Abstract
Background
Conditioned taste aversion (CTA) learning is a highly specialized form of conditioning found across taxa that leads to avoidance of an initially neutral stimulus, such as taste or odor, that is associated with, but is not the cause of, a detrimental health condition. The present study examines if honey bees (Apis mellifera L.) develop ethanol-induced CTA.
Methods
Restrained bees were first administered a sucrose solution that was cinnamon scented, lavender scented, or unscented, and contained either 0%, 2.5%, 5%, 10%, or 20% ethanol. Then, 30 minutes later, we used a proboscis extension response (PER) conditioning procedure where the bees were taught to associate either cinnamon odor, lavender odor, or an air-puff with repeated sucrose feedings. For some bees, the odor of the previously consumed ethanol solution was the same as the odor associated with sucrose in the conditioning procedure. If bees are able to learn ethanol-induced CTA, they should show an immediate low level of response to odors previously associated with ethanol.
Results
We found that bees did not develop CTA despite the substantial inhibitory and aversive effects ethanol has on behavior. Instead, bees receiving a conditioning odor that was previously associated with ethanol showed an immediate high level of response. While this demonstrates bees are capable of one-trial learning common to CTA experiments, this high level of response is the opposite of what would occur if the bees developed a CTA. Responding on subsequent trials also showed a general inhibitory effect of ethanol. Finally, we found that consumption of cinnamon extract reduced the effects of ethanol.
Conclusions
The honey bee’s lack of learned avoidance to ethanol mirrors that seen in human alcoholism. These findings demonstrate the usefulness of honey bees as an insect model for ethanol consumption.
Keywords: honey bee, conditioned taste aversion, taste aversion learning, alcohol, cinnamon
Conditioned taste aversion (CTA) learning is a specialized form of associative conditioning found across taxa that leads to avoidance of an initially neutral stimulus, such as taste or odor, that is associated with, but is not the cause of, a detrimental health condition. The original work on taste aversion learning was conducted with rats, pairing a saccharin solution with gamma radiation. A single, 6-hour radiation exposure while rats drank the solution caused an immediate aversion that persisted for over 40 days (Garcia et al., 1955). This early finding led to a large body of research investigating CTA as well as its evolutionary function (Rozin & Kalat, 1971).
Generally, CTA learning is considered a form of respondent conditioning where an initially neutral conditioned stimulus (CS), such as the taste, odor or appearance of food becomes associated with an unconditioned stimulus (US), an unpleasant illness (Klosterhalfen & Klosterhalfen, 1985; Logue, 1979). Although CTAs are pervasive across taxa (see the supplementary materials for a review), research shows it has some unusual and defining features. First, CTAs can occur after a small number of exposures to aversive stimuli, with many experiments producing behavioral alterations after a single trial (Garcia & Koelling, 1966). The tendency for one-trial acquisition is perhaps the most unique characteristic of CTA, leading many to suggest it is a special form of biologically prepared learning (Seligman, 1970). Second, the development of CTAs can occur despite very long delays between ingestion and the onset of gastrointestinal distress. While most experiments show that optimal conditioning occurs with CS-US delays of only a few seconds (Kimble, 1961), CTAs can occur even with 24-hour delays (Etscorn & Stephens, 1973).
An alternative explanation considers CTA learning to be a form of operant conditioning where the behavior of consuming a specific item is punished by subsequent illness (Fouquet et al., 2001; Li et al., 2013). In some aspects, CTAs show similarities to other forms of aversive operant conditioning (Klosterhalfen & Klosterhalfen, 1985; Logue, 1979). For example, escape/avoidance procedures may also show one-trial learning (Bolles, 1970). The long delay between the behavior and punishment in the operant perspective of CTA may be explained in terms of stimuli, such as aftertaste, that bridge the gap between initial consumption and illness (Bitterman, 1975; 1976). More research is needed to clarify the role of both respondent and operant conditioning as mechanisms of CTA.
Human alcoholism presents an enigma when considering evolutionary theories of CTA. Like other species, humans are known to display CTAs only to specific substances (Riley & Tuck, 1985). However, while ethanol-induced aversions are common (Logue et al., 1981), human alcoholics may not develop an aversion, despite the detrimental effects of ethanol (for a review of effects of alcohol abuse in humans see American Psychiatric Association, 2013). This dichotomy in ethanol-induced CTAs can also be observed in rodent models. While rodents often do show ethanol-induced aversions (Cappell et al., 1973; Chester et al., 2003; Roma et al., 2008), the extent that aversions are developed vary by strain (Broadbent et al., 2002; Risinger & Cunningham, 1995), and some genetically modified rodents show reduced aversions (Blednov et al. 2011; Hill et al., 2003). Given the variability within commonly studied species, comparisons across species may be useful for understanding the factors that affect ethanol-induced CTA. In this paper, we investigate if honey bees (Apis mellifera L.) develop ethanol-induced CTA similar to many nonalcoholic humans and rodent models, or if ethanol-induced CTA is absent or reduced, as in human alcoholics and specialty or modified rodent strains.
Honey bees are ideal invertebrate subjects to investigate ethanol-induced CTA. In terms of psychological ability, honey bees possess ample discrimination and learning capabilities for conditioned aversions. They can discriminate food sources based on a variety of cues, including olfactory and taste (de Brito Sanchez, 2011; Wright et al., 2005). The bee’s response to food, the proboscis extension response (PER), is also highly adaptable and sensitive to both appetitive and aversive consequences (Smith et al., 1991) making it a good indicator of conditioned aversions. Honey bees are also excellent invertebrate models of ethanol consumption (see the supplementary materials for a review). In the following experiment, we investigate ethanol-induced CTA in honey bees using a PER conditioning method. The bees are trained to associate a neutral odor with sucrose. For some bees, that odor was also previously associated with an ethanol solution. If bees are capable of developing an ethanol-induced CTA, we expect that associating an odor with ethanol will inhibit responses the next time that odor is presented.
Materials and Methods
Subjects
Honey bee (Apis mellifera L., n = 640) foragers were collected from an outdoor feeder, containing a 2 M sucrose solution, located 30 m from a single-hive apiary. Foragers collected in this manner are 20–30 days old (Seeley, 1982, 1995; Winston & Neilson-Punnett, 1982), have experience associating odors with reward and learning to navigate (Giurfa, 2007), and are useful in many types of appetitive and aversive learning experiments, in both laboratory and field experiments (Abramson et al., 2015; Craig et al., 2014; Dinges et al., 2013). As there was only one hive in the area, all subjects were assumed to be experimentally naïve foragers from the same hive.
Each bee was captured in a glass vial, and then anesthetized in an ice-water bath. After the bee became inactive, it was removed from the vial and placed into a metal harness. A strip of duct tape was placed between the bee’s head and thorax, and then secured to the sides of the harness. After the bees warmed and became active, each bee was administered a 2 M sucrose solution until the bee no longer extended its proboscis. The bees were left in the harness until the experiment began the next morning. This procedure ensures that subjects have similar levels of motivation to feed when the experiment begins.
Procedure
Pre-conditioning
Thirty minutes prior to the start of the PER conditioning procedure, we used a small glass syringe to feed each bee 10 µl of a 2 M sucrose solution with an odor and ethanol concentration that varied as an experimental parameter. The solution that bees received was cinnamon scented, lavender scented, or unscented and contained 0%, 2.5%, 5%, 10% or 20% ethanol. Thus, there were 5 ethanol concentrations at 3 odor alternatives, creating 15 total solutions. We used two distinct scents as a counterbalance for any innate effects of the scents, or the possibility they might react with ethanol. These specific scents were selected because prior research indicated they produce similarly neutral responses (Abramson et al., 1997, 2010, 2015). We refer to the scent of the sucrose as the pre-conditioning stimulus (PS).
We used the range of 0% to 20% ethanol because the literature shows substantial dose-dependent effects on learning and behavior (e.g., Abramson et al., 2015; Mustard el al., 2008). Generally, low ethanol doses (2.5% to 5%) show little effect on behavior 30 minutes after ingestion (Abramson et al., 2015). High ethanol doses (20% and greater) inhibit learning and may incapacitate bees (Abramson et al., 2015; Mustard el al., 2008). The intermediate dose (10%) shows mixed results, with a major factor being the time between consumption and testing. For example, Abramson et al. (2015) used a 30-minute delay, while Mustard et al. (2008) used a 2-hour delay. Including a large range of doses thus provides the best opportunity to observe ethanol’s effect in a CTA paradigm. Bees that did not feed were discarded from the experiment to ensure that all subjects had similar feeding motivation (see Abramson et al., 1997). See the supplementary material for specific details on sucrose solution preparation.
