Abstract
Background
Breast cancer is the leading cause of oncological mortality among women. Efficient detection of cancer cells in an early stage and potent therapeutic agents targeting metastatic tumors are highly needed to improve survival rates. Emerging evidence indicates that lncRNAs (long noncoding RNAs) are critical regulators of fundamental cellular processes in a variety of tumors including breast cancer. The functional details of these regulatory elements, however, remain largely unexplored.
Methods
In this study, lncRNA ROR (linc-ROR) was examined by real-time PCR in different breast cancer cell lines and breast tumor tissues/non-tumor tissues were collected from both breast cancer patients and healthy controls. Linc-ROR was knockdown in breast cancer cell lines and the effects on cell proliferation, migration and invasion were tested both in vitro and in vivo tumor model. Effects of linc-ROR knockdown on TGF-β signaling pathway were investigated by Western blot.
Results
Our studies have suggested that linc-ROR, a critical factor for embryonic stem cell maintenance, probably acts as an oncogenic factor in breast cancer cells, causing poor prognostic outcomes. Overexpression of linc-ROR seems to be responsible for promoting proliferation and invasion of cancer cells as well as tumor growth in nude mice. The regulatory action of linc-ROR can affect the activity of the TGF-β signaling pathway, which has been proven critical for mammary development and breast cancer.
Conclusions
The results have highlighted the potential importance of linc-ROR in the progression of advanced breast cancer, and thus will stimulate efforts in the development of novel diagnostic and therapeutic strategies.
Keywords: Long noncoding RNA, ROR, TGF-β, Breast cancer
Background
Breast cancer is the most common cancer and the leading cause of oncologic mortality for women worldwide [1]. The primary risk factors of this disease include age, high hormone level, race, economic status, and iodine deficiency in diet [2]. Early diagnosis is key to successful treatment of breast cancer. Diagnostic procedures often include clinical examination, mammography, ultrasound, magnetic resonance imaging and biopsy. Standard treatment often requires complete tissue removal, chemotherapy, radiotherapy, and hormone therapy [3]. Many studies aimed to demonstrate the gene expression profiles of breast cancer have led to the recognition that the disease is highly heterogeneous and newly discovered molecular markers have allowed categorization of the disease into several biological subtypes, ultimately enabling the optimization of clinical practices in many aspects, such as prediction of prognosis and treatment responses [4–6]. Despite the overall advancement in the field, metastatic relapse of the disease at the advanced stage remains a major problem.
The non-protein-coding regions in human genome have been well recognized as important regulatory and functional units, most of which encode long non-coding RNAs (lncRNAs) [7]. Like, protein-coding mRNA, many lncRNAs are transcribed by RNA polymerase II and undergo maturation through 5′ cap modification, 3′ polyadenylation and splicing. On the other hand, many features of lncRNAs can distinguish them from protein-coding mRNAs. Generally, lncRNAs lack coding potential, expressed at relatively lower levels, prone to localize in the nucleus and evolve faster [7]. Accumulating evidence has supported involvement of a broad spectrum of lncRNAs in a variety of disease states including oncogenesis [8]. In breast cancer research, both oncogenic and tumor suppressive lncRNAs have been identified. They are involved in the regulation of gene expression at various levels that ultimately affect cancer cell growth, apoptosis, migration, invasion and stemness maintenance [9]. Notably, several lncRNAs modulating the TGF-β (transforming growth factor beta) signaling pathway, a central function for mammary development, have been found aberrantly expressed in breast cancer [10, 11].
