Abstract
Most ingested ethanol is metabolized in the liver to acetaldehyde and then to acetate, which can be oxidized by the brain. This project assessed whether chronic exposure to alcohol can increase cerebral oxidation of acetate. Through metabolism, acetate may contribute to long-term adaptation to drinking. Two groups of adult male Sprague-Dawley rats were studied, one treated with ethanol vapor and the other given room air. After 3 weeks the rats received an intravenous infusion of [2-13C]ethanol via a lateral tail vein for 2h. As the liver converts ethanol to [2-13C]acetate, some of the acetate enters the brain. Through oxidation the 13C is incorporated into the metabolic intermediate α-ketoglutarate, which is converted to glutamate, glutamine, and GABA. These were observed by magnetic resonance spectroscopy and found to be 13C-labeled primarily through the consumption of ethanol-derived acetate. Brain glutamine, glutamate, and GABA 13C enrichments, normalized to 13C-acetate enrichments in the plasma, were higher in the chronically treated rats than in the ethanol-naïve rats, suggesting increased cerebral uptake and oxidation of circulating acetate. Chronic ethanol exposure increased incorporation of systemically derived acetate into brain glutamine, glutamate, and GABA, key neurochemicals linked to brain energy metabolism and neurotransmission.
Keywords: Ethanol, Acetate, Brain Metabolism, NMR, Rats
Introduction
Alcohol is metabolized in the periphery, particularly in the liver (Norberg et al. 2003, Lundquist et al. 1962), by conversion to an intermediate, acetaldehyde, and then to acetate (Manzo-Avalos & Saavedra-Molina 2010). More than 90% of acetate produced in the liver is released to the blood (Jucker et al. 1998), and it travels to other organs, including the brain, for use as an energy substrate (Patel et al. 2010) and in fatty acid and cholesterol biosynthesis (Hellman et al. 1954, Natali et al. 2007). Consumption of enough ethanol (EtOH) to achieve blood alcohol levels of 50 mg/dL raises plasma acetate levels to 1–2 mM (Nuutinen et al. 1985, Peng et al. 2007), and more ethanol does not further raise the levels (Lundquist et al. 1973, Lundquist et al. 1962, Mascord et al. 1992, Nuutinen et al. 1985). Alcohol-dependent individuals show elevated blood acetate up to 24 hours after the last drink (Pronko et al. 1997), and non-dependent heavy drinkers show elevated plasma acetate even with a breath alcohol of 0 (Jiang et al. 2013). Acetate oxidation via acetyl-CoA synthetase (AceCS) generates adenosine (Kiviluoma et al. 1989, Kiselevski et al. 2003), whose levels are increased during EtOH intake. The increased extraneuronal adenosine levels are highly related with cerebellar ataxia, sleep, alcohol preference and reinforcement, and glutamate signaling (Asatryan et al. 2011). Thus, the metabolic fate of EtOH as acetate might have significant energetic and behavioral consequences.
In the absence of EtOH, acetate contributes negligibly to brain metabolism, because acetate levels are low, produced primarily by intestinal flora (Waniewski & Martin 1998). However, it has been hypothesized that brain acetate metabolism may increase during chronic EtOH consumption, perhaps contributing to neuroadaptation and dependence on EtOH (Derr et al. 1981, Israel et al. 1994, Roach & Reese 1972, Volkow et al. 2006). The rationale for this hypothesis is based on several findings:
1). Blood acetate rises during EtOH consumption.
During hepatic oxidation of EtOH, blood acetate concentrations rise 3- to 5-fold to the 1–2mM range (Sarkola et al. 2002, Carmichael et al. 1991). The zero-order kinetics of EtOH elimination imply a constant rate of acetate production for nearly the entire duration of EtOH metabolism. Thus, blood acetate is maintained at a significantly elevated and stable concentration throughout the period of EtOH intoxication (Orrego et al. 1988). In alcoholics and heavy drinkers, the rate of EtOH metabolism is more rapid, resulting in acetate plateau levels approximately 2-fold higher in alcohol-tolerant individuals than in controls (Korri et al. 1985), suggesting greater availability of acetate as a substrate for brain metabolism. Therefore, EtOH metabolism results in an abundant supply of acetate in the blood.
2). Blood acetate is consumed by the brain and lowers glucose oxidation.
Pawlosky et al (Pawlosky et al. 2010) found that elevations of blood EtOH and acetate concentrations decrease the rates of glucose oxidation and glucose phosphorylation in rats, and do so in proportion to the concentration of plasma acetate. The study of Pawlosky was consistent with previous experiments that showed that glucose metabolism decreases in brain during EtOH exposure in rats (Learn et al. 2003) and that carbon from EtOH is oxidized in the brains of hamsters(Roach & Reese 1971). In humans, Volkow et al (Volkow et al. 2006) reported that modest doses (0.25 to 0.5 g/kg) of EtOH reduced the rate of brain glucose utilization by 10–23%, with greater reductions seen in alcoholics compared to control subjects (Volkow et al. 1990). A further study found that alcohol acutely decreases brain glucose uptake, while increasing acetate uptake, and did so more in heavy drinkers (Volkow et al. 2013). Such findings suggest that heavy chronic alcohol exposure may increase brain utilization of acetate, as shown recently in non-alcohol-dependent human heavy drinkers (Jiang et al. 2013).
3). EtOH exposure increases enzyme capacity for acetate oxidation.
A 36% increase in cortical AceCS activity in homogenates of rat cortex occurs following 7 days of EtOH treatment (Kiselevski et al. 2003), consistent with an adaptive increase in acetate metabolism by brain during chronic EtOH exposure.
Taken together, those three lines of evidence support the hypothesis that the brain may adapt to chronic EtOH intake, in part, by increasing its metabolism of acetate.
We investigated the metabolism of EtOH and its product acetate after chronic EtOH vapor treatment, using 13C-labeled EtOH administered intravenously to label products of acetate metabolism in the brain, which were measured by 13C MRS. Our objectives were to quantify the contribution of EtOH-derived acetate to oxidative brain metabolism, and to determine whether chronic EtOH exposure would change the contribution.
Methods and Materials
Metabolic Fate of EtOH-Derived 13C Acetate
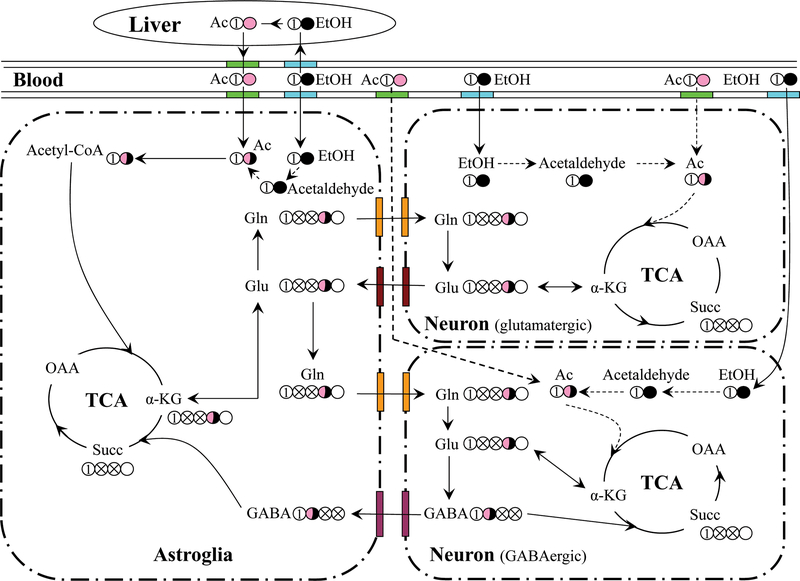
Acetate crosses the blood-brain barrier (BBB) more slowly than does glucose (Deelchand et al. 2009, Oldendorf 1973, Patel et al. 2010) and is metabolized exclusively by astroglia (Fig. 1) (Waniewski & Martin 1998). Indeed, a recent study shows that acetate is excluded from a portion of the brain that approximates the neuronal volume (Patel et al. 2010), and the acetate transporter that is absent on neurons but present on astrocytes appears to account for the selectivity (Waniewski & Martin 1998). Acetate can supply a substantial fraction of fuel for the brain during hypoglycemia in rats (Gruetter et al. 2001, Jiang et al. 2009) and humans (Mason et al. 2006, Lebon et al. 2002). 13C-labeled tracers such as [2-13C]acetate provide positional information that allows one to trace the metabolic paths that lead to labeling of different positions of neurochemicals, such as glutamate (Glu), glutamine (Gln), and γ-aminobutyric acid (GABA) (Fig. 1) (Patel et al. 2010, Zimatkin et al. 1998, Cohen et al. 1980, Haberg et al. 1998, Hassel & Sonnewald 1995).