Conditioning
The conditioning procedure began 30 minutes after the pre-conditioning feeding. Several studies on hemolymph ethanol level indicate this is ample time for bees to be affected by ethanol consumption (Bozic et al., 2007; Maze et al. 2006). See Figure S1 in the supplementary material for hemolymph ethanol levels 30 minutes after consumption of 0% to 45% ethanol solutions. We followed a standard PER conditioning protocol (Abramson et al., 2015; Bitterman et al., 1983). In this procedure, an olfactory conditioned stimulus (CS) is repeatedly associated with a sucrose feeding, the unconditioned stimulus (US). After several CS/US pairings, proboscis extension is elicited by the previously neutral CS.
During the conditioning procedure, bees received one of three CS delivered manually through a syringe: 1) a puff of cinnamon odor to the antenna, 2) a puff of lavender odor to the antennae, or 3) a puff of air to the antennae. See the supplementary materials for specific details on CS odor preparation. Previous research showed that this method produces reliable results consistent with automated techniques (Abramson & Boyd, 2001). During each of the 12 conditioning trials, the subject was placed in front of an exhaust fan to eliminate any potentially lingering odors. After a few moments of acclimation, we used a non-overlap procedure, where the CS (3 seconds) terminated before the US (2 seconds) with an intertrial interval (ITI) between CS presentations of 10 minutes. The US was presented by first touching a subject’s antennae with a strip of filter paper containing 2 M sucrose. Subjects were then allowed to feed from the filter paper for 2 seconds. At the end of the 2-second feeding, the subject was removed from the exhaust fan for a 10-minute ITI, and another subject was placed in front of the exhaust fan in preparation for its trial. During each trial, responses to the CS were recorded manually. If the subject extended its proboscis during the CS presentation, a 1 was recorded. If the bee did not extend its proboscis during the CS presentation, a 0 was recorded. Unconditioned responses to sucrose were similarly recorded. Approximately 15 bees, from multiple experimental conditions, were run in this manner each day.
Groups
Bees were split into several groups based on 1) the ethanol pre-conditioning dose (0%, 2.5%, 5%, 10%, or 20%), 2) the pre-conditioning stimulus (cinnamon scented, lavender scented, or unscented), and 3) the CS used in the conditioning procedure (cinnamon, lavender, or air). Bees that received sucrose in the pre-conditioning procedure with the same scent that they later received as an odor CS in the conditioning procedure were grouped together in the same-stimulus group. Bees that received sucrose in the pre-conditioning procedure with a different scent than they later received as an odor CS in the conditioning procedure were grouped together in the different-stimulus group. For example, bees that received cinnamon-scented sucrose during pre-conditioning and cinnamon odor as a CS were placed in the same-stimulus group, while bees that received cinnamon-scented sucrose during pre-conditioning and lavender odor as a CS were placed in the different-stimulus group. CTA learning predicts that bees fed ethanol will have a lower rate of conditioned response on the first trial in the same-stimulus group than in the different-stimulus group. However, the unconditioned feeding response should be similar between both groups.
We also used two experimental controls. First, the air-control group received an air-puff as a CS. The purpose of the air-control group was to investigate if the bees’ response to the CS is due to the association of the CS with sucrose (US), instead of merely an effect of repeated exposure to cinnamon or lavender odor. Bees in the air-control and different-stimulus groups are both exposed to a novel stimulus (air-puff or odor) that is associated with sucrose during each trial, and thus should show a similar acquisition of conditioned response.
Second, the unscented-sucrose control group received unscented sucrose at 0% ethanol and either a cinnamon or lavender CS. The purpose of unscented-sucrose control group was to investigate the possibility that consumption of cinnamon or lavender during the pre-conditioning procedure would have an effect on acquisition of conditioned response. The unscented-sucrose control group was thus compared to the 0% different-stimulus group to see if the addition of cinnamon or lavender to the pre-conditioning solution affected learning.
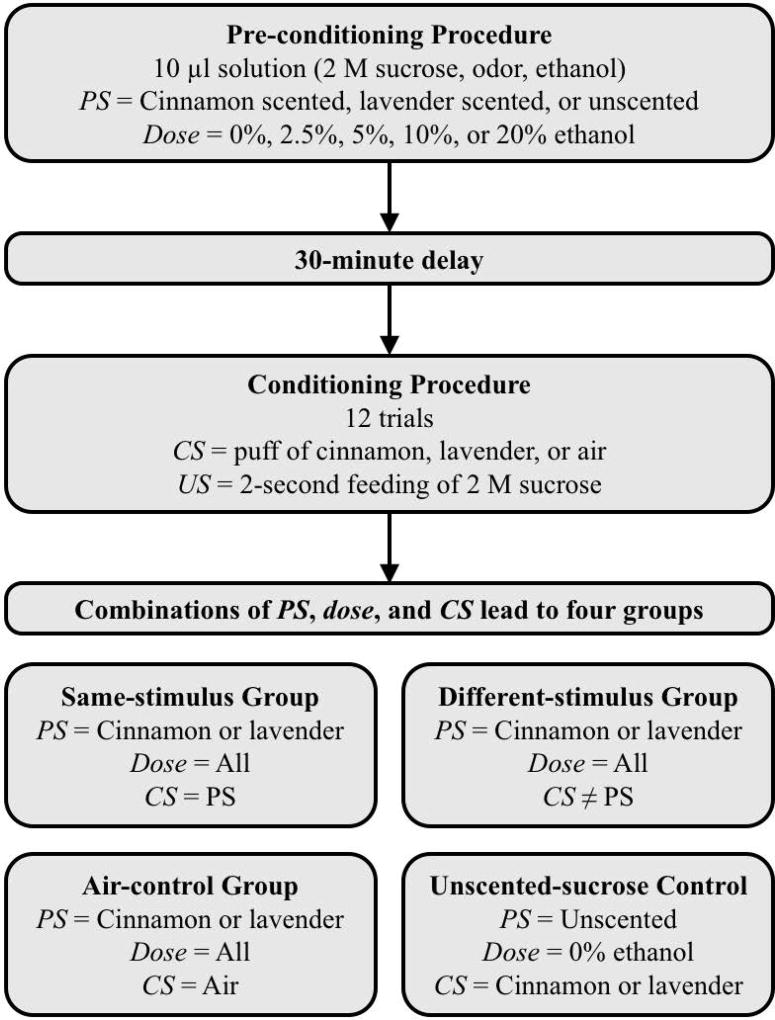
This categorization of subjects resulted in 16 groups of 40 bees each; 5 same-stimulus groups at each dose, 5 different-stimulus groups at each dose, 5 air-control groups at each dose, and 1 unscented-sucrose group at 0% ethanol. A summary of the experimental design can be seen in Table 1 and Figure 1.
Table 1.
| Experimental Design | ||||
|---|---|---|---|---|
|
| ||||
| Group | PS | CS | EtOH Dose | n |
| Same-stimulus | Cinnamon | Cinnamon | 0% | 20 |
| Same-stimulus | Lavender | Lavender | 0% | 20 |
| Same-stimulus | Cinnamon | Cinnamon | 2.5% | 20 |
| Same-stimulus | Lavender | Lavender | 2.5% | 20 |
| Same-stimulus | Cinnamon | Cinnamon | 5% | 20 |
| Same-stimulus | Lavender | Lavender | 5% | 20 |
| Same-stimulus | Cinnamon | Cinnamon | 10% | 20 |
| Same-stimulus | Lavender | Lavender | 10% | 20 |
| Same-stimulus | Cinnamon | Cinnamon | 20% | 20 |
| Same-stimulus | Lavender | Lavender | 20% | 20 |
|
| ||||
| Different-stimulus | Cinnamon | Lavender | 0% | 20 |
| Different-stimulus | Lavender | Cinnamon | 0% | 20 |
| Different-stimulus | Cinnamon | Lavender | 2.5% | 20 |
| Different-stimulus | Lavender | Cinnamon | 2.5% | 20 |
| Different-stimulus | Cinnamon | Lavender | 5% | 20 |
| Different-stimulus | Lavender | Cinnamon | 5% | 20 |
| Different-stimulus | Cinnamon | Lavender | 10% | 20 |
| Different-stimulus | Lavender | Cinnamon | 10% | 20 |
| Different-stimulus | Cinnamon | Lavender | 20% | 20 |
| Different-stimulus | Lavender | Cinnamon | 20% | 20 |
|
| ||||
| Air-control | Cinnamon | Air | 0% | 20 |
| Air-control | Lavender | Air | 0% | 20 |
| Air-control | Cinnamon | Air | 2.5% | 20 |
| Air-control | Lavender | Air | 2.5% | 20 |
| Air-control | Cinnamon | Air | 5% | 20 |
| Air-control | Lavender | Air | 5% | 20 |
| Air-control | Cinnamon | Air | 10% | 20 |
| Air-control | Lavender | Air | 10% | 20 |
| Air-control | Cinnamon | Air | 20% | 20 |
| Air-control | Lavender | Air | 20% | 20 |
|
| ||||
| Unscented-sucrose | None | Cinnamon | 0% | 20 |
| Unscented-sucrose | None | Lavender | 0% | 20 |
Figure 1.
