Abstract
Diabetic retinopathy is one of the most serious microvascular complications induced by hyperglycemia via five major pathways, including polyol, hexosamine, protein kinase C, and angiotensin II pathways and the accumulation of advanced glycation end products. The hyperglycemia-induced overproduction of reactive oxygen species (ROS) induces local inflammation, mitochondrial dysfunction, microvascular dysfunction, and cell apoptosis. The accumulation of ROS, local inflammation, and cell death are tightly linked and considerably affect all phases of diabetic retinopathy pathogenesis. Furthermore, microvascular dysfunction induces ischemia and local inflammation, leading to neovascularization, macular edema, and neurodysfunction, ultimately leading to long-term blindness. Therefore, it is crucial to understand and elucidate the detailed mechanisms underlying the development of diabetic retinopathy. In this review, we summarized the existing knowledge about the pathogenesis and current strategies for the treatment of diabetic retinopathy, and we believe this systematization will help and support further research in this area.
1. Introduction
Diabetes mellitus (DM) is a metabolic disease characterized by hyperglycemia, due to the defects in insulin secretion and impaired insulin resistance. Diabetes, the long-term high blood sugar condition, leads to the damaging of various tissues, especially the eyes, kidneys, heart, and blood vessels, and it may aggravate other functional disorders. Diabetic retinopathy (DR) represents one of the most serious microvascular complications, in which the main pathological changes include retinal inflammation, increased vascular permeability, and abnormal angiogenesis on the surface of the retina. Previously, five classic pathways were shown to be implicated in the development of diabetic complications: polyol pathway activation, induction of the hexosamine pathway, activation of angiotensin II pathways, increase in the advanced glycosylation end product (AGE) levels in response to the activation of the cell-dependent receptors, and the activation of protein kinase C (PKC) due to the high glucose-induced peroxide overexpression [1]. Mitochondrial damage and oxidative stress are important factors affecting the development of DR [2], as they induce the production of reactive oxygen species (ROS) and the apoptosis of endothelial cells and pericytes.
Landmark clinical trials during the 1980s demonstrated that laser photocoagulation can effectively prevent the loss of vision in the patients with proliferative DR or diabetic macular edema (DME) [3, 4]. The progress of the image modalities, especially optical coherence tomography (OCT) and fluorescein angiography (FA), plays an important role in monitoring and diagnosing the disease progression and complications. Furthermore, the introduction of the intraocular administration of anti-vascular endothelial growth factor (VEGF) agents was a revolution in the management of DR, leading to the possibility of reversing the visual outcome [3–5]. Current clinical trials suggest that the anti-VEGF therapy may represent a first-line therapy for proliferative DR treatment [6]. However, although DR has been studied for a number of years in vitro and in vivo, the detailed mechanisms underlying DR pathogenesis and progression remain unclear, especially concerning the observed mitochondrial dysfunction and oxidative stress. In this review article, we summarized currently investigated mechanisms and treatments, hoping to provide a stronger foundation for the future development of targeted approaches.
2. Clinical Features of DR
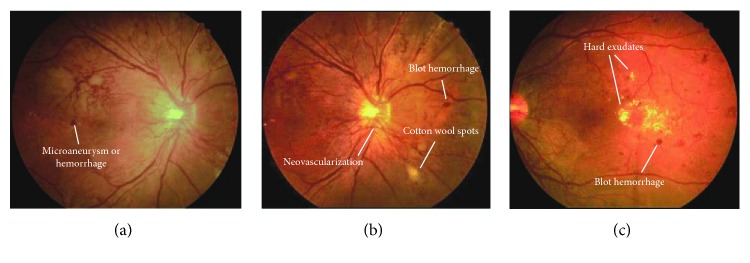
Based on the fundus manifestations during DR progression, different stages of DR can be recognized: mild, moderate, severe nonproliferative, and proliferative DR [7]. In the nonproliferative DR, microaneurysms can be observed, together with some intraretinal hemorrhage and flame-shape hemorrhage, intraretinal microvascular abnormalities (IRMA), and venous caliber changes, while the proliferative DR is characterized by the presence of pathologic neovascularization (Figure 1). Proliferative DR can be further classified according to the location of the new vessels, which can be found either on the optic disc or elsewhere. Neovascularization is usually accompanied by vitreous hemorrhage, traction retinal detachment, iris neovascularization (rubeosis), and angle neovascularization with intraocular pressure elevation (neovascular glaucoma). These lesions can be found years after the diagnosis of type I DM, but they are found at the time of type II DM diagnosis [8]. An important additional category of the DR cases is diabetic macular edema (DME), which represents the most important cause of the vision loss in patients with DR. DME occurs in the DR cases with different severity of disease, even in the mild nonproliferative DR [7]. DME can be categorized into mild cases, located at the posterior pole, but distant from the center of the macula; moderate cases, where the edema is located in the macula but not the center; and severe cases, which involve the center.
Figure 1.
Clinical feature of diabetic retinopathy, including microaneurysm, microhemorrhage, cotton wool spots, neovascularization, and hard exudates.
3. Mechanisms Underlying the Development and Progression of DR
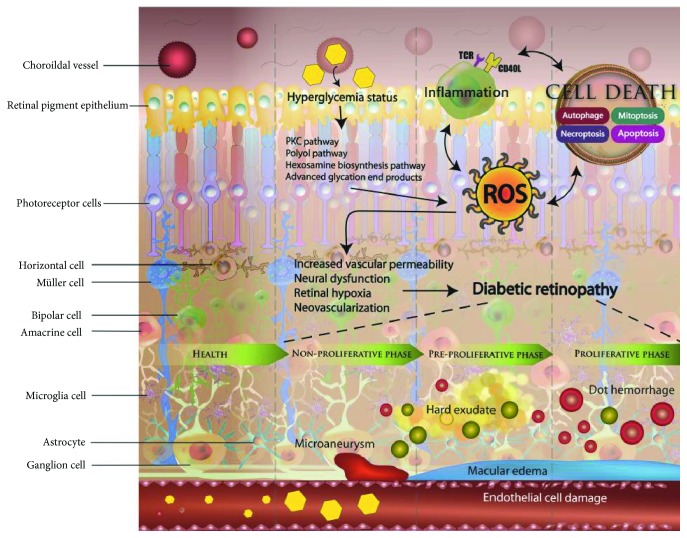
DR is a multifactorial disease, characterized by hyperglycemia, leukostasis, microvascular damage, microinflammation, increased vascular permeability, vascular occlusion, local ischemia, and general neurodegeneration. Persistent hyperglycemia induces cellular metabolism imbalance, including excessive glucose oxidation, ROS production, local inflammation, and endothelial cell death. The detailed mechanisms underlying the accumulation of oxidative stress remain unclear. Recent studies demonstrated that five major pathways are involved in the pathogenesis of this disease, including polyol and angiotensin II pathways, AGE, PKC, and the hexosamine biosynthesis pathways [9–13]. Oxidative stress activates local inflammation and cell death. Endothelial cells on the retinal capillaries, responsible for the balancing of vascular permeability, are damaged by hyperglycemia, which further leads to fluid leakage and accumulation in the retina due to the breakdown of tight junctions between cells. Endothelial cell apoptosis, necrosis, necroptosis, and mitosis lead to local inflammation and microvascular dysfunction in the retina, which further causes blindness. The generation of ROS, inflammation, and cell death form a vicious cycle, promoting the development of DR. Furthermore, microvascular occlusions and hemorrhage activate ischemic signaling, which is followed by neovascularization through the expressions of VEGF [14, 15]. However, the neovascularized vessels are fragile, and their abnormal structure allows hemorrhaging. Progressive hemorrhage induces an increase in the VEGF expression, chronic inflammation, and retinal neurodegeneration. Neurodegeneration, inflammation, and vascular dysfunction occur in parallel and are closely interconnected (Figure 2).
Figure 2.
Illustration showing different mechanisms underlying diabetic retinopathy. During hyperglycemia, the excessive production of reactive oxygen species (ROS) via polyol pathway, advanced glycation end product (AGE) pathway, and protein kinase C (PKC) pathway can lead to the development of local inflammation and cell death. This vicious cycle increases vascular permeability, neural dysfunction, retinal hypoxia, and neovascularization. Neurodegeneration, inflammation, and vascular dysfunction operate in parallel and closely, which ultimately leads to the development of diabetic retinopathy.
Although several pathological mechanisms were reported, the details remain unclear. In this review, we focused on the increased oxidative stress, which occurs due to the polyol pathway activation leading to sorbitol accumulation, production of AGEs, activation of the PKC pathway, inflammation, and cell death.