Fig. 1:
Model for cerebral metabolism of [2-13C]acetate and [2-13C]ethanol showing incorporation of 13C into the astroglial, glutamatergic, and GABAergic TCA cycles. Acetate is transported into astroglia and possibly neurons, converted to acetyl-CoA, which enters the TCA cycle, labeling Glu-C4. Labeled Glu can leave the glutamatergic neuron through neurotransmitter release and is rapidly converted to Gln in astroglia. Gln is then transported back to glutamatergic neurons as a precursor for neurotransmitter Glu, thus completing the Glu-Gln cycle. Carbons that traverse a complete turn of the TCA cycle are scrambled due to the molecular symmetry of succinate, and thus Glu and Gln-C2 and C3 are labeled equally on the second and subsequent turns (crossed circles). GABAergic neurons synthesize GABA-C2 from Glu-C4, whereas GABA derived from Glu-C2 and C3 is labeled in C3 and C4. Once released, neurotransmitter GABA has two potential fates: reuptake by GABAergic neurons for reuse and/or direct oxidation in the GABAergic neuron, or uptake and oxidation in astroglia. To the extent that ethanol might be oxidized intracerebrally, ethanol enters astroglia and neurons and can be converted first to acetaldehyde and then to acetate, which is then oxidized. The oxidation of this intracerebrally generated acetate might occur in neurons as well as astrocytes, if the acetate is generated intraneuronally. Abbreviations: α-KG: α-ketoglutarate; OAA: Oxaloacetate; Succ: Succinate; Gln: Glutamine; Glu: Glutamate; GABA: γ-aminobutyric acid.
Animal Procedures
All experiments were carried according to protocols approved by the Yale Animal Care and Use Committee. Adult male Sprague-Dawley rats (Charles River Labs, Inc.) ages 44–48 days were maintained on a 12h/12h light-dark cycle, with food (Teklad Global 18% Protein Rodent Diet 2018, Harlan Laboratories, Madison, Wisconsin) and water available ad libitum. They acclimated for two days before the procedures began. To familiarize the rats with human interaction and minimize stress during blood draws and on the day of the infusion, they were handled daily. Room air or EtOH was administered to rats in vapor chambers (Wang et al. 2012). The food was weighed daily to allow the determination of food consumption by the rats over the course of EtOH or air exposure.
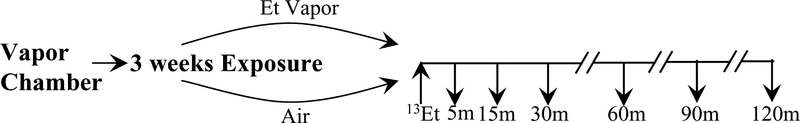
All animals were treated according to the protocol in Fig. 2. Nine rats were treated with EtOH vapor 8hr/day (9am to 5pm during the day time, in the light cycle) for 3 weeks in vapor chambers, and nine control rats were administered room air in vapor chambers. For the exposed rats, the initial EtOH vapor concentration was 15.5 mg/L and gradually increased to ~25 mg/L over one week. This inhalation model is associated with minimal hepatic dysfunction (Diluzio & Stege 1979). To monitor blood alcohol concentrations, 200 μL samples were drawn from the saphenous vein weekly after 7 hours of the day’s exposure. The animals were awake and moving freely, except for the brief times when they were picked up and held gently for 10–20 seconds to draw the sample. After centrifugation, the plasma alcohol level was measured (Analox GM7, Analox Instruments, Inc., Lunenburg, MA).
Fig. 2:
Experimental design. 9 rats were treated with either ~25 mg/L EtOH vapor or room air for 8 hr/day for 3 weeks in vapor chambers. On the experimental day [2-13C]EtOH was infused via tail vein according to a pharmacologic model (Ramchandani & O’Connor 2006) to reach 21.7mM (100 mg/dL) plasma EtOH by 15 minutes and maintained for the rest of the 2-hour infusion, followed by euthanasia via focused microwave irradiation. During the infusion, blood samples were drawn from a saphenous vein at 5, 15, 30, 60, 90 and 120 minutes. Tissue and blood samples were prepared for NMR analysis. Brain metabolite 13C enrichments were normalized by the final plasma acetate enrichments.
The purpose of the 3-week EtOH administration was to test if chronic EtOH exposure could induce acetate and/or EtOH consumption by the brain. Our finding of elevated acetate consumption in chronic heavy drinkers was obtained after overnight fasting (Jiang et al. 2013), and we likewise fasted these animals before the infusion. On the day of 13C-EtOH administration, anesthesia was initiated with 3% isoflurane and lowered to a maintenance dose of 1.5–2.5% for 1–2 minutes to catheterize a lateral tail vein with a 23-G needle connected to PE-50 tubing (Instech Laboratories, Inc., Plymouth Meeting, PA) (Wang et al. 2010). The tail vein catheterization failed in one EtOH-treated rat, so that group had only 8 animals. After catheterization, the rats recovered for 30 minutes, moving and walking freely in the cage. Then [2-13C]EtOH up to 1.373 mg/kg was infused through the tail catheter using a pharmacologically modeled infusion protocol that accounts for body weight to estimate liver activity, body water, etc. (Ramchandani & O’Connor 2006) to raise the blood alcohol level to 21.7mM (100 mg/dL) by 15 minutes and maintain it stable for the remainder of two hours. Blood samples (~100μl) were collected from the saphenous vein at time points 0, 5, 15, 30, 60, 90, and 120 minutes and used to ascertain plasm concentrations and 13C enrichments of acetate and EtOH, including verification that an isotopic steady state was achieved. After the infusion, the rats were euthanized with focused microwave irradiation (Mayne et al. 1999). This technique fixes the rat brain within 1–2 seconds and inactivates enzymes so that metabolite measurements closely reflect conditions in vivo. The fixed brain was removed and dissected into 13 regions for NMR analysis as described previously (Wang et al. 2010). The names of the regions and their abbreviations are given in Fig. 4.
Fig. 4:
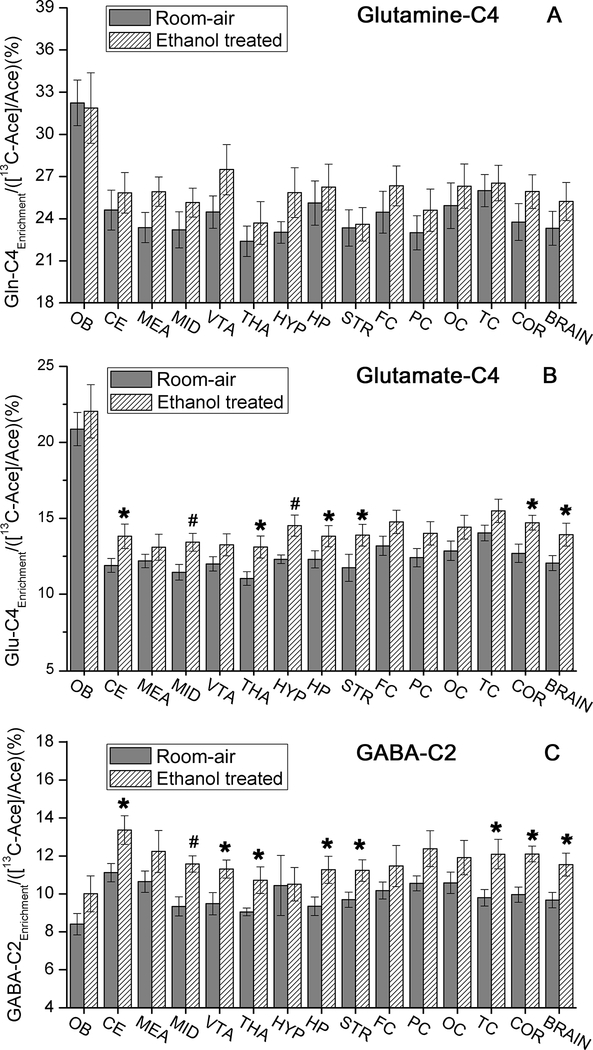
The normalized 13C enrichments of (A) glutamine-C4, (B) glutamate-C4, and (C) GABA-C2 in different brain regions, overall cortex, and the whole brain, normalized by the concentration of [2-13C]acetate in the plasma for room-air (9 rats) and ethanol treated (8 rats) groups. Note: COR: overall cortex; OB: olfactory bulb; CE: cerebellum; MEA: medulla; MID: midbrain; VTA: Ventral tegmental area; THA: Thalamus; HYP: hypothalamus; HP: hippocampus; STR: striatum; FC: frontal cortex; PC: parietal cortex; OC: occipital cortex; TC: temporal cortex, *p<0.05, #p<0.01.