Diagram of the experimental methods. Subjects first entered a pre-conditioning procedure and consumed a sucrose solution scented with the pre-conditioning stimulus (PS) that contained 0% to 20% ethanol. After a 30-minute delay, subjects entered a 12-trial conditioning procedure. During the conditioning procedure subjects received a conditioned stimulus (CS) odor, followed by a sucrose unconditioned stimulus (US) feeding. Combinations of PS, dose and CS lead to the four groups of the experiment.
Analysis
We analyzed both unconditioned and conditioned responses using a repeated measures logistic regression via generalized estimating equations (GEE; Hardin & Hilbe, 2003) with a logistic link. This regression analysis allows us to make statistical comparisons between groups as well as make predictions based on observed data. The logistic aspect of the regression is well suited for learning data with binary responses, such as the presence or absence of proboscis extension. While other techniques, such as analysis of variance or linear regression, provide good approximations for binary data, logistic regression is specialized for this type of data. The repeated measures aspect of the regression controls for repeated measures from each subject across the 12 conditioning trials using an exchangeable dependence structure. This is important as the response of each subject on later trials may be related to their responses on previous trials. Overall, this method is well-suited for learning data with binary responses and has been used by several laboratories to study learning in bees (e.g., Mustard, et al. 2008; Riddell & Mallon, 2005; Simone-Finstrom et al., 2010; Wright et al., 2010). For a comparison between repeated measures logistic regression and other statistical methods to analyze learning in bees, see Hartz et al. (2001). See the supplementary material for a detailed discussion of this technique. Our analyses were conducted through the Stats Models package of SciPy (Jones et al., 2001), a free scientific analysis package for the Python programming language (http://www.python.org).
Results
Initial Group Comparison
We investigated differences in responding between the same-stimulus, different-stimulus, and air-control groups using separate models for both unconditioned response (UR) and conditioned response (CR). For both models, we included the parameters group (same-stimulus or air-control), dose, trial, group × dose interaction, group × trial interaction, dose × trial interaction, and group × dose × trial interaction. The different-stimulus group was included in the intercept, so that both same-stimulus and air-control groups could be easily compared to the different-stimulus group. The intercept can be considered the “starting point” of the model, thus, the same-stimulus group and air-control group parameters, as well as the group interactions refer to disparity between these groups and the different-stimulus group. This analysis considered group to be a categorical variable, while dose and trial were treated as continuous variables. For all parameters estimates reported in the following sections, positive values indicate that parameter (variable) increases the probability of response, while negative values indicate that parameter decreases the probability of response. The absolute value of the parameter estimates indicate the overall magnitude of the effect. For more details on interpreting logistic regression, see the supplementary materials.
The results of this analysis can be seen in Table 2. For UR, we found that the intercept was significant (estimate = 1.963, p = 0.000), and that both the same-stimulus and air-control groups were nonsignificant (p values > 0.4). This effect can also be seen by visually comparing the similarity in UR between the same-stimulus group (Figure 2), the different-stimulus group (Figure 3) and the air-control group (Figure 4). The effect of dose was also significant (estimate = −0.247, p = 0.000), indicating that the motivation or ability to respond decreased as dose increased. The effect of trial was significant (estimate = 0.131, p = 0.001), although increases in trial caused a smaller change in UR than dose. This small increase may be due to habituation to the restraint or a sobering effect. No interactions were significant. The finding that neither group, nor any group interactions were significant suggests that there were no differences in UR as a function of group. For all parameters, the effects are also easily seen when comparing the graphs. These findings are as expected, and show that motivation or ability to respond was similarly reduced by ethanol in all groups.
Table 2.
| Group Unconditioned Response Comparison | |||||
|---|---|---|---|---|---|
|
| |||||
| Parameter | Estimate | Standard Error | 95% Confidence Intervals | p-value | |
| Intercept | 1.963 | 0.381 | [1.216 | 2.710] | 0.000 |
| Same-stimulus group | −0.272 | 0.451 | [−1.156 | 0.612] | 0.547 |
| Air-control group | −0.343 | 0.450 | [−1.226 | 0.539] | 0.446 |
| Dose | −0.247 | 0.050 | [−0.346 | −0.148] | 0.000 |
| Trial | 0.131 | 0.041 | [0.051 | 0.212] | 0.001 |
| Same × Dose | 0.032 | 0.061 | [−0.088 | 0.152] | 0.602 |
| Air × Dose | 0.066 | 0.058 | [−0.048 | 0.181] | 0.255 |
| Same × Trial | −0.082 | 0.049 | [−0.178 | 0.015] | 0.097 |
| Air × Trial | −0.036 | 0.047 | [−0.129 | 0.057] | 0.445 |
| Dose × Trial | −0.002 | 0.004 | [−0.009 | 0.005] | 0.618 |
| Same × Dose × Trial | 0.005 | 0.005 | [−0.004 | 0.015] | 0.256 |
| Air × Dose × Trial | 0.000 | 0.004 | [−0.008 | 0.008] | 0.967 |
| Group Conditioned Response Comparison | |||||
|---|---|---|---|---|---|
|
| |||||
| Parameter | Estimate | Standard Error | 95% Confidence Intervals | p-value | |
| Intercept | −0.369 | 0.231 | [−0.822 | 0.084] | 0.110 |
| Same-stimulus group | 1.089 | 0.322 | [0.458 | 1.720] | 0.001 |
| Air-control group | −0.154 | 0.305 | [−0.753 | 0.444] | 0.613 |
| Dose | −0.137 | 0.033 | [−0.202 | −0.072] | 0.000 |
| Trial | 0.259 | 0.033 | [0.194 | 0.323] | 0.000 |
| Same × Dose | −0.052 | 0.050 | [−0.150 | 0.046] | 0.302 |
| Air × Dose | 0.027 | 0.042 | [−0.055 | 0.110] | 0.521 |
| Same × Trial | −0.169 | 0.041 | [−0.250 | −0.089] | 0.000 |
| Air × Trial | −0.005 | 0.043 | [−0.089 | 0.078] | 0.902 |
| Dose × Trial | −0.009 | 0.003 | [−0.015 | −0.002] | 0.009 |
| Same × Dose × Trial | 0.008 | 0.004 | [−0.000 | 0.017] | 0.064 |
| Air × Dose × Trial | 0.000 | 0.004 | [−0.009 | 0.008] | 0.935 |
Note. The different-stimulus group is included in the intercept. The same-stimulus group and air-control group are abbreviated in the interactions as same and air, respectively.
Figure 2.
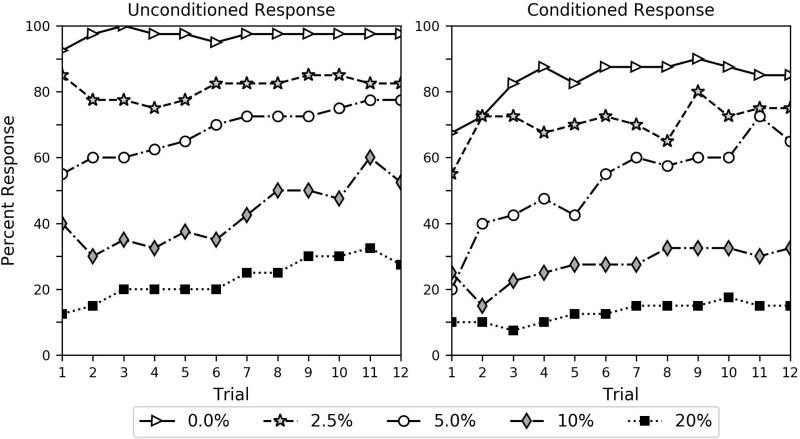
Percent of subjects in the same-stimulus group responding to the unconditioned and conditioned stimuli. The odor the bees received as a conditioned stimulus during the conditioning trials was the same odor that they received in the pre-conditioning solution. The key shows the percent ethanol dose the bees received in the 10 µl 2 M sucrose solution during the pre-conditioning procedure.