4. Oxidative Stress Roles in the DR Pathogenesis
DR development is a complex pathological process. Although the mechanisms underlying this have not been completely elucidated, oxidative stress was shown to represent a key factor in this process [16]. Clinical and experimental studies demonstrated that hyperglycemia represents the primary factor leading to the pathogenesis of diabetic complications [17]. In the ischemic state, oxidative stress, superoxide dismutase, glutathione, lipid peroxide, and malondialdehyde (MDA) levels were shown to increase, while those of antioxidants decreased, thus inducing the oxidative damage of the retina [17]. In vitro, increased superoxide levels were observed in hyperglycemic conditions and shown to be accompanied by an increase in the hydrogen peroxide content in retinal cells [18–20]. Oxidative stress can damage cell membrane integrity as well [21], inducing apoptosis, microvascular damage, and barrier damage and ultimately leading to DR development.
4.1. Polyol Pathway Activation
Polyol pathway activation represents one of the processes observed under the hyperglycemia-induced oxidative stress conditions during DR pathogenesis, and this pathway is known as the sorbitol-aldose reductase pathway as well [22, 23]. Here, glucose is reduced to sorbitol and subsequently oxidized to fructose, with the help of two enzymes: aldose reductase, which converts glucose into sorbitol, and sorbitol dehydrogenase, which oxidize sorbitol into fructose [22]. Aldose reductase and sorbitol dehydrogenase require nicotinamide adenine dinucleotide phosphate (NADPH) and nicotinamide adenine dinucleotide (NAD+) to convert glucose into fructose [24]. Under hyperglycemic conditions, polyol pathway activity increases, which is followed by a decrease in the levels of NADPH that can regenerate an intracellular antioxidant, GSH [22]. The overactivation of the polyol pathway leads to the accumulation of ROS, which induces oxidative stress in cells. Under the physiological conditions, hexokinase returns to the glycolytic pathway by phosphorylating fructose into fructose-6-phosphate. However, high serum glucose levels lead to an imbalance between glycogenesis and the glycolysis pathway, favoring the accumulation of sorbitol. The imbalance in the potential energy reduction process was reported in a study examining mitochondrial dysfunction during DR pathogenesis. An excess of glucose in diabetes is converted to sorbitol by aldose reductase, but sorbitol cannot easily penetrate cellular membrane. One part of sorbitol molecules is catalyzed by the sorbitol dehydrogenase, leading to the oxidation of fructose, which is difficult to process further [25]. Therefore, sorbitol and fructose accumulate in cells, leading to an increase in osmotic pressure, edema rupture, and membrane permeability damage. Considering the effects of aldose reductase on the retina, the DR pathogenesis is induced by aldose reductase activity together with the changes in the osmotic pressure caused by the accumulation of polyhydric alcohol and the second step of the sorbitol pathway, in which SDH catalyzes the oxidation of sorbitol to fructose [26]. The reduction of NAD+ into NADH, due to hypoxia and redox imbalance, increases intracellular NADH levels, leading to cell edema, structural alterations, metabolic disorders, and microvascular lesion [27].
4.2. Hexosamine Pathway Activation
In the hexosamine pathway, glucose is phosphorylated and converted into fructose-6-phosphate. Glutamine provides an amino group to fructose-6-phosphate, which leads to the formation of glucosamine 6-phosphate by fructose-6-phosphate amidotransferase (GFAT) [28]. Glucosamine 6-phosphate is acetylated and isomerized to N-acetylglucosamine 6-phosphate and finally converted to diphosphate uracil-N-acetylglucosamine (UDP-GlcNAc), which can form proteoglycans, glycolipids, and glycoproteins [29]. Glucosamine can be directly phosphorylated by hexokinase as well, leading to the generation of glucosamine 6-phosphate and conversion to UDP-GlcNAc, a substrate for post-transcriptional modification of intracellular factors [30]. The hexosamine pathway was reported to mediate the toxic effects of ROS in hyperglycemia [28–31]. In the presence of increased glucose levels, a large amount of ROS is generated, which may inhibit glyceraldehyde-3-phosphate dehydrogenase (GAPDH) activity, resulting in the influx of glycolytic products to the hexosamine pathway [10, 32]. Glucosamine produced by the activated hexosamine increases H2O2 production, which further results in an increased oxidation, changes in cell endothelium, increased vascular permeability, and angiogenesis. Inhibition of GAPDH induces the AGE pathway activity as well, through the interactions with intracellular methylglyoxal, leading to the increase in retinal oxidative stress [9, 33].
4.3. Activation of the PKC Pathway
The PKC pathway is considered a pathway with the key role in the pathogenesis of DR. Many studies demonstrated that the activation of the PKC pathway can lead to endothelial cell damaging by increasing endothelial permeability, changing NO bioavailability, reducing prostaglandin production, inducing VEGF expression, and inducing the production of thromboxane and endothelin-1 (ET-1) [34–37]. Hyperglycemic status induces the accumulation of ROS and synthesis of diacylglycerol (DAG), leading to the PKC pathway activation. Several PKC isoforms were shown to be activated during DR pathogenesis, such as PKC-α, -β, -δ, and -ε [38, 39]. PKC-β activation was shown to induce the release of NO, ET-1, and VEGF in endothelial cells, leading to an increase in the retinal vascular permeability and decrease in blood flow, causing macular edema. PKC-δ activation induces the formation of ROS and activates the p38 and MAPK pathway, which promotes the expression of SHP-1 and NF-κB, thus inhibiting the expression of platelet-derived growth factor (PDGF) and activating caspase signaling, which ultimately leads to pericyte loss and formation of microaneurysms. In vitro, the activity of diabetes-induced oxidative stress was shown to decrease following the administration of the PKC-β-specific inhibitor (LY53331), and the absence of the PKC-β isoform was shown to prevent ROS-mediated diabetic complications [40, 41]. Additionally, LY333531 treatment decreased PKC signaling levels, improving retinal vascular circulation [41, 42]. PKC pathway activation alters NO production through eNOS expression, directly affecting vascular tone and permeability and ultimately promoting endothelial dysfunction.
4.4. AGE Accumulation
Hyperglycemia leads to an increase in the nonenzymatic glycosylation of tissue macromolecules. AGEs are irreversibly cross-linked products, formed from strong glycating dicarbonyl compounds such as methylglyoxal and glyoxal [43]. The receptor for AGE (RAGE) plays an important role in the DR pathogenesis as well [44], as its activation mediates a wide range of biological effects, including ROS level increase, cytokine release, and cell function and death alterations. AGE and RAGE, accumulated in the retinal microvessels, interact directly with intracellular proteins, leading to endothelial dysfunction [45, 46]. Increased AGE accumulation induces pericyte apoptosis in the retina as well, through the activation of NF-κB [47]. In the bovine retinal capillary pericytes, following the treatment with AGE solution, pericyte apoptosis and a decrease in the antioxidant activity were observed [48]. AGEs can promote the release of cytokines and VEGF, affecting vascular endothelial permeability and self-regulation and inducing inflammation.
4.5. Angiotensin II (ANG-II) Induces Retinal Oxidative Damage
ANG-II is the product of renin-angiotensin system (RAS) that is involved in the regulation of the systemic and local blood pressures [49]. ANG-II plays important roles in both atherosclerosis and diabetes pathogeneses [50–52]. During DR pathogenesis, this molecule induces vasoconstriction, inflammation, oxidative stress, cellular dysfunctions, angiogenesis, and fibrosis [53, 54]. Additionally, it can activate NADPH enzyme levels as well, thus increasing the production of ROS and directly damaging endothelial cells [55, 56]. Previously, ANG-II was reported to induce the production of peroxynitrite in vascular endothelial cells and to promote PARP signaling activation, which in turn activates NF-κB and a release of many inflammatory cytokines, leading to the endothelial cell damage [57, 58]. These ANG-II effects can be prevented by the use of NADPH oxidase inhibitors [59, 60].
