Abstract
The substantial morbidity and mortality caused by invasive fungal pathogens, including Cryptococcus neoformans, necessitates increased understanding of protective immune responses against these infections. Our previous work using murine models of cryptococcal lung infection demonstrated that dendritic cells (DCs) orchestrate critical transitions from innate to adaptive immunity and that IL-10 signaling blockade improves fungal clearance. To further understand interrelationships between IL-10 production, fungal clearance, and the effect of IL-10 on lung DCs we performed a comparative temporal analysis of cryptococcal lung infection in wild type C57BL/6J mice (designated IL-10+/+) and IL-10−/− mice inoculated intratracheally with Cryptococcus neoformans (strain 52D). Early and sustained IL-10 production by lung leukocytes was associated with persistent infection in IL-10+/+ mice, whereas fungal clearance was improved in IL-10−/− mice during the late adaptive phase of infection. Numbers of monocyte-derived DCs, T cells, and alveolar and exudate macrophages were increased in lungs of IL-10−/− versus IL-10+/+ mice concurrent with evidence of enhanced DC type-1, Th1/Th17 CD4 T cell, and classical macrophage activation. Bone marrow-derived DCs stimulated with cryptococcal mannoproteins, a component of the fungal capsule, upregulated expression of IL-10 and IL-10 receptor, which promoted DC type-2 activation in an autocrine manner. Thus, our findings implicate fungal triggered autocrine IL-10 signaling and DC type-2 activation as important contributors to the development of non-protective immune effector responses which characterize persistent cryptococcal lung infection. Collectively, this study informs and strengthens the rationale for IL-10 signaling blockade as a novel treatment for fungal infections.
Keywords: IL-10, Cryptococcus, lung infection, dendritic cells, rodent
INTRODUCTION
Cryptococcus neoformans (C. neoformans) is a commonly encountered, encapsulated fungus that inhabits numerous environmental niches worldwide (1). Inhalation of C. neoformans causes primary lung infections, which, depending on the virulence of the organism and the host’s immune status, are either: a) successfully cleared; b) evolve into progressive infections characterized by lethal central nervous system dissemination; or c) persist in chronic form (2). Progressive cryptococcal infections constitute the second leading cause of AIDS-related mortality and are the second most common fungal infection in organ transplant patients (2, 3). Persistent cryptococcal lung infections can develop following initial anti-fungal therapy (4), and may result in bronchiectasis and chronic destruction of lung parenchyma (5). Albeit infrequently, clinically significant cryptococcal infections can occur in seemingly immunocompetent hosts suggesting the presence of unidentified immune defects (6, 7). Thus, better understanding of pathogen-host interactions that hinder development of protective immune responses against C. neoformans is critical for designing new immunologically-based therapies.
Studies using murine models have demonstrated that clearance of cryptococci from the lung requires a cell-mediated, adaptive, Th1 and Th17-type immune response (8–10). This protective immune response is associated with classical macrophage activation, which promotes effective intracellular fungal killing (11–14). In contrast, pathogen or host-derived factors promoting a Th2 immune response, characterized by secretion of IL-4, IL-5 and IL-13, induce alternatively-activated macrophages, which are incapable of fungal eradication (15–17).
IL-10, a potent immunosuppressive and anti-inflammatory cytokine, is crucial for maintaining peripheral immunological tolerance and containing inflammation-associated tissue damage (18, 19) as evidenced by studies demonstrating high mortality rates of IL-10 deficient mice due to severe inflammation in response to lipopolysaccharides (LPS) administration (20–22) or infection with certain pathogens (23–25). In contrast, maladaptive early or/and excessive IL-10 secretion can counteract effective host immune responses and lead to persistent or progressive infections (26–28). Current evidence suggests that IL-10 impairs host defenses against C. neoformans. In patients with cryptococcosis, high levels of IL-10 in peripheral blood samples correlate with fungemia, disseminated disease, and early mortality (29–31), and previous work by our research group has shown limited evidence that fungal clearance is impaired in IL-10 deficient mice with cryptococcal lung infection (32). Moreover, we have recently demonstrated that IL-10 signaling blockade, via an antibody targeting the IL-10 receptor, reduces both lung and brain cryptococcal burden without causing adverse effects (33). Anti-IL-10 receptor antibody therapy proved effective when administered either early or late during the course of established cryptococcal infection and appeared to promote Th1 and Th17 immune responses (33).
The objective of the current study was to better delineate the immunomodulatory role of IL-10 in the pathophysiology of cryptococcal lung infection and to further understand the therapeutic efficacy of IL-10 blockade. Our findings reveal that IL-10 production by lung leukocytes impairs fungal clearance during the late adaptive phase of the immune response. IL-10 production correlates with reduced numbers of myeloid and lymphoid effector cells, skewed dendritic cell (DC) activation towards a DC type-2 phenotype, enhanced Th2 and regulatory T cell (Treg) polarization, and augmented alternative/M2 macrophage activation. We further identify in vitro a novel, autocrine IL-10 signaling pathway triggered by C. neoformans capsular mannoproteins (MPs) which may promote non-protective DC type-2 activation. Thus, our findings enhance our understanding of immunoregulatory networks which impair fungal clearance and advance the rationale for IL-10 blockade as a potential therapy for the treatment of fungal lung infections.
MATERIALS AND METHODS
Mice
Wild type (IL-10+/+) C57BL/6J mice were obtained from the Jackson Laboratory (Bar Harbor, ME). IL-10−/− breeding pairs on the C57BL/6J genetic background were purchased from the Jackson Laboratory and bred on site. Mice were housed under specific pathogen-free conditions in the Animal Care Facility at the Ann Arbor Veterans Affairs Health System. All animal studies were approved by the Veterans Affairs Institutional Animal Care and Use Committee. Mice were 6–10 weeks of age at the time of infection.
C. neoformans Culture
C. neoformans strain 52D was obtained from the American Type Culture Collection (catalogue number 24067; Manassas, VA) and grown to a late logarithmic phase (~72 h) at 37°C in Sabouraud dextrose broth (Difco by Becton Dickinson, Sparks, MD) on a shaker. At the end of culture, yeasts were harvested, washed in Phosphate Buffered Saline (PBS; Gibco by Life Technologies, Grand Island, NY) and counted in the presence of Trypan Blue using a hemocytometer. C. neoformans was used for intratracheal inoculation of mice immediately after counting.
Surgical intratracheal inoculation
Mice were anesthetized by intraperitoneal injection of ketamine (100 mg/kg; Fort Dodge Laboratories, Fort Dodge, IA) and xylazine (5 mg/kg; Lloyd Laboratories, Shenandoah, IA). Through a small midline neck incision, the strap muscles were parted and retracted laterally to expose the trachea. Intratracheal inoculation was performed under direct vision using a 30 gauge needle attached to a 1 ml syringe mounted on a repetitive pipette (stepper, Tridak, Brookfield, CT). An inoculum of 104 organisms (30 µL) was injected into the trachea. Skin was closed using cyanoacrylate adhesive.
Tissue collection and processing
Lungs were perfused in situ via the right heart using PBS (~10 ml) until pulmonary vessels became clear. Lung lobes were then harvested, minced and placed in digestion buffer (5 ml/lung) containing 5% complete medium [CM; RPMI medium 1640, fetal bovine serum (5%), penicillin-streptomycin (1%), MEM Non-Essential Amino Acids Solution (1%), and sodium pyruvate (1%); all from Gibco by Life Technologies], deoxyribonuclease I (250 Kunitz units/lung; Sigma-Aldrich, St. Louis, MO) and collagenase type I (0.1%; Gibco by Life Technologies). Lungs were mechanically homogenized twice using a gentleMacs dissociator (Miltenyi Biotec, Auborn, CA), and enzymatically digested between homogenization cycles at 37°C for 35 minutes on a rocker. After erythrocyte lysis by ACK (KD Medical, Columbia, MD), cells were washed and filtered over a 100 µm mesh. Dead cells were removed by centrifugation over a Percoll (Sigma-Aldrich) gradient. Viable, lung-derived cells in each sample were enumerated in the presence of Trypan Blue using a hemocytometer. Each lung was processed and analyzed individually.
Colony Forming Unit (CFU) assay
To quantify fungal burden in the lung, aliquots (10 µl) of lung digests were plated in serial 10-fold dilutions on Sabouraud dextrose agar (BBL by Becton Dickinson) plates in duplicates and incubated at room temperature for 2 days. Colonies were counted under a microscope, and the number of CFU/lung was calculated. All colonies displayed a smooth phenotype.
Histologic evaluation of lung sections
Lungs were inflated in situ with 50% optimal cutting temperature (OCT) compound (Tissue Tek, Sakura Finetek, Torrance, CA) in PBS via the trachea. Lung lobes were then harvested and frozen in OCT. Twenty µm sections were stained using hematoxylin-eosin (H&E) and viewed by light microscopy. Images were acquired using the Digital Microphotography system DFX1200 with ACT-1 software (Nikon Co, Tokyo, Japan).