The blood samples were centrifuged at 18,000g for 10 minutes at 2–4 °C, and plasma was removed and stored, with the brain samples, at −80° C for later analysis.
Preparation of Brain Tissue for NMR Study
The frozen samples were placed into a 7-mL homogenizer with 300 μl 0.1M HCl/MEtOH in the wet ice bath and ground. Ice-cold 60% EtOH (1.5 mL) was added with 50μL of 10mM [2-13C]glycine as an internal standard, and the mixture was homogenized. The homogenizer was rinsed with 1.5 mL ice-cold 60% EtOH, and the total liquid was centrifuged at 18,000g for 30 minutes at 2–4 °C. The supernatant was frozen with liquid nitrogen and lyophilized. The lyophilized extracts were dissolved with D2O buffer [(0.06 mL D2O with 0.54 mL buffer (K2HPO4 and KH2PO4, 2mM formate as a chemical shift reference, pH = 7.0))]. The solution was centrifuged at 18,000g for 30 minutes, and the supernatant was removed for 1H-13C NMR analysis.
Preparation of Plasma Samples for NMR Study
The frozen plasma was thawed and centrifuged at 18,000g for 5 minutes. Between 10 and 50μl plasma, depending on the blood sample volume, was withdrawn and mixed to a total volume of 300μl with water, and an additional 60μl D2O, 240μl buffer with 12.5mM phosphate (90mM K2HPO4 and 35mM KH2PO4) and 5mM sodium formate were added. The mixture was transferred to a 5-mL NMR tube for 1H NMR analysis (Rothman et al. 1985).
Plasma and Brain Tissue Extract Analysis
The samples from each rat were measured individually at 11.75T on a Bruker AVANCE vertical bore NMR spectrometer using 1H-13C heteronuclear editing (Rothman et al. 1985). Briefly, the heteronuclear editing method consists of the acquisition of two spin-echo measurements, one with a broad-band inversion pulse applied at the 13C frequency, and the other without the inversion pulse. The difference between the spectra represents protons bound to 13C (at twice the true intensity), while the subspectrum without the inversion pulse represents the protons for 13C-labeled and the unlabeled compounds (i.e., the total concentrations). 13C decoupling was applied during the acquisition of the free-induction decays to collapse 13C-1H couplings, reducing spectral complexity while increasing sensitivity. An 8-ms echo time, 20-s repetition time, 12-ppm sweep width, and 32,768 complex data points were used.
Calculation of Fractional Enrichments of the Metabolites
Metabolite quantification was performed by comparison of the metabolite resonance areas to that of edited 13C-glycine. Because spectra were run in the fully relaxed configuration (TR=20s), no correction for saturation was applied. The metabolite enrichments were determined for each dissected region but also averaged over all neocortical regions and over the whole brain minus the olfactory bulb.
The plasm acetate levels used in this study lie in a range in which the 13C enrichments of Glu and Gln-C4 depend linearly on the [2-13C]acetate enrichment in the plasma (31), so differences in plasma acetate levels and enrichments will lead to different brain labeling even in the absence of differences in transport and oxidation kinetics. To minimize such differences, the metabolite enrichments were normalized by the plasma [2-13C]acetate enrichments.
Statistical Analysis
Given the relatively small sample size, comparisons between groups were analyzed using nonparametric methods. Glu, Gln, and GABA levels averaged across the whole brain and the whole cortex were compared between groups using the Wilcoxon two-sample test. Metabolite levels within individual regions were compared between groups using the nonparametric approach for repeated measures data (Brunner et al. 2002), where the data were first ranked, and then fitted using a mixed effects model with an unstructured variance-covariance matrix and p-values adjusted for ANOVA-type statistics (ATS). In these models, treatment group was included as a between-subjects factor and region (see 13 regions) was included as a within-subjects factor. Owing to the small sample, both un-adjusted and Bonferroni-adjusted p-values are reported. The latter adjustments were made within but not across metabolite (i.e., adjustment for 13 tests for regional comparisons and 2 tests for whole brain and cortex comparisons. All results presented are means ±SEM.
Results
Animals
When the chamber air flow was first turned on, whether for EtOH or room air, most rats showed elevated activity for a few seconds but no signs of discomfort. The rats ran for several seconds, and then walked around the cage, sniffing the sides of the cage for 30–60 seconds, which was likely caused by the change in air pressure in the cages. The rats in the ethanol-exposed group showed slightly more activity, walking for about 30 seconds longer than their air-exposed counterparts. Both groups then returned to their pre-vapor behavioral state. Because the rats showed no signs of discomfort (no voicing, no spikey fur, no discharge around the eyes, no lethargy or sitting hunched in a corner, no tremors or increased rate of respiration), their behavior was only observed qualitatively.
The plasma EtOH measurements averaged 34.6 ± 2.1mM (159 ± 10 mg/dL) during the three weeks. Considering the fact that in vapor chambers blood ethanol levels each day rise linearly over the hours of exposure used here (Gilpin et al. 2009), the average level of ethanol concentration was 17.3 mM (79.5mg/dL), which is similar to current driving limits in the U.S.. The initial weights were 183.7 ± 2.6 g and 181.1 ± 1.2 g for the EtOH-treated and room air groups, respectively. The amount of chow that the rats consumed daily differed between the groups, averaging of 14.6 ± 2.8 g and 22.4 ± 1.9 g (p<0.0001) for the EtOH-treated and room air groups, respectively, and regression analysis showed a significant increase in the room-air rats’ consumption from 20 ± 0.2g to 24 ± 4g (p<0.0001), but not in EtOH-treated rats (p=0.68). After three weeks of exposure, their body weights increased to 263.9±6.6g and 307.2±8.2g for the treated and room air groups, respectively, with a significant effect of EtOH exposure (p<0.001).
Plasma Analysis
[2-13C]EtOH:
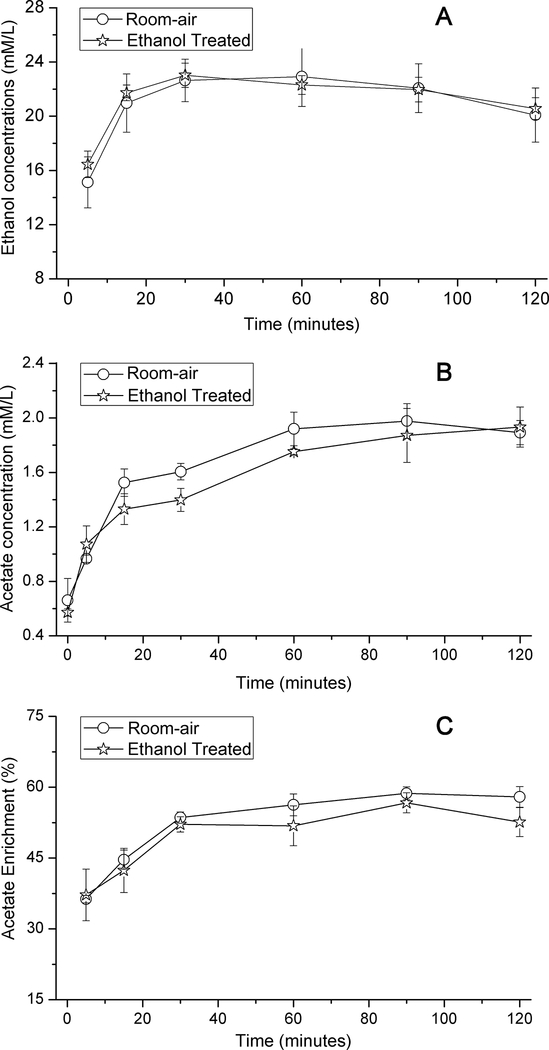
For EtOH-treated and room air exposed rats, the infusion protocol increased the blood alcohol concentration to 21–23 mM (97–106 mg/dL) within 15 minutes (Fig. 3a) and maintained that level for the duration of the 2-hour exposure.
Fig. 3:
Time courses of the concentration of (A) plasma ethanol and (B) acetate, and (C) enrichment of [2-13C]acetate for room-air (9 rats) and ethanol treated (8 rats) groups.