5. Mitochondrial Dysfunction Roles in the DR Pathogenesis
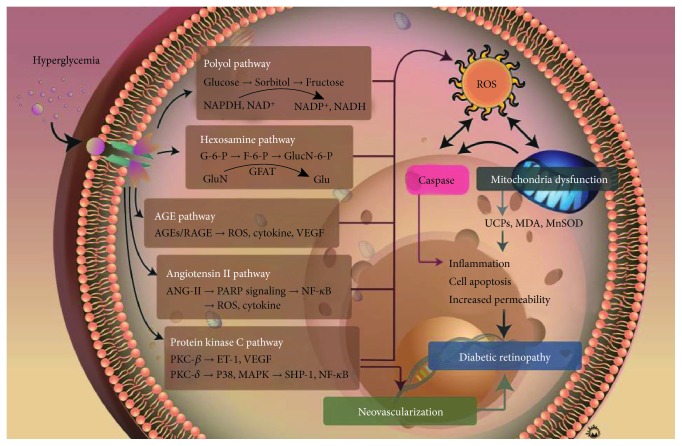
Mitochondria are the primary source of cellular energy, involved in metabolic processes and respiration [15]. Their main role is adenosine triphosphate (ATP) production, cell metabolism control, and apoptosis regulation [61], and their dysfunction severely affects tissue homeostasis. ROS [62], superoxide dismutase, and hydroxyl radicals are mainly formed in the mitochondria. Under hyperglycemic conditions, ROS is overproduced in the retina, leading to an increase in oxidative processes and the disturbance in the mitochondrial functions, which may lead to the retinal capillary cell apoptosis [63–65]. Oxidative stress increase during hyperglycemia damages the structure and function of mitochondria [63]. The main alterations in the expression levels and activity are associated with these molecules: mitochondrial superoxide dismutase (MnSOD) [66–68], catalase (CAT) [69, 70], MDA [71–73], uncoupling proteins (UCPs) [18, 74, 75], aldose reductase, AGEs, glutathione peroxidase, nitrotyrosine (NT) [15, 76, 77], and 8-hydroxyguanosine (8-OHG and 8-OHdG) [78–80]. The UCPs, MDA, and MnSOD have been investigated the most. UCPs belong to the mitochondrial anion carrier gene family, and the uncoupling refers to the separation of ATP synthesis and mitochondrial respiration, which is achieved through proton leakage [81]. UCP functions include the reduction in the electrochemical gradient by increasing the proton leakage through the mitochondrial inner membrane, thereby reducing ROS production. Previous studies demonstrated that five isoforms of UCPs are expressed in bovine retinal microvascular endothelial cells and pericytes. UCP1, UCP2, and MnSOD were shown to be expressed in high-glucose environment [82]. Retinal neuron apoptosis in DR was observed and shown to be associated with a decrease in MnSOD expression [83, 84]. Mitochondrial morphology is altered in these processes as well, and their expansion can be observed in the retina of diabetic rats [20, 85]. Endothelial cells and pericytes gradually lose their original morphological features and become heterogeneous with irregular arrangement, finally leading to retinal cell apoptosis [86]. These processes induce mitochondrial ROS production, endothelial cell and pericyte apoptosis, and, ultimately, DR pathogenesis (Figure 3).
Figure 3.
Mechanisms underlying hyperglycemia-induced oxidative stress increase that is involved in diabetic retinopathy pathogenesis.
6. Angiogenesis and VEGF Roles during DR Pathogenesis
As early as the 1950s, scholars suggested that the DR development may be associated with retinal ischemia and hypoxia-induced neovascularization, which was first confirmed in 1994 [87, 88]. Two subtypes of VEGF exist, and VEGF2 stimulates the proliferation and migration of endothelial cells to form new blood vessels that may enable ocular microvascular leakage in the proliferative DR (PDR) patients [89]. Recent studies also found that many molecular signaling pathways associated with VEGF in patients with PDR have varying degrees of disruption, resulting in an imbalance of intravitreal angiogenesis [90–92]. The expression of placental growth factor (P1GF) in the vitreous cavity of patients with PDR is significantly increased, which further enhances VEGF signaling [90]. Additionally, the expression of connective tissue growth factor (CTGF) in the vitreous cavity of patients was shown to be significantly upregulated, and it accelerates the fibrosis process and acts in synergy with VEGF. CTGF plays an important role in the process of fibrogenesis of neovascular membrane and retinal detachment as well [91]. VEGF induces an increased expression of intracellular adhesion molecule-1 (ICAM-1), leading to the stasis and aggregation of white blood cells in the retina, gradually destroying the blood-retinal barrier and causing the damage and death of vascular endothelial cells, which ultimately leads to the formation of capillaries with no perfusion area. With the DR progression, the concentrations of VEGF and ICAM-1 in the vitreous cavity increase as well, and their levels were shown to correlate significantly [93]. VEGF also stimulates the migration of endothelial progenitor cells (EPCs) from the bone marrow to the retina and accelerates the neovascularization by inducing the release of the stem cell factor (SCF) [94, 95]. VEGF may interfere with the balance in the levels of tissue plasminogen activator (t-PA) and plasminogen activator inhibitor (PAI). The overexpression of t-PA and PAI induces extracellular matrix destruction and the degradation of the basement membrane of vascular endothelial cells [96, 97]. Increased VEGF levels may induce the expression of various inflammatory factors, such as transforming growth factor beta-1 (TGF-β1) and interleukin 6 (IL-6) that accelerate PDR progression [98]. Heparan sulfate is an important component of the blood-retinal barrier stroma. Abu et al. [92] demonstrated that heparanase concentrations in the vitreous cavity of the PDR patients are significantly higher compared with those in the controls, suggesting that heparanase accelerates the decomposition of heparan sulfate and the destruction of the blood-retinal barrier, together with inducing VEGF expression and promoting neovascularization. Furthermore, they demonstrated that heparan sulfate expression alterations were more severe in younger patients, which, to some extent, explains why the PDR progression is more difficult to control in younger patients with the anti-VEGF therapy [92, 99, 100].
7. DR Treatment Strategies
Vision loss is the most severe consequence of DR, and it can be managed using different approaches, including intraocular anti-VEGF agents and steroids for the treatment of DME, panretinal laser photocoagulation aimed at proliferative DR treatment, and surgery for vitreous hemorrhage and traction retinal detachment.
7.1. Laser Photocoagulation
Panretinal photocoagulation has been developed since the 1960s and represents a standard treatment for proliferative DR and DME. The principle of laser photocoagulation activity is based on the thermal effects that alleviate retinal ischemia and regulate the hemodynamics of retinal circulation. Improved retinal hypoxia and hemodynamic changes result in the regression of neovascularization and macular edema due to the reduction in the levels of VEGF and other inflammatory factors [101].
The landmark Diabetic Retinopathy Study (DRS) reported the reduction of severe vision loss in the patients with the high-risk proliferative DR and severe nonproliferative DR from 33% to 13% over 5 years following the prompt application of the panretinal photocoagulation [102]. Furthermore, the Early Treatment Diabetic Retinopathy Study (ETDRS) demonstrated that focal laser photocoagulation reduced by 50% the risk of moderate vision loss in patients with the clinically significant DME [102, 103]. Panretinal photocoagulation remains the mainstay of the proliferative DR treatment and should be especially considered for the patients with poor compliance and poor metabolic control, prior to the cataract surgery and severe cataract development, which may limit the applicability of the laser photocoagulation treatment. However, some side effects of this therapy exist, including a moderate decrease in vision, scotoma development, and secondary neovascularization development.
7.2. Treatment with the Intraocular Anti-VEGF Agents
In the 2000s, intraocular injection of anti-VEGF agents was introduced as a new treatment modality for DME, and this approach represents one of the most important approaches recently developed in ophthalmology [104]. Currently, three anti-VEGF treatments are used: ranibizumab (Lucentis, Genentech, South San Francisco, CA, USA), bevacizumab (Avastin, Genentech), and aflibercept (Eylea, Regeneron, Tarrytown, NY, USA).
For the treatment of DME, anti-VEGF currently represents the first-line therapy, which has replaced the focal macular laser treatment. The RESTORE study reported that the ranibizumab monotherapy or ranibizumab in combination with laser treatment demonstrated superior visual acuity gain over standard laser alone in patients with DME [105]. The results showed that the administration of ranibizumab and the deferred laser is superior to that of ranibizumab with prompt laser. For approximately 50% of the eyes treated with intravitreal ranibizumab, no further therapies were required over 5 years. These results suggest that the anti-VEGF treatment lowers the injection frequency with time, while the macular focal laser plays a less important role in the DME treatment [106, 107]. The Ranibizumab for Diabetic Macular Edema (RIDE and RISE) trials examined the effects of monthly ranibizumab injections at 0.3 mg and 0.5 mg, with the 5-year follow-up, and demonstrated that the early and regular application of ranibizumab lowers the risk of proliferative DR development. Furthermore, the use of ranibizumab was observed to prevent retinal nonperfusion [108].
