Corticotropin-releasing factor (CRF) is a neuropeptide in the brain and body that coordinates hormonal, sympathetic, and behavioral responses to stressors. Since its discovery in 1981 (1), literally thousands of articles have been published that support its role in these three functional domains. CRF controls corticotropin secretion and in turn glucocorticoid activation in the face of acute stressor exposure via its actions as a releasing factor in the paraventricular nucleus of the hypothalamus. Vale and colleagues first demonstrated that CRF initiates the hypothalamic-pituitary-adrenal (HPA) axis neuroendocrine stress response by binding CRF1 receptors on anterior pituitary corticotropes to release adrenocorticotropic hormone (1). CRF controls sympathetic activation via its actions as a neurotransmitter in the brainstem (2), and CRF via CRF1 receptors controls behavioral responses to stressors, from activation to freezing, anxiety-like responses, and fear conditioning via its actions as a neurotransmitter in the extended amygdala [for review, see Zorrilla et al. (3)]. CRF and CRF1 receptors are widely distributed in stress-responsive brain regions, including the neocortex, central extended amygdala, medial septum, hippocampus, thalamus, cerebellum, and autonomic midbrain and hindbrain nuclei (1). CRF in the gastrointestinal system plays a key role in modulating gastrointestinal motility and as such may play a key role in stress-related physiological disorders, such as irritable bowel syndrome (4). In human psychiatric disorders, CRF has been implicated in anxiety and depressive disorders and addiction, again largely based on preclinical animal models (3) and correlational measures of CRF in cerebrospinal fluid and postmortem brains (5). Much of the preclinical work that implicates endogenous CRF in biological function has relied on both peptide and small-molecule antagonists. In rodents, CRF antagonists have profound effects in blocking physiological and behavioral responses to stressors (1,3).
Nevertheless, the translation of preclinical research to the human condition has been marked by a nearly universal lack of efficacy of small-molecule CRF receptor antagonists in treating human psychiatric disease (6). This lack of efficacy has been attributed to a range of issues, including brain penetration, receptor occupancy, and species differences. Particularly vexing has been the overwhelming data that show that in animal preclinical studies, small-molecule CRF1 receptor antagonists have robust effects in blocking stress-induced anxiety-like responses, stress-like withdrawal responses during acute withdrawal and protracted abstinence in drug and alcohol dependence, excessive drug intake associated with dependence, and the stress-induced reinstatement of drug seeking (3,6). Nonetheless, virtually no positive results have been reported in human double-blind clinical trials of the efficacy of CRF antagonists in psychiatric disorders such as anxiety, depression, and posttraumatic stress disorder, and no positive results have been reported with CRF antagonists in human laboratory studies of addiction to date.
The study by Kalin et al. (5) used nonhuman primates, and their results may represent a major breakthrough in the translation of CRF from rodents to primates, thus indicating that nonhuman primate models are critical for understanding the mechanisms that underlie human psychopathology and that such models may provide more clear insights into the function of brain CRF.
Kalin et al. (5) previously established a nonhuman primate model of anxious temperament for studying the early-life risk of developing anxiety and depression (7). Their studies and others identified the central nucleus of the amygdala (CeA) as an essential component of the neural substrates of anxious temperament. In the anxious temperament studies, infant rhesus monkeys dramatically altered their behavior when a human intruded into their environment. When the human stared at the infant, it engaged in aggressive gestures and vocalizations, but when the human averted his or her gaze, the infant froze. Such behaviors were hypothesized to be defensive, representing attempts by the infant to protect itself in a threatening situation. The excessive expression of such behavior might reflect human psychopathology that is characterized by excessive or inappropriate fear responses (7).
