Abstract
Purpose
Studies investigating efficacy and safety of bevacizumab in pterygium have increased and reported controversial results. Thus, we updated this meta-analysis to clarify the issue.
Methods
Studies were selected through search of the databases Embase, PubMed, Web of Science, and the Cochrane Central Register of Controlled Trials (CENTRAL) from their inception up until June 2017. The pooled risk ratio (RR) and 95% confidence interval (CI) were calculated for recurrence and complication rates by using random effects model.
Results
1045 eyes in 18 randomized controlled trials (RCTs) enrolled. Overall, the pooled estimate showed a statistically significant effect of bevacizumab on the reduction of recurrence (RR 0.74, 95% CI 0.56–0.97, P=0.03). Subgroup analyses presented significant results beneficial to bevacizumab (primary pterygium group, RR 0.53, 95% CI 0.33–0.83, P=0.006; conjunctival autograft group, RR 0.48, 95% CI 0.25–0.91, P=0.02; and follow-up longer than 12 months group, RR 0.36, 95% CI 0.13–0.99, P=0.05). No statistically significant difference was observed in complication rates.
Conclusions
Application of bevacizumab showed a statistically significant decrease in recurrence rate following removal of primary pterygia, or in cases with conjunctival autograft, or with follow-up longer than 12 months, while complications were not increased.
1. Introduction
Pterygium is one of the most common ocular surface diseases, which is characterized by the fibrovascular conjunctiva tissue proceeding from the bulbar conjunctiva towards the cornea. It limits eye movements and causes dry eye, irritation, foreign body sensation, and even decrease of visual acuity [1]. The primary treatment for pterygium is surgery, and the major problem of the treatment is the high recurrence rate, varying between 38% and 88% in bare sclera, 5%–30% in conjunctival autograft, and 0%–15% in limbal conjunctival autograft [2]. Many adjuvant therapies have been developed to reduce recurrence including mitomycin C [3, 4], 5-FU [5–7], and radiotherapy [8, 9].
In 2001, expression of vascular endothelial growth factor (VEGF) was firstly demonstrated in pterygia [10]. Pterygia present higher levels of VEGF compared with normal conjunctiva [11–16]. This brings about speculation that anti-VEGF drugs may be useful for pterygia patients. Bevacizumab is a recombinant human monoclonal antibody against VEGF, which is approved by FDA treating neoplasms. Many randomized controlled trials (RCTs) were performed to assess the safety and efficacy of bevacizumab in management of pterygium, showing conflicting conclusions [17–34]. A meta-analysis of 9 RCTs was conducted in 2014 [35], and the result showed that topical or subconjunctival bevacizumab had no statistically significant effect on preventing pterygium recurrence. However, the result was not been consistently supported by another 9 new RCTs published after 2014 [26–34]. The conclusion might be altered by the addition of 9 new studies. Therefore, we performed an additional meta-analysis to further evaluate the impact of bevacizumab on the recurrence and complication rates in the treatment of pterygium.
2. Methods
2.1. Search Strategy
The databases of Embase, PubMed, Web of Science, and the Cochrane Central Register of Controlled Trials (CENTRAL) were searched from their inception up until June 2017. Details of the search strategies were described in the Search Strategy file. Endnote software was used to exclude the duplications. Titles and abstracts were scrutinized to deduct apparently irrelevant studies. Full texts were retrieved and assessed for qualification. A manual search was executed by checking the reference lists of all retrieved studies and reviewing articles to distinguish studies not found by the electronic searches. Language was not restricted.
2.2. Inclusion and Exclusion Criteria
The articles were considered qualified if the studies fulfilled the following inclusion criteria: (1) participants: pterygium patients (including primary pterygium, impending recurrent pterygium, and recurrent pterygium); (2) intervention: topical or subconjunctival bevacizumab, regardless of operation or not. The dose of bevacizumab, follow-up periods, or length of fibrovascular growth passing the corneal limbus were not confined; (3) comparison: bevacizumab and control; (4) outcomes: recurrence and/or complication rates; and (5) publication type: RCT. RCTs without exact raw data available for extraction were excluded.