Linc-ROR (lncRNA ROR) was initially discovered as a modulator for reprogramming of human induced pluripotent stem cells and proven to have strong impact on self-renewal and differentiation of human embryonic stem cells [12, 13]. Since then, dysregulation of linc-ROR has been found in a variety of tumors [14]. In many breast cancer cell lines and tissues, linc-ROR is dramatically upregulated and has been implicated to contribute to malignancy and treatment resistance of advanced breast cancer [15–19]. The oncogenic function of linc-ROR has been linked to the regulation of multiple signaling pathways, which are presumably important for the development and progression of cancers. However, a complete picture of the regulatory network associated with linc-ROR is still missing. This has hampered the application of linc-ROR as a diagnostic biomarker and development of new therapeutic approaches.
We found that linc-ROR was overexpressed in both breast cancer cell lines and patient tissues. The high expression levels of linc-ROR ware associated with poor prognostic outcomes. Further characterization suggested the role of linc-ROR in enhancing proliferation and invasion of breast cancer cells as well as promoting tumor growth in nude mice. We also found that the expression levels of linc-ROR affected several factors in the TGF-β pathway suggesting that the regulatory link between linc-ROR and TGF-β is potentially important for progression of advanced breast cancer. The results provided new insights into the signaling networks associated with the oncogenic action of linc-ROR and might help develop new diagnostic and therapeutic strategies.
Materials and methods
Breast tissue samples
Collection of patient samples was approved by the Ethics Committee of Taihe Hospital affiliated to Hubei University of Medicine. The breast tissues were obtained from 94 breast cancer patients who had surgery in the Taihe Hospital between 2015 and 2017. The patients were 31–59 years old (45 ± 13.5 years old on average) without smoking history. Their breast cancer conditions were diagnosed by two pathologists following the American Society of Clinical Oncology guidelines. Both tumor and the adjacent normal tissues were collected. The normal tissues were at least 2 cm away from the edges of the tumors and contained no obvious tumor cells. All the tissue samples were obtained from fresh surgical specimens, flash-frozen in liquid nitrogen, and stored at − 80 °C.
Cell lines and culture conditions
The human breast cancer cell lines (MDA-MB-231 and MCF-7) and the normal mammary fibroblast cell line (Hs578Bst) were purchased from Beijing Zhongyuan Ltd. and Shanghai Kexing Biotech Ltd. respectively. The MDA-MB-231 cells were maintained in L-15 medium, while the MCF-7 and Hs578Bst cells were maintained in DMEM (Dulbecco’s Modified Eagle’s Medium). All culture media were supplemented with 10% FBS (fetal bovine serum), 100 U/mL of penicillin, and 100 μg/mL of streptomycin, unless otherwise specified. All the cells were incubated at 37 °C in 5% CO2.
Cell transfection
The siRNA specifically targeting ROR (si-ROR) and the scrambled control (si-nc) were synthesized by Shanghai GenePharma Co. Ltd. The RNAs were transfected into cells using the Lipofectamine RNAiMAX reagents (Thermo Fisher Scientific, USA) according to the manufacturer’s protocol. The si-nc and si-ROR sequence are below. si-nc: UUCUC CGAAC GUGUC ACGU; si-ROR: GGAGA GGAAG CCUGA GAGU.
RNA extraction and qRT-PCR
RNA was isolated from tissues or cells using the TRIzol reagent (Thermo Fisher Scientific) and quantitatively analyzed by qRT-PCR (quantitative reverse transcription polymerase chain reaction). Briefly, RNA was reverse transcribed using the SuperScript First Strand cDNA System kit (Thermo Fisher Scientific) and the DNA was subjected to quantitative PCR in a Thermo Fisher Scientific 7300 Real-Time PCR System. The RNA level of GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used as an internal control. The delta Ct (threshold cycle) method was used to quantitate gene expression levels, while the delta–delta Ct method was used to determine the relative fold changes in gene expression.
The primers used are listed below. ROR:5′-CTCCAGCTATGCAGACCACTC-3′;5′-GTGACGCCTGACCTGTTGAC-3′. GAPDH:5′-AATGGACAACTGGTCGTGGAC-3′,5′-CCCTCCAGGGGATCTGTTTG-3′.