Figure 3.
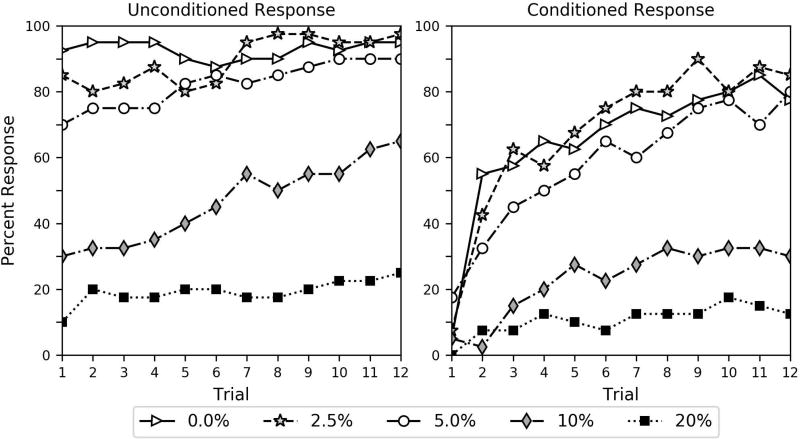
Percent of subjects in the different-stimulus group responding to the unconditioned and conditioned stimuli. The odor the bees received as a conditioned stimulus during the conditioning trials was a different odor than they received in the pre-conditioning solution. The key shows the percent ethanol dose the bees received in the 10 µl 2 M sucrose solution during the pre-conditioning procedure.
Figure 4.
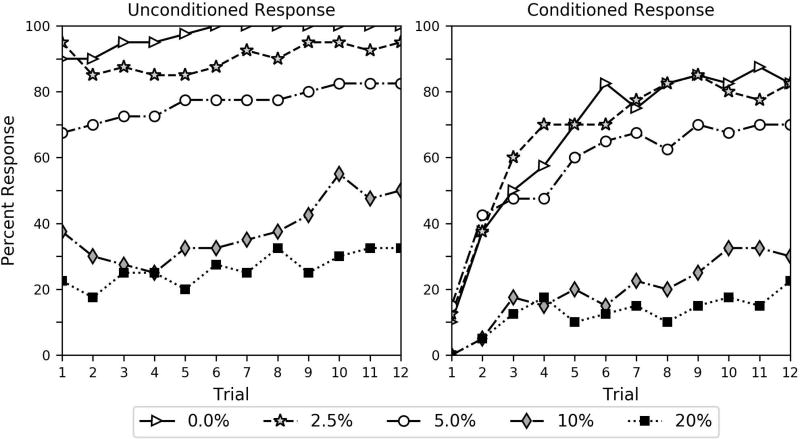
Percent of subjects in the air-control group responding to the unconditioned and conditioned stimuli. For these bees, an unscented air-puff functioned as the conditioned stimulus during the conditioning trials. The key shows the percent ethanol dose the bees received in the 10 µl 2 M sucrose solution during the pre-conditioning procedure.
Table 2 also shows a comparison of CR across groups, while the percent of bees producing CR in each group can be seen in Figures 2, 3 and 4. Additionally, Figure S2 provides a direct visual comparison of CR on the first trial. For CR, we found that the intercept was not significant (estimate = -0.369, p = 0.110), that the same same-stimulus group was significant (estimate = 1.089, p = 0.001), and that the air-control group was not significant (estimate = −0.154, p = 0.613). This finding indicates that bees in the same-stimulus group responded significantly more to the CS than bees in the different-stimulus group. This overall group effect is without consideration of dose or trial (these parameters are discussed below), and shows that associating an odor with ethanol in the pre-conditioning procedure did not inhibit responses as would be predicted by CTA. The analysis also found no differences between the different-stimulus group and the air-control group, suggesting that the bees were responding to CS odors due to their association with the sucrose US, and not because cinnamon or lavender odor innately elicits responding.
The effect of dose was significant (estimate = −0.137, p = 0.000), indicating that ethanol consumption reduced the probability of CR. This alone does not suggest that ethanol affected learning, rather it shows that ethanol reduces some aspect of CR, such as learning, ability, or motivation. The effect of trial was also significant (estimate = 0.259, p = 0.000), and shows the overall learning effect (increase in CR) across the 12 trials. Additionally, the interaction of dose and trial was significant (estimate = −0.009, p = 0.009). Although this is a relatively small effect, it shows that the ability to learn (increasing CR across trials) is reduced as ethanol dose increases. No interactions involving group and dose were significant, indicating that each group was effected similarly by ethanol dose. Taken together, our analysis shows that ethanol reduces CR (dose effect), and that ethanol reduces learning (dose and trial interaction).
The interaction of the same-stimulus group and trial (estimate = −0.169, p = 0.000) was significant. Note that the parameter estimate for this interaction is negative, while the overall trial effect is positive. This shows that learning (increase in CR across trials) was significantly lower for the same-stimulus group than the different-stimulus group. This can also be seen by comparing the acquisition curves in Figures 2 and 3. The reduced learning effect in the same-stimulus group occurs because these bees learned the association during the preconditioning procedure instead of during the 12 conditioning trials. This can also be seen in terms of the high level of CR on the first trial in Figure 2 and Figure S2. If the bees learned an ethanol-induced CTA, they would not show such a high level of CR on the first trial. Indeed, our bees respond exactly the opposite as would be predicted by CTA. Additionally, the immediate CR in the same-stimulus group also shows that the bees were responding specifically to the similarity of the odor CS and PS. Although bees may learn to associate the air-puff during the CS delivery with sucrose, an air-puff was not present during the pre-conditioning procedure.
The CR of the different-stimulus group is remarkably distinct from what was observed for the same-stimulus group. Again, note that the analysis in Table 2 shows that not only is CR significantly different as a function of group (same-stimulus group parameter estimate = 1.089, p = 0.001), but also that the groups are affected significantly different as a function of trial (different-stimulus trial estimate = 0.259, p = 0.000; disparity between same-stimulus and different-stimulus trial parameter estimate = −0.169, p = 0.000). The contrast in acquisition curves can also be clearly observed in Figures 2 and 3. These findings again demonstrate that the bees in the different-stimulus group learned to associate the novel odor with the sucrose feeding across the 12 conditioning trials while the bees in the same-stimulus group already learned the association during the pre-conditioning procedure.
Regression plots displaying the statistical predictions for probability of CR and UR for the same-stimulus, different-stimulus and air-control groups can be seen in Figures S3, S4, and S5. These plots are visual representations of the analysis shown in Table 2. Generally, the regression lines mirror the observed data. The major difference is that the regression does not illustrate the powerful one-trial learning effect we observed. Adding additional parameters to the model may account for these effects. However, there is no clear a priori theory to support additional parameters, and post hoc attempts at model fitting would not greatly benefit our understanding of the bees’ behavior.
As ethanol dose had a substantial effect, we conducted additional analyses directly comparing CR of the same-stimulus and different-stimulus groups at each dose. This analysis considered dose to be a categorical variable, and is discussed in detail in the supplementary material. The overall findings of the individual dose analysis support our previous analysis (Table 2) and provide evidence that our bees did not learn an ethanol-induced CTA. At no dose was acquisition of CR in the same-stimulus group inhibited compared to that of the different-stimulus group, as would be predicted by CTA.
Unscented-sucrose Comparison
As an additional control, we compared the CR of the unscented-sucrose control group to that of the different-stimulus 0% group. This comparison was used to see if consuming cinnamon or lavender scented sucrose during the pre-conditioning procedure affected CR. We did not use the same-stimulus 0% group for this comparison as the one-trial learning effect observed in the same-stimulus group made comparisons to acquisition curves difficult. To make the comparison between CR of unscented-sucrose group and the different-stimulus 0% group we divided the different-stimulus 0% group into two subgroups; those that received cinnamon as a PS, and those that received lavender as a PS. Then, we included a subgroup parameter (cinnamon PS or lavender PS) as the sole parameter in the model, with the unscented-sucrose group contained in the intercept. This allowed the CR curves of bees fed cinnamon or lavender scented sucrose to be compared to those of bees fed unscented-sucrose. Our analysis found no significant parameters (intercept p = 0.159, cinnamon p = 0.514, lavender p = 0.114), indicating that consuming cinnamon or lavender scented sucrose did not have an effect on CR.
