and silver crustaceans and their larvae and in some Teleosts (in French). Rev. Int. Oceanogr. Med 43, 79–95 (1976). [Google Scholar]
- Arnot JA, Gobas FA, 2003. A generic QSAR for assessing the bioaccumulation potential of organic chemicals in aquatic food webs. QSAR Comb. Sci 22 (3), 337–345. [Google Scholar]
- Nishiuchi Y, Asano K, 1979. Toxicity of agricultural chemicals to some freshwater organisms 59. Suisan Zoshoku 27 (1), 48–55. [Google Scholar]
- Barata C, Baird DJ,Markich SJ, 1998. Influence of genetic and environmental factors on the tolerance of Daphnia magna Straus to essential and non-essential metals. Aquat. Toxicol 42 (2), 115–137. [Google Scholar]
- Baudouin MF, Scoppa P, 1974. Acute toxicity of various metals to freshwater zooplankton. Bull. Environ. Contam. Toxicol 12 (6), 745–751. [DOI] [PubMed] [Google Scholar]
- Benson R, Conerly OD, Sander W, Batt AL, Boone S, Furlong ET, Glassmeyer ST, Kolpin DW, Mash HE, Schenck KM, Simmons JE, 2017. Human health screening and public health significance of contaminants of emerging concern detected in public water supplies. Sci. Total Environ 579, 1643–1648 (in this issue). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley RE, Dean JW, Hollister TA, LeBlanc GA, Sauter S, 1978. Laboratory Evaluation of the Toxicity of Elemental Phosphorus (P4) to Aquatic Organisms U.S. Army Med. Res. Dev. Command, Washington, D.C. [Google Scholar]
- Biesinger KE, Christensen GM, 1972. Effects of various metals on survival, growth, reproduction, and metabolism of Daphnia magna. J. Fish. Res. Board Can 29 (12), 1691–1700. [Google Scholar]
- Birge WJ, Black JA, Ramey BA, 1981. The reproductive toxicology of aquatic contaminants. Hazard Assess. Chem. Curr. Dev 1, 59–115. [Google Scholar]
- Black JA, Westerman AG, Hudson JE, 1980. Aquatic toxicity tests on inorganic elements occurring in oil shale. In: Gale C (Ed.), Oil Shale Symposium: Sampling, Analysis and Quality Assurance EPA-600/9-80-022, U.S. EPA, Cincinnati, OH, pp. 519–534. [Google Scholar]
- Boethling RS, Howard PH,Meylan WM, 2004. Finding and estimating chemical property data for environmental assessment. Environ. Toxicol. Chem 23 (10), 2290–2308. [DOI] [PubMed] [Google Scholar]
- Borgmann U, Couillard Y, Doyle P, Dixon DG, 2005. Toxicity of sixty-three metals and metalloids to Hyalella azteca at two levels of water hardness. Environ. Toxicol. Chem 24 (3), 641–652. [DOI] [PubMed] [Google Scholar]
- Caldwell DJ,Mastrocco F, Anderson PD, Länge R, Sumpter JP, 2012. Predicted-no-effect concentrations for the steroid estrogens estrone, 17β-estradiol, estriol, and 17α-ethinylestradiol. Environ. Toxicol. Chem 31 (6), 1396–1406. [DOI] [PubMed] [Google Scholar]
- Core Team R, 2013. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: (URL http://www.R-project.org/). [Google Scholar]
- Di Toro DM, Allen H, Bergman H, Meyer J, Paquin P, Santore R, 2001. A biotic ligand model of the acute toxicity of metals: I. Technical basis. Environ. Toxicol. Chem 20 (10), 2383–2396. [PubMed] [Google Scholar]
- Earl JE,Whiteman HH, 2009. Effects of pulsed nitrate exposure on amphibian development. Environ. Toxicol. Chem 28 (6), 1331–1337. [DOI] [PubMed] [Google Scholar]
- Fletcher GL, Hoyle RJ, Horne DA, 1970. Yellow phosphorus pollution: its toxicity to seawater-maintained brook trout (Salvelinus fontinalis) and smelt (Osmerus mordax). J. Fish. Board Can 27 (8), 1379–1384. [Google Scholar]
- Glassmeyer ST, Furlong ET, Kolpin DW, Batt AL, Benson R, Boone JS, Conerly O, Donohue MJ, King DN, Kostich MS, Mash HE, Pfaller SL, Schenck KM, Simmons JE, Varughese EA, Vesper SJ, Villegas EN, Wilson VS, 2017. Nationwide reconnaissance of contaminants of emerging concern in source and treated Drinking waters of the United States. Sci. Total Environ 579, 1629–1642 (in this issue). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch AC, Blaustein AR, 2000. Combined effects of UV-B, nitrate, and low pH reduce the survival and activity level of larval cascades frogs (Rana cascadae). Arch. Environ. Contam. Toxicol 39 (4), 494–499. [DOI] [PubMed] [Google Scholar]
- Hem JD, 1985. Study and Interpretation of the Chemical Characteristics of Natural Water (Water Supply Paper 2254) Department of the Interior, US Geological Survey. [Google Scholar]
- Huggett DB, Cook JC, Ericson JF, Williams RT, 2003. A theoretical model for utilizing mammalian pharmacology and safety data to prioritize potential impacts of human pharmaceuticals to fish. Hum. Ecol. Risk. Assess 9 (7), 1789–1799. [Google Scholar]
