Abstract
Single-molecule force spectroscopy and modeling have revealed that the adhesion molecule vinculin and F-actin form a catch bond that is dependent on the direction of forces along the actin filament. This may underlie the mechanisms by which cells sense directional physical cues.
In the past decade, advances in mass spectrometry, structural biology, force spectroscopy and imaging tools have contributed to great progress in understanding how cells feel and respond to strain, shear stress, and extracellular matrix stiffness in a process termed cellular mechano-transduction. However, it is well established that cells sense not only the magnitude, but also the direction of physical cues, by mechanisms that remain mysterious: flow-mediated shear stress on endothelia induces inflammatory or atheroprotective signaling depending on the flow direction [1]; left–right asymmetry of vertebrates is established by directional fluid flow in the ventral node of developing embryos [2]; several cell and tissue types re-orient their cytoskeletons and polarize relative to the direction of applied strain or shear stress [3]; and cell migration up extracellular matrix stiffness gradients is thought to mediate development and cancer metastasis [4]. In a recent study, Dunn and colleagues [5] provide important new insight into the molecular-scale basis of the cellular response to directional physical cues by showing differential bond dynamics and strength between two critical mechanotransduction proteins, actin and vinculin, depending on the direction of applied force.
Many cellular responses to physical cues are mediated by interactions between transmembrane integrins and their extracellular ligands [6]. Integrins transmit mechanical information across the cell membrane via a series of protein-protein interactions between the extracellular ligand and the actin cytoskeleton. Transmission of mechanical cues by integrins is transduced into cytoskeletal and adhesion remodeling, tuning cellular adhesion strength to counter mechanical perturbations and coordinate intracellular signaling pathways.
The molecular basis of force-induced adhesion strengthening via integrins has been attributed to either force-dependent recruitment of additional adhesion proteins (i.e. increased avidity) or force-mediated increase in bond strength and lifetime between individual proteins (i.e. increased affinity) [7]. Although mechanical regulation of avidity and affinity is most often considered in the context of integrin clustering and activation, similar principles also apply to other adhesion proteins.
A well-studied example of force-induced avidity changes is the strengthening of the integrin-actin connection via force-mediated increase in the number of talin-vinculin-actin interactions. Talin mediates a relatively weak link between integrin and actin by binding both proteins simultaneously [8]. When force is applied across this link, talin unfolds, revealing several binding sites for the actin-binding protein vinculin [9]. The integrin-actin linkage is thus thought to be strengthened by increasing the number of talin-actin connections through the recruitment of multiple vinculins. A well-characterized example of force-induced affinity increase is the integrin-ligand catch bond [10]. Force applied to activated, ligand-bound integrins has been shown to increase bond lifetime by nearly an order of magnitude across a range of different integrin family members. The structural basis of integrin-ligand catch bonding through force-regulated allostery has also begun to be elucidated [11,12]. However, in spite of these mechanistic insights into force-mediated strengthening of integrin-based adhesion, the regulation of adhesion protein avidity or affinity by force has never been shown to be directionally sensitive, and thus our understanding of many important direction-dependent mechanosensitive processes in biology has remained stunted.
In their recent paper, Dunn and colleagues [5] used a single-molecule, dual optical tweezers based technique to show that vinculin-actin binding is enhanced by force in a direction-dependent manner, providing the first demonstration of a directionally sensitive catch bond. In their assay, an actin filament suspended between beads held in calibrated optical traps is moved across immobilized vinculin molecules. This results in vinculin-actin binding-unbinding events that can be subjected to precise loads and measured with high resolution. The authors observed the canonical characteristics of catch bonding between vinculin and actin, with bond lifetime increasing as the force on the actin filament was increased. However, this was only seen in about half the interactions measured. Because the actin filament has an inherent polarity, they hypothesized that the catch bond could be dependent on the direction of force relative to the polarity of the filament. Indeed, by inverting actin orientation by a 180° rotation of single filament-vinculin interactions, they found that applied force increased bond lifetime for only one filament orientation.
To determine the preferred force orientation to activate the catch bond, the authors determined the polarity of actin filaments using myosin VI motors, which generate force toward the ‘minus’ (pointed) end of the filament [13]. They moved the same filament from immobilized myosin VI molecules where polarity was determined to immobilized vinculin molecules and performed measurements of the effects of force on the vinculin-actin interaction. This revealed that the longer bond lifetimes corresponded to force towards the minus end of the actin filament, while shorter lifetimes corresponded to force towards the ‘plus’ (barbed) end. Thus, the inherent polarity of the actin filament mediates the asymmetric vinculin-actin catch bond.