[2-13C]Acetate from [2-13C]EtOH:
By 15 minutes, the infusion of [2-13C]EtOH raised the percent enrichment of acetate to 46.0±3.6% and 46.5±3.5% (p=0.911), in the treated and room-air groups, respectively, with respective concentrations of 1.33±0.11mM and 1.53±0.10mM (p=0.219) (Fig. 3b and c). After 30 minutes, the percent enrichment reached ~55% for both groups. The elevated plasma acetate concentration during EtOH metabolism, together with the high fractional enrichment, confirmed the availability of EtOH-derived acetate (Jucker et al. 1998) as substrate for brain metabolism within minutes of EtOH administration.
Other 13C-labeled plasma metabolites:
The vast majority of the acetate produced from EtOH by the liver is released to the blood (Jucker et al. 1998), and in fasted animals intrahepatic metabolism of the remainder leads to labeling of lactate, ketone bodies, and glucose. Accordingly, 13C appeared to a small extent in [3-13C]lactate, [1-13C]glucose, and [2 or 4-13C] β-Hydroxybutyrate (Table 1). No differences were seen between groups for these compounds (p>0.20), which indicated that three weeks exposure are insufficient to alter hepatic production of acetate. In brain slices, conversion of lactate and glucose to small amounts of acetate has been observed (Rae et al. 2012), but the production in those studies can be estimated to contribute between 0.002 and 0.003% of 13C label in acetate.
Table 1:
The concentrations and percent enrichments of 13C in lactate, glucose, and β-hydroxybutyrate in the plasma. No differences were found for these compounds between the room-air and vapor-treated groups. Samples volumes during the infusion were too small to detect glucose, whose C1 resonance lies near that of water. No significant group differences were detected for concentrations or 13C enrichments (p > 0.20).
| time (min) | Lactate |
Glucose |
β-Hydroxybutyrate |
|||
|---|---|---|---|---|---|---|
| [ ](mM) | C-3FE(%) | [ ](mM) | C-1FE(%) | [ ](mM) | C-2FE(%) | |
| 3 weeks air | ||||||
| 0 | 2.47±0.19 | N.D. | N.D. | N.D. | 1.83±0.09 | 2.76±0.64 |
| 5 | 2.40±0.23 | 0.89±0.20 | N.D. | N.D. | 1.72±0.08 | 3.51±1.08 |
| 15 | 2.14±0.18 | 1.33±0.16 | N.D. | N.D. | 1.88±0.17 | 7.06±1.43 |
| 30 | 1.92±0.21 | 2.14±0.25 | N.D. | N.D. | 1.94±0.10 | 7.28±0.71 |
| 60 | 1.71±0.17 | 3.37±0.42 | N.D. | N.D. | 2.26±0.14 | 10.58±1.50 |
| 90 | 1.66±0.16 | 4.21±0.43 | N.D. | N.D. | 2.31±0.15 | 10.12±1.00 |
| 120 | 2.04±0.10 | 3.84±0.26 | 5.59±0.34 | 2.02±0.40 | 2.16±0.17 | 9.37±0.86 |
| 3 weeks ethanol | ||||||
| 0 | 2.30±0.22 | N.D. | N.D. | N.D. | 1.75±0.15 | 3.76±0.79 |
| 5 | 2.29±0.08 | 1.57±0.45 | N.D. | N.D. | 1.74±0.09 | 5.64±2.69 |
| 15 | 2.29±0.21 | 2.17±0.43 | N.D. | N.D. | 1.91±0.16 | 7.29±1.63 |
| 30 | 2.00±0.11 | 2.69±0.42 | N.D. | N.D. | 1.88±0.09 | 8.27±1.74 |
| 60 | 2.10±0.21 | 2.68±0.15 | N.D. | N.D. | 1.97±0.08 | 10.14±1.90 |
| 90 | 1.82±0.13 | 3.85±0.57 | N.D. | N.D. | 2.16±0.10 | 10.94±0.55 |
| 120 | 2.01±0.18 | 4.43±0.49 | 5.37±0.64 | 2.33±0.11 | 2.24±0.07 | 11.22±1.08 |
Note: N.D.: not determined.
Regional Analysis of Brain Metabolites
Having established the availability in plasma of 13C-labeled substrates for brain metabolism, we examined 13C incorporation into brain Glu, Gln, and GABA (Fig. 4). With the tail vein infusion, 13C-EtOH plateaued at ~21.7 mM (100 mg/dL) for the room-air and treated groups. The enrichments of the 13C neurochemicals relative to plasma [2-13C]acetate (Fig. 4 and Table 1S) show Gln-C4 with the greatest labeling in all regions, followed by Glu-C4 and GABA-C2, consistent with the metabolic pathways in Fig. 1. For Glu-C4 and Gln-C4, 13C-labeling was greatest in the OB. The EtOH-treated group had higher Glu-C4 enrichment in the cerebellum (p=0.031), midbrain (p=0.004), thalamus (p=0.027), hypothamalums (p=0.009), hippocampus (p=0.047), and striatum (p=0.048), with trend-levels for the frontal, temporal, and parietal cortices (p=0.07–0.08). None of these differences maintained significance after adjustment for the 13 comparisons made. Several brain regions also showed greater 13C-labeling of GABA-C2 in the EtOH-treated group: cerebellum (p=0.021), midbrain (p=0.001), ventral tegmental area (p=0.029), thalamus (p=0.024), hippocampus (p=0.03), striatum (p=0.028), and temporal cortex (p=0.011). Only the observed difference in the midbrain maintained significance after adjustment for 13 tests (p=0.014). It should be noted that the signal-to-noise ratios for Glu, Gln, and GABA were ~200, ~100, and ~30, respectively, much tighter than the within-group variations of each region. The variations reflect typical biological variability in 13C enrichments (Patel et al. 2005, Wang et al. 2010, Chateil et al. 2001). Therefore, the regions were also averaged as cortex and whole brain. The regional enrichments of Glu-C4 and GABA-C2 were significantly greater in the treated animals (Fig. 4) for both averaged tissues (un-adjusted p = 0.018 – 0.024; adjusted p = 0.037 – 0.048).
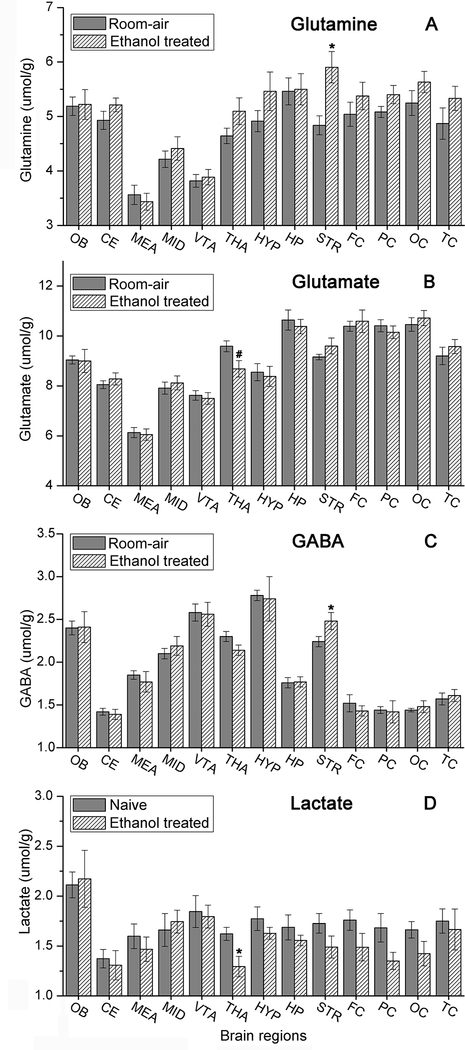
Fig. 5 and Table 2S show the different concentrations of Glu, Gln and GABA in various regions of the brain. The levels of brain lactate in Fig. 5D show that brain fixation occurred within 1–2 seconds. EtOH raised GABA and Gln in the STR (p=0.025 and 0.046) and decreased Glu in the THA (p=0.006), although with a Bonferroni correction of 13, significance was not retained. The striatal increase of GABA and Gln was the opposite of that seen with nicotine (Wang et al. 2010), perhaps a neurochemical sign of compensatory actions between nicotine and alcohol (Greenstein et al. 2010).
Fig. 5.
The total concentration of (A) glutamine, (B) glutamate, (C) GABA, and (D) lactate in the 13 brain regions for room-air (9 rats) and ethanol treated (8 rats) groups. (*p<0.05, #p<0.01).