2.3. Outcome Measurements
The primary outcome measurements were recurrence and complication rates. Recurrence was diagnosed when any fibrovascular growth crossed the limbus and extended over the cornea to any distance by slit-lamp examination. The number of recurrences was estimated at the endpoint of the follow-up in each study. Complications such as lacrimation, inflammation, photophobia, conjunctival erythema, conjunctival flap edema, conjunctival graft loss, subconjunctival hemorrhage, corneal dellen, severe conjunctival or corneal scarring, and systemic complications were counted. The number of complications at the last documenting time during the follow-up in each study was calculated.
2.4. Data Extraction
The data were extracted by two reviewers (Yi Sun and Bowen Zhang) independently. Discrepancies were resolved by discussion to reach a consensus between the investigators. The information collected from each study included the first author's last name, year of publication, study design, location and duration of the study, sample size including sex, age, and diagnoses, type of treatment and control, route of administration, and dose of bevacizumab.
2.5. Risk of Bias Assessment
Two reviewers (Yi Sun and Bowen Zhang) separately evaluated the risk of bias in each study according to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions 5.3. The authors reviewed the studies and assigned a value of “high,” “low,” or “unclear” to the following items: (1) selection bias (Was there sufficient generation of the randomization sequence and allocation concealment?); (2) performance and detection bias (Was there blinding of participants, personnel, and outcome assessors?); (3) attrition bias (Were there incomplete outcome data and how to deal with this?); (4) reporting bias (Was there evidence of reporting outcome selectively?); and (5) other sources of bias (Were there any other potential threats to validity?). Any disagreement was discussed until a consensus was reached.
2.6. Statistical Analysis
The recurrence and complication rates were handled as dichotomous variables measured as the risk ratio (RR) with a 95% confidence interval (CI). Due to the diversity in sample size and the differences in clinical characteristics among the studies, it was presumed that heterogeneity existed even when no statistical significance was observed. Therefore, the data were pooled using a random effects model. Statistical heterogeneity among the studies was assessed by calculating a Cochran Q statistic and an I 2 statistic. Subgroup analysis and sensitivity analysis for the recurrence rate were carried out to evaluate the impact of the following factors on the results: (a) participants: primary pterygium, impending recurrent pterygium, and recurrent pterygium; (b) intervention: topical use or subconjunctival injection of bevacizumab; type of operation or not; and (c) follow-up periods: ≤6 months, 6~12 months, and ≥12 months. We explored asymmetry in funnel plots to detect publication biases. The analysis was performed using RevMan 5.3 (The Cochrane Collaboration, Copenhagen, Denmark).
3. Results
3.1. Literature Search
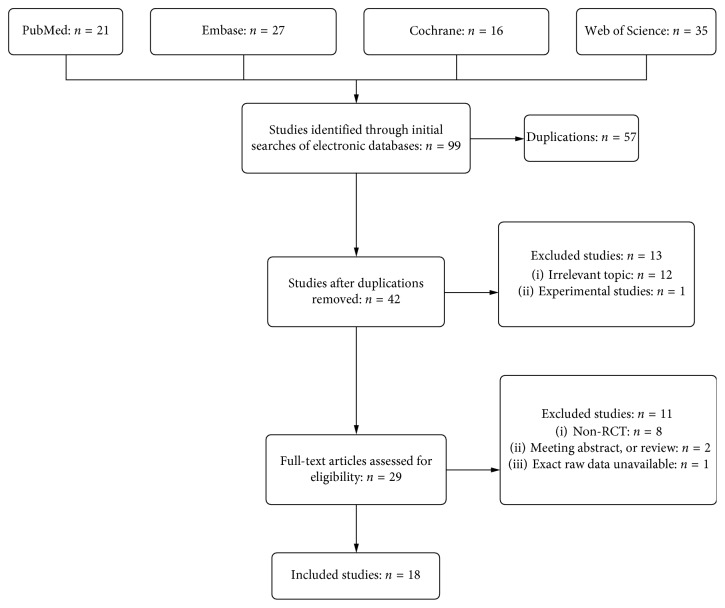
Literature search and selection process are summarized in Figure 1. A total of 99 articles were initially enrolled. After removing duplications, the abstracts of the remaining studies were inspected, and 29 articles with possibly relevant trials were further identified in full texts. Eighteen randomized controlled trials (RCTs) were deemed eligible after a full text screening and were finally included in this meta-analysis.