Generation and pulsing of bone marrow-derived dendritic cells (BM-DCs)
C. neoformans-derived (C. neoformans-) MPs were isolated and purified as previously described (34).
Erythrocyte-depleted bone marrow cells from flushed marrow cavities of femurs and tibias of naïve wild type (IL-10+/+) or IL-10 deficient (IL-10−/−) mice were cultured in 10% CM supplemented with 20 ng/ml granulocyte macrophage colony-stimulating factor (GM-CSF; Pepro Tech, Inc., Rocky Hill, NJ) at a concentration of 1×106 cells/ml at 37ºC and 5% CO2 for 7 days. The culture was replenished with cytokine on day 3. At the end of culture, BM-DCs were harvested, enriched using 14.5% metrizamide (Nyegaard & Co., Oslo, Norway)-CM gradients and washed twice with PBS. BM-DCs [1×106 cells/ml of 10% CM with GM-CSF (20 ng/ml)] were then cultured in the presence of either C. neoformans-MPs (50 µg/ml), LPS (1 μg/ml; List Biological Laboratories, Campbell, California), or vehicle (PBS) at 37ºC and 5% CO2 for 24 hours. In some experiments, either a blocking anti-IL-10 receptor antibody (clone 1B1.3a, BioLegend, San Diego, CA, 20 µg/ml), an isotype-matched control antibody (rat IgG1k, clone RTK207, BioLegend), or recombinant mouse IL-10 (ThermoFisher Scientific, Rockford, IL, 3 ng/ml) were added to C. neoformans-MPs-pulsed BM-DC cultures.
Measurement of cytokine secretion
Lung-derived cells, obtained from mice as described above, were cultured at a concentration of 5×106 cells/ml of 10% CM in 12-well plates for 24 hours at 37ºC and 5% CO2. At the end of culture, supernatants were harvested, spun down to remove cells, and stored at −80°C. IL-10 concentration in supernatants was determined in triplicates using an enzyme-linked immunosorbent assay (ELISA; BD Biosciences, San Jose, CA) following the manufacturer’s protocol.
Supernatants of pulsed BM-DCs were harvested, spun down and stored at −80°C. IL-10 and IL-12p70 concentration were quantified in triplicates using a cytometric bead array (LEGENDplex mouse inflammation panel, BioLegend) according to the manufacturer’s instructions. Data were acquired using an LSR II flow cytometer equipped with FACSdiva software (both from BD Biosciences).
Measurement of gene expression
RNA was extracted from pulsed BM-DCs using TRIzol reagent (Invitrogen by Life Technologies) and DNase treated using the Turbo DNA-free Kit (Ambion by Life Technologies). Transcript levels were quantified using a one-step qRT-PCR (QuantiTect Sybr Green Kit, Qiagen, Valencia, CA) with Gapdh serving as an endogenous reference. Reactions were run in triplicates using a StepOne Plus real-time PCR system (Applied Biosystems). Primers were synthesized by Sigma-Aldrich based on the sequences listed in Supplementary Table I. Primers for il10, il10ra and Arginase 1 (QuantiTect Primer Assays) were obtained from Qiagen. Data were analyzed using the 2-ΔΔCT method.
Cell staining and flow cytometry analysis
Fluorochrome-conjugated antibodies used for cell staining are listed in Supplementary Table II. Cells were first stained with a fixable viability dye (Zombie aqua, BioLegend) following the manufacturer’s protocol. After blocking Fc receptors using anti-CD16/32 antibody (clone 93, BioLegend), cells were stained for cell surface markers and then fixed with 2% formaldehyde (ThermoFisher Scientific) in PBS. Intracellular staining was performed using Intracellular Fixation and Permeabilization or Foxp3/Transcription Factor staining buffer set (both from eBioscience by ThermoFisher Scientific, Waltham, MA) according to the manufacturer’s instructions. To detect intracellular IL-12 production in DCs, lung leukocytes (1×106 cells/ml of 10% CM) were incubated in the presence of brefeldin A and monensin (both at 1µl/ml; BioLegend) at 37ºC and 5% CO2 for 5 hours prior to cell staining. To detect intracellular interferon γ (IFNγ), IL-17A, and IL-13 production in CD4+ T cells, lung leukocytes were stimulated with Cell Activation Cocktail (2 µl/ml of PMA and ionomycin, BioLegend) during incubation with Brefeldin A and monensin. Samples stained with isotype-matched control antibodies were included in all experiments.
Data were acquired using an LSR II or LSRFortessa flow cytometer (both from BD Biosciences) and analyzed using FlowJo software (Treestar, Ashland, Oregon). At least 150,000 events in the CD45+ gate were acquired per lung sample. To determine the number of cells in each population of interest in each sample, the corresponding percentage was multiplied by the total number of viable CD45+ cells in that sample. The latter value was calculated for each sample as the product of the percentage of viable CD45+ cells and the original hemocytometer count of total viable cells identified within that sample.
Statistical analysis
Data were evaluated by unpaired, two-tailed t-test, corrected for multiple comparisons using the Holm-Sidak method in certain experiments, or one-way analysis of variance (ANOVA) followed by Tukey’s test for multiple comparisons. P values < 0.05 were considered statistically significant.
RESULTS
IL-10 promotes persistence of cryptococcal lung infection
To investigate temporal relationships between IL-10 and fungal clearance, we infected wild-type (IL-10+/+) C57BL/6J and IL-10−/− (C57BL/6J genetic background) mice with C. neoformans, strain 52D, via intratracheal inoculation and then quantified IL-10 production by lung leukocytes and fungal lung burden using CFU assays throughout 6 weeks post infection (wpi). Cryptococcal infection in IL-10+/+ mice triggered early and sustained IL-10 production by lung leukocytes (Figure 1A, squares). As expected, no IL-10 production above the detection limit of the assay was observed in samples obtained from IL-10−/− mice. Interestingly, despite early induction of IL-10, fungal lung burden in the two genotypes was similar during the first 3 wpi (Figure 1B). In contrast, whereas cryptococcal burden remained persistently elevated in IL-10+/+ mice, fungal clearance was markedly improved in the lungs of IL-10−/− mice at 4 and 6 wpi.
Figure 1.
IL-10 impairs fungal clearance at the late adoptive phase of the immune response. IL-10+/+ or IL-10−/− mice were infected with C. neoformans via intratracheal inoculation. At the indicated time points post infection, lungs were harvested from cohorts of mice and dispersed into single cell suspensions. (A) IL-10 concentration in supernatants of lung-derived cultured cells was quantified using ELISA. (B) Fungal lung burden was assessed using CFU assays. Zero week post infection (wpi) designates uninfected mice. The dotted line designates the minimal assay detection level. Cumulative data from 2 (A) or 3 (B) independent experiments are presented as mean ± SEM of 4–12 mice. IL-10+/+ mice, black squares (A) or bars (B); IL-10−/− mice, circles (A) or white bars (B). ** P < 0.01, *** P < 0.001 comparing IL-10+/+ at the designated time point to IL-10+/+ at time point 0 (A) or IL-10−/− to IL-10+/+ at the same time point (B); t-test corrected for multiple comparisons using the Holm-Sidak method. (C-F) Representative lung sections of IL-10+/+ (C and E) or IL-10−/− (D and F) mice prior to (C, D) and at 6 wpi (E and F). Sections were stained with H&E and examined by light microscopy at original magnification of X400. Lungs of uninfected IL-10−/− mice (D) appeared similar to wild type controls (C). At 6 wpi, numerous cryptococci and sparse alveolar immune infiltrates were seen in lungs of IL-10+/+ mice (E). Many of these cryptococci were located extracellularly (thin arrows) rather than intracellularly (thick arrows). Lungs of infected IL-10−/− mice (F) displayed extensive dense alveolar immune infiltrates and few cryptococci, most of which appeared to be intracellular.
Histologic examination of lung sections obtained from uninfected IL-10−/− mice disclosed no apparent pathology (Figure 1C and D). At 6 wpi, lungs of IL-10+/+ mice harbored numerous cryptococci, located both extra- and intracellularly, along with sparse alveolar immune infiltrates (Figure 1E). In contrast, lungs of infected IL-10−/− mice displayed extensive, dense immune infiltrates and few cryptococci, most of which appeared to be intracellular (Figure 1F). Thus, IL-10 production by lung leukocytes is strongly associated with persistence of murine cryptococcal lung infection.
IL-10 inhibits accumulation of myeloid effector cells in the lung during cryptococcal infection
In order to precisely examine leukocyte subsets in C. neoformans-infected lungs, we designed an 11-channel antibody panel targeting cell surface markers and developed a new flow cytometry gating strategy (Figure S1) based on modifications of our previous work (35) and others (36). Implementation of this gating strategy allowed us to identify and enumerate the following myeloid subsets of CD45+ lung leukocytes in the lungs of uninfected and infected mice: 1) neutrophils (Ly6G+ CD11b+); 2) eosinophils (Ly6Glow Siglec F+ CD11b+ CD11c−); 3) CD103+ conventional DC (cDC)(Ly6G− Siglec F− CD11c+ CD24+ CD103+ CD11b−); 4) CD11b+ cDC (Ly6G− Siglec F− CD11c+ CD24+ CD103− CD11b+); 5) monocyte-derived DC (mDC)(Ly6G− Siglec F− CD11c+ CD24− MHC Class II+ autofluorescence-); 6) Ly6c+ monocytes (Ly6G− Siglec F− CD11c− Ly6C+ CD11b+); 7) monocyte-derived exudate macrophages (Ly6G− Siglec F− CD11c+ CD24− MHC Class II+ autofluorescence+); and 8) alveolar macrophages (Ly6G− Siglec F+ CD11c+).