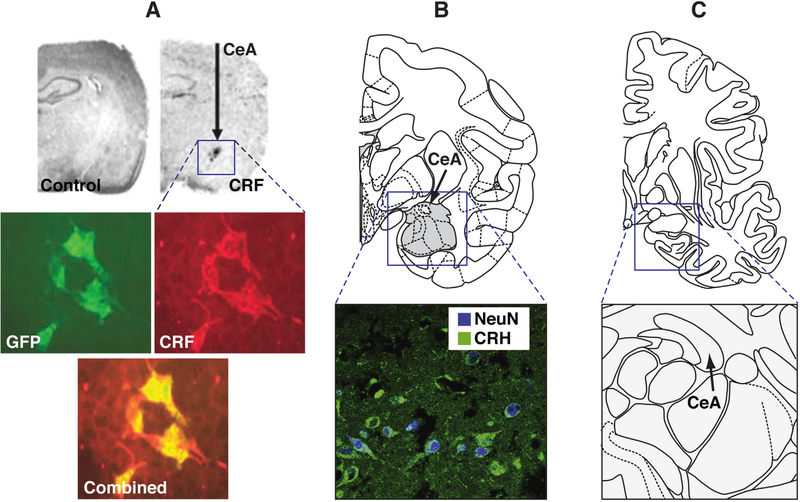
CRF is expressed in the CeA in both rodents and primates, and CRF overexpression using a lentivirus construct in the CeA in adult female rats increased the acoustic startle response and depressive-like behavior in the forced swim test when testing began 2 weeks after viral injection (8) (Figure 1). A later study found that at 4 months after lentiviral overexpression of CRF in the CeA, female mice exhibited lower stress-induced anxietylike behavior, effects that were attributable to possible downregulation of CRF1 receptors (9). In the study by Kalin et al. (5), viral vector technology in young rhesus monkeys and assessments of anxiety and multimodal neuroimaging were combined to explore the consequences of a chronic increase in CRF in the CeA. Five monkeys received bilateral dorsal amygdala infusions of adeno-associated virus serotype 2 that contained a CRF construct. The monkeys’ cage mates served as control subjects that did not undergo surgery. Two months after the viral infusions, anxious temperament, regional brain metabolism, resting functional magnetic resonance imaging, and diffusion tensor imaging (fractional anisotropy) were assessed.
Figure 1.
(A) Corticotropin-releasing factor (CRF) was constitutively overexpressed in the central nucleus of the amygdala (CeA) using a DNA plasmid that encoded a lentiviral construct. (Top) In situ hybridization showed that the lentiviral cytomegalovirus increased CRF transcript expression in the rat CeA vs. a control virus. (Bottom) Coexpression of green fluorescent protein (GFP) and CRF in lenti-CRF-infected human embryonic kidney 293 cells was verified by double-immunofluorescence staining with both an anti-GFP antibody (green) and anti-CRF antibody (red). The photograph below shows a combined overlay. Reproduced with permission from Keen-Rhinehart et al. (8). (B) Endogenous CRF expression in the primate CeA. (Top) Diagram of a coronal section through the amygdala of a primate brain showing the increased expression of CRF after infusions of adeno-associated virus serotype 2 that contained a CRF construct. (Bottom) A moderate amount of endogenous CRF immunoreactivity (green) was observed in neurons, as defined by the overlap with neuronal nuclear antigen (NeuN) expression (blue). Reproduced with permission from Kalin et al. (5). (C) Human amygdala. (Top) Diagram of a coronal section of the human brain showing the amygdala. (Bottom) The arrow indicates the CeA in humans. To date, I could find no evidence of a brain mapping study of CRF immunoreactivity in the human amygdala. Reproduced with permission from Herringa et al. (10). CRH, corticotropin-releasing hormone.
Dorsal amygdala CRF overexpression significantly increased anxious temperament and metabolism in the dorsal amygdala (Figure 1). Changes in metabolism in other anxious temperament– related regions and measures of functional and structural connectivity revealed an increase in metabolism in posterior regions of the orbital proisocortex, anterior insula, and hippocampus and decreases in fractional anisotropy in the medial thalamus that encompassed portions of the central medial thalamic nucleus, paracentral thalamic nucleus, and magnocellular division of the ventral anterior thalamic nucleus. Using measures of white matter integrity, the authors found evidence of decreases in fractional anisotropy in the medial thalamus that encompassed portions of the central medial thalamic nucleus, paracentral thalamic nucleus, and magnocellular division of the ventral anterior thalamic nucleus. The authors hypothesized that CRF overexpression in the dorsal amygdala could lead to greater activation of medial thalamic CRF1 receptors because primate studies have shown that these regions contain CRF-immunoreactive cell bodies and fibers and relatively high densities of CRF1 receptors. Altogether, these results indicate CRF-induced activation of the neurocircuitry that is associated with an anxious temperament.