Figure 1.
Flow diagram for the literature search and selection process.
3.2. Characteristics and Quality Assessment of the Included Studies
Characteristics of included studies are summarized in Table 1. In total, 18 RCTs were included in this review [17–34]. 17 studies were published in English and 1 in Chinese. 1045 eyes were enrolled: 561 in the bevacizumab group and 484 in the control group. Quality assessment was conducted according to Cochrane Handbook for Systematic Reviews of Interventions 5.3. The risks of biases in these studies are shown in supplementary data file (available here).
Table 1.
Characteristics of the included randomized clinical trials.
| Author (year) | Location | No. of eyes (Bev/Con) | Administration route of bevacizumab | Mean age (Bev/Con, y) | Type of pterygium | Follow-up (m) | Treatment method |
|---|---|---|---|---|---|---|---|
| Fallah (2010) | Iran | 26/28 | Topical | 49.96/51.61 | Impending recurrent | 3~6 | Nonsurgery |
| Razeghinejad (2010) | Iran | 15/15 | Subconjunctival | 45.8/41.6 | Primary | 8 vs 7.4 | Conjunctival autograft |
| Banifatemi (2011) | Iran | 22/22 | Subconjunctival | 41.95/44.13 | Primary | 1 | Conjunctival autograft |
| Enkvetchakul (2011) | Thailand | 34/40 | Subconjunctival | 51.5/49 | Primary | 6 | Nonsurgery |
| Shenasi (2011) | Iran | 33/33 | Subconjunctival | 58.67/55.94 | Primary | 9 | Bare sclera |
| Shahin (2012) | Egypt | 20/21 | Subconjunctival | 58.40/57.58 | Primary | 8 | Conjunctivolimbal autograft |
| Lekhanont (2012) | Thailand | 60/20 | Subconjunctival | 48.98/48.27 | Impending recurrent | 3 | Nonsurgery |
| Ozgurhan (2013) | Turkey | 22/22 | Topical | 48.4/50.5 | Recurrent | 6 | Conjunctival autograft |
| Xu (2013) | China | 40/40 | Subconjunctival | 44/41 | Primary | 12 | Conjunctivolimbal autograft |
| Nava-Castaneda, A (2014) | Mexico | 33/16 | Subconjunctival | 48.75/47.8 | Primary | 12 | Conjunctival autograft |
| Karalezli (2014) | Turkey | 42/46 | Topical | 58.82/53.04 | Primary | 29.3 VS 28.5 | Conjunctival autograft |
| Razeghinejad(2014) | Iran | 20/21 | Subconjunctival | 41.95/44.13 | Primary | 6 | Conjunctival autograft |
| Ozsutcu(2014) | Turkey | 30/30 | Subconjunctival | 43.25/41.68 | Primary | 9 | Conjunctival autograft |
| Kasetsuwan(2015) | Thailand | 12/10 | Topical | 50.7/59.3 | Primary | 3 | Bare sclera |
| Hwang(2015) | Korea | 36/33 | Topical | 71.3/73.4 | Primary | 6 | Bare sclera |
| Singh(2015) | India | 30/30 | Subconjunctival | 37.33 | Primary | 3 | Conjunctival autograft |
| Bekibele(2016) | Nigeria | 26/27 | Subconjunctival | 49.2/52.0 | Primary | 18.35 | Conjunctiva autograft |
| Motarjemizadeh(2016) | Iran | 60/30 | Topical | 39.47/40.97 | Primary | 12 | Bare sclera |
Bev: bevacizumab; Con, control; y, year; m, month.