To quantitatively describe the direction-dependent binding states, the authors used a two-state catch bond model [14]. Here, the transition rate between states is proportional to the magnitude of force, the path length and the angle between force and reaction path. This model not only captured the authors’ data well but also predicted that, when vinculin interacts with actin in a weakly bound state, it transitions to the strongly bound state. Additionally, the effect of force on the strongly bound state was to stabilize this bond. Finally, to test whether this asymmetric vinculin-actin catch bond could underlie the mechanisms for directional cell function, the authors used computational modeling of actin dynamics coupling to integrin-talin through vinculin at the leading edge of a cell. Simulated randomly oriented actin filaments interacting with vinculin based on parameters from the two-state model resulted in ‘polarity sorting’ of actin with barbed ends facing the leading edge. This is consistent with the polarity of filaments observed in cells [15] and suggests that the asymmetric vinculin-actin catch bond can result in the establishment of long-range ordering and polarity of the actin cytoskeleton.
This study underscores the importance and significance of molecular-scale interactions in mechanotransduction. However, while the in silico results suggest the vinculin-actin catch bond could provide a mechanism for the directional response of cells, studies in cells and tissues will be required to test the relevant scales of this interaction and its physiological effects. This requires a better understanding of the structural mechanisms for vinculin-actin directional catch bonding to design separation-of-function mutations that specifically perturb this behavior.
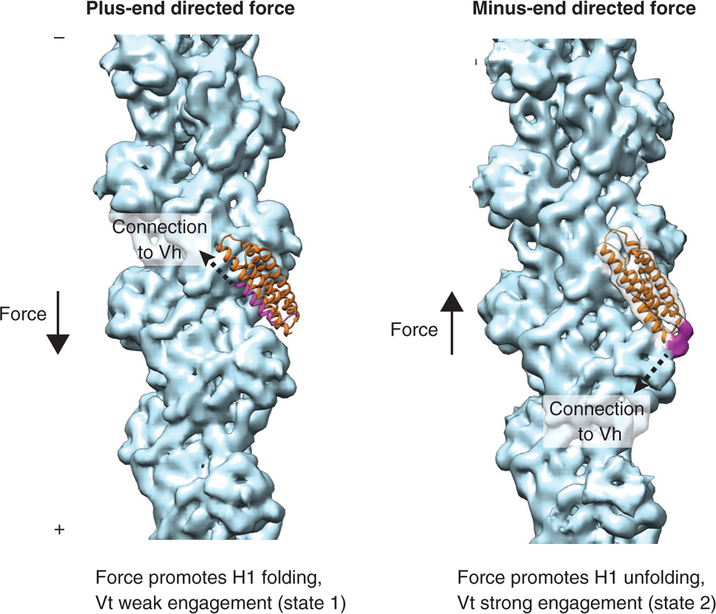
The authors report that the minimal actin-binding vinculin tail domain (Vt) retains directional catch bond activity, suggesting that hints could be drawn from a recent medium-resolution cryo-EM structure of the interface between this domain and actin [16]. Vt is a five-helix bundle [17] of which one helix, H1 (Figure 1, left, magenta), was found to unfold in the context of the Vt-actin complex, licensing structural rearrangements in the bundle to facilitate its actin binding and also forming a small additional interface on the filament surface (Figure 1, right, magenta). It is tempting to speculate that the state visualized by cryo-EM represents the strong binding state (state 2) observed by Dunn and colleagues [5]. Here (Figure 1, right), the connection between the talin-binding vinculin head domain (Vh) and Vt (dotted arrow) is directed toward the plus end of the actin filament, and a minus-end-directed force would pull H1 away from the bundle, reinforcing the unfolded state and a strong Vt-actin interaction. However, a plus-end-directed force (Figure 1, left) would pull H1 towards the bundle, tilting the equilibrium towards refolding and a weak Vt-actin interaction, putatively corresponding to state 1. As force-regulated protein unfolding has been reported to promote other protein-protein interactions that strengthen adhesion, notably the talin–vinculin interface [9], this could emerge as a general mechanistic theme.
Figure 1. Speculative mechanistic model of the two-state vinculin-actin catch bond.
Solid arrows represent force vectors, dotted arrows represent the approximate orientation of the connection between vinculin head (Vh) and vinculin tail (Vt) domains. Left: state 1, the weak binding state. Residues 896–1047 of the crystal structure of Vt in the absence of actin (PDB 1QKR) are displayed in ribbon representation, superimposed on the cryo-EM structure of the Vt-actin complex (not shown). H1 is magenta; H2–H4 are orange. A cryo-EM reconstruction of naked F-actin (EMD 6448) is displayed in light blue. Right: state 2, the strong binding state. Vt from the cryo-EM structure of the Vt-F-actin complex (PDB 3JBI) is displayed in orange ribbon representation. The cryo-EM density map of the complex (EMD 6446) is displayed with the following coloring: Vt helices 1–4, transparent grey; actin, light blue; density corresponding to unfolded H1, magenta.
The above model represents a case in which mechanical information is encoded by structural rearrangements in protein-protein interactions altering bond behavior. Another mechanism for encoding directional information could be through molecular ordering in adhesion clusters. Here, activation and ordering of molecules could be sensitive to force direction altering the overall geometry of interactions. Such molecular ordering in adhesions has been shown for talin and, more recently, for integrins [18–20]. Orientation of molecules could sterically limit protein-protein interactions, which in turn would alter the relative orientations between forces and sites of interactions and thus affect bond behavior.