Although multiple intraocular injections are required, the treatment protocol depends on the selected drug, dosage, and retreatment criteria, and different levels of visual improvement can be obtained. In the DRCR.net Protocol T and Cai and Bressler [109, 110] reports, bevacizumab, ranibizumab, and aflibercept were compared, and it was shown that all three drugs can improve vision and are well-tolerated. However, the intravitreal administration of bevacizumab was inferior to that of both aflibercept and ranibizumab. A subgroup analysis demonstrated that aflibercept has a superior effect on the vision improvement with poorer initial baseline BCVA (less than 69 letters) compared with ranibizumab and bevacizumab. The difference in the resulting visual acuity between aflibercept and ranibizumab was significant in the first year but decreased at 2 years [109, 111]. Additionally, the effects of the anti-VEGF therapy on the high-risk proliferative DR have not been clarified. Recently, the results of the DRCR.net Protocol S were presented, demonstrating that the intravitreal ranibizumab injection is not inferior to the panretinal laser photocoagulation in patients with the increased risk of proliferative DR. At 2 years, mean visual acuity improvement following the application of these two treatment modalities was shown to be similar. Area under the curve (AUC) analysis demonstrated the superiority of ranibizumab to panretinal photocoagulation, with the incidence of vitrectomy lower in the group receiving ranibizumab. Although Protocol S results confirmed only the noninferiority of ranibizumab over panretinal photocoagulation, the cost of consecutive injections was shown to be considerably lower, suggesting that the anti-VEGF therapy may represent a valuable alternative for the treatment of proliferative DR, while the combination of these therapies may be the most practical approach in the clinic [6].
More than 50% of patients with the proliferative DR were shown not to have increased VEGF levels in the vitreous fluid [88], which may explain why some proliferative DR patients are resistant to the anti-VEGF treatment. In this patient subgroup, proinflammatory cytokines most likely play pathological roles, and the application of intravitreal steroids or other cytokine inhibitors may be more plausible treatment options.
7.3. Intraocular Steroid Application
The molecular mechanisms involving inflammatory pathways have been proposed to underlie DME pathogenesis [112, 113]. In addition to the increase in VEGF expression, the secretion of different types of proinflammatory cytokines (TNF-α, IL-6, and IL-1β) represents a common occurrence in patients with the proliferative DR. Corticosteroids suppressing the inflammatory pathways are considered potential DME treatment options. Furthermore, inflammatory cells produce a number of angiogenic growth factors and cytokines, which can promote neovascularization [114]. Corticosteroids downregulate VEGF expression by reducing proinflammatory cytokine levels and regulating the activity of inflammatory cells. However, due to the adverse effects of corticosteroids, such as cataract development, increase in the intraocular pressure, and increased risk of endophthalmitis, and anti-VEGF therapy, they can be administered only to a selected group of patients, such as those with chronic DME.
In a number of trials, the benefits of intravitreal triamcinolone were shown to be inferior to those obtained by using laser treatment alone, during a 3-year follow-up. The DRCR.net also reported that the intravitreal triamcinolone effects lasted for less than 1 year, while the visual acuity outcome was not superior to that of the laser photocoagulation at 2 years [106].
Ozurdex (Allergan, Irvine, California, USA) is a biodegradable implant that slowly releases 0.7 mg of dexamethasone over 6 months. The Macular Edema: Assessment of Implantable Dexamethasone in Diabetes (MEAD) study evaluated the effectiveness of dexamethasone implants in patients with DME. A higher percentage of patients was shown to achieve more than 15-letter improvement over 3 years in comparison with that in the sham group (22.2% vs. 12%, P < 0.018) [115]. Iluvien (Alimera Sciences, Alpharetta, GA, USA), another sustained-release intravitreal implant, provides the therapeutic effects up to 36 months by slowly delivering micrograms of fluocinolone acetonide. The Fluocinolone Acetonide in Diabetic Macular Edema study evaluated the effectiveness of the low-dose (0.2 μg per day) and high-dose (0.5 μg per day) fluocinolone implants in patients who received at least one laser therapy, demonstrating the improvement of more than 15 letters in both fluocinolone implant-treated groups, compared with that in the control group. Furthermore, in the subgroup with chronic DME followed up for 3 years, a higher percentage of patients with Iluvien treatment showed a VA improvement of more than 15 letters than that in the control group (28.7% vs. 18.9%) [116].
7.4. Surgery
Vitrectomy is a standard treatment for severe proliferative DR, for patients who do not respond to the panretinal photocoagulation and anti-VEGF therapy or those with persistent vitreous hemorrhage or traction retinal detachment. The DRCR.net Protocol D study investigated the effectiveness of pars plana vitrectomy and membrane peeling in patients with DME and vitreomacular traction, reporting that approximately 40% of patients had improved visual acuity, whereas 22% of patients were shown to have poorer visual acuity outcome, with less than 10-letter gain [117]. Prolonged vitreous hemorrhage and retinal detachment involving macula are common complications of proliferative DR, and these patients should receive surgical treatment. If panretinal photocoagulation has been performed previously, the reabsorption of hemorrhage can be observed, but the surgery is indicated if vitreous hemorrhage persists for longer than 6 months [118, 119]. According to the results of the Diabetic Retinopathy Vitrectomy Study (DRVS), early vitrectomy is strongly suggested, especially in patients with type I diabetes, but no advantages were reported for the patients with type II diabetes [120].
Surgical intervention for the treatment of neovascular glaucoma is indicated in the presence of neovascularization and persistent elevation of intraocular pressure, even after the complete panretinal photocoagulation and anti-VEGF therapy. Glaucoma drainage implants (GDIs) have recently gained popularity for the treatment of neovascular glaucoma (NVG), and their success relies less on the control of intraocular inflammation and bleb failure rate [118, 119].
7.5. New Insights in the Pharmacological Management of DR
Several novel pharmacological therapies are currently developed to target the mechanisms underlying DR development and progression, and these are expected to change our approaches to the DR treatment. The main strategy is preventing the damage to the retinal microvasculature induced by the progression of diabetes. Experimental studies demonstrated that the PKC activity is significantly increased following the increase in blood sugar levels, which has been implicated in the pathogenesis of microvascular damage. Ruboxistaurin (LY333531), a specific inhibitor of PKC-β1 and -β2, has been shown to prevent microvascular complications and ischemia-associated neovascularization in the animal models of diabetes [40, 121]. Additional trials evaluating the effectiveness and safety of ruboxistaurin are currently being conducted [122–125]. Furthermore, the activity of somatostatin has been linked to the progression of DR, and early studies reported that the octreotide therapy in the patients with nonproliferative DR may decrease the need for the application of laser photocoagulation [126, 127]. However, these effects were not significantly partial in the patients with proliferative DR.
Systemic therapies were shown to reduce the risk of DR progression and to modulate retinal microvasculature via renin-angiotensin system (RAAS) and lipid metabolism. Fenofibrate is the third generation of fibric acid-derivative lipid-regulating drugs, with can prevent the progression of DR through multiple mechanisms, including the well-established lipid-lowering effect, the inhibition of the VEGF pathway, and the maintenance of the normal endothelial structure [128]. The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study reported the effects of fenofibrate on the cardiovascular system and the considerable decrease in the need for laser photocoagulation treatment and severe DR progression [129]. Enalapril, an angiotensin-converting enzyme (ACE) inhibitor, was also reported to decrease the neovascularization and progression of the diabetes complications, but it remains unclear whether these beneficial effects are due to the hypotensive activity [130]. However, many drugs are still in the phases I and II of clinical trials, and they may increase the effectiveness of the DR therapy in the future.
8. Conclusions
The pathogenesis of DR is complex and has not been completely elucidated. However, its incidence and severity are high. Current treatment methods are mainly aimed at the treatment of the DMR and advanced-stage proliferative DR. The advantages and disadvantages of the existing single and combined therapies must be evaluated as well. Therefore, the observation that the accumulation of ROS induces neurovascular dysfunction through mitochondrial failure, inflammation, and cell death which are the major mechanisms underlying DR development represents an important conceptual advance in this field.
Acknowledgments
This study was funded by grants RD106077 and RD106078 from the Show Chwan Memorial Hospital, Taiwan.
Contributor Information
Tzu-Ting Lai, Email: bestmind0402@gmail.com.
Chia-Jung Li, Email: nigel6761@gmail.com.
Conflicts of Interest
The authors declare no conflict of interest.
Authors' Contributions
Meng-Yu Wu and Tzu-Ting Lai contributed equally to this work.