Western blot analysis
The harvested cells lysed and the supernatants were quantitated by BCA assays using a Varioskan multimode microplate spectrophotometer (Thermo Scientific, USA). Equal amounts of proteins were subjected to 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE). The separated proteins were transferred on to nitrocellulose membranes. The membranes were blocked with 10% no-fat milk in phosphate buffered saline (PBS) at 37 °C for 1 h. Specific antibodies against TGF-β (ab31013, abcam), Smad2 (ab40855, abcam), α-SMA (#19245, CST) and GAPDH (ab9485, abcam) were added for overnight incubation at 4 °C. After the antibodies were removed, membranes were incubated with secondary antibodies (ab6721, abcam) for 1 h at 37 °C. Protein bands were detected with the Odyssey Infrared Imaging System (LI-COR Inc., USA). Protein levels were averaged from triplicates.
MTT assay
Cells were seeded into 96-well plates at a density of 5 × 103/well. The cells transfected with siRNA were incubated for 48 h before being resuspended and seeded into the 96-well plates. The MTT solution (20 μL) was added to the plates at 12-, 24-, 48-, and 72-h time points and the cells were further incubated for 4 h at 37 °C. The culture medium was then replaced by 150 μL DMSO (dimethyl sulfoxide) and the cells were oscillated for 15 min. The absorbance at 490 nm was determined with an enzyme-labeled analyzer.
Cell invasion assays
Cell invasion was analyzed using Transwell invasion chambers (Corning, USA). Briefly, 3 × 104 transfected cells in serum-free DMEM were added onto the upper chambers of 8 μm-diameter Transwell inserts pre-coated with Matrigel (R&D Systems, USA), and 0.5 mL DMEM with 10% FBS was added to the lower chamber. After 48 h incubation at 37 °C, cells on the bottom chamber were fixed with 70% ethanol, stained with 0.1% crystal violet, and photographed under an inverted microscope. The numbers of cells in five random fields per well were counted and averaged as the invading cell number.
Tumor xenograft model
The MCF-7 and MDA-MB-231 breast cancer cells were transfected with lenti-control (sh-nc) or lenti-shRNA-ROR (sh-ROR) (GeneChem, Shanghai, China) and selected for puromycin resistance (10 μg/mL). Female nude mice, 4–5 weeks of age, were purchased from Vital River Laboratory Animal Technology Ltd. (China). Stable MCF-7 and MDA-MB-231 cells (2 × 106 cells in 50% matrigel, BD Biosciences, USA) with or without shRNA transfection were subcutaneously injected into the right flank of nude mice. The formation and growth of tumor volume in the nude mice were monitored. At day 31, the mice were sacrificed and the tumors were isolated and weighted.
Tumor volume was calculated by the formula below.
The primer sequences for sh-nc and sh-ROR are listed below.
GATCCCCTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAATTTTTC.
GATCCCCCCTGAGAGTTGGCATGAATTTCAAGAGAATTCATGCCAACTCTCAGGTTTTTC.
Statistical analysis
All statistical analyses were carried out using the SPSS 17.0 software (SPSS Inc., USA). Most datasets were analyzed using Independent Samples t Test. The overall survival was estimated using the Kaplan–Meier method. The results with p < 0.05 were considered statistically significant.