Use of this flow cytometry-based approach facilitated our efforts to quantify myeloid cell subsets in the lungs of IL-10+/+ or IL-10−/− mice prior to and at weekly or biweekly intervals post C. neoformans infection for a period of 6 weeks. No significant differences in the total numbers of CD45+ cells, or any myeloid subsets, were identified between the two genotypes of uninfected mice (Figure 2A-I, week 0 post infection). During the first 3 wpi, numbers of CD45+ leukocytes in the lung increased in both genotypes relative to uninfected mice, but remained comparable between IL-10+/+ and IL-10−/− mice (Figure 2A). In contrast, numbers of CD45+ cells were elevated in IL-10−/− versus IL-10+/+ mice at 4 and 6 wpi (Figure 2A). Amongst granulocytes, the number of neutrophils in lungs of infected IL-10−/− mice was increased compared with IL-10+/+ mice at 2, 4 and 6 wpi (Figure 2B), whereas the number of eosinophils were reduced at 2, 3 and 4 wpi (Figure 2C). Comparison of CD103+ and CD11b+ conventional DCs yielded no significant differences between the two genotypes (Figure 2D and E, respectively). Notably, up to ~6.4 fold increase in the number of monocyte-derived DCs was observed in IL-10−/− versus IL-10+/+ mice at 2 and 6 wpi (Figure 2F). At 6 wpi, we also detected an increase in the number of Ly6C+ monocytes, exudate macrophages and alveolar macrophages in lungs of IL-10−/− versus IL-10+/+ mice (Figure 2G-I, respectively). Collectively, these findings show that IL-10 impairs accumulation of key effector myeloid cells in the lung during the late adaptive phase (week 4 to 6) of cryptococcal infection, which directly correlates with the improvement in fungal clearance observed in IL-10−/− mice at these time points post-infection (refer to Figure 1B).
Figure 2.
IL-10 impairs late accumulation of effector myeloid cells in lungs of C. neoformans-infected mice. IL-10+/+ or IL-10−/− mice were infected with C. neoformans via intratracheal inoculation. At the indicated time points post infection, lungs were harvested from cohorts of mice and dispersed into single cell suspensions. Zero wpi designates uninfected mice. Cells were stained and analyzed by flow cytometry as described in Figure S1. The number of CD45+ cells (A), neutrophils (B), eosinophils (C), CD103+ conventional DCs (D), CD11b+ conventional DCs (E), monocyte-derived DCs (F), Ly6C+ monocytes (G), exudate macrophages (H) and alveolar macrophages (I) per mouse lung was calculated. Cumulative data from 3 (A-C) or 2 (D-I) independent experiments are presented as mean ± SEM of 4–12 mice. IL-10+/+ mice, black bars; IL-10−/− mice, white bars. ** P < 0.01, *** P < 0.001; t-test corrected for multiple comparisons using the Holm-Sidak method.
IL-10 promotes lung DC type-2 activation during cryptococcal infection
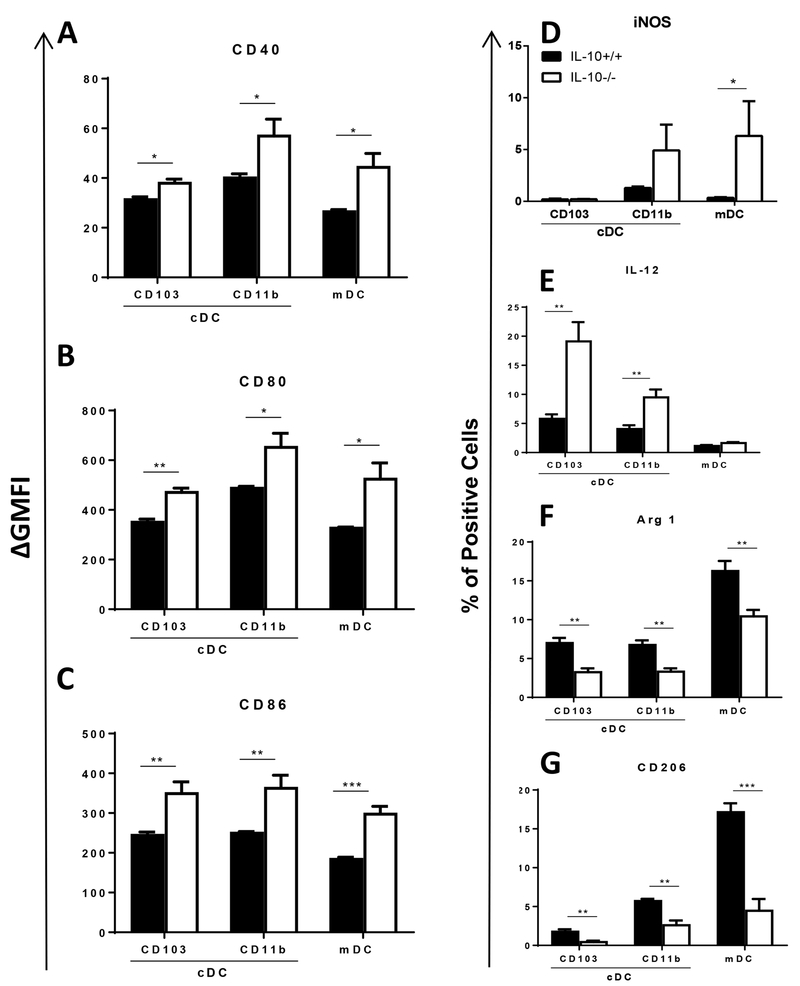
DCs are central to host defenses against cryptococcal lung infection as they orchestrate the critical transition from innate to adaptive immunity (reviewed in (37)). DC type-1 activation promotes protective adaptive immune responses leading to effective fungal clearance, whereas DC type-2 activation is non-protective and promotes persistent or progressive infections. We therefore next investigated the effects of IL-10 on the activation profile of three distinct myeloid DC subsets in the lung throughout the course of C. neoformans infection. Using the gating strategy described above (Figure S1), we evaluated cell surface expression of co-stimulatory molecules (CD40, CD80 and CD86) and intracellular expression of DC type-1 [inducible nitric oxide synthase (iNOS) and IL-12] and DC type-2 (arginase 1 and CD206) markers in three DC subsets (CD103+ conventional DCs, CD11b+ conventional DCs, and monocyte-derived DCs) in lungs of IL-10+/+ or IL-10−/− mice prior to and during cryptococcal lung infection. No statistically significant differences in DC activation phenotype were observed between genotypes in uninfected mice or at 3 wpi (data not shown). However, at 4 wpi, expression of CD40, CD80, and CD86 were increased on all three DC subsets in the lungs of IL-10−/− versus IL-10+/+ mice [Figure S2 (A-C) and Figure 3 (A-C)]; similar differences were identified on DCs obtained at 6 wpi (data not shown). The frequency of iNOS-expressing monocyte-derived DCs, but not conventional DCs, was increased in IL-10−/− versus IL-10+/+ mice (Figure S2D and 3D). In contrast, the frequency of IL-12+ conventional DCs, but not monocyte-derived DCs, was increased in IL-10−/− versus IL-10+/+ mice (Figure S2E and 3E). Whereas IL-10 had inhibitory effects on DC type-1 markers, both DC type-2 markers evaluated (arginase 1 and CD206) were expressed less frequent by lung DCs in IL-10−/− mice (Figure S2F-G and 3F-G) suggesting that IL-10 promotes DC type-2 activation. Altogether, our results show that during the late adaptive phase of cryptococcal infection, IL-10 downregulates DC expression of co-stimulatory molecules and DC type-1 markers while upregulating expression of DC type-2 markers, thereby skewing the polarization of lung DCs towards a phenotype associated with impaired fungal clearance. Interestingly, IL-10 modulated activation of all 3 lung DC subsets even though it decreased accumulation only of monocyte-derived DCs (refer to Figure 2D - F).
Figure 3.
IL-10 downregulates DC surface expression of co-stimulatory molecules and promotes DC type-2 activation in lungs of C. neoformans-infected mice. Lung-derived single cell suspensions harvested 3 (F) or 4 (A-E and G) wpi from IL-10+/+ or IL-10−/− mice were stained and analyzed by flow cytometry to identify CD103+ conventional DCs, CD11b+ conventional DCs and monocyte-derived DCs. (A-C) Cell surface expression of CD40 (A), CD80 (B) and CD86 (C) on these DC subpopulations was assessed by determining the ∆ geometric mean fluorescence intensity (GMFI) of each sample relative to an isotype-matched control antibody-stained sample. (D-G) The percentage of cells expressing iNOS (D), IL-12 (E), arginase 1 (F) and CD206 (G) in the indicated DC subpopulation was assessed using intracellular staining. (A-G) Bar graph data are presented as mean ± SEM of 4–5 mice per group. IL-10+/+ mice, black bars; IL-10−/− mice, white bars. * P < 0.05, ** P < 0.01, *** P < 0.001; t-test corrected for multiple comparisons using the Holm-Sidak method. Similar results were obtained in 2 or 3 independent experiments. cDC, conventional DC; mDC, monocyte-derived DC. cDC, conventional DC; mDC, monocyte-derived DC; Arg 1, arginase 1.