3.3. Meta-Analysis
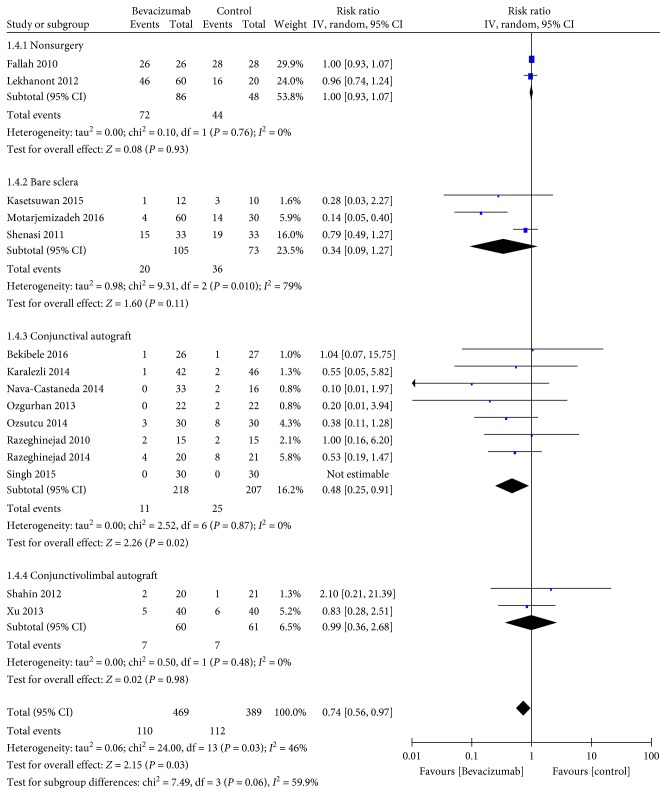
15 studies reported recurrences. Definitions of pterygium recurrence of the included randomized clinical trials are shown in Table 2. Overall recurrence rate of this meta-analysis was summarized in supplementary data file. The pooled results demonstrated that bevacizumab significantly reduced the pterygium recurrence (RR 0.74, 95% CI 0.56–0.97, P=0.03; P heterogeneity = 0.03, I 2 = 46%). Subgroup analysis for the recurrence rate based on the pterygium types showed a statistically significant decrease in recurrence rate in the primary pterygium group (RR 0.53, 95% CI 0.33–0.83, P=0.006; P heterogeneity = 0.21, I 2 = 25%), while not in the recurrent pterygium group (RR 1.00, 95% CI 0.93–1.07, P=0.91; P heterogeneity = 0.55, I 2 = 0%) (Figure 2). Similarly, significant results in favor of bevacizumab were found in the conjunctival autograft group (RR 0.48, 95% CI 0.25–0.91, P=0.022; P heterogeneity = 0.87, I 2 = 0%) (Figure 3) and the follow-up longer than 12 months group (RR 0.36, 95% CI 0.13–0.99, P=0.05; P heterogeneity = 0.15, I 2 = 41%) (Figure 4). There was no statistically significant difference between the topical bevacizumab group (RR 0.38, 95% CI 0.12–1.23, P=0.11; P heterogeneity = 0.002, I 2 = 76%) and the subconjunctival bevacizumab group (RR 0.87, 95% CI 0.70–1.07, P=0.18; P heterogeneity = 0.64, I 2 = 0%) (supplementary data file).
Table 2.
Definition of pterygium recurrence of the included randomized clinical trials.
| Author (year) | Definition of recurrence |
|---|---|
| Fallah (2010) | Fibrovascular tissue stretching onto cornea |
| Razeghinejad (2010) | Fibrovascular tissue extending more than 1.5 mm across limbus |
| Shenasi (2011) | Fibrovascular growth crossing limbus and extending over the cornea to any distance |
| Shahin (2012) | 4 grades classified |
| Lekhanont (2012) | Fibrovascular tissue invading cornea or when the lesion was categorized as grade 4 |
| Ozgurhan (2013) | No specific definition |
| Xu (2013) | Fibrovascular tissue invading cornea |
| Nava-Castaneda, A (2014) | 4 grades classified |
| Karalezli (2014) | Fibrovascular growth passing the corneal limbus by more than 1mm |
| Razeghinejad (2014) | More than 1.5 mm of fibrovascular tissue overgrowth on cornea and any fibrovascular tissue crossing limbus |
| Ozsutcu (2014) | Any fibrovascular growth of conjunctival tissue extending more than 1.5 mm across limbus |
| Kasetsuwan (2015) | 4 grades classified |
| Singh (2015) | 4 grades classified |
| Bekibele (2016) | Growth of fibrovascular tissue 1 mm or more into cornea |
| Motarjemizadeh (2016) | New vessels or fibrovascular connective tissues crossing corneal limbus |
Figure 2.