Results
High expression of linc-ROR is linked to breast cancer
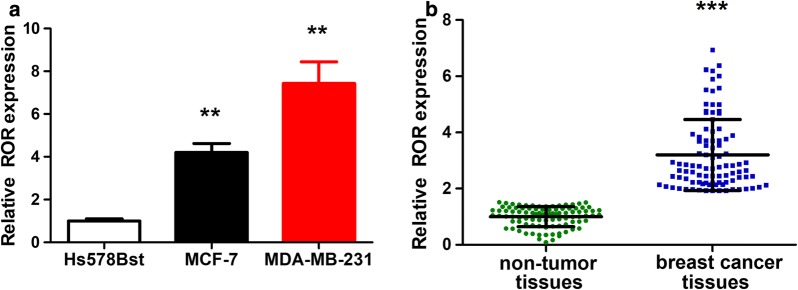
Dysregulation of linc-ROR has been found in a variety of tumors including breast cancer. We compared the expression levels of linc-ROR in cells cultured in vitro and found that the expression in two breast cancer cell lines (MCF-7 and MDA-MB-231) were higher than that in the normal mammary fibroblast cell line (Hs578Bst) (Fig. 1a). In order to find clinical relevance, we collected tumor tissues and the adjacent normal tissues from 94 patients and statistically compared the expression levels of linc-ROR between these two groups (Fig. 1b). Indeed, linc-ROR was upregulated in the tumor tissues. The results suggested that high expression of linc-ROR might be important in breast cancer.
Fig. 1.
Upregulation of linc-ROR in breast cancer cell lines and tumor tissues. a Bar plot comparing the linc-ROR RNA levels in different cell lines. The MCF-7 and MDA-MB-231 cells are breast cancer cells, while the Hs578Bst cells are normal mammary fibroblast cells. The linc-ROR RNA levels were quantitated by qRT-PCR. GAPDH RNA was used as an internal control. Data were averaged from triplicate experiments. b Statistical comparison of linc-ROR expression levels in patient tissue samples. Linc-ROR RNA levels were quantitated from breast tumor tissues and the adjacent normal tissues using the same method described above. **p < 0.01, ***p < 0.001, vs the control
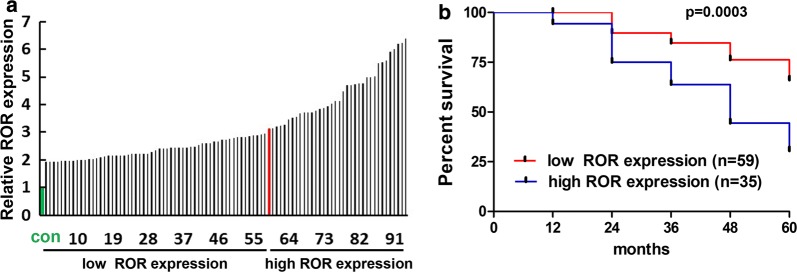
We tried to further assess the expression of linc-ROR in clinical prognosis of breast cancer patients. We first divided the 94 patients into two groups, the low- and high-expression groups, according to the linc-ROR levels in the tumor tissues. The demarcation was set at a patient with 3.2-fold increase in linc-ROR expression (Fig. 2a). When the 60-month survival fractions were compared, the high-expression groups showed faster decline in survival (Fig. 2b). This reinforced our hypothesis that linc-ROR may be an oncogenic factor for the development of breast cancer.
Fig. 2.
Comparison of prognostic survival rates between Linc-ROR low- and high-expression groups. a Schematic presentation of two patient groups with low- and high-expression levels of linc-ROR. The expression levels of all 94 patients (black bars) were ordered from low to high. The control expression level (green bar) was normalized to 1. The demarcation was set to the patient (red bar) with 3.2-fold increase in the level of linc-ROR. b Kaplan–Meier overall survival curves of the low- and high-expression groups. Breast cancer patients with high expression of linc-ROR showed a poorer prognosis than those with low expression
Linc-ROR is required for the enhanced proliferation and invasion of breast cancer cells
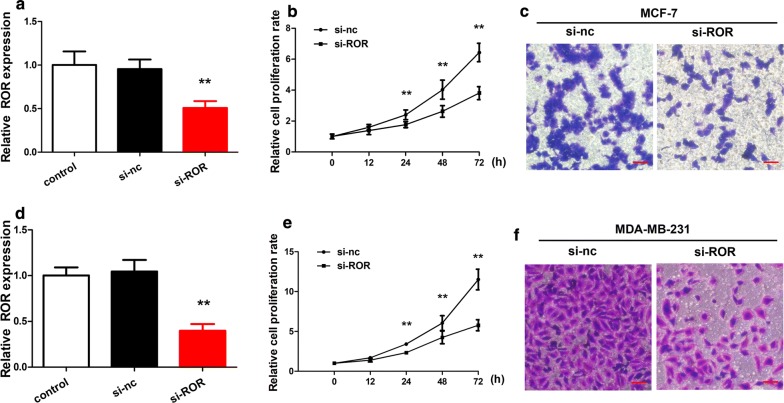
We examined the importance of linc-ROR in the MCF-7 and MDA-MB-231 breast cancer cells by analyzing the proliferation and invasion of those cells in vitro. When the level of linc-ROR was reduced by introduction of interference RNA targeting the linc-ROR transcripts (si-ROR) in both types of cancer cells, the proliferation and invasion of those cells were partially inhibited, supporting the potential importance of linc-ROR for the tumorigenic phenotypes of the cancer cells (Fig. 3).