IL-10 inhibits accumulation and skews polarization of lung T cells during cryptococcal infection
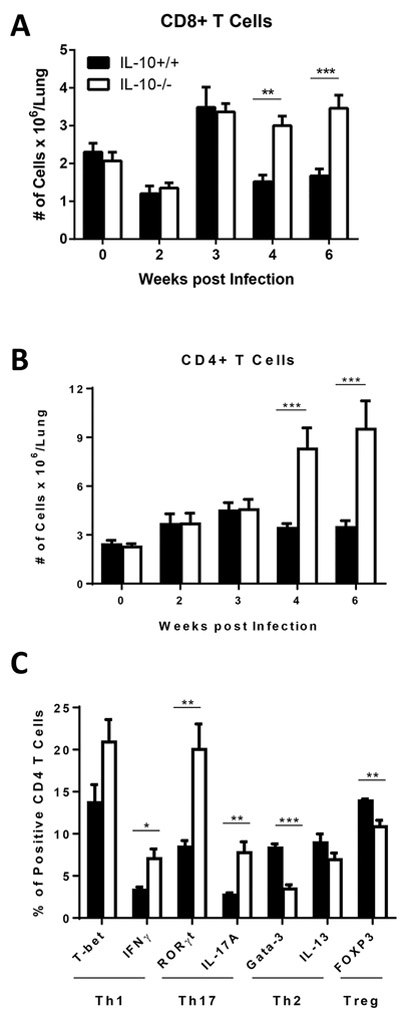
Having identified IL-10 effects on lung DCs, we next examined the effects of IL-10 on lung T cell accumulation and CD4 T cell polarization during the course of C. neoformans infection. We used flow cytometry to identify (Figure S1N and O) and quantify CD8 and CD4 T cells in the lungs of IL-10+/+ or IL-10−/− mice in a manner similar to that described for myeloid cells. Our results demonstrate that the number of CD8 and CD4 T cells in the lung did not differ significantly between the two genotypes in uninfected mice or during early infection at 2 and 3 wpi (Figure 4A and B). However, the number of both T cell populations increased ~2 to ~3 fold in IL-10−/− versus IL-10+/+ mice during late infection at 4 and 6 wpi.
Figure 4.
IL-10 impairs late accumulation of T cells and skews polarization of CD4 T cells in lungs of C. neoformans-infected mice. IL-10+/+ and IL-10−/− mice were infected with C. neoformans via intratracheal inoculation. (A and B) At the indicated time points post infection, lungs were harvested from cohorts of mice and dispersed into single cell suspensions. Zero wpi designates uninfected mice. Cells were stained and analyzed by flow cytometry to identify T cell subsets as described in Figure S1. The number of CD8+ (A) and CD4+ (B) T cells per mouse lung was calculated. Cumulative data are presented as mean ± SEM of 2 (A) or 3 (B) independent experiments, n = 4–12 mice. IL-10+/+ mice, black bars; IL-10−/− mice, white bars. ** P < 0.01, *** P < 0.001; t test corrected for multiple comparisons using the Holm-Sidak method. (C) Lung-derived single cell suspensions harvested 4 wpi from IL-10+/+ or IL-10−/− mice were stained and analyzed by flow cytometry to identify CD4+ T cells. The percentage of CD4+ T cells expressing T-bet, IFNγ, RORγt, IL-17A, Gata-3, IL-13 or FOXP3 was assessed using intracellular staining. Bar graph data are presented as mean ± SEM of 4 mice per group. * P < 0.05, ** P < 0.01, *** P < 0.00; t test.
We further characterized lung CD4 T cells based on their intracellular expression of signature transcription factors and cytokines to identify Th1 (T-bet or IFNγ expressing), Th17 (RORγt or IL-17A expressing), Th2 (Gata-3 or IL-13 expressing) or Treg (FOXP3 expressing) cells (Figure S3 and Figure 4C). In lungs of uninfected mice, we observed minimal evidence of T cell polarization and a small reduction in IFNγ+ CD4 T cells in IL-10−/− relative to IL-10+/+ mice, but no other differences in T cell polarization profiles were identified between the two genotypes (Figure S3B). In contrast, prominent differences in lung CD4 T cell polarization were detected at 2, 3, 4 and 6 wpi between IL-10−/− and IL-10+/+ mice (Figure S3A, C-E and Figure 4C). Specifically, we observed increased Th1 and Th17 polarization and decreased Th2 and Treg polarization in the lungs of IL-10−/− relative to IL-10+/+ mice. Collectively, our findings indicate that IL-10 impairs accumulation of CD4 and CD8 T cells in the lung during the late adaptive phase of cryptococcal infection and promotes a shift in the balance of CD4 T cell polarization in favor of non-protective Th2 immune responses.
IL-10 promotes alternative macrophage activation during cryptococcal infection
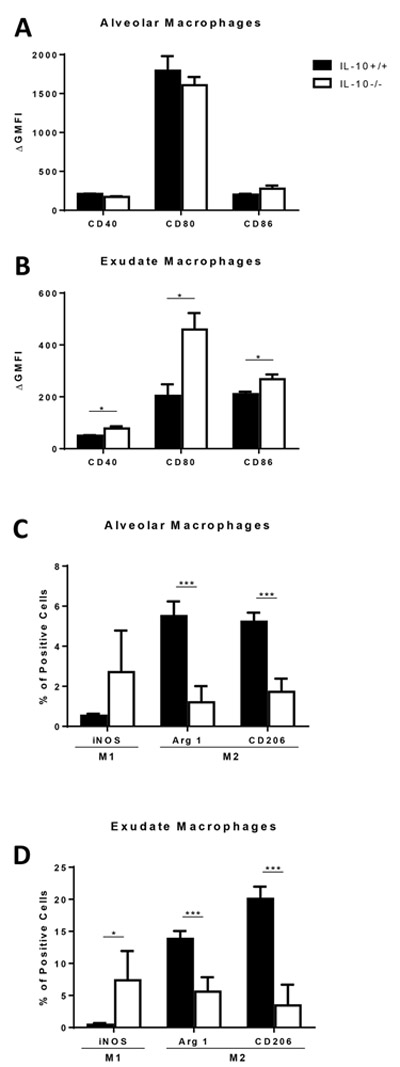
We next sought to evaluate whether these observed effects of IL-10 on T cell polarization were associated with differential macrophage activation in response to cryptococcal lung infection. Prior studies have shown that classically activated, M1-polarized, macrophages are associated with effective fungicidal activity, whereas alternatively activated, M2-polarized, macrophages are linked with intracellular yeast proliferation (9, 13, 38–41). This difference in effector function can be correlated with increased costimulatory molecule expression and upregulated iNOS expression in M1 macrophages versus decreased costimulatory molecule expression and upregulated arginase 1 and CD206 expression in M2 macrophages (42). We therefore assessed pulmonary macrophage cell surface expression of co-stimulatory molecules (CD40, CD80 and CD86) and intracellular expression of iNOS, arginase and CD206 during the course of C. neoformans infection in IL-10+/+ or IL-10−/− mice using the gating strategy described in Figure S1. Expression of all these markers was low or undetected in uninfected mice and did not differ significantly between the two genotypes (data not shown). At 6 wpi, however, CD40, CD80 and CD86 cell surface expression was increased in exudate macrophages, but similar in alveolar macrophages, of IL-10−/− versus IL-10+/+ mice (Figure S4A, B and 5A, B). The frequency of iNOS-expressing exudate macrophages, but not alveolar macrophages, was also significantly increased in IL-10−/− versus IL-10+/+ mice (Figure S4C top row and 5C, D left bars). Arginase 1 and CD206 were expressed less frequent in both alveolar and exudate macrophages of IL-10−/− versus IL-10+/+ mice (Figure S4C middle and bottom row and 5C, D middle and right bars). Similar albeit less prominent differences in macrophage activation profiles were observed at 4 wpi (data not shown). These findings demonstrate that IL-10 skews both lung macrophage populations towards alternative activation during late cryptococcal lung infection. Notably, M1 activation of lung macrophages in IL-10−/− mice temporally coincided with their increased accumulation in the lung (refer to Figure 2H, I) and with effective fungal clearance (refer to Figure 1B).
Figure 5.
IL-10 facilitates alternative activation of macrophages in lungs of C. neoformans-infected mice. Lung-derived single cell suspensions harvested 6 wpi from IL-10+/+ or IL-10−/− mice were stained and analyzed by flow cytometry to identify alveolar and exudate macrophages. (A and B) Cell surface expression of CD40, CD80 and CD86 on alveolar (A) and exudate (B) macrophages was assessed by determining the ∆ geometric mean fluorescence intensity (GMFI) of each sample relative to an isotype-matched control antibody-stained sample. (C and D) The percentage of cells expressing iNOS, arginase 1 and CD206 in the indicated macrophage subpopulations was assessed using intracellular staining. (A-D) Bar graph data are presented as mean ± SEM of 4 or 5 mice per group. IL-10+/+ mice, black bars; IL-10−/− mice, white bars. * P < 0.05, *** P < 0.001; t test. Similar results were obtained in 2 independent experiments. MQ, macrophages; Arg 1, arginase 1.