Dose and Pre-conditioning Stimulus Interaction
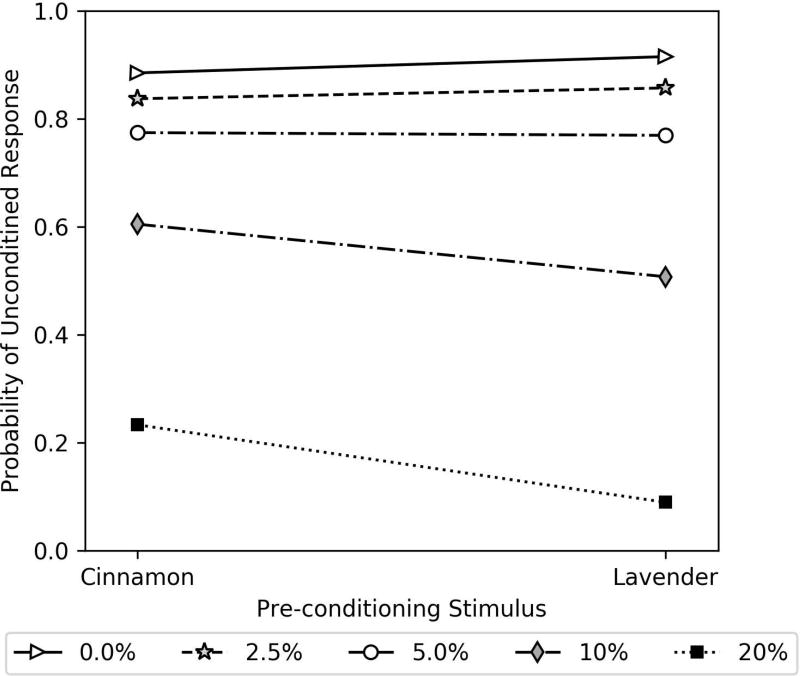
In our initial inspections of the data, we observed an interesting interaction between the ethanol dose and the PS in the same-stimulus, different-stimulus, and air-control groups. Although these effects appeared small, they were also consistent. To further explore this interaction, we pooled UR data from all same-stimulus, different-stimulus and air-control groups and conducted an additional analysis. We focused on the UR instead of the CR as there was little theoretical basis to suggest a difference in CR, and because UR has less change in response as a function of trial. Analyzing the UR, therefore, provided the best opportunity to investigate the dose and PS interaction. We expected, and found, no significant differences between groups in our initial model (p values > 0.3), so we revised the model to only include the parameters dose, PS, and the interaction of dose and PS. The statistical results of this analysis can be seen in Table 3, and the effect of cinnamon as a pre-conditioning stimulus at higher ethanol doses can be clearly seen in the regression lines plotted in Figure 5. Although there is little difference between probability of unconditioned response at 0%, 2.5% and 5% ethanol, the probability of response is greater for cinnamon at the 10% and 20% doses. The analysis in Table 3 reveal that the interaction between dose and PS is significant (estimate = −0.0733, p = 0.015).
Table 3.
| All Groups Unconditioned Response PS / Dose Analysis | |||||
|---|---|---|---|---|---|
|
| |||||
| Parameter | Estimate | Standard Error | 95% Confidence Intervals | p-value | |
| Intercept | 2.0423 | 0.172 | [1.705 | 2.379] | 0.000 |
| Dose | −0.1617 | 0.018 | [−0.197 | −0.126] | 0.000 |
| PS | 0.3378 | 0.261 | [−0.174 | 0.850] | 0.196 |
| PS × Dose | −0.0733 | 0.030 | [−0.132 | −0.014] | 0.015 |
Note. Pre-conditioning stimulus (PS) was coded as: 0-cinnamon, 1-lavender. The cinnamon PS was therefore included in the intercept.
Figure 5.
Regression lines of probability of unconditioned response, for all groups combined, as a function of pre-conditioning stimulus and ethanol dose. The key shows the percent ethanol dose the bees received in the 10 µl 2 M sucrose solution during the pre-conditioning procedure.
Discussion
Although we followed the traditional CTA method closely, we found no evidence that honey bees can learn ethanol-induced CTAs. Our bees received a single association of cinnamon or lavender odor with ethanol, and responses to the odors were tested 30 minutes after ethanol ingestion. If honey bees can develop ethanol-induced CTA, the bees in the same-stimulus group should have displayed an aversion. Instead, the opposite effect was observed. The bees in the same-stimulus group showed a high level of conditioned response on the first trial; this also demonstrates that honey bees are capable of one-trial learning common to CTA experiments. For all groups, higher doses of ethanol substantially decreased the probability of responding as well as slightly reduced learning ability, adding to the large body of literature that shows the detrimental effects of ethanol on bees. However, despite use of traditional methods, and demonstration of one-trial learning, our experiment did not show ethanol-induced CTA. We observed these effects both when considering ethanol dose as a continuous variable, and when separately analyzing each ethanol dose.
State Dependent Learning
One interesting finding of many substance-use experiments is that associations learned under the influence of a drug are sometimes remembered more effectively when the drug is present than when it is absent. Such “state-dependent learning” in response to ethanol has even been observed in simple organisms such as nematodes (Bettinger & McIntire, 2004). Thus, state-dependent learning is a potential complication for many substance use studies. Although our experiment does not explicitly test for state-dependent learning, some evidence of state-dependent learning might be apparent in the increase in response rate as a function of trial in the same-stimulus group, particularly at the 5% ethanol dose.
Bees in the same-stimulus group received a single association of ethanol, sucrose and odor during the pre-conditioning procedure. Then, 30 minutes later during the first conditioning trial, the bees are under the influence of ethanol, and thus in a different state than the pre-conditioning procedure. However, with increasing trial, the effects of ethanol may decrease, leading to later trials being a greater match of state to the original sober state when the association was learned. State-dependent learning suggests that performance increases as the performance state more closely matches the learning state. This may explain, along with general habituation and sobering effects, some increase in response across trial observed in the same-stimulus group, especially at the 5% dose. Future investigations will be required in order to adequately distinguish the effects of state-dependent learning, habituation to restraints, and general sobering effects.
Behavioral Ecology of Honey Bees
Although we did not find evidence of ethanol-induced CTA, honey bees are known to display aversions to some toxins. However, aversions are not always observed when expected. For example, selenium can be found in nectar, in the form of selenate or selenomethionine, in regions with selenium-rich soils (Presser, 1994; Quinn et al., 2011; Tuzen et al., 2007), and a surplus of selenium is toxic and can cause developmental deformities (Wu, 2004), changes in protein folding, and DNA damage (Schrauzer, 2000; Spallholz, 1997). Although both selenate and selenomethionine are toxic to honey bees, only selenomethionine inhibits proboscis extension (Hladun et al., 2012). In another example, Wright et al. (2010) demonstrated that bees easily learn to discriminate between odors associated with quinine and sucrose, but not between odors associated with amygdalin and sucrose. These patterns in honey bee food avoidance have led some to suggest the ability of bees to recognize toxins is controversial (de Brito Sanchez et al., 2005; de Brito Sanchez et al., 2014; Desmedt et al., 2016). However, this pattern is not unique to bees. Even humans learn CTA only in response to specific substances (Riley & Tuck, 1985).
The difficulty honey bees have in some CTA tasks may be affected by their highly specialized behavioral ecology. Honey bees are a eusocial species and are often described as a super-organism (Southwick, 1983; Seeley, 1989). A honey bee hive is actually a group-level adaptive unit (Seeley, 1997) and selection pressures may be very strong at the colony level but not at the individual level (Seeley, 1995). Most species studied in CTA research only have selection pressures at the individual level, thus distinctions in biologically prepared learning ability, such as CTA learning (Seligman, 1970), may mirror distinctions in types of biological selection pressures.
For honey bees, behavioral and cognitive abilities may not be adapted towards individual survival, but instead towards colony survival. Although bees possess many sophisticated psychological capabilities (Giurfa et al., 2001; Abramson et al., 2015), they may rely on colony-level “distributed cognition” (Giurfa, 2007) to solve some problems. Thus, when focusing on the behavior of individual bees, research occasionally demonstrates the limits of their psychological adaptability. For example, we found that bees are unable to learn the offset of an event as a CS (Abramson, Nolf, Mixon & Wells, 2010), and that they respond inefficiently in fixed interval schedules of reinforcement (Craig et al., 2014). Similarly, honey bees may have difficulty learning CTAs as a result of dependence on colony-level processes.