- HydroQual, Inc., 2007. The Biotic Ligand Model Windows Interface. Version 2.2.1: User’s Guide and Reference Manual HydroQual, Inc., Mahwah, NJ. [Google Scholar]
- Hyne RV, Padovan A, Parry DL, Renaud SM, 1993. Increased fecundity of the cladoceran Moinodaphnia macleayi on a diet supplemented with a green alga, and its use in uranium toxicity tests. Mar. Freshw. Res 44 (3), 389–399. [Google Scholar]
- Kimball GL, 1978. The effects of lesser known metals and one organic to fathead minnows (Pimephales promelas) and Daphnia magna Manuscript, Department of Entomology, Fish and Wildlife, Univ. of Minnesota, Minneapolis. [Google Scholar]
- Knudtson BK, 1979. Acute toxicity of vanadium to two species of freshwater fish. Bull. Environ. Contam. Toxicol 23 (1), 95–99. [DOI] [PubMed] [Google Scholar]
- Kostich MS, Batt AL, Lazorchak JM, 2014. Concentrations of prioritized pharmaceuticals in effluents from 50 large wastewater treatment plants in the US and implications for risk estimation. Environ. Pollut 184, 354–359. [DOI] [PubMed] [Google Scholar]
- Länge R, Dietrich D, 2002. Environmental risk assessment of pharmaceutical drug substances and conceptual considerations. Toxicol. Lett 131 (1–2), 97–104. [DOI] [PubMed] [Google Scholar]
- Maddock BG, Taylor D, 1976. The acute toxicity of dissolved elemental phosphorus to cod (Gadus morhua). Water Res 10 (4), 289–294. [Google Scholar]
- Meylan WM, Howard PH, 2005. Estimating octanol–air partition coefficients with octanol–water partition coefficients and Henry’s law constants. Chemosphere 61 (5), 640–644. [DOI] [PubMed] [Google Scholar]
- Meylan WM, Howard PH, Boethling RS, 1996. Improvedmethod for estimating water solubility from octanol/water partition coefficient. Environ. Toxicol. Chem 15 (2), 100–106. [Google Scholar]
- Pickett JB, Specht WL, Keyes JL, 1993. Acute and Chronic Toxicity of Uranium Compounds to Ceriodaphnia–Daphnia dubia (no. WSRC-RP-92-995) Westinghouse Savannah River Co., Aiken, SC (United States). [Google Scholar]
- Reish DJ, 1970. The effects of varying concentrations of nutrients, chlorinity, and dissolved oxygen on polychaetous annelids. Water Res 4 (11), 721–735. [Google Scholar]
- Reuschenbach P, Silvani M, Dammann M, Warnecke D, Knacker T, 2008. ECOSAR model performance with a large test set of industrial chemicals. Chemosphere 71 (10), 1986–1995. [DOI] [PubMed] [Google Scholar]
- Saxton MA,Morrow EA, Bourbonniere RA,Wilhelm SW, 2011. Glyphosate influence on phytoplankton community structure in Lake Erie. J. Great Lakes Res 37 (4), 683–690. [Google Scholar]
- Suzuki K, 1959. The toxic influence of heavy metal salts upon mosquito larvae. J. Fac. Sci. Hokkaido Univ. Ser. VI. Zool 14 (2), 196–209. [Google Scholar]
- Toth D, Tomasovicova D, 1979. Effect of pesticides on survival of Tetrahymena pyriformis in Danube water. Biol. Ser C Vseobecna biologia
- USEPA, 2012. Methodology Document for The Ecological Structure–Activity Relationship Model (ECOSAR) Class Program United States Environmental Protection Agency, Washington, DC, USA. [Google Scholar]
- USEPA, 2013a. ECOTOX user guide: ECOTOXicology database system. Version 4.0 (URL http:/www.epa.gov/ecotox/).
- USEPA, 2013b. Estimation Programs Interface Suite™ for Microsoft® Windows, v 4.11 United States Environmental Protection Agency, Washington, DC, USA. [Google Scholar]
- USEPA, 2013c. Pesticide ecotoxicity database. Environmental Fate and Effects Division
- U.S. EPA, Washington, D.C: (Accessed 28 March 2014, URL http://www.ipmcenters.org/Ecotox/). [Google Scholar]
- Vedamanikam VJ, Shazilli NAM, 2008. Comparative toxicity of nine metals to two Malaysian aquatic dipterian larvae with reference to temperature variation. Bull. Environ. Contam. Toxicol 80 (6), 516–520. [DOI] [PubMed] [Google Scholar]
- Wallen IE, Greer WC, Lasater R, 1957. Toxicity to “Gambusia affinis” of certain pure chemicals in turbid waters. Sewage Ind. Waste 695–711.
- Weininger D, 1988. SMILES, a chemical language and information system. 1. Introduction to methodology and encoding rules. J. Chem. Inf. Comput. Sci 28 (1), 31–36. [Google Scholar]
- Williams PP, 1978. In vitro susceptibility of Mycoplasma hyopneumoniae and Mycoplasma hyorhinis to fifty-one antimicrobial agents. Antimicrob. Agents Chemother 14 (2), 210–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