Subgroup analysis for the recurrence rates according to types of pterygium (n = 15, the remainder 3 studies without recurrence).
Figure 3.
Subgroup analysis for the recurrence rates according to the treatment (n = 15, the remainder 3 studies without recurrence).
Figure 4.
Subgroup analysis for the recurrence rates according to the follow-up time (n = 15, the remainder 3 studies without recurrence).
17 studies reporting complications were analyzed. There was no statistically significant difference between bevacizumab group and control group (RR 0.87, 95% CI 0.66–1.13, P=0.30; P heterogeneity = 0.52, I 2 = 0%) (supplementary data file). Further analysis of the subconjunctival hemorrhage rate showed that a statistically significant difference was not found between groups (RR 1.50, 95% CI 0.63–3.59, P=0.36; P heterogeneity = 0.69, I 2 = 0%) (supplementary data file).
Publication bias for recurrence rates and complications was checked by evaluating funnel plots (supplementary data file).
4. Discussion
This meta-analysis, updated with 1045 eyes in 18 RCTs showed that bevacizumab would significantly reduce pterygium recurrence rate after surgery in either case of primary pterygium or use of conjunctival autograft or follow-up longer than 12 months. Complications of bevacizumab were not increased compared with the control.
An earlier meta-analysis performed by Hu indicated that bevacizumab had no statistically significant effect on preventing pterygium recurrence [35]. Hu included 9 RCTs, of which 7 reported recurrence and 8 reported complications, whereas in our current meta-analysis, we report raw data on recurrences in 15 and complications in 17 studies. The inclusion of more trials and more cases renders our analysis more statistically significant.
According to Prabhasawat [36], corneal recurrence with fibrovascular tissue covering the excision area and invading the cornea (grade 4) was the true recurrence. However, the definition of recurrence adopted in literatures varied. The inconsistent definition of pterygium recurrence in the included studies (Table 2) implied that the conclusion of the meta-analysis should be interpreted prudently. Study by Razeghinejad defined recurrence as any fibrovascular growth of conjunctival tissue extending more than 1.5 mm across the limbus [37]. In addition, data of recurrence in table 3 of the literature were found incorrect. Thus, the study was excluded. Moreover, the significant effect of bevacizumab on decreasing recurrence in the follow-up longer than 12 months group would suggest that longer follow-up in the future studies could further favor the effect.
RR for the overall recurrence rate was 0.74, with 95% CI [0.56, 0.97]. After removal of the study by Motarjemizadeh [31], I 2 decreased to 0% and RR was 0.98, with 95% CI [0.92, 1.05], but it did not affect the conclusive result in subgroup analysis on the pterygium type or administration route of bevacizumab. Therefore, sensitivity analysis was unstable and the heterogeneity was mainly caused by this study. However, there was no reason to exclude the study after comprehensive reading of the full text.
There was no statistically significant difference in overall complications and subconjunctival hemorrhage between bevacizumab group and control group, showing the safety of bevacizumab. The sensitivity analysis for the complication was stable. It is different from the previous meta-analysis by Hu [35], who reported the bevacizumab group was associated with a higher risk of developing subconjunctival hemorrhage.
The funnel plot for the recurrence and complication rates displayed asymmetry. This could be due to factors other than publication bias, including poor methodological quality, true heterogeneity, artefactual variation, and chance.
Our study had several potential limitations. First, the heterogeneity may result from different administration route of bevacizumab, different type of pterygium, surgeon's experience, and follow-up duration. Second, sensitivity analyses of the recurrence rate were not stable. Therefore, caution is required in their interpretation and more research is still needed.