Fig. 3.
Linc-ROR knockdown reduced cell proliferation and invasion. a Comparison of the linc-ROR levels in the cultured MCF-7 breast cancer cells with or without linc-ROR knockdown. Linc-ROR was knocked down by the si-ROR interference RNA. Cells were also untransfected (control) or transfected with a scrambled negative-control siRNA (si-nc) for comparison. b Time courses of MCF-7 proliferation. Cell proliferation rates were compared between the cells transfected with si-ROR or si-nc. The proliferation rates were quantitated every 12 h over 3 days. Linc-ROR knockdown by si-ROR led to slower proliferation. c Comparison of MCF-7 cell invasion. The cells transfected with si-ROR or si-nc were assessed in transwell assays. The scale bars are 100 μm. The data points were averaged from triplicates. **p < 0.01 vs the control. d–f Comparison of proliferation and invasion for the MDA-MB-231 breast cancer cells transfected with si-ROR or si-nc. The panels are arranged as a–c
Knocking down of linc-ROR renders tumor growth
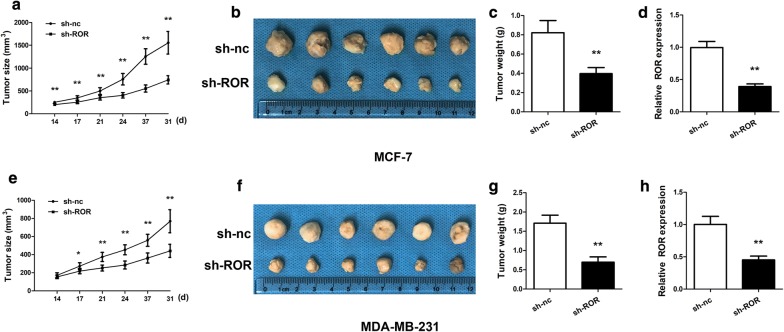
We also injected both types of cancer cells into nude mice and compared the growth of tumors in the animals. When si-ROR were transfected into the cancer cells to suppress the expression of linc-ROR, the growth of the tumors formed from the injected cells was apparently slower during the 1-month measurement period. After the tumors were isolated from the terminated animals, the weights of the tumors were also compared. Indeed, knocking down linc-ROR in both types of cancer cells rendered formation of smaller tumors (Fig. 4). The results suggested that the linc-ROR is potentially critical for the development of breast cancer.
Fig. 4.