BM-DCs stimulated with C. neoformans-MPs secrete IL-10 and up-regulate cell surface expression of the IL-10 receptor
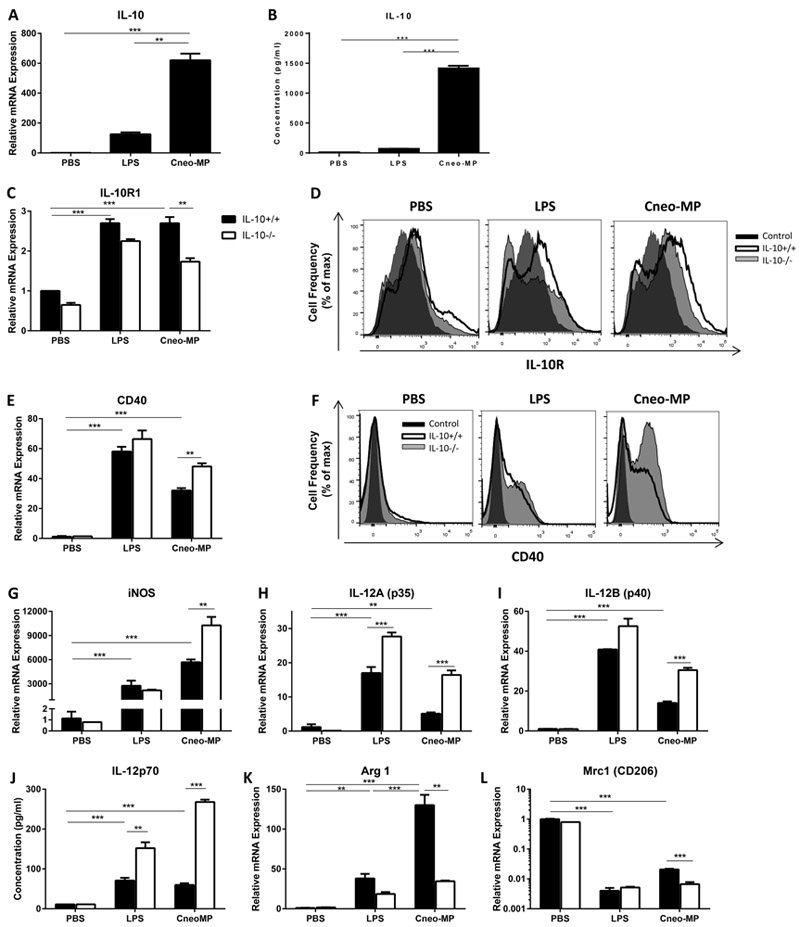
Our data identified IL-10 signaling as a central molecular mechanism impairing numerous effector immune responses against cryptococcal lung infection. Yet, questions pertaining to the trigger and source of IL-10 production, as well as the manner by which IL-10 shapes the nature of adaptive immunity in this model remained unanswered. Given the major role of DCs in eliciting adaptive immunity, we further focused our attention on these cells by assessing their in vitro response to C. neoformans-MPs, an important antigenic component of the fungal capsule known to generate adaptive immunity (43–46). BM-DCs generated from IL-10+/+ (IL-10+/+ BM-DCs) or IL-10−/− (IL-10−/− BM-DCs) mice were pulsed with C. neoformans-MPs for 24 hours and subsequently assessed for IL-10 gene expression by qRT-PCR and for IL-10 protein production by cytometric bead array. Control cultures included BM-DCs pulsed with PBS (vehicle, negative control) and with LPS (positive control for DC type-1 activation). Our results demonstrate that C. neoformans-MPs-pulsed IL-10+/+ BM-DCs up-regulated IL-10 mRNA expression by ~600 fold relative to PBS-pulsed IL-10+/+ BM-DCs (Figure 6A) and concomitantly increased IL-10 protein secretion (Figure 6B). In contrast, LPS-pulsing yielded much lower IL-10 production.
Figure 6.
BM-DCs pulsed with cryptococcal MPs up-regulate expression of IL-10 and IL-10 receptor, which promotes DC type-2 activation. BM-DCs generated from IL-10+/+ (A-L) and IL-10−/− (C-L) mice were pulsed with C. neoformans-MPs, LPS or PBS (vehicle) for 24 hours. (A, C, E, G-I and K-L) Transcript levels of IL-10 (A), IL-10R1 (C), CD40 (E), iNOS (G), IL-12A (H), IL-12B (I), Arginase 1 (K) and Mrc1 (CD206, L) in pulsed BM-DCs were quantified by qRT-PCR analysis. Expression level was normalized to GAPDH and expressed relative to the mean expression of PBS-pulsed IL-10+/+ DCs. (B and J) Concentration of IL-10 (B) and IL-12 (J) in supernatants of pulsed BM-DCs was quantified using a cytometric bead array. (A-C, E, G-L) Data are reported as mean ± SEM of triplicates. ** P < 0.01, *** P< 0.001; one-way ANOVA followed by Tukey’s test for multiple comparisons (A and B), t test corrected for multiple comparisons using the Holm-Sidak method for comparing IL-10−/− to IL-10+/+ using the same pulsing condition or two-way ANOVA followed by Tukey’s test for multiple comparisons for comparing IL-10+/+ DCs pulsed with C. neoformans-MPs or LPS versus PBS. Similar results were obtained in 2 independent experiments. IL-10+/+ BM-DC, black bars; IL-10−/− BM-DC, white bars. (D and F) Pulsed BM-DCs were stained using a fluorochrome-conjugated antibody targeting the IL-10 receptor (D) or CD40 (F) and analyzed by flow cytometry. Histogram overlays show cell surface expression of the IL-10 receptor (D) or CD40 (F). Control antibody staining, black histogram; target antibody staining of IL-10+/+ cells, thick line clear histogram; target antibody staining of IL-10−/− cells, thin line shaded histogram. MP, Mannoprotein ; IL-10R, IL-10 receptor.
We proceeded to evaluate the expression of IL-10 receptor by C. neoformans-MPs-pulsed BM-DCs. The IL-10 receptor is a heterodimer composed of IL-10R1 and IL-10R2 subunits (47). Cellular expression of IL-10R1, the high affinity ligand-binding subunit, is regulated by immune cell activation, whereas expression of IL-10R2, the accessory subunit for signaling, is constitutive and unaltered in response to activation (48). Analysis by qRT-PCR (Figure 6C) and flow cytometry (Figure 6D) showed that pulsing of IL-10+/+ BM-DCs with C. neoformans-MPs increased both IL-10R1 transcript level (Figure 6C, black bars) and IL-10 receptor cell surface expression (Figure 6D, thick line clear histogram) relative to PBS-pulsed BM-DCs. As expected, IL-10R2 transcript level did not change in response to antigen pulsing (data not shown). Interestingly, C. neoformans-MPs-pulsed IL-10−/− BM-DCs expressed less IL-10R1 transcripts (Figure 6C, white bars) and cell surface IL-10 receptor (Figure 6D, thin line shaded histograms) compared with IL-10+/+ BM-DCs. Collectively, these findings indicate that in vitro exposure of BM-DCs to cryptococcal-MPs promotes abundant IL-10 production and further upregulation of its receptor.
Autocrine IL-10 signaling in BM-DCs in response to C. neoformans-MPs stimulation promotes DC type-2 activation
Our final objective was to determine whether autocrine IL-10 signaling in C. neoformans-MPs-pulsed BM-DCs can skew their activation profile towards a DC type-2 phenotype. We therefore first assessed the activation profile of C. neoformans-MPs-pulsed IL-10−/− versus IL-10+/+ BM-DCs by comparing their expression level of co-stimulatory molecules (CD40, CD80 and CD86), DC type-1 (iNOS and IL-12) and DC type-2 (arginase 1 and CD206) markers. Analysis by qRT-PCR demonstrated that C. neoformans-MPs-pulsing of IL-10+/+ BM-DCs increased CD40, CD80 and CD86 transcript level relative to PBS-pulsing (data not shown), however only CD40 transcript level was increased in C. neoformans-MPs-pulsed IL-10−/− versus IL-10+/+ BM-DCs (Figure 6E). Flow cytometric analysis validated these findings on the cell surface at the protein level (Figure 6F). C. neoformans-MPs-pulsing of IL-10+/+ BM-DCs increased iNOS, IL-12A, and IL-12B transcript level relative to PBS-pulsed BM-DCs and the transcript level of all 3 genes was increased in C. neoformans-MPs-pulsed IL-10−/− versus IL-10+/+ BM-DCs (Figure 6G-I). Quantification of IL-12 concentration in supernatants of pulsed BM-DCs using a cytometric bead array confirmed an increase in IL-12p70 production at the protein level (Figure 6J). Arginase 1 transcript level increased by ~130 fold in C. neoformans-MPs-pulsed IL-10+/+ BM-DCs compared with PBS-pulsed IL-10+/+ BM-DCs (Figure 6K). Importantly, a substantial reduction in both arginase 1 and Mrc1 (the gene encoding CD206) transcript level was detected in C. neoformans-MPs-pulsed IL-10−/− versus IL-10+/+ BM-DC (Figure 6K, L). Altogether, these results show that the presence of IL-10 in C. neoformans-MPs-pulsed BM-DCs downregulates CD40, iNOS and IL-12 expression, while up-regulating arginase1 and Mrc1 expression, thereby promoting BM-DC type-2 activation.
To investigate whether these IL-10 effects are mediated via autocrine signaling, we pulsed IL-10+/+ BM-DCs with C. neoformans-MPs in the presence of a blocking anti-IL-10 receptor antibody or an isotype-matched control antibody. We also added recombinant mouse IL-10 to cultures of IL-10−/− BM-DCs pulsed with C. neoformans-MPs. Additional control cultures included BM-DCs generated from both genotypes and pulsed with PBS or C. neoformans-MPs. Analysis by qRT-PCR of C. neoformans-MPs-pulsed IL-10+/+ BM-DCs revealed that blockade of IL-10 signaling increased CD40, iNOS, IL-12A and IL-12B gene expression relative to control antibody, whereas Arginase 1 transcript level decreased (Figure 7A-D black bars). The reduction in Mrc 1gene expression did not reach statistical significance (Figure 7F black bars). The addition of exogenous IL-10 to C. neoformans-MPs-pulsed IL-10−/− BM-DC cultures reduced CD40 and IL-12B gene expression (Figure 7A, D white bars). Changes in transcript level of all other genes analyzed matched the expected trend but did not reach statistical significance (Figure 7B, C, E and F white bars). Collectively, our data suggest that in vitro autocrine IL-10 signaling triggered by C. neoformans-MPs promotes BM-DC type-2 activation.