References
- 1.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 2.Tarr J. M., Kaul K., Chopra M., Kohner E. M., Chibber R. Pathophysiology of diabetic retinopathy. ISRN Ophthalmology. 2013;2013:13. doi: 10.1155/2013/343560.343560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonetti D. A., Klein R., Gardner T. W. Diabetic retinopathy. The New England Journal of Medicine. 2012;366(13):1227–1239. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- 4.Cheung N., Mitchell P., Wong T. Y. Diabetic retinopathy. The Lancet. 2010;376(9735):124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 5.Frank R. N. Diabetic retinopathy. The New England Journal of Medicine. 2004;350(1):48–58. doi: 10.1056/NEJMra021678. [DOI] [PubMed] [Google Scholar]
- 6.Writing Committee for the Diabetic Retinopathy Clinical Research, Gross J. G., Glassman A. R., et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA. 2015;314(20):2137–2146. doi: 10.1001/jama.2015.15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilkinson C. P., Ferris F. L., Klein R. E., 3rd, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110(9):1677–1682. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 8.Sim D. A., Keane P. A., Rajendram R., et al. Patterns of peripheral retinal and central macula ischemia in diabetic retinopathy as evaluated by ultra-widefield fluorescein angiography. American Journal of Ophthalmology. 2014;158(1):144–153.e1. doi: 10.1016/j.ajo.2014.03.009. e141. [DOI] [PubMed] [Google Scholar]
- 9.Beisswenger P. J., Howell S. K., Smith K., Szwergold B. S. Glyceraldehyde-3-phosphate dehydrogenase activity as an independent modifier of methylglyoxal levels in diabetes. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2003;1637(1):98–106. doi: 10.1016/S09254439(02)00219-3. [DOI] [PubMed] [Google Scholar]
- 10.Du X. L., Edelstein D., Rossetti L., et al. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(22):12222–12226. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engerman R. L., Kern T. S., Larson M. E. Nerve conduction and aldose reductase inhibition during 5 years of diabetes or galactosaemia in dogs. Diabetologia. 1994;37(2):141–144. doi: 10.1007/s001250050084. [DOI] [PubMed] [Google Scholar]
- 12.Koya D., King G. L. Protein kinase C activation and the development of diabetic complications. Diabetes. 1998;47(6):859–866. doi: 10.2337/diabetes.47.6.859. [DOI] [PubMed] [Google Scholar]
- 13.Stauble B., Boscoboinik D., Tasinato A., Azzi A. Modulation of activator protein-1 (AP-1) transcription factor and protein kinase C by hydrogen peroxide and D-alpha-tocopherol in vascular smooth muscle cells. European Journal of Biochemistry. 1994;226(2):393–402. doi: 10.1111/j.1432-1033.1994.tb20064.x. [DOI] [PubMed] [Google Scholar]
- 14.Caldwell R. B., Bartoli M., Behzadian M. A., et al. Vascular endothelial growth factor and diabetic retinopathy: role of oxidative stress. Current Drug Targets. 2005;6(4):511–524. doi: 10.2174/1389450054021981. [DOI] [PubMed] [Google Scholar]
- 15.Kowluru R. A. Diabetic retinopathy: mitochondrial dysfunction and retinal capillary cell death. Antioxidants & Redox Signaling. 2005;7(11-12):1581–1587. doi: 10.1089/ars.2005.7.1581. [DOI] [PubMed] [Google Scholar]
- 16.Madsen-Bouterse S. A., Zhong Q., Mohammad G., Ho Y. S., Kowluru R. A. Oxidative damage of mitochondrial DNA in diabetes and its protection by manganese superoxide dismutase. Free Radical Research. 2010;44(3):313–321. doi: 10.3109/10715760903494168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cade W. T. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Physical Therapy. 2008;88(11):1322–1335. doi: 10.2522/ptj.20080008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cui Y., Xu X., Bi H., et al. Expression modification of uncoupling proteins and MnSOD in retinal endothelial cells and pericytes induced by high glucose: the role of reactive oxygen species in diabetic retinopathy. Experimental Eye Research. 2006;83(4):807–816. doi: 10.1016/j.exer.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 19.Du Y., Miller C. M., Kern T. S. Hyperglycemia increases mitochondrial superoxide in retina and retinal cells. Free Radical Biology & Medicine. 2003;35(11):1491–1499. doi: 10.1016/j.freeradbiomed.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 20.Kowluru R. A., Abbas S. N. Diabetes-induced mitochondrial dysfunction in the retina. Investigative Ophthalmology & Visual Science. 2003;44(12):5327–5334. doi: 10.1167/iovs.03-0353. [DOI] [PubMed] [Google Scholar]
- 21.Bonnefont-Rousselot D. Glucose and reactive oxygen species. Current Opinion in Clinical Nutrition and Metabolic Care. 2002;5(5):561–568. doi: 10.1097/00075197-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Lorenzi M. The polyol pathway as a mechanism for diabetic retinopathy: attractive, elusive, and resilient. Experimental Diabetes Research. 2007;2007:10. doi: 10.1155/2007/61038.61038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altmann C., Schmidt M. The role of microglia in diabetic retinopathy: inflammation, microvasculature defects and neurodegeneration. International Journal of Molecular Sciences. 2018;19(1):p. 110. doi: 10.3390/ijms19010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunlop M. Aldose reductase and the role of the polyol pathway in diabetic nephropathy. Kidney International Supplement. 2000;77:S3–12. doi: 10.1046/j.1523-1755.2000.07702.x. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt R. E., Dorsey D. A., Beaudet L. N., et al. A potent sorbitol dehydrogenase inhibitor exacerbates sympathetic autonomic neuropathy in rats with streptozotocin-induced diabetes. Experimental Neurology. 2005;192(2):407–419. doi: 10.1016/j.expneurol.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 26.Ferreira F. N., Crispim D., Canani L. H., Gross J. L., dos Santos K. G. Association study of sorbitol dehydrogenase -888G>C polymorphism with type 2 diabetic retinopathy in Caucasian-Brazilians. Experimental Eye Research. 2013;115:140–143. doi: 10.1016/j.exer.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 27.Ellis E. A., Guberski D. L., Hutson B., Grant M. B. Time course of NADH oxidase, inducible nitric oxide synthase and peroxynitrite in diabetic retinopathy in the BBZ/WOR rat. Nitric oxide. 2002;6(3):295–304. doi: 10.1006/niox.2001.0419. [DOI] [PubMed] [Google Scholar]
- 28.Jones D. R., Keune W.-J., Anderson K. E., Stephens L. R., Hawkins P. T., Divecha N. The hexosamine biosynthesis pathway and O-GlcNAcylation maintain insulin-stimulated PI3K-PKB phosphorylation and tumour cell growth after short-term glucose deprivation. The FEBS Journal. 2014;281(16):3591–3608. doi: 10.1111/febs.12879. [DOI] [PubMed] [Google Scholar]
- 29.Buse M. G. Hexosamines, insulin resistance and the complications of diabetes: current status. American Journal of Physiology Endocrinology and Metabolism. 2006;290(1):E1–E8. doi: 10.1152/ajpendo.00329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schleicher E. D., Weigert C. Role of the hexosamine biosynthetic pathway in diabetic nephropathy. Kidney International Supplement. 2000;77:S13–S18. doi: 10.1046/j.1523-1755.2000.07703.x. [DOI] [PubMed] [Google Scholar]
- 31.Fantus I. G., Goldberg H. J., Whiteside C. I., Topic D. The hexosamine biosynthesis pathway. In: Cortes P., Mogensen C. E., editors. The Diabetic Kidney. Totowa, NJ, USA: Humana Press; 2006. pp. 117–133. (Contemporary Diabetes). [DOI] [Google Scholar]
- 32.Du X., Matsumura T., Edelstein D., et al. Inhibition of GAPDH activity by poly (ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. Journal of Clinical Investigation. 2003;112(7):1049–1057. doi: 10.1172/JCI18127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 34.Amadio M., Bucolo C., Leggio G. M., Drago F., Govoni S., Pascale A. The PKCbeta/HuR/VEGF pathway in diabetic retinopathy. Biochemical Pharmacology. 2010;80(8):1230–1237. doi: 10.1016/j.bcp.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 35.Das Evcimen N., King G. L. The role of protein kinase C activation and the vascular complications of diabetes. Pharmacological Research. 2007;55(6):498–510. doi: 10.1016/j.phrs.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 36.Noh H., King G. L. The role of protein kinase C activation in diabetic nephropathy. Kidney International Supplement. 2007;72:S49–S53. doi: 10.1038/sj.ki.5002386. [DOI] [PubMed] [Google Scholar]