Discussion
Previous reports have shown that the brain consumes acetate that is converted from EtOH by the liver. In this study, we assessed whether chronic exposure to EtOH can increase the cerebral oxidative capacity for acetate, as seen in human chronic heavy drinkers (Volkow et al. 2013, Jiang et al. 2013). Indeed, three weeks of exposure to EtOH vapor did increase acetate consumption, consistent with previous reports of increased uptake of acetate (Volkow et al. 2013, Jiang et al. 2013) and decreased uptake of glucose in the presence of EtOH (Volkow et al. 2006, Pawlosky et al. 2010)
Availability of EtOH-Derived Acetate
When alcohol is converted to the intermediate acetaldehyde and then to acetate, the step from acetaldehyde to acetate is irreversible, while the equilibrium between EtOH and acetaldehyde favors a lower acetaldehyde level. The irreversible step from acetaldehyde to acetate continuously draws away acetaldehyde, and so also contributes to keeping the concentration of acetaldehyde much lower than the levels of EtOH and acetate (Umulis et al. 2005). The liver consumes some of the acetate via acetyl-coA by AcetylCoA synthetase (AceCS), which is critical for increased histone acetylation at promoter regions of proinflammatory genes and consequent enhancement of the inflammatory response in acute alcoholic hepatitis (Kendrick et al. 2010). The brain contains appreciable quantities of AceCS (Yip et al. 1991) and so may also be subject to inflammation, similarly to the liver. However, because the capacity of the liver to consume acetate is limited (Jucker et al. 1998), most hepatically generated acetate is released to the blood, serving as a potential fuel for other organs, including the brain. In the present study, upon injection of 13C-EtOH, plasma acetate concentrations and 13C-enrichments rose quickly, which confirmed the availability of EtOH-derived acetate (Jucker et al. 1998) as a substrate for brain metabolism within minutes of EtOH administration. Both groups of animals had significant acetate availability following EtOH administration.
A potential alternate way for EtOH to provide energy for the brain is the intracerebral conversion to acetaldehyde from EtOH (Fig. 1), although in the brain this pathway is believed to operate significantly more slowly than acetate oxidation (Zimatkin et al. 1998, Warner & Gustafsson 1994, Aragon et al. 1992).
Acetate Consumption with Chronic Exposure
The consumption of EtOH-derived acetate by the brain was consistent with previous observations in rodents (Learn et al. 2003, Roach & Reese 1971, Pawlosky et al. 2010) and humans (Volkow et al. 1990, Volkow et al. 2013, Volkow et al. 2006) that showed that in the presence of EtOH, the brain consumes acetate and decreases its use of glucose. Of particular interest is Pawlosky’s observation that the decrease in glucose phosphorylation is proportional to the concentration of acetate in the blood (Pawlosky et al. 2010). The finding in this study that the capacity of the brain to consume acetate was increased by chronic EtOH exposure is consistent with reports of increased acetate consumption in human heavy drinkers (Volkow et al. 2013, Jiang et al. 2013) and of increased enzyme activities in EtOH-treated rats (Kiselevski et al. 2003). Furthermore, the chronic ethanol treatment may result in upregulation of BBB acetate transport (Jiang et al. 2013), which could be caused by elevations of ethanol-derived acetate and/or exposure to ketone bodies due to reduced food intake (Pifferi et al. 2011, Leino et al. 2001). The consistency of the observations in humans with this and previous rodent work indicates that rats have sufficiently similar metabolic responses to EtOH to serve as useful models to study EtOH’s effects on acetate metabolism.
Practical Implications for Heavy Drinking
Although the body mass index in humans is slightly higher with more intense drinking (French et al. 2010), and heavy drinkers have higher blood glucose levels when sober (Leggio et al. 2009), drinking while fasting can acutely cause hypoglycemia (Brecher & Lehti 1996, Maddison 1968, Huang & Sjoholm 2008, Juhlin-Dannfelt 1977), due to stimulation of insulin secretion (Huang & Sjoholm 2008) and decreased gluconeogenesis (Krebs et al. 1969). Compounding low blood sugar, there is evidence that some alcoholics have depressed counter-regulatory responses to hypoglycemia (Wright 1978). Thus in an abusing or dependent population that is malnourished, the additional fuel could provide some reward in the form of energetic benefit. The availability of acetate and the increased capacity of the brain to consume it provide the potential for acetate to serve as a compensatory caloric reward that encourages a drinker to continue a drinking session, particularly in times of failure to eat. The caloric perspective remains a hypothesis that requires a separate study to evaluate it.
An additional promoter of EtOH consumption may be the elevation of adenosine: Acetate oxidation produces adenosine (Kiviluoma et al. 1989), and EtOH itself raises extracellular adenosine (Mailliard & Diamond 2004, Asatryan et al. 2011), which is sedating, with properties similar to EtOH intoxication (Carmichael et al. 1991, Carmichael et al. 1993). The EtOH-based elevation of blood acetate persists for up to 24 hours (Pronko et al. 1997), so heavy drinkers are chronically exposed to high levels of acetate, and some dependent drinkers likely have constant elevations of acetate and, therefore, adenosine. If the capacity for acetate consumption is higher, as seen in the chronically treated rats, then any adenosine effects can be expected to be enhanced. Faced with a persistent elevation of adenosine, the brain likely adapts, and during withdrawal the loss of adenosine may contribute to symptoms (Volkow et al. 2013). Although this proposal remains a theory, adenosinergic changes have been reported in dependence and withdrawal, with potential use in facilitating detoxification (Jarvis & Becker 1998, De Witte et al. 2003).
Greater 13C Enrichments in the Olfactory Bulb
The OB had higher 13C enrichments than the other regions. The difference may arise from a more open BBB than in other brain regions (Dobrogowska & Vorbrodt 1999, Ueno et al. 1996). With respect to the BBB, acetate transport occurs at a rate that is on the order of consumption (Patel et al. 2010, Deelchand et al. 2009), so a more permissive BBB is expected to raise the brain acetate level. Because the intracerebral acetate concentration is near the KM for its utilization (Patel et al. 2010, Deelchand et al. 2009, Mason et al. 2006), one would expect a higher brain acetate level to increase the rate of acetate consumption.
The Efficacy of the Focused Microwave Irradiation
Lactate is highly sensitive to ischemia, rising many-fold within seconds of death if brain fixation is not rapid (Nilsson et al. 1975). The microwave technique assures a death faster than other methods (<2 seconds), does not provoke distress in the animal (Mayne et al. 1999), and nearly all types of enzymes are deactivated. Accordingly, the lactate concentrations (Fig. 5D) agree closely with concentrations reported for lactate in Wistar rats 1–2 seconds after death (Nilsson et al. 1975), confirming minimal post-mortem metabolism. The concentration of GABA is also subject to post-mortem changes, but a delay of 1–2 seconds in tissue fixation has a negligible effect (Erdo 1984), as is also true for Glu and Gln (Petroff et al. 1988). Therefore, the microwave-fixed tissue closely reflected metabolism immediately prior to sacrifice.
Weight of the animals and caloric intake
EtOH-treated rats consumed ~7.8g chow less per day than the room-air rats, equivalent to 101 kJ less energy (13 kJ/g, Harlan Laboratories) and did not increase their food intake over the weeks of the study, but oxidation of EtOH can provide energy. During exposure to EtOH vapor, the rat blood alcohol level in a vapor chamber linear increases gradually over several hours of exposure (Gilpin et al. 2009), and in the present study reached a concentration of ~32.6 mM (150 mg/dL) in the blood. Considering the 68.1% body water in rats (Foy & Schnieden 1960), an EtOH elimination rate of 0.75mg/dl/min with zero-order kinetics (Gilpin et al. 2009), and the growth of the exposed animals from 181 to 264g over the three-week procedure, the EtOH cleared daily by each rat can be estimated as 773mg, with a potential caloric value of 23 kJ/day (Coyle 1995). Thus, the EtOH potentially ameliorated some of the weight loss due to the lower food consumption in the exposed group.