Figure 7.
Autocrine IL-10 signaling induced by cryptococcal MPs skews the polarization of BM-DCs. BM-DCs generated from IL-10+/+ mice were pulsed with C. neoformans-MPs in the presence or absence of a blocking anti-IL-10 receptor antibody or an isotype-matched control antibody. In addition, BM-DCs generated from IL-10−/− mice were pulsed with C. neoformans-MPs in the presence or absence of exogenous recombinant mouse IL-10. BM-DCs derived from both genotypes and pulsed only with PBS (vehicle) served as additional controls. After 24 hours of culture, transcript levels of CD40 (A), iNOS (B), IL-12A (C), IL-12B (D), Arginase 1 (E) and Mrc1 (CD206, F) in pulsed BM-DCs were quantified by qRT-PCR. Expression level was normalized to GAPDH and expressed relative to the mean expression of PBS-pulsed IL-10+/+DCs. Bar graph data are reported as mean ± SEM of triplicates. IL-10+/+BM-DC, black bars; IL-10−/−BM-DC, white bars. * P < 0.05, ** P < 0.01, *** P < 0.001; one-way ANOVA followed by Tukey’s multiple comparison test.
DISCUSSION
Invasive fungal lung infections constitute an important global health concern as currently available therapy is lengthy, toxic, and often ineffective. In this study, our comparative temporal analysis of IL-10+/+ and IL-10−/− mice infected with C. neoformans strain 52D demonstrates that persistent lung infection in IL-10+/+ mice is associated with: 1) sustained IL-10 production by lung leukocytes; 2) reduced accumulation of monocyte-derived DCs, CD4 and CD8 T cells, alveolar and exudate macrophages; 3) DC type-2 and alternative macrophage activation; and 4) suppressed Th1 and Th17 along with enhanced Th2 and Treg polarization. Our complementary in vitro studies suggest that these important effects of IL-10 on adaptive immunity may result from autocrine IL-10-mediated DC type-2 activation in response to C. neoformans-MPs. Collectively, these findings advance our understanding of the cellular and molecular mechanisms through which IL-10 promotes fungal persistence and strengthen the rationale for blocking IL-10 signaling as a novel immunotherapy for cryptococcal and related fungal lung infections.
Our findings demonstrate that persistent cryptococcal lung infection induces early and sustained IL-10 secretion by pulmonary leukocytes. We show that BM-DCs stimulated in vitro by C. neoformans-MPs abundantly secrete IL-10 suggesting that lung DCs may be a cellular source of IL-10 secretion although other well recognized potential cellular sources of IL-10 (18), such as macrophages and certain subsets of T cells, can also account for the observed high levels of IL-10 production during cryptococcal infection. Further in vivo studies are required to identify the major cellular source of IL-10 during cryptococcal lung infection and to elucidate direct versus indirect cellular targets of IL-10-mediated effects.
Our immunophenotyping of lung DC subsets showed that IL-10 downregulates expression of co-stimulatory molecules and DC type-1 markers whereas it upregulates expression of DC type-2 markers during the adaptive phase of cryptococcal infection. Although numerous host and (or) pathogen derived factors may influence this phenotypic change in vivo, our in vitro data suggest that these effects may be explained, at least in part, by exposure of lung DCs to C. neoformans MPs. We show that BM-DCs stimulated with C. neoformans-MPs display a mixed activation profile, as they upregulate expression of co-stimulatory molecules (CD40, CD80 and CD86) and DC type-1 (iNOS and IL-12) markers, but also expression of arginase 1. Our findings further demonstrate that autocrine IL-10 signaling in these cells reduces expression of CD40, iNOS and IL-12, while increasing expression of arginase 1, thereby skewing BM-DCs toward a type-2 activation profile. Possible downstream mediators of autocrine IL-10 signaling in BM-DCs include STAT 3 and STAT 1 (49). Interestingly, CD206, one of the DC type-2 markers regulated by IL-10 signaling, is also an important cell surface mannose receptor known to bind cryptococcal MPs (34). Although not the focus of the current study, it will be interesting to investigate additional interrelationships between the IL-10 pathway and mannose receptors in future experiments.
In this study, we detected reduced lung fungal burden in IL-10 deficient versus wild type mice only at the late adaptive phase (week 4 and 6) of cryptococcal infection. These findings are in contrast to earlier reports showing that IL-10 negatively impacts innate cell-based mechanisms of resistance against other pathogens, such as Candida albicans (50) and Mycobacterium bovis (51). Highlighting the complexity of IL-10 immunomodulatory effects, resistance to Listeria monocytogenes in IL-10 deficient mice was found to be mediated by both innate and adaptive immunity (52). Blackstock et al (53) reported that the survival rate of IL-10 deficient mice infected by the intratracheal route with C. neoformans strain NU-2 was higher than wild type C57BL/6J controls, and similar findings were demonstrated by Beenhouwer et al (54) using intravenous inoculation of C. neoformans strain 52D. However, the mechanisms underlying these observations remained elusive until Hernandez et al (32) showed that IL-10 impairs clearance of cryptoccoci from the lung in a manner dependent upon generation of cellular adaptive immunity, consisting of both CD4 and CD8 T cells. Our results corroborate and extend these findings by demonstrating that IL-10 skews polarization of lung CD4 T cells throughout the adaptive phase (week 2–6) of cryptococcal infection. We show here that IL-10 suppresses Th1 and Th17 immune responses while simultaneously promoting Th2 and Treg responses. Both Th1 and Th17 T cells are known to be essential for eliciting a protective immune response against C. neoformans as they release IFN-γ and IL-17A, respectively, which induce classical/M1 activation of fungicidal macrophages (8–10, 55). In contrast, Th2 T cells secrete IL-4, IL-5 and IL-13, which lead to alternative/M2 activation of non-fungicidal macrophages (15–17, 56). Notably, Tregs have been shown to preferentially inhibit Th2 immune responses, and thereby reduce lung fungal burden during cryptococcal infection (57, 58). Thus, collectively our findings further explain IL-10’s capacity to sustain a mixed population of T helper subsets that promotes persistence of cryptococcal lung infection.
Our results provide insights that may be useful in guiding efforts to develop novel immunotherapies for invasive fungal lung infections. In the current study, we show that lack of IL-10 improves key immune effector functions during cryptococcal lung infection. Strikingly similar findings, including increased accumulation of Th1 and Th17 cells, monocyte-derived DCs, and exudate macrophages in the lung, were observed after treatment of C. neoformans-infected C57BL/6 mice with an anti-IL-10 receptor blocking antibody (33). More recently, we have shown that antibody-mediated blockade of Programmed Cell Death Protein-1 (PD-1), a critical immune checkpoint signaling pathway, also promotes clearance of established fungal lung infection (59). Our data suggest an interrelationship between the IL-10 and the PD-1 immunoregulatory pathways, as PD-1 blockade downregulated IL-10 and IL-5 gene expression in lung leukocytes and activated Th1 and Th17 CD4 T cells by upregulating cell surface expression of OX40 (59). Lastly, our current observation that BM-DCs secrete substantial amounts of IL-10 upon MPs exposure may explain findings reported by Herring et al. (60), which evaluated the therapeutic efficacy of adoptively-transferred C. neoformans-MPs-pulsed wild-type BM-DCs administered prior to intratracheal infection of mice with C. neoformans strain 52D. Contrary to their initial hypothesis, this approach augmented secretion of IL-10, IL-4 and IL-5 by lung leukocytes and increased fungal lung burden relative to control mice. Our current findings suggest that this approach may have been ineffective because MPs stimulated IL-10 production by DCs, which in an autocrine manner promoted DC type-2 activation and lead to enhanced Th2 responses. Thus, although MPs have emerged as the major antigens eliciting T cell responses to C. neoformans (43, 61–63) and have been viewed as potential immunostimulants (46, 64), our current results suggest a more cautionary approach may be warranted since MPs may trigger IL-10 secretion in DCs and promote undesirable effects on DC polarization.
In summary, systematic examination of the interrelationship between DCs and IL-10 signaling during the course of persistent cryptococcal lung infection implicates that fungal-induced IL-10 signaling skews lung DCs toward a non-protective DC type-2 activation profile which may promote additional impairments in adaptive immunity. Whereas numerous cryptococcal antigens may negatively affect DC phenotype, here we identify cryptococcal MPs as a potent stimulant for IL-10 production in vitro and further define an autocrine pathway through which IL-10 can promote non-protective DC type-2 activation. Since increased IL-10 levels have been implicated in the failure to clear other clinically-relevant fungal infections (65–69), these findings broadly enhance our understanding of host-pathogen interactions and may prove beneficial in the development of novel immunotherapies for infectious diseases.
Supplementary Material
Acknowledgments
Supported by a Merit Review Award (J.J.O.) from the Biomedical Laboratory Research and Development Service, Department of Veterans Affairs and a NIH NHLBI T32 Multidisciplinary Research Training Program in Lung Diseases (J.A.R.).