Honey bees also have an unusual method of food distribution and storage that may reduce the potential benefits of CTAs. Bees distribute food around the hive, minimizing the exposure any individual has with a toxin. Only foragers, the last role in the life of worker bees, may be exposed to substantial levels of toxin. Some harmful compounds may also evaporate or deteriorate during the honey production or storage process, further limiting the colony’s exposure to toxins. Sealed honey is also unlikely to ferment or become toxic due to its hygroscopic nature, therefore bees are also unlikely to encounter toxins in the honey storage. (For a detailed description of the honey production process, see Seeley, 1995). Considering the unique manner in which bees preserve and consume food, it is possible that they do not display the same types of food-avoidance mechanisms as other species.
Absence of CTA has also been demonstrated in one other highly specialized species: vampire bats (Ratcliffe et al., 2003). While the authors were able to demonstrate that three generalist species (one insectivore and two frugivores) readily acquired CTAs, the common vampire bat did not. Ratcliffe et al. suggest this is because of the highly specialized diet of vampire bats. Vampire bats are monophagous and unlikely to encounter toxins in their food. Thus, there is little reason for them to evolve the ability to develop CTAs, and they would not have an alternative food if they learned to avoid blood. It is possible that both honey bees and vampire bats show unusual responses in CTA experiments due to unusual selection pressures, compared to most species studied.
Cinnamon and Ethanol Interactions
We discovered a surprising dose-dependent effect of the pre-conditioning stimulus. For our bees, consuming the cinnamon oil during the pre-conditioning procedure reduced the effects of ethanol ingestion. This is the first effect of this type reported in honey bees. Recent research has found some interactions between cinnamon and ethanol in vertebrates. For example, ingestion of cinnamon extract protects against the early stages of alcoholic liver disease in mice (Kanuri et al., 2009), and may reduce intestinal bacterial endotoxins that are also implicated in chronic alcohol-induced liver damage (Azumi et al., 1997). Cinnamon also appears to have a number of anti-inflammatory, antimicrobial, antioxidant, antitumor, cardiovascular, cholesterol-lowering, and immunomodulatory effects in humans and laboratory animals (Gruenwald & Armbruester, 2010). This finding is worth follow-up research, as invertebrates such as honey bees can be a useful behavioral and physiological model for human alcoholism (Abramson et al., 2007); honey bees may be ideal subjects to investigate the physiology of the interaction of cinnamon extract ingestion and ethanol consumption. This unexpected finding also speaks to the importance of using multiple counterbalanced stimuli, as well as investigating differences between presumed equivalent stimuli.
Conclusion
Our research is the first to show that honey bees do not acquire ethanol-induced CTAs. This is an important finding as it suggests a divergence in the learning abilities of honey bees and traditional vertebrate laboratory species. Although CTA appears to be a highly conserved process, our research suggests it may be absent in species with unique feeding behaviors or life histories. Future research should consider how systematic this lack of toxin aversion is, and should focus on multiple procedures and toxins. Ultimately, this work has implications for the effect of natural environmental toxins and pesticides on honey bees, a species that is especially important to human agriculture.
Our research also has implications for use of honey bees as an invertebrate model for alcoholism. Although honey bees are greatly affected by ethanol consumption, they do not appear to develop ethanol-induced CTAs. This is also seen in human alcoholics, who may repeatedly ingest substantial doses of ethanol, even after repeated intoxications. Honey bees are therefore an excellent model of alcoholism to complement model organisms that have an innate preference for ethanol, such as fruit flies (Devineni & Heberlein, 2009; McKenzie & Parsons, 1972), or model organisms that are often selectively bred to display alcoholism, such as rodents (Blednov et al., 2011). Additionally, like humans, ethanol use can be lowered by Antabuse consumption in honey bees (Abramson et al., 2003). A next step in the CTA research might be to see if Antabuse and ethanol can be used together to create strong, long lasting aversions through single associations using the honey bee model. Future research should consider such techniques to reduce ethanol consumption, as well as potential ethanol-cinnamon interactions. Additional research may also benefit from considering the pharmacodynamics of ethanol, as ethanol affects important neurotransmitters involved in learning, memory and motivation such as GABA, glutamate, serotonin, and dopamine (see the supplementary materials for a discussion; Di Chiara, 1997; Lovinger, 1999; Malenka et al., 2009).
We hope that our findings stimulate additional comparative alcohol consumption research, with special consideration for reporting what might normally be considered “negative results.” Documenting the lack of behavioral or cognitive abilities is an important component of comparative psychology (Avarguès-Weber & Giurfa, 2013) that is necessary to avoid the common publication bias and “file-drawer” effect that plagues the field of psychology in general (Rosenthal, 1979).
Supplementary Material
Hemolymph EtOH levels, in millimoles, of honey bees 30 minutes after consuming a 10 μl solution of 0 to 45% EtOH.
Percent of subjects in the same-stimulus, different-stimulus, and air-control groups responding to the conditioned stimulus on the first trial. The key shows the percent EtOH dose the bees received in the 10 µl 2 M sucrose solution during the preconditioning procedure.
Regression lines showing the statistical predictions of probability of unconditioned and conditioned response by subjects in the same-stimulus group. The key shows the percent EtOH dose the bees received in the 10 µl 2 M sucrose solution during the preconditioning procedure.
Regression lines showing the statistical predictions of probability of unconditioned and conditioned response by subjects in the different-stimulus group. The key shows the percent EtOH dose the bees received in the 10 µl 2 M sucrose solution during the preconditioning procedure.
Regression lines showing the statistical predictions of probability of unconditioned and conditioned response by subjects in the air-control group. The key shows the percent EtOH dose the bees received in the 10 µl 2 M sucrose solution during the preconditioning procedure.
Supplementary material.
Acknowledgments
This material was based upon work was supported in part by National Science Foundation grants DBI 0552717, DBI 1263327, OISE 1545803, GRF 1144467, and by National Institutes of Health grant P20GM103499.
Contributor Information
Christopher A. Varnon, Department of Psychology, Converse College.
Christopher W. Dinges, Department of Psychology, Oklahoma State University.
Timothy E. Black, Department of Psychology, Oklahoma State University.
Harrington Wells, Department of Biological Science, University of Tulsa.
Charles I. Abramson, Department of Psychology, Oklahoma State University.