Despite these limitations, the evidence from the updated meta-analysis shows that bevacizumab application following pterygium surgery provides a statistically significant decrease in recurrence rate in cases of primary pterygium, or use of conjunctival autograft, or follow-up longer than 12 months without an increase in complications. Further study of the long-term efficacy of bevacizumab on reducing pterygium recurrence based on the definition of true recurrence (grade 4) will be needed.
Acknowledgments
The study was supported by Guangdong Natural Science Foundation (2016A030313208).
Disclosure
The funding organization played no role in the design or conduct of this study.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Authors' Contributions
Yi Sun and Bowen Zhang contributed equally.
Supplementary Materials
The supplemental data file mainly described risk of bias assessment, the overall effect of Bevacizumab on reducing recurrence rates, the insignificant difference of recurrence rates between topical and subconjunctival bevacizumab, the complications without increase, and the publication bias.
References
- 1.Chui J., Di Girolamo N., Wakefield D., Coroneo M. T. The pathogenesis of pterygium: current concepts and their therapeutic implications. Ocular Surface. 2008;6:24–43. doi: 10.1016/s1542-0124(12)70103-9. [DOI] [PubMed] [Google Scholar]
- 2.Hacıoğlu D., Erdöl H. Developments and current approaches in the treatment of pterygium. International Ophthalmology. 2017;37:1073–1081. doi: 10.1007/s10792-016-0358-5. [DOI] [PubMed] [Google Scholar]
- 3.Verma N., Garap J. A., Maris R., Kerek A. Intraoperative use of mitomycin C in the treatment of recurrent pterygium. Papua and New Guinea Medical Journal. 1998;41:37–42. [PubMed] [Google Scholar]
- 4.Mastropasqua L., Carpineto P., Ciancaglini M., Enrico Gallenga P. Long term results of intraoperative mitomycin C in the treatment of recurrent pterygium. British Journal of Ophthalmology. 1996;80:288–291. doi: 10.1136/bjo.80.4.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bekibele C. O., Baiyeroju A. M., Olusanya B. A., Ashaye A. O., Oluleye T. S. Pterygium treatment using 5-FU as adjuvant treatment compared to conjunctiva autograft. Eye. 2008;22:31–34. doi: 10.1038/sj.eye.6702480. [DOI] [PubMed] [Google Scholar]
- 6.Akarsu C., Taner P., Ergin A. 5-fluorouracil as chemoadjuvant for primary pterygium surgery: preliminary report. Cornea. 2003;22:522–526. doi: 10.1097/00003226-200308000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Pikkel J., Porges Y., Ophir A. Halting pterygium recurrence by postoperative 5-fluorouracil. Cornea. 2001;20:168–171. doi: 10.1097/00003226-200103000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Simşek T., Günalp I., Atilla H. Comparative efficacy of β-irradiation and mitomycin-C in primary and recurrent pterygium. European Journal of Ophthalmology. 2001;11:126–132. doi: 10.1177/112067210101100204. [DOI] [PubMed] [Google Scholar]
- 9.Ajayi B. G., Bekibele C. O. Evaluation of the effectiveness of post-operative beta-irradiation in the management of pterygium. African Journal of Medicine and Medical Sciences. 2002;31:9–11. [PubMed] [Google Scholar]
- 10.Lee D. H., Cho H. J., Kim J. T., Choi J. S., Joo C. K. Expression of vascular endothelial growth factor and inducible nitric oxide synthase in pterygia. Cornea. 2001;20:738–742. doi: 10.1097/00003226-200110000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Gumus K., Karakucuk S., Mirza G. E., Akgun H., Arda H., Oner A. O. Overexpression of vascular endothelial growth factor receptor 2 in pterygia may have a predictive value for a higher postoperative recurrence rate. British Journal of Ophthalmology. 2014;98:796–800. doi: 10.1136/bjophthalmol-2012-301944. [DOI] [PubMed] [Google Scholar]