Linc-ROR knockdown reduced tumor growth in nude mice. a Time-course comparison of tumor growth from the MCF-7 cells. The cells were transfected with si-ROR or si-nc and injected into nude mice. After 14 days, tumor sizes were measured twice a week over 3 weeks. b Image of tumors harvested at day 31. c Comparison of averaged tumor weights. The tumors grown from the MCF-7 cells transfected with si-ROR were smaller. d Relative ROR expression of tumors was confirmed. The expression of ROR of tumors from the MCF cells transfected with si-ROR was inhibited. e–h Comparison of tumors derived from the MDA-MB-231 cells, which were transfected with si-ROR or si-nc and injected into nude mice. The panels are arranged as a–c. **p < 0.01 vs the control
Linc-ROR knockdown inhibits the activation of the TGF-β signaling pathway
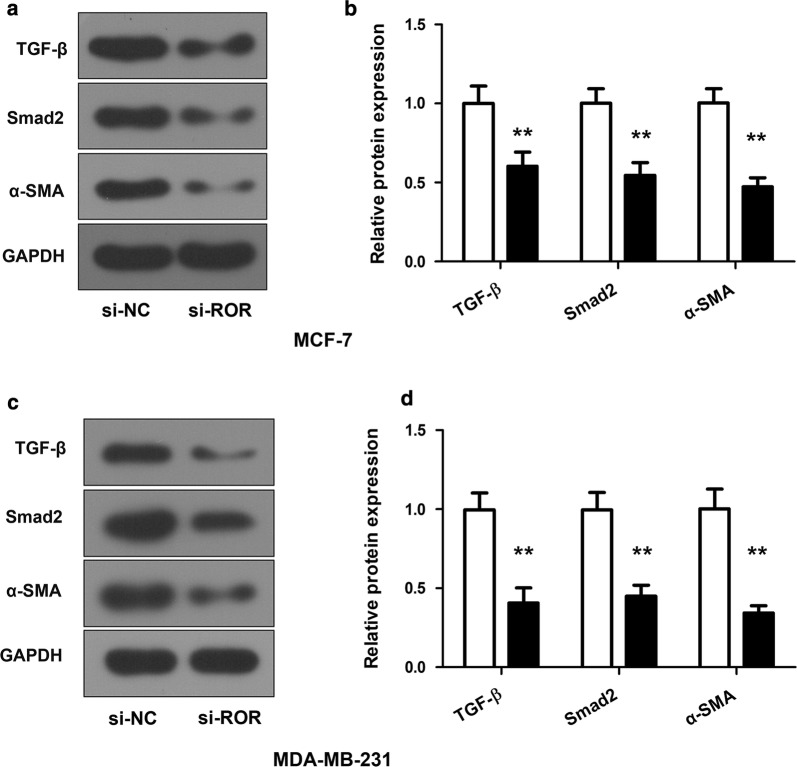
In order to understand how linc-ROR may support the development of breast cancer, we analyzed its impact on TGF-β, a critical signaling factor orchestrating mammary epithelial development and contributing to cancer progression in the advanced stages. When linc-ROR was knocked down in the MCF-7 and MDA-MB-231 breast cancer cells, the expression levels of TGF-β were also diminished. As a result, the downstream factors, such as Smad2 and α-SMA, were also downregulated (Fig. 5). These results indicated that linc-ROR likely modulates the TGF-β signaling pathway to trigger the expression of a series of factors involved in the progression of breast cancer.
Fig. 5.
Linc-ROR modulates the TGF-β signaling pathway. a Western blot probing TGF-β, Smad2 and α-SMA in the MCF-7 cells. The cells were transfected with si-ROR or si-nc. GAPDH was blotted as an internal reference. b Quantitative comparison of the expression levels of TGF-β, Smad2 and α-SMA. The protein expression levels in the MCF-7 cells transfected with si-nc were normalized to 1. The cells transfected with si-ROR showed reduced expression of all three proteins. c, d Comparison of the protein expression levels of TGF-β, Smad2 and α-SMA in the MDA-MB-231 cells. The panels were arranged as a, b. Data were Mean ± SD derived from three independent experiments. *p < 0.05 vs the control
Discussion
Our study has confirmed aberrant overexpression of linc-ROR in both breast cancer cell lines and patient tumors, demonstrating its strong correlation with poor prognostic outcomes. The results, together with those from many previous studies, have thus reinforced the hypothesis that this lncRNA is an oncogenic factor that can promote the development and progression of breast cancer [15–19]. Our results have further suggested that the TGF-β signaling pathway may be a downstream target of linc-ROR, responsible for promoting breast cancer. This is line with the previous conclusion that TGF-β is a central signaling molecule in mammary development as well as tumorigenesis [10].