- 37.Ishii H., Koya D., King G. L. Protein kinase C activation and its role in the development of vascular complications in diabetes mellitus. Journal of molecular medicine. 1998;76(1):21–31. doi: 10.1007/s109-1998-8101-y. [DOI] [PubMed] [Google Scholar]
- 38.Idris I., Gray S., Donnelly R. Protein kinase C activation: isozyme-specific effects on metabolism and cardiovascular complications in diabetes. Diabetologia. 2001;44(6):659–673. doi: 10.1007/s001250051675. [DOI] [PubMed] [Google Scholar]
- 39.Inoguchi T., Battan R., Handler E., Sportsman J. R., Heath W., King G. L. Preferential elevation of protein kinase C isoform beta II and diacylglycerol levels in the aorta and heart of diabetic rats: differential reversibility to glycemic control by islet cell transplantation. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(22):11059–11063. doi: 10.1073/pnas.89.22.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishii H., Jirousek M. R., Koya D., et al. Amelioration of vascular dysfunctions in diabetic rats by an oral PKC beta inhibitor. Science. 1996;272(5262):728–731. doi: 10.1126/science.272.5262.728. [DOI] [PubMed] [Google Scholar]
- 41.Yokota T., Ma R. C., Park J. Y., et al. Role of protein kinase C on the expression of platelet-derived growth factor and endothelin-1 in the retina of diabetic rats and cultured retinal capillary pericytes. Diabetes. 2003;52(3):838–845. doi: 10.2337/diabetes.52.3.838. [DOI] [PubMed] [Google Scholar]
- 42.Park J. Y., Ha S. W., King G. L. The role of protein kinase C activation in the pathogenesis of diabetic vascular complications. Peritoneal dialysis international. 1999;19(Supplement 2):S222–S227. [PubMed] [Google Scholar]
- 43.Glomb M. A., Monnier V. M. Mechanism of protein modification by glyoxal and glycolaldehyde, reactive intermediates of the Maillard reaction. The Journal of Biological Chemistry. 1995;270(17):10017–10026. doi: 10.1074/jbc.270.17.10017. [DOI] [PubMed] [Google Scholar]
- 44.Hudson B. I., Stickland M. H., Futers T. S., Grant P. J. Effects of novel polymorphisms in the RAGE gene on transcriptional regulation and their association with diabetic retinopathy. Diabetes. 2001;50(6):1505–1511. doi: 10.2337/diabetes.50.6.1505. [DOI] [PubMed] [Google Scholar]
- 45.Stitt A. W. The role of advanced glycation in the pathogenesis of diabetic retinopathy. Experimental and Molecular Pathology. 2003;75(1):95–108. doi: 10.1016/S0014-4800(03)00035-2. [DOI] [PubMed] [Google Scholar]
- 46.Yamagishi S., Matsui T. Advanced glycation end products (AGEs), oxidative stress and diabetic retinopathy. Current Pharmaceutical Biotechnology. 2011;12(3):362–368. doi: 10.2174/138920111794480534. [DOI] [PubMed] [Google Scholar]
- 47.Katagiri M., Shoji J., Inada N., Kato S., Kitano S., Uchigata Y. Evaluation of vitreous levels of advanced glycation end products and angiogenic factors as biomarkers for severity of diabetic retinopathy. International Ophthalmology. 2017;38(2):607–615. doi: 10.1007/s10792-017-0499-1. [DOI] [PubMed] [Google Scholar]
- 48.Yokoi M., Yamagishi S. I., Takeuchi M., et al. Elevations of AGE and vascular endothelial growth factor with decreased total antioxidant status in the vitreous fluid of diabetic patients with retinopathy. The British Journal of Ophthalmology. 2005;89(6):673–675. doi: 10.1136/bjo.2004.055053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Funatsu H., Yamashita H. Pathogenesis of diabetic retinopathy and the renin-angiotensin system. Ophthalmic and Physiological Optics. 2003;23(6):495–501. doi: 10.1046/j.1475-1313.2003.00134.x. [DOI] [PubMed] [Google Scholar]
- 50.Chu K. Y., Leung P. S. Angiotensin II in type 2 diabetes mellitus. Current Protein & Peptide Science. 2009;10(1):75–84. doi: 10.2174/138920309787315176. [DOI] [PubMed] [Google Scholar]
- 51.Ribeiro-Oliveira A., Nogueira A. I., Pereira R. M., Boas W. W. V., dos Santos R. A. S., e Silva A. C. S. The renin–angiotensin system and diabetes: an update. Vascular Health and Risk Management. 2008;4(4):787–803. doi: 10.2147/vhrm.s1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu M.-Y., Li C.-J., Hou M.-F., Chu P.-Y. New insights into the role of inflammation in the pathogenesis of atherosclerosis. International Journal of Molecular Sciences. 2017;18(10):p. 2034. doi: 10.3390/ijms18102034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sjolie A. K., Chaturvedi N. The retinal renin-angiotensin system: implications for therapy in diabetic retinopathy. Journal of Human Hypertension. 2002;16:S42–S46. doi: 10.1038/sj.jhh.1001438. [DOI] [PubMed] [Google Scholar]
- 54.Williams B. Angiotensin II, VEGF, and diabetic retinopathy. The Lancet. 1998;351(9105):837–838. doi: 10.1016/S0140-6736(05)78974-1. [DOI] [PubMed] [Google Scholar]
- 55.Seagle B. L., Gasyna E. M., Mieler W. F., Norris J. R., Jr. Photoprotection of human retinal pigment epithelium cells against blue light-induced apoptosis by melanin free radicals from Sepia officinalis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(45):16644–16648. doi: 10.1073/pnas.0605986103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kowluru R. A. Effect of advanced glycation end products on accelerated apoptosis of retinal capillary cells under in vitro conditions. Life Sciences. 2005;76(9):1051–1060. doi: 10.1016/j.lfs.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 57.van Dijk E. H., Duits D. E., Versluis M., et al. Loss of MAPK pathway activation in post-mitotic retinal cells as mechanism in MEK inhibition-related retinopathy in cancer patients. Medicine. 2016;95(18, article e3457) doi: 10.1097/md.0000000000003457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cai Y., Li W., Tu H., et al. Curcumolide reduces diabetic retinal vascular leukostasis and leakage partly via inhibition of the p38MAPK/NF-κB signaling. Bioorganic & Medicinal Chemistry Letters. 2017;27(8):1835–1839. doi: 10.1016/j.bmcl.2017.02.045. [DOI] [PubMed] [Google Scholar]
- 59.Lai T. H., Wu P. H., Wu W. B. Involvement of NADPH oxidase and NF-κB activation in CXCL1 induction by vascular endothelial growth factor in human endometrial epithelial cells of patients with adenomyosis. Journal of Reproductive Immunology. 2016;118:61–69. doi: 10.1016/j.jri.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 60.Drummond G. R., Sobey C. G. Endothelial NADPH oxidases: which NOX to target in vascular disease? Trends in Endocrinology and Metabolism. 2014;25(9):452–463. doi: 10.1016/j.tem.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 61.Scheffler I. E. A century of mitochondrial research: achievements and perspectives. Mitochondrion. 2001;1(1):3–31. doi: 10.1016/S1567-7249(00)00002-7. [DOI] [PubMed] [Google Scholar]
- 62.Santos J. M., Tewari S., Kowluru R. A. A compensatory mechanism protects retinal mitochondria from initial insult in diabetic retinopathy. Free Radical Biology & Medicine. 2012;53(9):1729–1737. doi: 10.1016/j.freeradbiomed.2012.08.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Madsen-Bouterse S. A., Mohammad G., Kanwar M., Kowluru R. A. Role of mitochondrial DNA damage in the development of diabetic retinopathy, and the metabolic memory phenomenon associated with Its progression. Antioxidants & Redox Signaling. 2010;13(6):797–805. doi: 10.1089/ars.2009.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kowluru R. A. Mitochondria damage in the pathogenesis of diabetic retinopathy and in the metabolic memory associated with its continued progression. Current Medicinal Chemistry. 2013;20(26):3226–3233. doi: 10.2174/09298673113209990029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mohammad G., Kowluru R. A. Novel role of mitochondrial matrix metalloproteinase-2 in the development of diabetic retinopathy. Investigative Ophthalmology & Visual Science. 2011;52(6):3832–3841. doi: 10.1167/iovs.10-6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haghighi S. F., Salehi Z., Sabouri M. R., Abbasi N. Polymorphic variant of MnSOD A16V and risk of diabetic retinopathy. Molekuliarnaia Biologiia. 2015;49(1):114–118. doi: 10.7868/s002689841501005x. [DOI] [PubMed] [Google Scholar]
- 67.Kanwar M., Chan P. S., Kern T. S., Kowluru R. A. Oxidative damage in the retinal mitochondria of diabetic mice: possible protection by superoxide dismutase. Investigative Ophthalmology & Visual Science. 2007;48(8):3805–3811. doi: 10.1167/iovs.06-1280. [DOI] [PubMed] [Google Scholar]