References
- Abramson CI, Aquino IS, Silva MC, Price JM. Learning in the Africanized honey bee: Apis mellifera L. Physiol Behav. 1997;62:657–674. doi: 10.1016/S0031-9384(97)00194-7. [DOI] [PubMed] [Google Scholar]
- Abramson CI, Boyd BJ. An automated apparatus for conditioning proboscis extension in honeybees (Apis mellifera L.) Entomolo Sci. 2001;36:78–92. doi: 10.1673/031.010.12201. [DOI] [Google Scholar]
- Abramson CI, Craig DPA, Varnon CA, Wells H. The effect of ethanol on reversal learning in honey bees (Apis mellifera anatolica): Response inhibition in a social insect model. Alcohol. 2015;49:245–258. doi: 10.1016/j.alcohol.2015.02.005. [DOI] [PubMed] [Google Scholar]
- Abramson CI, Fellows GW, Browne BL, Lawson A, Ortiz RA. Development of an ethanol model using social insects: II. Effect of Antabuse on consumatory responses and learned behavior of the honey bee (Apis mellifera L.) Psychol Rep. 2003;92(2):365–378. doi: 10.2466/pr0.2003.92.2.365. [DOI] [PubMed] [Google Scholar]
- Abramson CI, Giray T, Mixson TA, Nolf SL, Wells H, Kence A, Kence M. Proboscis conditioning experiments with honeybees, Apis mellifera caucasica, with butyric acid and DEET mixture as conditioned and unconditioned stimuli. J Insect Sci. 2010;10:122. doi: 10.1673/031.010.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramson CI, Nolf SL, Mixon TA, Wells H. Can honey bees learn the removal of a stimulus as a conditioning cue? Ethology. 2010;118(6):843–854. doi: 10.1111/j.1439-0310.2010.01796.x. [DOI] [Google Scholar]
- Abramson CI, Wells H, Bozic J. A social insect model for the study of ethanol induced behavior: The honey bee. In: Yoshida R, editor. Trends in Alcohol Abuse and Alcoholism Research. Nova Science; New York: 2007. pp. 197–218. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. 5. American Psychiatric Association; Virginia: 2013. [Google Scholar]
- Avarguès-Weber A, Giurfa M. Conceptual learning by miniature brains. Proc R Soc Lond. 2013;280(1772):2013190. doi: 10.1098/rspb.2013.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azumi S, Tanimura A, Tanamoto K. A novel inhibitor of bacterial endotoxin derived from cinnamon bark. Biochem and Biophys Res Commun. 1997;234:506–510. doi: 10.1006/bbrc.1997.6668. [DOI] [PubMed] [Google Scholar]
- Bettinger JC, McIntire SL. State-dependency in C. elegans. Genes Brain Behav. 2004;3:266–272. doi: 10.1111/j.1601-183X.2004.00080.x. [DOI] [PubMed] [Google Scholar]
- Bitterman ME. The comparative analysis of learning. Sci. 1975;188:699–709. doi: 10.1126/science.188.4189.699. [DOI] [PubMed] [Google Scholar]
- Bitterman ME. Flavor aversion studies. Sci. 192:266–269. (I976) [Google Scholar]
- Bitterman ME, Menzel R, Fietz A, Schäfer S. Classical conditioning of proboscis extension in honeybees (Apis mellifera) J Comp Psychol. 1983;97:107–119. [PubMed] [Google Scholar]
- Blednov YA, Borghese CM, McCracken ML, Benavidez JM, Geil CR, Osterndorff-Kahanek E, Werner DF, Lyer S, Swihart A, Harrison NL, Homanics GE, Harris RA. Loss of ethanol conditioned taste aversion and motor stimulation in knockin mice with ethanol-insensitive α2-containing GABAA receptors. J Pharmacol Exp Ther. 2011;336(1):145–154. doi: 10.1124/jpet.110.171645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolles RC. Species-specific defense reactions and avoidance learning. Psychol Rev. 1970;77:32–48. [Google Scholar]
- Bozic J, DiCesare J, Wells H, Abramson CI. Ethanol levels in honeybee hemolymph resulting from alcohol ingestion. Alcohol. 2007;41:281–284. doi: 10.1016/j.alcohol.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Broadbent J, Muccino KJ, Cunningham CL. Ethanol-induced conditioned taste aversion in 15 inbred mouse strains. Behav Neurosci. 2002;116(1):138–148. doi: 10.1037/0735-7044.116.1.138. [DOI] [PubMed] [Google Scholar]
- Chester JA, Lumeng L, Li T, Grahame NJ. High– and low–alcohol-preferring mice show differences in conditioned taste aversion to alcohol. Alcohol Clin Exp Res. 2003;27:12–18. doi: 10.1111/j.1530-0277.2003.tb02714.x. [DOI] [PubMed] [Google Scholar]
- Cappell H, LeBlanc AE, Endrenyi L. Aversive conditioning by psychoactive drugs: Effects of morphine, alcohol and chlordiazepoxide. Psychopharmacologia. 1973;29(3):239–246. doi: 10.1007/BF00414038. [DOI] [PubMed] [Google Scholar]
- Craig DPA, Varnon CA, Sokolowski MBC, Wells H, Abramson CI. An assessment of fixed interval timing in free-flying honey bees (Apis mellifera ligustica): An analysis of individual performance. PloS One. 2014;9:e101262. doi: 10.1371/journal.pone.0101262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G. Alcohol and dopamine. Alcohol Health Res World. 1997;21(2):108–114. [PMC free article] [PubMed] [Google Scholar]
- de Brito Sanchez MG. Taste perception in honey bees. Chem Senses. 2011;36(8):675–692. doi: 10.1093/chemse/bjr040. [DOI] [PubMed] [Google Scholar]
- de Brito Sanchez MG, Giurfa M, de Paula Mota TR, Gauthier M. Electrophysiological and behavioural characterization of gustatory responses to antennal ‘bitter’ taste in honeybees. Euro J Neurosci. 2005;22:3161–3170. doi: 10.1111/j.1460-9568.2005.04516.x. doi: EJN4516 10.1111/j.1460-9568.2005.04516.x. [DOI] [PubMed] [Google Scholar]
- de Brito Sanchez MG, Lorenzo E, Su S, Lui F, Zhan Y, Giurfa M. The tarsal taste of honey bees: behavioral and electrophysiological analyses. Front Behav Neurosci. 2014;8:1–16. doi: 10.3389/fnbeh.2014.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmedt L, Hotier L, Giurfa M, Velarde R, de Brito Sanchez MG. Absence of food alternatives promotes risk-prone feeding of unpalatable substances in honey bees. Sci Rep. 2016;18(6):31809. doi: 10.1038/srep31809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devineni AV, Heberlein U. Preferential ethanol consumption in Drosophila models features of addiction. Curr Biol. 2009;19:2126–2132. doi: 10.1016/j.cub.2009.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinges CW, Avalos A, Abramson CI, Craig DPA, Austin ZM, Varnon CA, Dal FN, Giray T, Wells H. Aversive conditioning in honey bees (Apis mellifera anatolica): A comparison of drones and workers. J Exp Biol. 2013;216:4124–4134. doi: 10.1242/jeb.090100. [DOI] [PubMed] [Google Scholar]
- Etscorn F, Stephens R. Establishment of conditioned taste aversions with a 24-hour CS-US interval. Physio Psychol. 1973;1:251–253. [Google Scholar]
- Fouquet N, Oberling P, Sandner G. Differential effect of free intake versus oral perfusion of sucrose in conditioned taste aversion in rats. Physiol Behav. 2001;74:465–474. doi: 10.1016/s0031-9384(01)00585-6. doi: S0031938401005856. [DOI] [PubMed] [Google Scholar]
- Garcia J, Kimeldorf DJ, Koelling RA. Conditioned aversion to saccharine resulting from exposure to gamma radiation. Science. 1955;122:157–158. doi: 10.1126/science.122.3179.1089. [DOI] [PubMed] [Google Scholar]
- Garcia J, Koelling RA. Relation of cue to consequence in avoidance learning. Psychon Sci. 1966;4:123–124. doi: 10.3758/BF03342209. [DOI] [Google Scholar]
- Giurfa M. Behavioral and neural analysis of associative learning in the honeybee: a taste from the magic well. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2007;193(8):801–24. doi: 10.1007/s00359-007-0235-9. [DOI] [PubMed] [Google Scholar]
- Giurfa M, Zhang S, Jenett A, Menzel R, Srinivasan MV. The concepts of 'sameness' and 'difference' in an insect. Nature. 2001;410:930–933. doi: 10.1038/35073582. [DOI] [PubMed] [Google Scholar]
- Gruenwald J, Armbruester N. Cinnamon and health. Crit Rev Food Sci Nutr. 2010;50:822–834. doi: 10.1080/10408390902773052. [DOI] [PubMed] [Google Scholar]
- Hardin J, Hilbe JM. Generalized Estimating Equations. Boca Raton, FL: Chapman & Hall; 2003. [Google Scholar]
- Hartz SM, Ben-Shahar Y, Tyler M. Logistic growth curve analysis in associative learning data. Anim Cogn. 2001;4:185–189. doi: 10.1007/s100710000075. [DOI] [Google Scholar]
- Hill KG, Alva H, Blednov YA, Cunningham CL. Reduced ethanol-induced conditioned taste aversion and conditioned place preference in GIRK2 null mutant mice. Psychopharmacology. 2003;169(1):108–114. doi: 10.1007/s00213-003-1472-4. [DOI] [PubMed] [Google Scholar]