The significance of this study is important on a number of fronts. First, the study demonstrates the feasibility of translating rodent mechanistic studies that directly manipulate gene function in the brain to primates and opens the door to an exciting new vista in primate research. Second, the study implicates overactive extrahypothalamic CRF systems in the pathophysiology of excessive anxiety-like behavior in primates, but perhaps more importantly puts a focus on a particular pathophysiology that is associated with conditions of excessive or inappropriate fear responses. This endophenotype could guide further measures of CRF receptor antagonists in human clinical studies. Third, both the similarities and differences between rodents and primates (e.g., a key role for CRF2 receptors in the amygdala in primates vs. rodents) may provide insights into the frustration of directly translating rodent to human studies. As speculated by the authors, perhaps CRF2 receptors play a more prominent role than previously thought. Fourth, the study provides a translational roadmap that may become critically important for translating what has been learned in rodent studies to human psychopathology (Figure 1). Notably, however, I could find no brain mapping study of CRF immunoreactivity in the postmortem human brain (Figure 1). Primate studies that combine molecular manipulations and behavioral phenotyping in rodents and multimodal neuroimaging measures in humans merit strong consideration as we begin to exploit the technical advances of the brain initiatives that are being launched at the National Institutes of Health, in Europe, and around the world.
Acknowledgments and Disclosures
The author has no financial disclosures to declare beyond his primary employment at the National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health. The author reports no biomedical financial interests or potential conflicts of interest.
References
- 1.Bale TL, Vale WW (2004): CRF and CRF receptors: Role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol 44: 525–557. [DOI] [PubMed] [Google Scholar]
- 2.Kovács KJ (2013): CRH: The link between hormonal-, metabolic- and behavioral responses to stress. J Chem Neuroanat 54:25–33. [DOI] [PubMed] [Google Scholar]
- 3.Zorrilla EP, Logrip ML, Koob GF (2014): Corticotropin releasing factor: A key role in the neurobiology of addiction. Front Neuroendocrinol 35: 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taché Y, Brunnhuber S (2008): From Hans Selye’s discovery of biological stress to the identification of corticotropin-releasing factor signaling pathways: Implication in stress-related functional bowel diseases. Ann N Y Acad Sci 1148:29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalin NH, Fox AS, Kovner R, Riedel MK, Fekete EM, Roseboom PH, et al. (2016): Overexpressing corticotropin-releasing factor in the primate amygdala increases anxious temperament and alters its neural circuit. Biol Psychiatry 80:345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koob GF, Zorrilla EP (2012): Update on corticotropin-releasing factor pharmacotherapy for psychiatric disorders: A revisionist view. Neuropsychopharmacol Rev 37:308–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalin NH, Shelton SE (1989): Defensive behaviors in infant rhesus monkeys: Environmental cues and neurochemical regulation. Science 243:1718–1721. [DOI] [PubMed] [Google Scholar]
- 8.Keen-Rhinehart E, Michopoulos V, Toufexis DJ, Martin EI, Nair H, Ressler KJ, et al. (2009): Continuous expression of corticotropin releasing factor in the central nucleus of the amygdala emulates the dysregulation of the stress and reproductive axes. Mol Psychiatry 14: 37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Regev L, Neufeld-Cohen A, Tsoory M, Kuperman Y, Getselter D, Gil S, Chen A (2011): Prolonged and site-specific over-expression of corticotropin-releasing factor reveals differential roles for extended amygdala nuclei in emotional regulation. Mol Psychiatry 16: 714–728. [DOI] [PubMed] [Google Scholar]
- 10.Herringa RJ, Roseboom PH, Kalin NH (2006): Decreased amygdala CRF-binding protein mRNA in post-mortem tissue from male but not female bipolar and schizophrenic subjects. Neuropsychopharmacology 31:1822–1831. [DOI] [PubMed] [Google Scholar]