Abbreviations:
- DC
dendritic cell
- C. neoformans
Cryptococcus neoformans
- MPs
mannoproteins
- Treg
regulatory T cell
- CFU
colony forming unit
- BM-
bone marrow derived
- iNOS
inducible nitric oxide synthase
- wpi
weeks post infection
REFERENCES
- 1.Levitz SM 1991. The ecology of Cryptococcus neoformans and the epidemiology of cryptococcosis. Rev Infect Dis. 13: 1163–1169. [DOI] [PubMed] [Google Scholar]
- 2.Li SS, and Mody CH. 2010. Cryptococcus. Proc Am Thorac Soc. 7: 186–196. [DOI] [PubMed] [Google Scholar]
- 3.Rajasingham R, Smith RM, Park BJ, Jarvis JN, Govender NP, Chiller TM, Denning DW, Loyse A, and Boulware DR. 2017. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis. 17: 873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bozzette SA, Larsen RA, Chiu J, Leal MA, Jacobsen J, Rothman P, Robinson P, Gilbert G, McCutchan JA, Tilles J, and et al. 1991. A placebo-controlled trial of maintenance therapy with fluconazole after treatment of cryptococcal meningitis in the acquired immunodeficiency syndrome. California Collaborative Treatment Group. N Engl J Med. 324: 580–584. [DOI] [PubMed] [Google Scholar]
- 5.Olszewski MA, Zhang Y, and Huffnagle GB. 2010. Mechanisms of cryptococcal virulence and persistence. Future Microbiol. 5: 1269–1288. [DOI] [PubMed] [Google Scholar]
- 6.Chen S, Sorrell T, Nimmo G, Speed B, Currie B, Ellis D, Marriott D, Pfeiffer T, Parr D, and Byth K. 2000. Epidemiology and host- and variety-dependent characteristics of infection due to Cryptococcus neoformans in Australia and New Zealand. Australasian Cryptococcal Study Group. Clin Infect Dis. 31: 499–508. [DOI] [PubMed] [Google Scholar]
- 7.Choi YH, Ngamskulrungroj P, Varma A, Sionov E, Hwang SM, Carriconde F, Meyer W, Litvintseva AP, Lee WG, Shin JH, Kim EC, Lee KW, Choi TY, Lee YS, and Kwon-Chung KJ. 2010. Prevalence of the VNIc genotype of Cryptococcus neoformans in non-HIV-associated cryptococcosis in the Republic of Korea. FEMS Yeast Res. 10: 769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wozniak KL, Ravi S, Macias S, Young ML, Olszewski MA, Steele C, and Wormley FL. 2009. Insights into the mechanisms of protective immunity against Cryptococcus neoformans infection using a mouse model of pulmonary cryptococcosis. PLoS One. 4: e6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Wang F, Tompkins KC, McNamara A, Jain AV, Moore BB, Toews GB, Huffnagle GB, and Olszewski MA. 2009. Robust Th1 and Th17 immunity supports pulmonary clearance but cannot prevent systemic dissemination of highly virulent Cryptococcus neoformans H99. Am J Pathol. 175: 2489–2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murdock BJ, Huffnagle GB, Olszewski MA, and Osterholzer JJ. 2014. Interleukin-17A enhances host defense against cryptococcal lung infection through effects mediated by leukocyte recruitment, activation, and gamma interferon production. Infect Immun. 82: 937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arora S, Olszewski MA, Tsang TM, McDonald RA, Toews GB, and Huffnagle GB. 2011. Effect of cytokine interplay on macrophage polarization during chronic pulmonary infection with Cryptococcus neoformans. Infect Immun. 79: 1915–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardison SE, Herrera G, Young ML, Hole CR, Wozniak KL, and Wormley FL Jr. 2012. Protective immunity against pulmonary cryptococcosis is associated with STAT1-mediated classical macrophage activation. J Immunol. 189: 4060–4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardison SE, Ravi S, Wozniak KL, Young ML, Olszewski MA, and Wormley FL Jr. 2010. Pulmonary infection with an interferon-gamma-producing Cryptococcus neoformans strain results in classical macrophage activation and protection. Am J Pathol. 176: 774–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leopold Wager CM, Hole CR, Wozniak KL, Olszewski MA, Mueller M, and Wormley FL Jr. 2015. STAT1 signaling within macrophages is required for antifungal activity against Cryptococcus neoformans. Infect Immun. 83: 4513–4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arora S, Hernandez Y, Erb-Downward JR, McDonald RA, Toews GB, and Huffnagle GB. 2005. Role of IFN-gamma in regulating T2 immunity and the development of alternatively activated macrophages during allergic bronchopulmonary mycosis. J Immunol. 174: 6346–6356. [DOI] [PubMed] [Google Scholar]
- 16.Jain AV, Zhang Y, Fields WB, McNamara DA, Choe MY, Chen GH, Erb-Downward J, Osterholzer JJ, Toews GB, Huffnagle GB, and Olszewski MA. 2009. Th2 but not Th1 immune bias results in altered lung functions in a murine model of pulmonary Cryptococcus neoformans infection. Infect Immun. 77: 5389–5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller U, Stenzel W, Kohler G, Werner C, Polte T, Hansen G, Schutze N, Straubinger RK, Blessing M, McKenzie AN, Brombacher F, and Alber G. 2007. IL-13 induces disease-promoting type 2 cytokines, alternatively activated macrophages and allergic inflammation during pulmonary infection of mice with Cryptococcus neoformans. J Immunol. 179: 5367–5377. [DOI] [PubMed] [Google Scholar]
- 18.Saraiva M, and O’Garra A. 2010. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 10: 170–181. [DOI] [PubMed] [Google Scholar]
- 19.Cyktor JC, and Turner J. 2011. Interleukin-10 and immunity against prokaryotic and eukaryotic intracellular pathogens. Infect Immun. 79: 2964–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berg DJ, Kuhn R, Rajewsky K, Muller W, Menon S, Davidson N, Grunig G, and Rennick D. 1995. Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxic shock and the Shwartzman reaction but not endotoxin tolerance. J Clin Invest. 96: 2339–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pils MC, Pisano F, Fasnacht N, Heinrich JM, Groebe L, Schippers A, Rozell B, Jack RS, and Muller W. 2010. Monocytes/macrophages and/or neutrophils are the target of IL-10 in the LPS endotoxemia model. Eur J Immunol. 40: 443–448. [DOI] [PubMed] [Google Scholar]
- 22.Siewe L, Bollati-Fogolin M, Wickenhauser C, Krieg T, Muller W, and Roers A. 2006. Interleukin-10 derived from macrophages and/or neutrophils regulates the inflammatory response to LPS but not the response to CpG DNA. Eur J Immunol. 36: 3248–3255. [DOI] [PubMed] [Google Scholar]
- 23.Gazzinelli RT, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kuhn R, Muller W, Trinchieri G, and Sher A. 1996. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J Immunol. 157: 798–805. [PubMed] [Google Scholar]
- 24.Jankovic D, Kullberg MC, Feng CG, Goldszmid RS, Collazo CM, Wilson M, Wynn TA, Kamanaka M, Flavell RA, and Sher A. 2007. Conventional T-bet(+)Foxp3(−) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J Exp Med. 204: 273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sewnath ME, Olszyna DP, Birjmohun R, ten Kate FJ, Gouma DJ, and van Der Poll T. 2001. IL-10-deficient mice demonstrate multiple organ failure and increased mortality during Escherichia coli peritonitis despite an accelerated bacterial clearance. J Immunol. 166: 6323–6331. [DOI] [PubMed] [Google Scholar]
- 26.McKinstry KK, Strutt TM, Buck A, Curtis JD, Dibble JP, Huston G, Tighe M, Hamada H, Sell S, Dutton RW, and Swain SL. 2009. IL-10 deficiency unleashes an influenza-specific Th17 response and enhances survival against high-dose challenge. J Immunol. 182: 7353–7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun K, Torres L, and Metzger DW. 2010. A detrimental effect of interleukin-10 on protective pulmonary humoral immunity during primary influenza A virus infection. J Virol. 84: 5007–5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, and Oldstone MB. 2006. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 12: 1301–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lortholary O, Improvisi L, Rayhane N, Gray F, Fitting C, Cavaillon JM, and Dromer F. 1999. Cytokine profiles of AIDS patients are similar to those of mice with disseminated Cryptococcus neoformans infection. Infect Immun. 67: 6314–6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh N, Husain S, Limaye AP, Pursell K, Klintmalm GB, Pruett TL, Somani J, Stosor V, del Busto R, Wagener MM, and Steele C. 2006. Systemic and cerebrospinal fluid T-helper cytokine responses in organ transplant recipients with Cryptococcus neoformans infection. Transpl Immunol. 16: 69–72. [DOI] [PubMed] [Google Scholar]
- 31.Scriven JE, Graham LM, Schutz C, Scriba TJ, Wilkinson KA, Wilkinson RJ, Boulware DR, Urban BC, Lalloo DG, and Meintjes G. 2016. A Glucuronoxylomannan-Associated Immune Signature, Characterized by Monocyte Deactivation and an Increased Interleukin 10 Level, Is a Predictor of Death in Cryptococcal Meningitis. J Infect Dis. 213: 1725–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hernandez Y, Arora S, Erb-Downward JR, McDonald RA, Toews GB, and Huffnagle GB. 2005. Distinct roles for IL-4 and IL-10 in regulating T2 immunity during allergic bronchopulmonary mycosis. J Immunol. 174: 1027–1036. [DOI] [PubMed] [Google Scholar]