- Hladun K, Smith BH, Mustard JA, Morton RR, Trumble JT. Selinium toxicity to honey bee (Apis mellifera L.) pollinators: Effects on behavior and survival. PLoS one. 2012;7(4):e34137. doi: 10.1371/journal.pone.0034137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E, Oliphant E, Peterson P. SciPy: Open Source Scientific Tools for Python. 2001 Retrieved from http://www.scipy.org/
- Kanuri G, Weber S, Volynets V, Spruss A, Bischoff SC, Bergheim I. Cinnamon extract protects against acute alcohol-induced liver steatosis in mice. J Nutr. 2009;139(3):482–487. doi: 10.3945/jn.108.100495. [DOI] [PubMed] [Google Scholar]
- Kimble GA. Hilgard and Marquis' conditioning and learning. Appleton Century-Crofts; New York: 1961. [Google Scholar]
- Klosterhalfen S, Klosterhalfen W. Conditioned taste aversion and traditional learning. Psychol Res. 1985;47:71–94. doi: 10.1007/BF00309122. [DOI] [PubMed] [Google Scholar]
- Li K, Hsiao S, Li J. Conditioned taste aversion as instrumental punishment. J Exp Psychol Anim Behav Process. 2013;39:294–297. doi: 10.1037/a0031822. [DOI] [PubMed] [Google Scholar]
- Logue AW. Taste aversion and the generality of the laws of learning. Psychol Bull. 1979;86(2):276–296. [Google Scholar]
- Logue AW, Ophir I, Strauss KE. The acquisition of taste aversion in humans. Behav Res Ther. 1981;19(4):319–333. doi: 10.1016/0005-7967(81)90053-x. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. 5-HT3 receptors and the neural action of alcohols: An increasingly exciting topic. Neurochem Int. 1999;35(2):125–130. doi: 10.1016/s0197-0186(99)00054-6. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nestler EJ, Hyman SE. Reinforcement and addictive disorders. In: Sydor A, Brown RY, editors. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience. McGraw-Hill Medical; New York: 2009. pp. 364–375. [Google Scholar]
- Maze IS, Wright GA, Mustard JA. Acute ethanol ingestion produces dose-dependent effects on motor behavior in the honey bee (Apis mellifera) J Insect Physiol. 2006;52(11–12):1243–1253. doi: 10.1016/j.jinsphys.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie JA, Parsons PA. Alcohol tolerance: an ecological parameter in the relative success of Drosophila melanogaster and Drosophila simulans. Oecologia. 1972;10:373–388. doi: 10.1007/BF00345738. [DOI] [PubMed] [Google Scholar]
- Mustard JA, Edgar EA, Mazade RE, Wu C, Lillvis JL, Wright A. Acute ethanol ingestion impairs appetitive olfactory learning and odor discrimination in the honey bee. Neurobiol Learn Mem. 2008;90(4):633–643. doi: 10.1016/j.nlm.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presser TS. The Kesterson Effect. Environ Manage. 1994;18:437–454. [Google Scholar]
- Quinn CF, Prins CN, Freeman JL, Gross AM, Hantzis LJ, Reynolds RJ, Yang S, Covey PA, Banuelos GS, Pickering IJ, Fakra SC, Marcus MA, Arathi HS, Pilon-Smits EA. Selenium accumulation in flowers and its effects on pollination. New Phytol. 2011;192:727–737. doi: 10.1111/j.1469-8137.2011.03832.x. [DOI] [PubMed] [Google Scholar]
- Ratcliffe JM, Fenton MB, Galef BG., Jr An exception to the rule: Common vampire bats do not learn taste aversions. Anim Behav. 2003;65:385–389. [Google Scholar]
- Riddell CE, Mallon EB. Insect psychoneuroimmunology: Immune response reduces learning in protein starved bumblebees (Bombus terrestris) Brain Behav Immun. 2005;20:135–138. doi: 10.1016/j.bbi.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Riley AL, Tuck DL. Conditioned taste aversions: A behavioral index of toxicity. Ann N Y Acad Sci. 1985;443:272–292. doi: 10.1111/j.1749-6632.1985.tb27079.x. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Cunningham CL. Genetic differences in ethanol-induced conditioned taste aversion after ethanol preexposure. Alcohol. 1995;12(6):535–539. doi: 10.1016/0741-8329(95)00040-2. [DOI] [PubMed] [Google Scholar]
- Roma PG, Rinker JA, Serafine KM, Chen SA, Barr CS, Cheng K, Rice KC, Riley AL. Genetic and early environmental contributions to alcohol’s aversive and physiological effects. Pharmacol Biochem Behav. 2008;91:134–139. doi: 10.1016/j.pbb.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. File drawer problem and tolerance for null results. Psychol Bull. 1979;86:638–41. doi: 10.1037/0033-2909.86.3.638. [DOI] [Google Scholar]
- Rozin P, Kalat JW. Specific hungers and poison avoidance as adaptive specializations of learning. Psychol Rev. 1971;78:459–486. doi: 10.1037/h0031878. [DOI] [PubMed] [Google Scholar]
- Schrauzer GN. Selenomethionine: A review of its nutritional significance, metabolism and toxicity. J Nutr. 2000;130:1653–1656. doi: 10.1093/jn/130.7.1653. [DOI] [PubMed] [Google Scholar]
- Seeley TD. Adaptive significance of the age polyethism schedule in honeybee colonies. Behav Ecol Sociobiol. 1982;11:287–293. doi: 10.1007/BF00299306. [DOI] [Google Scholar]
- Seeley TD. The honey bee colony as a superorganism. Am Sci. 1989;77:546–553. [Google Scholar]
- Seeley TD. The wisdom of the hive: The social physiology of honey bee colonies. Harvard University Press; Cambridge, Massachusetts: 1995. [Google Scholar]
- Seeley TD. Honey bee colonies are group-level adaptive units. Am Nat. 1997;150:S22–S23. doi: 10.1086/286048. [DOI] [PubMed] [Google Scholar]
- Seligman MEP. On the generality of the laws of learning. Psychol Rev. 1970;77:406–418. [Google Scholar]
- Simone-Finstrom M, Gardner J, Spivak M. Tactile learning in resin foraging honeybees. Behav Ecol Sociobiol. 2010;64:1609–1617. doi: 10.1007/s00265-010-0974-4. [DOI] [Google Scholar]
- Smith BH, Abramson CI, Tobin TR. Conditional withholding of proboscis extension in honeybees (Apis mellifera) during discriminative punishment. J Comp Psychol. 1991;105:345–356. doi: 10.1037/0735-7036.105.4.345. [DOI] [PubMed] [Google Scholar]
- Southwick EE. The honey bee cluster as a homeothermic superorganism. Comp Biochem Physiol A Physiol. 1983;75:641–645. [Google Scholar]
- Spallholz JE. Free radical generation by selenium compounds and their prooxidant toxicity. Biomed Environ Sci. 1997;10:260–270. [PubMed] [Google Scholar]
- Tuzen M, Silici S, Mendil D, Soylak M. Trace element levels in honeys from different regions of Turkey. Food Chem. 2007;103:325–330. doi: 10.1016/j.foodchem.2006.07.053. [DOI] [Google Scholar]
- Winston ML, Neilson-Punnett E. Factors determining temporal division of labor in honeybees. Can J Zool. 1982;60(11):2947–2952. doi: 10.1139/z82-372. [DOI] [Google Scholar]
- Wright GA, Lutmerding A, Dudareva N, Smith BH. Intensity and the ratios of compounds in the scent of snapdragon flowers affect scent discrimination by honey bees (Apis mellifera) J Comp Physiol A. 2005;191(2):105–114. doi: 10.1007/s00359-004-0576-6. [DOI] [PubMed] [Google Scholar]
- Wright GA, Mustard JA, Simcock NK, Ross-Taylor AAR, McNicholas LD, Popescu A, Marion-Poll F. Parallel reinforcement pathways for conditioned food aversions in the honeybee. Curr Biol. 2010;20:2234–2240. doi: 10.1016/j.cub.2010.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L. Review of 15 years of research on ecotoxicology and remediation of land contaminated by agricultural drainage sediment rich in selenium. Ecotoxicol Environ Saf. 2004;57:257–269. doi: 10.1016/S0147-6513(03)00064-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hemolymph EtOH levels, in millimoles, of honey bees 30 minutes after consuming a 10 μl solution of 0 to 45% EtOH.
Percent of subjects in the same-stimulus, different-stimulus, and air-control groups responding to the conditioned stimulus on the first trial. The key shows the percent EtOH dose the bees received in the 10 µl 2 M sucrose solution during the preconditioning procedure.
Regression lines showing the statistical predictions of probability of unconditioned and conditioned response by subjects in the same-stimulus group. The key shows the percent EtOH dose the bees received in the 10 µl 2 M sucrose solution during the preconditioning procedure.
Regression lines showing the statistical predictions of probability of unconditioned and conditioned response by subjects in the different-stimulus group. The key shows the percent EtOH dose the bees received in the 10 µl 2 M sucrose solution during the preconditioning procedure.
Regression lines showing the statistical predictions of probability of unconditioned and conditioned response by subjects in the air-control group. The key shows the percent EtOH dose the bees received in the 10 µl 2 M sucrose solution during the preconditioning procedure.
Supplementary material.