- 12.Fukuhara J., Kase S., Ohashi T., et al. Expression of vascular endothelial growth factor C in human pterygium. Histochemistry and Cell Biology. 2013;139:381–389. doi: 10.1007/s00418-012-1019-z. [DOI] [PubMed] [Google Scholar]
- 13.Di Girolamo N., Coroneo M. T., Wakefield D. Active matrilysin (MMP-7) in human pterygia: potential role in angiogenesis. Investigative Ophthalmology & Visual Science. 2001;42:1963–1968. [PubMed] [Google Scholar]
- 14.Di Girolamo N., Chui J., Coroneo M. T., Wakefield D. Pathogenesis of pterygia: role of cytokines, growth factors, and matrix metalloproteinases. Progress in Retinal and Eye Research. 2004;23:195–228. doi: 10.1016/j.preteyeres.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Jin J., Guan M., Sima J., et al. Decreased pigment epithelium-derived factor and increased vascular endothelial growth factor levels in pterygia. Cornea. 2003;22:473–477. doi: 10.1097/00003226-200307000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Aspiotis M., Tsanou E., Gorezis S., et al. Angiogenesis in pterygium: study of microvessel density, vascular endothelial growth factor, and thrombospondin-1. Eye. 2007;21:1095–1101. doi: 10.1038/sj.eye.6702495. [DOI] [PubMed] [Google Scholar]
- 17.Razeghinejad M. R., Hosseini H., Ahmadi F., Rahat F., Eghbal H. Preliminary results of subconjunctival bevacizumab in primary pterygium excision. Ophthalmic Research. 2010;43:134–138. doi: 10.1159/000252980. [DOI] [PubMed] [Google Scholar]
- 18.Shenasi A., Mousavi F., Shoa-Ahari S., Rahimi-Ardabili B., Fouladi R. F. Subconjunctival bevacizumab immediately after excision of primary pterygium: the first clinical trial. Cornea. 2011;30:1219–1222. doi: 10.1097/ico.0b013e31820ca63f. [DOI] [PubMed] [Google Scholar]
- 19.Ozgurhan E. B., Agca A., Kara N., Yuksel K., Demircan A., Demirok A. Topical application of bevacizumab as an adjunct to recurrent pterygium surgery. Cornea. 2013;32:835–838. doi: 10.1097/ico.0b013e3182772d4e. [DOI] [PubMed] [Google Scholar]
- 20.Nava-Castañeda A., Olvera-Morales O., Ramos-Castellon C., Garnica-Hayashi L., Garfias Y. Randomized controlled trial of conjunctival autografting combined with subconjunctival bevacizumab for primary pterygium treatment: one year follow-up. Clinical & Experimental Ophthalmology. 2014;42:235–241. doi: 10.1111/ceo.12140. [DOI] [PubMed] [Google Scholar]
- 21.Lekhanont K., Patarakittam T., Thongphiew P., Suwan-apichon O., Hanutsaha P. Randomized controlled trial of subconjunctival bevacizumab injection in impending recurrent pterygium: a pilot study. Cornea. 2012;31:155–161. doi: 10.1097/ico.0b013e3182151e0e. [DOI] [PubMed] [Google Scholar]
- 22.Fallah M. R., Khosravi K., Hashemian M. N., Beheshtnezhad A. H., Rajabi M. T., Gohari M. Efficacy of topical bevacizumab for inhibiting growth of impending recurrent pterygium. Current Eye Research. 2010;35:17–22. doi: 10.3109/02713680903395273. [DOI] [PubMed] [Google Scholar]
- 23.Shahin M. M., Elbendary A. M., Elwan M. M. Intraoperative subconjunctival bevacizumab as an adjunctive treatment in primary pterygium: a preliminary report. Ophthalmic Surgery, Lasers, and Imaging. 2012;43:459–466. doi: 10.3928/15428877-20120802-02. [DOI] [PubMed] [Google Scholar]
- 24.Banifatemi M., Razeghinejad M. R., Hosseini H., Gholampour A. Bevacizumab and ocular wound healing after primary pterygium excision. Journal of Ocular Pharmacology and Therapeutics. 2011;27:17–21. doi: 10.1089/jop.2010.0094. [DOI] [PubMed] [Google Scholar]