Limitations and Future Directions
A potential ambiguity in this study is the intracerebral oxidation of EtOH, which has been long debated (Zimatkin & Buben 2007, Mukherji et al. 1975, Zimatkin et al. 1998, Deng & Deitrich 2008, Towne 1964). Even if the ethanol can be oxidized intracerebrally, the process is believed to be slow (Zimatkin et al. 1998, Xiang & Shen 2011, Warner & Gustafsson 1994, Aragon et al. 1992), but could potentially have contributed to Glu and Gln. According to published kinetics of ethanol metabolism in preparations of brain tissue (Warner & Gustafsson 1994, Banay-Schwartz et al. 1992, Inoue & Lindros 1982, Kiessling 1962a), 13C ethanol might occur at a rate of ~0.012 mmol/min/g. For a TCA rate of ~1–2 μmol/min/g in awake, freely moving rats (Wang et al. 2010, Bryan et al. 1983) one would predict 13C labeling of ~0.5–1% from ethanol, but to make that measurement in vivo would require a different approach. We found no published information about the impact of ethanol exposure on brain ethanol oxidation, so there is a possibility that some of the increased labeling came from induced intracerebral oxidation. It would have been ideal to measure the first product of ethanol oxidation in the brain, acetaldehyde, but its concentration is very low (Kiessling 1962b) and in this study was removed by the extraction procedures and lyophilization. A direct measurement of forward flow of EtOH oxidation remains to be shown in the living brain.
Another possible confound is that EtOH treatment in rats can result in reduced weight gain (Jucker et al. 1994), consistent with the present study. It cannot be ruled out that the observed elevation in what is probably acetate consumption might be related to the lower weight gain, although the similarity to findings in humans and rats suggests otherwise (Volkow et al. 2013, Jiang et al. 2013, Pawlosky et al. 2010).
This study was planned to assess metabolic adaptation of cerebral energy metabolism with chronic alcohol exposure, not dependence, to see if ethanol exposure was sufficient to induce greater carbon consumption from EtOH. We purposely chose to use a duration and intensity of exposure that was significantly less than that applied previously to induce dependence (Ferko & Bobyock 1977), so as to evaluate the impact of something like daily heavy drinking in the period before dependence. The duration of the exposure was unlikely to induce dependence, but it was enough to increase brain metabolism of EtOH-derived acetate. It may be that systemic acetate production and elimination are affected by chronic exposure (Nuutinen et al. 1985, Kozawa et al. 2007) and could contribute to the observed increase in the capacity of the brain to use acetate. In future studies it would be useful to measure blood acetate levels not only during the acute administration, but also during the chronic period of vapor exposure, to assess whether systemic metabolism of alcohol changed over time and might contribute to the observed increase in the capacity of the brain to consume acetate.
The small sample size limits the ability to interpret the regional data from the 13 brain areas that were examined in this study. A larger number of animals or follow-up studies that focused on particular brain regions like the STR would provide greater certainty in the assessment of ethanol exposure on their metabolism.
Important subsequent steps for this line of research are mechanistic in nature. Other mechanisms important for alcohol dependence and detoxification are whether changes in acetate consumption are accompanied by decreased glucose phosphorylation and enzyme activities related to glucose metabolism. For example, if the brain switches its enzymatic milieu to facilitate the consumption of acetate, then in early sobriety a return to more normal oxidation of glucose could be delayed. It would also be useful to disambiguate the source of increased capacity for acetate consumption with pair-fed animals to discriminate the impact of less caloric intake from repeated exposure to ethanol and acetate.
Conclusions
Our data show that three weeks of EtOH vapor exposure in rats was sufficient to increase cerebral metabolism of EtOH-derived acetate, labeling the major brain amino acid pools of Gln, Glu, and GABA. The increased capacity to consume acetate is consistent with the notion that ethanol-derived acetate can partially displace cerebral metabolism of glucose (Volkow et al. 2006, Volkow et al. 1990, Pawlosky et al. 2010, Learn et al. 2003, Roach & Reese 1971, Volkow et al. 2013). To assess the possibility of intracerebral oxidation of ethanol itself will require further work.
Supplementary Material
Acknowledgments
The research was supported by NIH grants R21 AA018210 (GM) and R21 AA019803 (GM), and P30 NS052519, and National Natural Science Foundation of China (NSFC) 21105116 (JW). The authors thank Sean O’Connor for providing the pharmacokinetic model and guidance in its use to derive the ethanol infusion protocol. We also acknowledge Brandon J Dorry for instruction on blood collection and information about the rat chow, and Golam Chowdhury for instruction on the use of the microwave machine.
These results were presented, in part, at the Annual Meetings of Research Society on Alcoholism, June 23–27, 2012, San Francisco, California.
Abbreviation:
- EtOH
Ethanol
- BBB
Blood-brain barrier
- AceCS
AcetylCoA synthetase
Footnotes
Financial Disclosures
KLB reports ownership of Pfizer common stock. All other authors report no biomedical financial interests or potential conflicts of interest.
References
- Aragon CM, Rogan F and Amit Z (1992) Ethanol metabolism in rat brain homogenates by a catalase-H2O2 system. Biochem. Pharmacol 44, 93–98. [DOI] [PubMed] [Google Scholar]
- Asatryan L, Nam HW, Lee MR, Thakkar MM, Saeed Dar M, Davies DL and Choi D-S (2011) Implication of the purinergic system in alcohol use disorders. Alcohol. Clin. Exp. Res 35, 584–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banay-Schwartz M, Kenessey A, DeGuzman T, Lajtha A and Palkovits M (1992) Protein content of various regions of rat brain and adult and aging human brain. Age 15, 51–54. [DOI] [PubMed] [Google Scholar]
- Brecher AS and Lehti MD (1996) A hypothesis linking hypoglycemia, hyperuricemia, lactic acidemia, and reduced gluconeogenesis in alcoholics to inactivation of glucose-6-phosphatase activity by acetaldehyde. Alcohol 13, 553–557. [DOI] [PubMed] [Google Scholar]
- Brunner E, Domhof S and Langer F (2002) Nonparametric analysis of longitudinal data in factorial experiments. New York, NY: John Wiley and Sons. [Google Scholar]
- Bryan RMJ, Hawkins RA, Mans AM, Davis DW and Page RB (1983) Cerebral glucose utilization in awake unstressed rats. Am. J. Physiol 244, C270–C275. [DOI] [PubMed] [Google Scholar]
- Carmichael FJ, Israel Y, Crawford M, Minhas K, Saldivia V, Sandrin S, Campisi P and Orrego H (1991) Central nervous system effects of acetate: Contribution to the central effects of ethanol. J. Pharmacol. Exp. Ther 259, 403–408. [PubMed] [Google Scholar]
- Carmichael FJ, Orrego H and Israel Y (1993) Acetate-induced adenosine mediated effects of ethanol. Alcohol Alcohol Suppl. 2, 411–418. [PubMed] [Google Scholar]
- Chateil J-F, Biran M, Thiaudière E, Canioni P and Merle M (2001) Metabolism of [1-13C]glucose and [2-13C]acetate in the hypoxic rat brain. Neurochem. Int 38, 399–407. [DOI] [PubMed] [Google Scholar]
- Cohen G, Sinet PM and Heikkila R (1980) Ethanol oxidation by rat-brain invivo. Alcohol. Clin. Exp. Res 4, 366–370. [DOI] [PubMed] [Google Scholar]
- Coyle EF (1995) Fat metabolism during exercise. Sports Science Exchange 8, 59–65. [Google Scholar]
- De Witte P, Pinto E, Ansseau M and Verbanck P (2003) Alcohol and withdrawal: from animal research to clinical issues. Neurosci. Biobehav. Rev 27, 189–197. [DOI] [PubMed] [Google Scholar]
- Deelchand DK, Shestov AA, Koski DM, Ugurbil K and Henry PG (2009) Acetate transport and utilization in the rat brain. J. Neurochem 109, 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X. s. and Deitrich RA (2008) Putative role of brain acetaldehyde in ethanol addiction. Curr. Drug Abuse Rev 1, 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derr RF, Draves K and Derr M (1981) Abatement by acetate of an ethanol withdrawal syndrome. Life Sci. 29, 1787–1790. [DOI] [PubMed] [Google Scholar]