- 33.Murdock BJ, Teitz-Tennenbaum S, Chen GH, Dils AJ, Malachowski AN, Curtis JL, Olszewski MA, and Osterholzer JJ. 2014. Early or late IL-10 blockade enhances Th1 and Th17 effector responses and promotes fungal clearance in mice with cryptococcal lung infection. J Immunol. 193: 4107–4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mansour MK, Schlesinger LS, and Levitz SM. 2002. Optimal T cell responses to Cryptococcus neoformans mannoprotein are dependent on recognition of conjugated carbohydrates by mannose receptors. J Immunol. 168: 2872–2879. [DOI] [PubMed] [Google Scholar]
- 35.Chen GH, Teitz-Tennenbaum S, Neal LM, Murdock BJ, Malachowski AN, Dils AJ, Olszewski MA, and Osterholzer JJ. 2016. Local GM-CSF-Dependent Differentiation and Activation of Pulmonary Dendritic Cells and Macrophages Protect against Progressive Cryptococcal Lung Infection in Mice. J Immunol. 196: 1810–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Misharin AV, Morales-Nebreda L, Mutlu GM, Budinger GR, and Perlman H. 2013. Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. Am J Respir Cell Mol Biol. 49: 503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eastman AJ, Osterholzer JJ, and Olszewski MA. 2015. Role of dendritic cell-pathogen interactions in the immune response to pulmonary cryptococcal infection. Future Microbiol. 10: 1837–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Osterholzer JJ, Surana R, Milam JE, Montano GT, Chen GH, Sonstein J, Curtis JL, Huffnagle GB, Toews GB, and Olszewski MA. 2009. Cryptococcal urease promotes the accumulation of immature dendritic cells and a non-protective T2 immune response within the lung. Am J Pathol. 174: 932–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osterholzer JJ, Chen GH, Olszewski MA, Zhang YM, Curtis JL, Huffnagle GB, and Toews GB. 2011. Chemokine receptor 2-mediated accumulation of fungicidal exudate macrophages in mice that clear cryptococcal lung infection. Am J Pathol. 178: 198–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Voelz K, Lammas DA, and May RC. 2009. Cytokine signaling regulates the outcome of intracellular macrophage parasitism by Cryptococcus neoformans. Infect Immun. 77: 3450–3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davis MJ, Tsang TM, Qiu Y, Dayrit JK, Freij JB, Huffnagle GB, and Olszewski MA. 2013. Macrophage M1/M2 polarization dynamically adapts to changes in cytokine microenvironments in Cryptococcus neoformans infection. MBio. 4: e00264–00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.MacMicking J, Xie QW, and Nathan C. 1997. Nitric oxide and macrophage function. Annu Rev Immunol. 15: 323–350. [DOI] [PubMed] [Google Scholar]
- 43.Levitz SM, Nong S, Mansour MK, Huang C, and Specht CA. 2001. Molecular characterization of a mannoprotein with homology to chitin deacetylases that stimulates T cell responses to Cryptococcus neoformans. Proc Natl Acad Sci U S A. 98: 10422–10427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoy JF, Murphy JW, and Miller GG. 1989. T cell response to soluble cryptococcal antigens after recovery from cryptococcal infection. J Infect Dis. 159: 116–119. [DOI] [PubMed] [Google Scholar]
- 45.Chaka W, Verheul AF, Vaishnav VV, Cherniak R, Scharringa J, Verhoef J, Snippe H, and Hoepelman AI. 1997. Induction of TNF-alpha in human peripheral blood mononuclear cells by the mannoprotein of Cryptococcus neoformans involves human mannose binding protein. J Immunol. 159: 2979–2985. [PubMed] [Google Scholar]
- 46.Pietrella D, Cherniak R, Strappini C, Perito S, Mosci P, Bistoni F, and Vecchiarelli A. 2001. Role of mannoprotein in induction and regulation of immunity to Cryptococcus neoformans. Infect Immun. 69: 2808–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walter MR 2014. The molecular basis of IL-10 function: from receptor structure to the onset of signaling. Curr Top Microbiol Immunol. 380: 191–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moore KW, de Waal Malefyt R, Coffman RL, and O’Garra A. 2001. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 19: 683–765. [DOI] [PubMed] [Google Scholar]
- 49.Sabat R, Grutz G, Warszawska K, Kirsch S, Witte E, Wolk K, and Geginat J. 2010. Biology of interleukin-10. Cytokine Growth Factor Rev. 21: 331–344. [DOI] [PubMed] [Google Scholar]
- 50.Vazquez-Torres A, Jones-Carson J, Wagner RD, Warner T, and Balish E. 1999. Early resistance of interleukin-10 knockout mice to acute systemic candidiasis. Infect Immun. 67: 670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murray PJ, and Young RA. 1999. Increased antimycobacterial immunity in interleukin-10-deficient mice. Infect Immun. 67: 3087–3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dai WJ, Kohler G, and Brombacher F. 1997. Both innate and acquired immunity to Listeria monocytogenes infection are increased in IL-10-deficient mice. J Immunol. 158: 2259–2267. [PubMed] [Google Scholar]
- 53.Blackstock R, Buchanan KL, Adesina AM, and Murphy JW. 1999. Differential regulation of immune responses by highly and weakly virulent Cryptococcus neoformans isolates. Infect Immun. 67: 3601–3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beenhouwer DO, Shapiro S, Feldmesser M, Casadevall A, and Scharff MD. 2001. Both Th1 and Th2 cytokines affect the ability of monoclonal antibodies to protect mice against Cryptococcus neoformans. Infect Immun. 69: 6445–6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen GH, McDonald RA, Wells JC, Huffnagle GB, Lukacs NW, and Toews GB. 2005. The gamma interferon receptor is required for the protective pulmonary inflammatory response to Cryptococcus neoformans. Infect Immun. 73: 1788–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gordon S 2003. Alternative activation of macrophages. Nat Rev Immunol. 3: 23–35. [DOI] [PubMed] [Google Scholar]
- 57.Schulze B, Piehler D, Eschke M, von Buttlar H, Kohler G, Sparwasser T, and Alber G. 2014. CD4(+) FoxP3(+) regulatory T cells suppress fatal T helper 2 cell immunity during pulmonary fungal infection. Eur J Immunol. 44: 3596–3604. [DOI] [PubMed] [Google Scholar]
- 58.Wiesner DL, Smith KD, Kotov DI, Nielsen JN, Bohjanen PR, and Nielsen K. 2016. Regulatory T Cell Induction and Retention in the Lungs Drives Suppression of Detrimental Type 2 Th Cells During Pulmonary Cryptococcal Infection. J Immunol. 196: 365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roussey JA, Viglianti SP, Teitz-Tennenbaum S, Olszewski MA, and Osterholzer JJ. 2017. Anti-PD-1 Antibody Treatment Promotes Clearance of Persistent Cryptococcal Lung Infection in Mice. J Immunol. 199: 3535–3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Herring AC, Falkowski NR, Chen GH, McDonald RA, Toews GB, and Huffnagle GB. 2005. Transient neutralization of tumor necrosis factor alpha can produce a chronic fungal infection in an immunocompetent host: potential role of immature dendritic cells. Infect Immun. 73: 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang C, Nong SH, Mansour MK, Specht CA, and Levitz SM. 2002. Purification and characterization of a second immunoreactive mannoprotein from Cryptococcus neoformans that stimulates T-Cell responses. Infect Immun. 70: 5485–5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Levitz SM, and North EA. 1997. Lymphoproliferation and cytokine profiles in human peripheral blood mononuclear cells stimulated by Cryptococcus neoformans. J Med Vet Mycol. 35: 229–236. [DOI] [PubMed] [Google Scholar]
- 63.Orendi JM, Verheul AF, De Vos NM, Visser MR, Snippe H, Cherniak R, Vaishnav VV, Rijkers GT, and Verhoef J. 1997. Mannoproteins of Cryptococcus neoformans induce proliferative response in human peripheral blood mononuclear cells (PBMC) and enhance HIV-1 replication. Clin Exp Immunol. 107: 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pietrella D, Corbucci C, Perito S, Bistoni G, and Vecchiarelli A. 2005. Mannoproteins from Cryptococcus neoformans promote dendritic cell maturation and activation. Infect Immun. 73: 820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Clemons KV, Grunig G, Sobel RA, Mirels LF, Rennick DM, and Stevens DA. 2000. Role of IL-10 in invasive aspergillosis: increased resistance of IL-10 gene knockout mice to lethal systemic aspergillosis. Clin Exp Immunol. 122: 186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Costa TA, Bazan SB, Feriotti C, Araujo EF, Bassi E, Loures FV, and Calich VL. 2013. In pulmonary paracoccidioidomycosis IL-10 deficiency leads to increased immunity and regressive infection without enhancing tissue pathology. PLoS Negl Trop Dis. 7: e2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deepe GS Jr., and Gibbons RS. 2003. Protective and memory immunity to Histoplasma capsulatum in the absence of IL-10. J Immunol. 171: 5353–5362. [DOI] [PubMed] [Google Scholar]
- 68.Jimenez Mdel P, Walls L, and Fierer J. 2006. High levels of interleukin-10 impair resistance to pulmonary coccidioidomycosis in mice in part through control of nitric oxide synthase 2 expression. Infect Immun. 74: 3387–3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qureshi MH, Harmsen AG, and Garvy BA. 2003. IL-10 modulates host responses and lung damage induced by Pneumocystis carinii infection. J Immunol. 170: 1002–1009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.