- 25.Enkvetchakul O., Thanathanee O., Rangsin R., Lekhanont K., Suwan-Apichon O. A randomized controlled trial of intralesional bevacizumab injection on primary pterygium: preliminary results. Cornea. 2011;30:1213–1218. doi: 10.1097/ico.0b013e31821c9b44. [DOI] [PubMed] [Google Scholar]
- 26.Razeghinejad M. R., Banifatemi M. Subconjunctival bevacizumab for primary pterygium excision;a randomized clinical trial. Journal of Ophthalmic & Vision Research. 2014;9:22–30. [PMC free article] [PubMed] [Google Scholar]
- 27.Ozsutcu M., Ayintap E., Akkan J. C., Koytak A., Aras C. Repeated bevacizumab injections versus mitomycin C in rotational conjunctival flap for prevention of pterygium recurrence. Indian Journal of Ophthalmology. 2014;62:407–411. doi: 10.4103/0301-4738.120220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh P., Sarkar L., Sethi H. S., Gupta V. S. A randomized controlled prospective study to assess the role of subconjunctival bevacizumab in primary pterygium surgery in Indian patients. Indian Journal of Ophthalmology. 2015;63:779–784. doi: 10.4103/0301-4738.171508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kasetsuwan N., Reinprayoon U., Satitpitakul V. Prevention of recurrent pterygium with topical bevacizumab 0.05% eye drops: a randomized controlled trial. Clinical Therapeutics. 2015;37:2347–2351. doi: 10.1016/j.clinthera.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 30.Hwang S., Choi S. A comparative study of topical mitomycin C, cyclosporine, and bevacizumab after primary pterygium surgery. Korean Journal of Ophthalmology. 2015;29:375–381. doi: 10.3341/kjo.2015.29.6.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Motarjemizadeh Q., Aidenloo N. S., Sepehri S. A comparative study of different concentrations of topical bevacizumab on the recurrence rate of excised primary pterygium: a short-term follow-up study. International Ophthalmology. 2016;36:63–71. doi: 10.1007/s10792-015-0076-4. [DOI] [PubMed] [Google Scholar]
- 32.Bekibele C. O., Sarimiye T. F., Ogundipe A., Olaniyan S. 5-fluorouracil vs avastin as adjunct to conjunctival autograft in the surgical treatment of pterygium. Eye. 2016;30:515–521. doi: 10.1038/eye.2016.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Q. B., Zhu L. W., Xu G. Z. Comparative study of pterygium surgery combined with bevacizumab or mitomycin C. International Eye Science. 2013;13:2532–2534. [Google Scholar]
- 34.Karalezli A., Kucukerdonmez C., Akova Y. A., Koktekir B. E. Does topical bevacizumab prevent postoperative recurrence after pterygium surgery with conjunctival autografting? International Journal of Ophthalmology. 2014;7:512–516. doi: 10.3980/j.issn.2222-3959.2014.03.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu Q., Qiao Y., Nie X., Cheng X., Ma Y. Bevacizumab in the treatment of pterygium: a meta-analysis. Cornea. 2014;33:154–160. doi: 10.1097/ico.0000000000000037. [DOI] [PubMed] [Google Scholar]
- 36.Prabhasawat P., Barton K., Burkett G., Tseng S. C. Comparison of conjunctival autografts, amniotic membrane grafts, and primary closure for pterygium excision. Ophthalmology. 1997;104:974–985. doi: 10.1016/s0161-6420(97)30197-3. [DOI] [PubMed] [Google Scholar]
- 37.Razeghinejad R., Banifatemi M., Hosseini H. The effect of different doses of subconjunctival bevacizumab on the recurrence rate of excised primary pterygium. Bulletin of the Belgian Societies of Ophthalmology. 2013;322:13–20. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The supplemental data file mainly described risk of bias assessment, the overall effect of Bevacizumab on reducing recurrence rates, the insignificant difference of recurrence rates between topical and subconjunctival bevacizumab, the complications without increase, and the publication bias.