- Diluzio NR and Stege TE (1979) Influence of chronic ethanol vapor inhalation on hepatic parenchymal and Kupffer cell function. Alcohol. Clin. Exp. Res 3, 240–247. [DOI] [PubMed] [Google Scholar]
- Dobrogowska DH and Vorbrodt AW (1999) Quantitative immunocytochemical study of blood-brain barrier glucose transporter (GLUT-1) in four regions of mouse brain. J. Histochem. Cytochem 47, 1021–1029. [DOI] [PubMed] [Google Scholar]
- Erdo SL (1984) Postmortem increase of GABA levels in peripheral rat tissues: prevention by 3-mercapto-propionic acid. J. Neural Transm 60, 303–314. [DOI] [PubMed] [Google Scholar]
- Ferko AP and Bobyock E (1977) Induction of physical dependence in rats by ethanol inhalation without the use of pyrazole. Toxicol. Appl. Pharmacol 40, 269–276. [DOI] [PubMed] [Google Scholar]
- Foy JM and Schnieden H (1960) Estimation of total body water (virtual tritium space) in the rat, cat, rabbit, guinea-pig and man, and of the biological half-life of tritium in man. J. Physiol.-London 154, 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French MT, Norton EC, Fang H and Maclean JC (2010) Alcohol consumption and body weight. Health Econ. 19, 814–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Smith AD, Cole M, Weiss F, Koob GF and Richardson HN (2009) Operant behavior and alcohol levels in blood and brain of alcohol-dependent rats. Alcohol. Clin. Exp. Res 33, 2113–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenstein JE, Kassel JD, Wardle MC, Veilleux JC, Evatt DP, Heinz AJ, Roesch LL, Braun AR and Yates MC (2010) The Separate and Combined Effects of Nicotine and Alcohol on Working Memory Capacity in Nonabstinent Smokers. Exp. Clin. Psychopharmacol 18, 120–128. [DOI] [PubMed] [Google Scholar]
- Gruetter R, Seaquist ER and Ugurbil K (2001) A mathematical model of compartmentalized neurotransmitter metabolism in the human brain. Am. J. Physiol. - Endocrin. Metab 281, E100–112. [DOI] [PubMed] [Google Scholar]
- Haberg A, Qu H, Haraldseth O, Unsgard G and Sonnewald U (1998) In vivo injection of 1-C-13 glucose and 1,2-C-13 acetate combined with ex vivo C-13 nuclear magnetic resonance spectroscopy: A novel approach to the study of middle cerebral artery occlusion in the rat. J. Cereb. Blood Flow Metab 18, 1223–1232. [DOI] [PubMed] [Google Scholar]
- Hassel B and Sonnewald U (1995) Selective inhibition of the tricarboxylic acid cycle of GABAergic neurons with 3-nitropropionic acid in vivo. J. Neurochem 65, 1184–1191. [DOI] [PubMed] [Google Scholar]
- Hellman L, Rosenfeld RS and Gallagher TF (1954) Cholesterol synthesis from C-14-acetate in man. J. Clin. Invest 33, 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z and Sjoholm A (2008) Ethanol acutely stimulates islet blood flow, amplifies insulin secretion, and induces hypoglycemia via nitric oxide and vagally mediated mechanisms. Endocrinology 149, 232–236. [DOI] [PubMed] [Google Scholar]
- Inoue K and Lindros KO (1982) Subcellular distribution of human brain aldehyde dehydrogenase. J. Neurochem 38, 884–888. [DOI] [PubMed] [Google Scholar]
- Israel Y, Orrego H and Carmichael FJ (1994) Acetate-mediated effects of ethanol. Alcohol. Clin. Exp. Res. 18, 144–148. [DOI] [PubMed] [Google Scholar]
- Jarvis MF and Becker HC (1998) Single and repeated episodes of ethanol withdrawal increase adenosine A1, but not A2A, receptor density in mouse brain. Brain Res. 786, 80–88. [DOI] [PubMed] [Google Scholar]
- Jiang L, Gulanski BI, De Feyter HM et al. (2013) Increased Brain Uptake and Oxidation of Acetate in Heavy Drinkers. J Clin Investig 123, 1605–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Herzog RI, Mason GF, de Graaf RA, Rothman DL, Sherwin RS and Behar KL (2009) Recurrent Antecedent Hypoglycemia Alters Neuronal Oxidative Metabolism In Vivo. Diabetes 58, 1266–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jucker BM, Barnard ML and Shulman RG (1994) NMR investigation of the futile cycling of ethanol in chronic alcoholic rats. Alcohol. Clin. Exp. Res 18, 1377–1385. [DOI] [PubMed] [Google Scholar]
- Jucker BM, Lee JY and Shulman RG (1998) In vivo 13C NMR measurements of hepatocellular tricarboxylic acid cycle flux. J. Biol. Chem 273, 12187–12194. [DOI] [PubMed] [Google Scholar]
- Juhlin-Dannfelt A (1977) Ethanol effects of substrate utilization by the human brain. Scand. J. Clin. Lab. Investig 37, 443–449. [PubMed] [Google Scholar]
- Kendrick SFW, O’Boyle G, Mann J, Zeybel M, Palmer J, Jones DEJ and Day CP (2010) Acetate, the key modulator of inflammatory responses in acute alcoholic hepatitis. Hepatology 51, 1988–1997. [DOI] [PubMed] [Google Scholar]
- Kiessling KH (1962a) The occurrence of acetaldehyde in various parts of rat brain after alcohol injection, and its effect on pyruvate oxidation. 27, 367–368. [DOI] [PubMed] [Google Scholar]
- Kiessling KH (1962b) The occurrence of acetaldehyde in various parts of rat brain after alcohol injection, and its effect on pyruvate oxidation. Exp. Cell Res 27, 367–368. [DOI] [PubMed] [Google Scholar]
- Kiselevski Y, Oganesian N, Zimatkin S, Szutowicz A, Angielski S, Niezabitowski P, Uracz W and Gryglewski RJ (2003) Acetate metabolism in brain mechanisms of adaptation to ethanol. Med. Sci. Monit 9, 178–182. [PubMed] [Google Scholar]
- Kiviluoma KT, Peuhkurinen KJ and Hassinen IE (1989) Adenine nucleotide transport and adenosine production in isolated rat heart mitochondria during acetate metabolism. Biochim. Biophys. Acta Bioenerg 974, 274–281. [DOI] [PubMed] [Google Scholar]
- Korri UM, Nuutinen H and Salaspuro M (1985) Increased blood acetate: a new laboratory marker of alcoholism and heavy drinking. Alcohol. Clin. Exp. Res 9, 468–471. [DOI] [PubMed] [Google Scholar]
- Kozawa S, Yukawa N, Liu JY, Shimamoto A, Kakizaki E and Fujimiya T (2007) Effect of chronic ethanol administration on disposition of ethanol and its metabolites in rat. Alcohol 41, 87–93. [DOI] [PubMed] [Google Scholar]
- Krebs HA, Freedland RA, Hems R and Stubbs M (1969) Inhibition of hepatic gluconeogenesis by ethanol. Biochem. J 112, 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Learn JE, Smith DG, McBride WJ, Lumeng L and Li TK (2003) Ethanol effects on local cerebral glucose utilization in high-alcohol-drinking and low-alcohol-drinking rats. Alcohol 29, 1–9. [DOI] [PubMed] [Google Scholar]
- Lebon V, Petersen KF, Cline GW, Shen J, Mason GF, Dufour S, Behar KL, Shulman GI and Rothman DL (2002) Astroglial contribution to brain energy metabolism in humans revealed by 13C nuclear magnetic resonance spectroscopy: Elucidation of the dominant pathway for neurotransmitter glutamate repletion and measurement of astrocytic oxidative metabolism. J. Neurosci 22, 1523–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio L, Ray LA, Kenna GA and Swift RM (2009) Blood glucose level, alcohol heavy drinking, and alcohol craving during treatment for alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) Study. Alcohol. Clin. Exp. Res 33, 1539–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leino RL, Gerhart DZ, Duelli R, Enerson BE and Drewes LR (2001) Diet-induced ketosis increases monocarboxylate transporter (MCT1) levels in rat brain. Neurochem. Int 38, 519–527. [DOI] [PubMed] [Google Scholar]
- Lundquist F, Sestoft L, Damgaard SE, Clausen JP and Trap-Jensen J (1973) Utilization of acetate in the human forearm during exercise after ethanol ingestion. J. Clin. Investig 52, 3231–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist F, Winkler K, Munckpet S, Tygstrup N and Mellemgaard K (1962) Ethanol metabolism and production of free acetate in the human liver. J. Clin. Invest 41, 955–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddison LL (1968) Ethanol induced hypoglycemia. Adv Metab Disord 3, 85–109. [DOI] [PubMed] [Google Scholar]
- Mailliard WS and Diamond I (2004) Recent advances in the neurobiology of alcoholism: the role of adenosine. Pharmacol. Therapeut 101, 39–46. [DOI] [PubMed] [Google Scholar]