Vinculin is also a critical component of cell–cell junctions, and mechanisms discovered for integrin-based adhesions could additionally apply to responses to mechanical cues in multicellular contexts, including development and wound healing. The field can also look forward to mechanistic investigations beyond the context of vinculin or integrin-based adhesions, given that diverse cell–cell and cell–environment interactions are mediated by catch bonds, ranging from the immune system to bacterial adhesion systems [14].
REFERENCES
- 1.Wang C, Baker BM, Chen CS, and Schwartz MA (2013). Endothelial cell sensing of flow direction. Arterioscler. Thromb. Vasc. Biol 33, 2130–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirokawa N, Tanaka Y, Okada Y, and Takeda S (2006). Nodal flow and the generation of left-right asymmetry. Cell 125, 33–45. [DOI] [PubMed] [Google Scholar]
- 3.Livne A, Bouchbinder E, and Geiger B (2014). Cell reorientation under cyclic stretching. Nat. Commun 5, 3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charras G, and Sahai E (2014). Physical influences of the extracellular environment on cell migration. Nat. Rev. Mol. Cell Biol. 15, 813–824. [DOI] [PubMed] [Google Scholar]
- 5.Huang DL, Bax NA, Buckley CD, Weis WI, and Dunn AR (2017). Vinculin forms a directionally asymmetric catch bond with F-actin. Science 357, 703–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hynes RO (2002). Integrins: Bidirectional, allosteric signaling machines. Cell 110, 673–687. [DOI] [PubMed] [Google Scholar]
- 7.Moore SW, Roca-Cusachs P, and Sheetz MP (2010). Stretchy proteins on stretchy substrates: the important elements of integrin-mediated rigidity sensing. Dev. Cell 19, 194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang G, Giannone G, Critchley DR, Fukumoto E, and Sheetz MP (2003). Twopiconewton slip bond between fibronectin and the cytoskeleton depends on talin. Nature 424, 334–337. [DOI] [PubMed] [Google Scholar]
- 9.del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, and Sheetz MP (2009). Stretching single talin rod molecules activates vinculin binding. Science 323, 638–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kong F, García AJ, a. P. Mould, M.J. Humphries, and C. Zhu (2009). Demonstration of catch bonds between an integrin and its ligand. J. Cell Biol 185, 1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puklin-Faucher E, Gao M, Schulten K, and Vogel V (2006). How the headpiece hinge angle is opened: new insights into the dynamics of integrin activation. J. Cell Biol 175, 349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao T, Takagi J, Coller BS, Wang J-H, and Springer TA (2004). Structural basis for allostery in integrins and binding to fibrinogenmimetic therapeutics. Nature 432, 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spudich JA, and Sivaramakrishnan S (2010). Myosin VI: an innovative motor that challenged the swinging lever arm hypothesis. Nat. Rev. Mol. Cell Biol 11, 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas WE, Vogel V, and Sokurenko E (2008). Biophysics of catch bonds. Annu. Rev. Biophys 37, 399–416. [DOI] [PubMed] [Google Scholar]
- 15.Small JV, Isenberg G, and Cellis JE (1978). Polarity of actin at the leading edge of cultured cells. Nature 272, 638–639. [DOI] [PubMed] [Google Scholar]
- 16.Kim LY, Thompson PM, Lee HT, Pershad M, Campbell SL, and Alushin GM (2016). The structural basis of actin organization by vinculin and metavinculin. J. Mol. Biol 428, 10–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bakolitsa C, Cohen DM, Bankston LA, Bobkov AA, Cadwell GW, Jennings L, Critchley DR, Craig SW, and Liddington RC (2004). Structural basis for vinculin activation at sites of cell adhesion. Nature 430, 583–586. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Wang Y, Goh WI, Goh H, Baird MA, Ruehland S, Teo S, Bate N, Critchley DR, Davidson MW, et al. (2015). Talin determines the nanoscale architecture of focal adhesions. Proc. Natl. Acad. Sci. USA 112, E4864–E4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swaminathan V, Kalappurakkal JM, Mehta SB, Nordenfelt P, Moore TI, Nobuyasu K, Baker DA, Oldenbourg R, Tani T, Mayor S, et al. (2016). Actin retrograde flow actively aligns and orients ligand-engaged integrins in focal adhesions. bioRxiv, 10.1101/071852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nordenfelt P, Moore TI, Mehta SB, Kalappurakkal JM, Swaminathan V, Koga N, Lambert TJ, Baker D, Waters JC, Oldenbourg R, et al. (2016). Direction of actin flow dictates integrin LFA-1 orientation during leukocyte migration. bioRxiv, 10.1101/071936. [DOI] [PMC free article] [PubMed] [Google Scholar]