Many studies have recognized that the function of TGFβ in the progression of breast cancer can be different depending on the stage of cancer [10, 20, 21]. In normal conditions, TGF-β has been shown to inhibit cell cycle and promote apoptosis that together significantly contribute to the suppressive role in the initiation and progression of tumorigenesis [22]. In the late stages of tumor progression, however, TGF-β acts as a tumor promoter largely by inducing and promoting epithelial-to-mesenchymal transitions [23, 24]. Our study has indicated that overexpression of linc-ROR is required to constitutively upregulate critical factors in the TGF-β signaling pathway. This suggests that the oncogenic activity of linc-ROR is at least needed for the progression of breast cancer in the advanced stages, although it likely regulates a highly complex signaling network that can impact many other biological processes that ultimately contribute to multiple steps along the progression of the disease.
Although the survival rates have been gradually increased in some countries because of remarkable improvements in the understanding and management of breast cancer, the disease is still a major medical burden worldwide [25]. Major challenges include developing cost-effective diagnosis, treating metastatic cancer and overcoming drug resistance in targeted therapies.
Conclusion
Our data suggest that linc-ROR, in combination with other lncRNAs associated with breast cancer, may serve as a clinically beneficial biomarker for detecting neoplastic cells, differentiating the different stages of breast cancer and predicting prognostic outcomes. Furthermore, blocking the functional impact of linc-ROR may represent a novel therapeutic strategy that may help overcome a plethora of cellular mechanisms exploited to favor drug resistance.
Authors’ contributions
LH, JT and FC designed and carried out the study. HY, FY, MW, JL, JF, and GZ participated in experiments and statistical analysis. LH, JT and FC wrote the manuscript. JT and FC revised the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
All the data supporting our findings can be found in the “Results” section of the paper. Please contact authors for data request.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The Ethics Committee of Taihe Hospital affiliated to Hubei University of Medicine approved the protocol, and written informed consent was provided by patients and healthy controls involved.
Funding
This study was supported by Shiyan City Scientific Research and Development Project (14Y18).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
LingLi Hou, Email: mznnh02@163.com.
Jiancheng Tu, Phone: +86-027-67812888, Email: rgnjn65@163.com.
Fangxiong Cheng, Phone: +86-027-68831462, Email: hrveyq2@163.com.
Hongwei Yang, Email: tihkc31@163.com.
Fei Yu, Email: orkff56@163.com.
Minghua Wang, Email: fhaqsf4@163.com.
Jiubo Liu, Email: iinqf38@163.com.
Jinbo Fan, Email: lvihkw3@163.com.
Guojun Zhou, Email: qgxyj02@163.com.