- Manzo-Avalos S and Saavedra-Molina A (2010) Cellular and mitochondrial effects of alcohol consumption. Int J Environ Res Public Health 7, 4281–4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascord D, Smith J, Starmer GA and Whitfield JB (1992) Effects of increasing the rate of alcohol metabolism on plasma acetate concentration. Alcohol Alcohol. 27, 25–28. [PubMed] [Google Scholar]
- Mason GF, Petersen KF, Lebon V, Rothman DL and Shulman GI (2006) Increased brain monocarboxylic acid transport and utilization in type 1 diabetes. Diabetes 55, 929–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayne M, Shepel PN and Geiger JD (1999) Recovery of high-integrity mRNA from brains of rats killed by high-energy focused microwave irradiation. Brain Res. Protoc 4, 295–302. [DOI] [PubMed] [Google Scholar]
- Mukherji B, Kashiki Y, Ohyanagi H and Sloviter HA (1975) Metabolism of ethanol and acetaldehyde by isolated perfused rat-brain. J. Neurochem 24, 841–843. [PubMed] [Google Scholar]
- Natali F, Siculella L, Salvati S and Gnoni GV (2007) Oleic acid is a potent inhibitor of fatty acid and cholesterol synthesis in C6 glioma cells. J. Lipid Res 48, 1966–1975. [DOI] [PubMed] [Google Scholar]
- Nilsson B, Norberg K, Nordström C-H and Siesjö BK (1975) Rate of Energy Utilization in the Cerebral Cortex of Rats. Acta Physiol. Scand 93, 569–571. [DOI] [PubMed] [Google Scholar]
- Norberg A, Jones AW, Hahn RG and Gabrielsson JL (2003) Role of variability in explaining ethanol pharmacokinetics - Research and forensic applications. Clin. Pharmacokinet 42, 1–31. [DOI] [PubMed] [Google Scholar]
- Nuutinen H, Lindros K, Hekali P and Salaspuro M (1985) Elevated blood acetate as indicator of fast ethanol elimination in chronic alcoholics. Alcohol 2, 623–626. [DOI] [PubMed] [Google Scholar]
- Oldendorf W (1973) Carrier-mediated blood-brain barrier transport of short-chain monocarboxylic organic acids. Am. J. Physiol 224, 1450–1453. [DOI] [PubMed] [Google Scholar]
- Orrego H, Carmichael FJ and Israel Y (1988) New insights on the mechanism of the alcohol-induced increase in portal blood flow. Can. J. Physiol. Pharmacol 66, 1–9. [DOI] [PubMed] [Google Scholar]
- Patel AB, de Graaf RA, Mason GF, Rothman DL, Shulman RG and Behar KL (2005) The contribution of GABA to glutamate/glutamine cycling and energy metabolism in the rat cortex in vivo. P. Natl. Acad. Sci. USA 102, 5588–5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AB, de Graaf RA, Rothman DL, Behar KL and Mason GF (2010) Evaluation of cerebral acetate transport and metabolic rates in the rat brain in vivo using 1H-[13C]-NMR. J. Cereb. Blood Flow Metab 30, 1200–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlosky RJ, Kashiwaya Y, Srivastava S, King MT, Crutchfield C, Volkow N, Kunos G, Li TK and Veech RL (2010) Alterations in brain glucose utilization accompanying elevations in blood ethanol and acetate concentrations in the rat. Alcohol. Clin. Exp. Res 34, 375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng G-S, Chen Y-C, Tsao T-P, Wang M-F and Yin S-J (2007) Pharmacokinetic and pharmacodynamic basis for partial protection against alcoholism in Asians, heterozygous for the variant ALDH2*2 gene allele. Pharmacogenetics Genom. 17, 845–855. [DOI] [PubMed] [Google Scholar]
- Petroff OAC, Ogino T and Alger JR (1988) High-resolution proton magnetic resonance spectroscopy of rabbit brain regional metabolites levels and postmortem changes. J. Neurochem 51, 163–171. [DOI] [PubMed] [Google Scholar]
- Pifferi F, Tremblay S, Croteau E, Fortier M, Tremblay-Mercier J, Lecomte R and Cunnane SC (2011) Mild experimental ketosis increases brain uptake of C-11-acetoacetate and F-18-fluorodeoxyglucose: a dual-tracer PET imaging study in rats. Nutr. Neurosci 14, 51–58. [DOI] [PubMed] [Google Scholar]
- Pronko PS, Velichko MG, Moroz AR and Rubanovich NN (1997) Low-molecular-weight metabolites relevant to ethanol metabolism: correlation with alcohol withdrawal severity and utility for identification of alcoholics. Alcohol Alcohol. 32, 761–768. [DOI] [PubMed] [Google Scholar]
- Rae C, Fekete AD, Kashem MA, Nasrallah FA and Broer S (2012) Metabolism, compartmentation, transport and production of acetate in the cortical brain tissue slice. Neurochem. Res 37, 2541–2553. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA and O’Connor S (2006) Studying alcohol elimination using the alcohol clamp method. Alcohol Res. Health 29, 286–290. [PMC free article] [PubMed] [Google Scholar]
- Roach MK and Reese WN (1971) Effect of ethanol on glucose and amino acid metabolism in brain. Biochem. Pharmacol 20, 2805–2812. [DOI] [PubMed] [Google Scholar]
- Roach MK and Reese WN (1972) (2-14C) ethanol as a precursor of glutamine, glutamate, β-aminobutyric acid and aspartate in hamster brain in vivo. Biochem. Pharmacol 21, 2013–2019. [DOI] [PubMed] [Google Scholar]
- Rothman DL, Behar KL, Hetherington HP, den Hollander JA, Bendall MR, Petroff OA and Shulman RG (1985) 1H-Observe/13C-decouple spectroscopic measurements of lactate and glutamate in the rat brain in vivo. Proc. Natl. Acad. Sci. USA 82, 1633–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkola T, Iles MR, Kohlenberg-Mueller K and Eriksson CJP (2002) Ethanol, acetaldehyde, acetate, and lactate levels after alcohol intake in white men and women: Effect of 4-methylpyrazole. Alcohol. Clin. Exp. Res 26, 239–245. [PubMed] [Google Scholar]
- Towne JC (1964) Effect of ethanol and acetaldehyde on liver and brain monoamine oxidase. Nature 201, 709–710. [DOI] [PubMed] [Google Scholar]
- Ueno M, Dobrogowska DH and Vorbrodt AW (1996) Immunocytochemical evaluation of the blood-brain barrier to endogenous albumin in the olfactory bulb and pons of senescence-accelerated mice (SAM). Histochem. Cell Biol 105, 203–212. [DOI] [PubMed] [Google Scholar]
- Umulis DM, Gürmen NM, Singh P and Fogler HS (2005) A physiologically based model for ethanol and acetaldehyde metabolism in human beings. Alcohol 35, 3–12. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wolf AP et al. (1990) Acute effects of ethanol on regional brain glucose metabolism and transport. Psychiatry Res. Neuroimaging 35, 39–48. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Kim SW, Wang G-J et al. (2013) Acute alcohol intoxication decreases glucose metabolism but increases acetate uptake in the human brain. Neuroimage 64, 277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Franceschi D et al. (2006) Low doses of alcohol substantially decrease glucose metabolism in the human brain. Neuroimage 29, 295–301. [DOI] [PubMed] [Google Scholar]
- Wang J, Jiang LH, Du HY and Mason GF (2012) An ethanol vapor chamber system for small animals. J. Neurosci. Methods 208, 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Jiang LH, Jiang YF, Ma XX, Chowdhury GMI and Mason GF (2010) Regional metabolite levels and turnover in the awake rat brain under the influence of nicotine. J. Neurochem. 113, 1447–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waniewski RA and Martin DL (1998) Preferential utilization of acetate by astrocytes is attributable to transport. J. Neurosci. 18, 5225–5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner M and Gustafsson JA (1994) Effect of ethanol on cytochrome P450 in the rat brain. Proc. Natl. Acad. Sci. USA 91, 1019–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J (1978) Endocrine effects of alcohol. Clin. Enidocrinol. Metab 7, 351–367. [DOI] [PubMed] [Google Scholar]
- Xiang Y and Shen J (2011) In vivo detection of intermediate metabolic products of [1-13C] ethanol in the brain using 13C MRS. NMR Biomed. 24, 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip V, Carter JG, Pusateri ME, MeDougal DB and Lowry OH (1991) Distribution in Brain and Retina of Four Enzymes of Acetyl CoA Synthesis in Relation to Choline Acetyl Transferase and Acetylcholine Esterase. Neurochem Res 16, 629–635. [DOI] [PubMed] [Google Scholar]
- Zimatkin SM and Buben AL (2007) Ethanol oxidation in the living brain. Alcohol. Alcohol 42, 529–532. [DOI] [PubMed] [Google Scholar]
- Zimatkin SM, Liopo AV and Deitrich RA (1998) Distribution and kinetics of ethanol metabolism in rat brain. Alcohol. Clin. Exp. Res 22, 1623–1627. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.