- 68.Kowluru R. A., Atasi L., Ho Y. S. Role of mitochondrial superoxide dismutase in the development of diabetic retinopathy. Investigative Ophthalmology & Visual Science. 2006;47(4):1594–1599. doi: 10.1167/iovs.05-1276. [DOI] [PubMed] [Google Scholar]
- 69.Bek T. Mitochondrial dysfunction and diabetic retinopathy. Mitochondrion. 2017;36:4–6. doi: 10.1016/j.mito.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 70.Giordano C. R. Catalase therapy corrects oxidative stress-induced pathophysiology in incipient diabetic retinopathy. Investigative Ophthalmology & Visual Science. 2015;56(5):3095–3102. doi: 10.1167/iovs.14-16194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kumari S., Panda S., Mangaraj M., Mandal M. K., Mahapatra P. C. Plasma MDA and antioxidant vitamins in diabetic retinopathy. Indian Journal of Clinical Biochemistry. 2008;23(2):158–162. doi: 10.1007/s12291-008-0035-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kumawat M., Kharb S., Singh V., Singh N., Singh S. K., Nada M. Plasma malondialdehyde (MDA) and anti-oxidant status in diabetic retinopathy. Journal of the Indian Medical Association. 2014;112(1):29–32. [PubMed] [Google Scholar]
- 73.Turk A., Nuhoglu I., Mentese A., Karahan S. C., Erdol H., Erem C. The relationship between diabetic retinopathy and serum levels of ischemia-modified albumin and malondialdehyde. Retina. 2011;31(3):602–608. doi: 10.1097/IAE.0b013e3181ed8cd1. [DOI] [PubMed] [Google Scholar]
- 74.Liu J., Li J., Li W.-J., Wang C.-M. The role of uncoupling proteins in diabetes mellitus. Journal of Diabetes Research. 2013;2013:7. doi: 10.1155/2013/585897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rousset S., Alves-Guerra M.-C., Mozo J., et al. The biology of mitochondrial uncoupling proteins. Diabetes. 2004;53(Supplement 1):S130–S135. doi: 10.2337/diabetes.53.2007.S130. [DOI] [PubMed] [Google Scholar]
- 76.Kowluru R. A., Kanwar M., Chan P. S., Zhang J. P. Inhibition of retinopathy and retinal metabolic abnormalities in diabetic rats with AREDS-based micronutrients. Archives of ophthalmology. 2008;126(9):1266–1272. doi: 10.1001/archopht.126.9.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhan X., Du Y., Crabb J. S., Gu X., Kern T. S., Crabb J. W. Targets of tyrosine nitration in diabetic rat retina. Molecular & cellular proteomics. 2008;7(5):864–874. doi: 10.1074/mcp.M700417-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 78.Dong Q. Y., Cui Y., Chen L., Song J., Sun L. Urinary 8-hydroxydeoxyguanosine levels in diabetic retinopathy patients. European Journal of Ophthalmology. 2008;18(1):94–98. doi: 10.1177/112067210801800116. [DOI] [PubMed] [Google Scholar]
- 79.Pan H. Z., Zhang H., Chang D., Li H., Sui H. The change of oxidative stress products in diabetes mellitus and diabetic retinopathy. The British Journal of Ophthalmology. 2008;92(4):548–551. doi: 10.1136/bjo.2007.130542. [DOI] [PubMed] [Google Scholar]
- 80.Wakabayashi Y., Usui Y., Shibauchi Y., Uchino H., Goto H. Increased levels of 8-hydroxydeoxyguanosine in the vitreous of patients with diabetic retinopathy. Diabetes Research and Clinical Practice. 2010;89(3):e59–e61. doi: 10.1016/j.diabres.2010.05.032. [DOI] [PubMed] [Google Scholar]
- 81.Salvemini D., Wang Z. Q., Zweier J. L., et al. A nonpeptidyl mimic of superoxide dismutase with therapeutic activity in rats. Science. 1999;286(5438):304–306. doi: 10.1126/science.286.5438.304. [DOI] [PubMed] [Google Scholar]
- 82.Osorio-Paz I., Uribe-Carvajal S., Salceda R. In the early stages of diabetes, rat retinal mitochondria undergo mild uncoupling due to UCP2 activity. PLoS One. 2015;10(5, article e0122727) doi: 10.1371/journal.pone.0122727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li X., Zhang M., Zhou H. The morphological features and mitochondrial oxidative stress mechanism of the retinal neurons apoptosis in early diabetic rats. Journal of Diabetes Research. 2014;2014:8. doi: 10.1155/2014/678123.678123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.de Souza B. M., Brondani L. A., Bouças A. P., et al. Associations between UCP1 -3826A/G, UCP2 -866G/A, Ala55Val and Ins/Del, and UCP3 -55C/T polymorphisms and susceptibility to type 2 diabetes mellitus: case-control study and meta-analysis. PLoS One. 2013;8(1, article e54259) doi: 10.1371/journal.pone.0054259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barot M., Gokulgandhi M. R., Mitra A. K. Mitochondrial dysfunction in retinal diseases. Current Eye Research. 2011;36(12):1069–1077. doi: 10.3109/02713683.2011.607536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li C., Miao X., Li F., et al. Oxidative stress-related mechanisms and antioxidant therapy in diabetic retinopathy. Oxidative Medicine and Cellular Longevity. 2017;2017:15. doi: 10.1155/2017/9702820.9702820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Spranger J., Pfeiffer A. F. New concepts in pathogenesis and treatment of diabetic retinopathy. Experimental and Clinical Endocrinology & Diabetes. 2001;109(Supplement 2):S438–S450. doi: 10.1055/s-2001-18601. [DOI] [PubMed] [Google Scholar]
- 88.Aiello L. P., Avery R. L., Arrigg P. G., et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. The New England Journal of Medicine. 1994;331(22):1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 89.Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. Journal of Biochemistry and Molecular Biology. 2006;39(5):469–478. doi: 10.5483/bmbrep.2006.39.5.469. [DOI] [PubMed] [Google Scholar]
- 90.Kovacs K., Marra K. V., Yu G., et al. Angiogenic and inflammatory vitreous biomarkers associated with increasing levels of retinal ischemia. Investigative Ophthalmology & Visual Science. 2015;56(11):6523–6530. doi: 10.1167/iovs.15-16793. [DOI] [PubMed] [Google Scholar]
- 91.Klaassen I., van Geest R. J., Kuiper E. J., van Noorden C. J., Schlingemann R. O. The role of CTGF in diabetic retinopathy. Experimental Eye Research. 2015;133:37–48. doi: 10.1016/j.exer.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 92.Abu El-Asrar A. M., Alam K., Nawaz M. I., et al. Upregulated expression of heparanase in the vitreous of patients with proliferative diabetic retinopathy originates from activated endothelial cells and leukocytes. Investigative Ophthalmology & Visual Science. 2015;56(13):8239–8247. doi: 10.1167/iovs.15-18025. [DOI] [PubMed] [Google Scholar]
- 93.Jain A., Saxena S., Khanna V. K., Shukla R. K., Meyer C. H. Status of serum VEGF and ICAM-1 and its association with external limiting membrane and inner segment-outer segment junction disruption in type 2 diabetes mellitus. Molecular Vision. 2013;19:1760–1768. [PMC free article] [PubMed] [Google Scholar]
- 94.Li B., Sharpe E. E., Maupin A. B., et al. VEGF and PlGF promote adult vasculogenesis by enhancing EPC recruitment and vessel formation at the site of tumor neovascularization. The FASEB journal. 2006;20(9):1495–1497. doi: 10.1096/fj.05-5137fje. [DOI] [PubMed] [Google Scholar]
- 95.Adamiec-Mroczek J., Oficjalska-Mlynczak J. Assessment of selected adhesion molecule and proinflammatory cytokine levels in the vitreous body of patients with type 2 diabetes—role of the inflammatory-immune process in the pathogenesis of proliferative diabetic retinopathy. Graefe's Archive for Clinical and Experimental Ophthalmology. 2008;246(12):1665–1670. doi: 10.1007/s00417-008-0868-6. [DOI] [PubMed] [Google Scholar]
- 96.Wu S. L., Zhan D. M., Xi S. H., He X. L. Roles of tissue plasminogen activator and Its inhibitor in proliferative diabetic retinopathy. International journal of ophthalmology. 2014;7(5):764–767. doi: 10.3980/j.issn.2222-3959.2014.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Verma A., Shan Z., Lei B., et al. ACE2 and Ang-(1-7) confer protection against development of diabetic retinopathy. Molecular therapy. 2012;20(1):28–36. doi: 10.1038/mt.2011.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rusnak S., Vrzalova J., Sobotova M., Hecova L., Ricarova R., Topolcan O. The measurement of intraocular biomarkers in various stages of proliferative diabetic retinopathy using multiplex xMAP technology. Journal of Ophthalmology. 2015;2015:6. doi: 10.1155/2015/424783.424783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ma Y., Zhang Y., Zhao T., Jiang Y. R. Vascular endothelial growth factor in plasma and vitreous fluid of patients with proliferative diabetic retinopathy patients after intravitreal injection of bevacizumab. American Journal of Ophthalmology. 2012;153(2):307–313.e2. doi: 10.1016/j.ajo.2011.08.006. e302. [DOI] [PubMed] [Google Scholar]
- 100.Nishiguchi K. M., Ushida H., Tomida D., Kachi S., Kondo M., Terasaki H. Age-dependent alteration of intraocular soluble heparan sulfate levels and its implications for proliferative diabetic retinopathy. Molecular Vision. 2013;19:1125–1131. [PMC free article] [PubMed] [Google Scholar]
- 101.Bressler N. M., Beck R. W., Ferris F. L., 3rd Panretinal photocoagulation for proliferative diabetic retinopathy. The New England Journal of Medicine. 2011;365(16):1520–1526. doi: 10.1056/NEJMct0908432. [DOI] [PubMed] [Google Scholar]
- 102.The Diabetic Retinopathy Study Research Group. Photocoagulation treatment of proliferative diabetic retinopathy: clinical application of Diabetic Retinopathy Study (DRS) findings, DRS report number 8. Ophthalmology. 1981;88(7):583–600. doi: 10.1016/S0161-6420(81)34978-1. [DOI] [PubMed] [Google Scholar]
- 103.Early Treatment Diabetic Retinopathy Study Research Group. Early photocoagulation for diabetic retinopathy: ETDRS report number 9. Ophthalmology. 1991;98(5):766–785. doi: 10.1016/S0161-6420(13)38011-7. [DOI] [PubMed] [Google Scholar]
- 104.Wirostko B., Wong T. Y., Simo R. Vascular endothelial growth factor and diabetic complications. Progress in Retinal and Eye Research. 2008;27(6):608–621. doi: 10.1016/j.preteyeres.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 105.Mitchell P., Bandello F., Schmidt-Erfurth U., et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118(4):615–625. doi: 10.1016/j.ophtha.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 106.Elman M. J., Bressler N. M., Qin H., et al. Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2011;118(4):609–614. doi: 10.1016/j.ophtha.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Elman M. J., Ayala A., Bressler N. M., et al. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: 5-year randomized trial results. Ophthalmology. 2015;122(2):375–381. doi: 10.1016/j.ophtha.2014.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ip M. S., Domalpally A., Sun J. K., Ehrlich J. S. Long-term effects of therapy with ranibizumab on diabetic retinopathy severity and baseline risk factors for worsening retinopathy. Ophthalmology. 2015;122(2):367–374. doi: 10.1016/j.ophtha.2014.08.048. [DOI] [PubMed] [Google Scholar]
- 109.The Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. The New England Journal of Medicine. 2015;372(13):1193–1203. doi: 10.1056/nejmoa1414264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cai S., Bressler N. M. Aflibercept, bevacizumab or ranibizumab for diabetic macular oedema: recent clinically relevant findings from DRCR.net Protocol T. Current Opinion in Ophthalmology. 2017;28(6):636–643. doi: 10.1097/ICU.0000000000000424. [DOI] [PubMed] [Google Scholar]
- 111.Wells J. A., Glassman A. R., Ayala A. R., et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123(6):1351–1359. doi: 10.1016/j.ophtha.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Goldberg R. B. Cytokine and cytokine-like inflammation markers, endothelial dysfunction, and imbalanced coagulation in development of diabetes and its complications. The Journal of Clinical Endocrinology and Metabolism. 2009;94(9):3171–3182. doi: 10.1210/jc.2008-2534. [DOI] [PubMed] [Google Scholar]
- 113.Simo-Servat O., Hernandez C., Simo R. Usefulness of the vitreous fluid analysis in the translational research of diabetic retinopathy. Mediators of Inflammation. 2012;2012:11. doi: 10.1155/2012/872978.872978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zijlstra A., Seandel M., Kupriyanova T. A., et al. Proangiogenic role of neutrophil-like inflammatory heterophils during neovascularization induced by growth factors and human tumor cells. Blood. 2006;107(1):317–327. doi: 10.1182/blood-2005-04-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Danis R. P., Sadda S., Li X. Y., Cui H., Hashad Y., Whitcup S. M. Anatomical effects of dexamethasone intravitreal implant in diabetic macular oedema: a pooled analysis of 3-year phase III trials. The British Journal of Ophthalmology. 2016;100(6):796–801. doi: 10.1136/bjophthalmol-2015-306823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Campochiaro P. A., Brown D. M., Pearson A., et al. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology. 2012;119(10):2125–2132. doi: 10.1016/j.ophtha.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 117.Diabetic Retinopathy Clinical Research Network Writing Committee on behalf of the DRCR.netlow asterisk, Haller J. A., Qin H., et al. Vitrectomy outcomes in eyes with diabetic macular edema and vitreomacular traction. Ophthalmology. 2010;117:1087–1093.e3. doi: 10.1016/j.ophtha.2009.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sharma T., Fong A., Lai T. Y., Lee V., Das S., Lam D. Surgical treatment for diabetic vitreoretinal diseases: a review. Clinical & Experimental Ophthalmology. 2016;44(4):340–354. doi: 10.1111/ceo.12752. [DOI] [PubMed] [Google Scholar]
- 119.Berrocal M. H., Acaba L. A., Acaba A. Surgery for diabetic eye complications. Current Diabetes Reports. 2016;16(10):p. 99. doi: 10.1007/s11892-016-0787-6. [DOI] [PubMed] [Google Scholar]
- 120.The diabetic retinopathy vitrectomy study research group. Early vitrectomy for severe proliferative diabetic retinopathy in eyes with useful vision: results of a randomized trial—diabetic retinopathy vitrectomy study report 3. Archives of Ophthalmology. 1985;103:1644–1652. doi: 10.1016/S0161-6420(88)33015-0. [DOI] [PubMed] [Google Scholar]
- 121.Danis R. P., Bingaman D. P., Jirousek M., Yang Y. Inhibition of intraocular neovascularization caused by retinal ischemia in pigs by PKCbeta inhibition with LY333531. Investigative Ophthalmology & Visual Science. 1998;39(1):171–179. [PubMed] [Google Scholar]
- 122.Ruboxistaurin: LY 333531. Drugs in R & D. 2007;8(3):193–199. doi: 10.2165/00126839-200708030-00007. [DOI] [PubMed] [Google Scholar]
- 123.The PKC-DRS Study Group. The effect of ruboxistaurin on visual loss in patients with moderately severe to very severe nonproliferative diabetic retinopathy. Initial results of the Protein Kinase C β Inhibitor Diabetic Retinopathy Study (PKC-DRS) multicenter randomized clinical trial. Diabetes. 2005;54(7):2188–2197. doi: 10.2337/diabetes.54.7.2188. [DOI] [PubMed] [Google Scholar]
- 124.PKC-DRS2 Group. Effect of ruboxistaurin on visual loss in patients with diabetic retinopathy. Ophthalmology. 2006;113(12):2221–2230. doi: 10.1016/j.ophtha.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 125.Javey G., Schwartz S. G., Harry J., Flynn W., Aiello L. P., Sheetz M. J. Ruboxistaurin: review of safety and efficacy in the treatment of diabetic retinopathy. Clinical Medicine Insights: Therapeutics. 2010;2 doi: 10.4137/cmt.s5046. [DOI] [Google Scholar]
- 126.Grant M. B., Mames R. N., Fitzgerald C., et al. The efficacy of octreotide in the therapy of severe nonproliferative and early proliferative diabetic retinopathy: a randomized controlled study. Diabetes Care. 2000;23(4):504–509. doi: 10.2337/diacare.23.4.504. [DOI] [PubMed] [Google Scholar]
- 127.Krassas G. E., Tzotzas T., Papazisis K., Pazaitou-Panayiotou K., Boboridis K. The efficacy of somatostatin analogues in the treatment of diabetic retinopathy and thyroid eye disease. Clinical ophthalmology. 2007;1(3):209–215. [PMC free article] [PubMed] [Google Scholar]
- 128.Abcouwer S. F. Direct effects of PPARα agonists on retinal inflammation and angiogenesis may explain how fenofibrate lowers risk of severe proliferative diabetic retinopathy. Diabetes. 2013;62(1):36–38. doi: 10.2337/db12-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Keech A. C., Mitchell P., Summanen P. A., et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. The Lancet. 2007;370(9600):1687–1697. doi: 10.1016/S0140-6736(07)61607-9. [DOI] [PubMed] [Google Scholar]
- 130.Moravski C. J., Kelly D. J., Cooper M. E., et al. Retinal neovascularization is prevented by blockade of the renin-angiotensin system. Hypertension. 2000;36(6):1099–1104. doi: 10.1161/01.HYP.36.6.1099. [DOI] [PubMed] [Google Scholar]