References
- 1.Becker S. A historic and scientific review of breast cancer: the next global healthcare challenge. Int J Gynaecol Obstet. 2015;131(Suppl 1):S36–S39. doi: 10.1016/j.ijgo.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 2.Steiner E, Klubert D, Knutson D. Assessing breast cancer risk in women. Am Fam Physician. 2008;78(12):1361–1366. [PubMed] [Google Scholar]
- 3.Ataollahi MR, et al. Breast cancer and associated factors: a review. J Med Life. 2015;8(Spec Iss 4):6–11. [PMC free article] [PubMed] [Google Scholar]
- 4.Libson S, Lippman M. A review of clinical aspects of breast cancer. Int Rev Psychiatry. 2014;26(1):4–15. doi: 10.3109/09540261.2013.852971. [DOI] [PubMed] [Google Scholar]
- 5.Bao T, Davidson NE. Gene expression profiling of breast cancer. Adv Surg. 2008;42:249–260. doi: 10.1016/j.yasu.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaur H, et al. Next-generation sequencing: a powerful tool for the discovery of molecular markers in breast ductal carcinoma in situ. Exp Rev Mol Diagn. 2013;13(2):151–165. doi: 10.1586/erm.13.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Derrien T, et al. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 2012;22(9):1775–1789. doi: 10.1101/gr.132159.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen G, et al. LncRNADisease: a database for long-non-coding RNA-associated diseases. Nucleic Acids Res. 2013;41(Database issue):D983–986. doi: 10.1093/nar/gks1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y, Sharma S, Watabe K. Roles of lncRNA in breast cancer. Front Biosci (Schol Ed) 2015;7:94–108. doi: 10.2741/s427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moses H, Barcellos-Hoff MH. TGF-beta biology in mammary development and breast cancer. Cold Spring Harb Perspect Biol. 2011;3(1):a003277. doi: 10.1101/cshperspect.a003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu S, et al. Oncogenic long noncoding RNA landscape in breast cancer. Mol Cancer. 2017;16(1):129. doi: 10.1186/s12943-017-0696-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loewer S, et al. Large intergenic non-coding RNA-RoR modulates reprogramming of human induced pluripotent stem cells. Nat Genet. 2010;42(12):1113–1117. doi: 10.1038/ng.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, et al. Endogenous miRNA sponge lincRNA-RoR regulates Oct4, Nanog, and Sox2 in human embryonic stem cell self-renewal. Dev Cell. 2013;25(1):69–80. doi: 10.1016/j.devcel.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Pan Y, et al. The emerging roles of long noncoding RNA ROR (lincRNA-ROR) and its possible mechanisms in human cancers. Cell Physiol Biochem. 2016;40(1–2):219–229. doi: 10.1159/000452539. [DOI] [PubMed] [Google Scholar]
- 15.Chen YM, et al. Linc-ROR induces epithelial–mesenchymal transition and contributes to drug resistance and invasion of breast cancer cells. Tumour Biol. 2016;37(8):10861–10870. doi: 10.1007/s13277-016-4909-1. [DOI] [PubMed] [Google Scholar]
- 16.Hou P, et al. LincRNA-ROR induces epithelial-to-mesenchymal transition and contributes to breast cancer tumorigenesis and metastasis. Cell Death Dis. 2014;5:e1287. doi: 10.1038/cddis.2014.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng WX, et al. Linc-RoR promotes MAPK/ERK signaling and confers estrogen-independent growth of breast cancer. Mol Cancer. 2017;16(1):161. doi: 10.1186/s12943-017-0727-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao T, et al. Large intergenic non-coding RNA-ROR as a potential biomarker for the diagnosis and dynamic monitoring of breast cancer. Cancer Biomark. 2017;20(2):165–173. doi: 10.3233/CBM-170064. [DOI] [PubMed] [Google Scholar]
- 19.Eades G, et al. lincRNA-RoR and miR-145 regulate invasion in triple-negative breast cancer via targeting ARF6. Mol Cancer Res. 2015;13(2):330–338. doi: 10.1158/1541-7786.MCR-14-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bachman KE, Park BH. Duel nature of TGF-beta signaling: tumor suppressor vs. tumor promoter. Curr Opin Oncol. 2005;17(1):49–54. doi: 10.1097/01.cco.0000143682.45316.ae. [DOI] [PubMed] [Google Scholar]
- 21.Massague J. TGFbeta signalling in context. Nat Rev Mol Cell Biol. 2012;13(10):616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heldin CH, Landstrom M, Moustakas A. Mechanism of TGF-beta signaling to growth arrest, apoptosis, and epithelial-mesenchymal transition. Curr Opin Cell Biol. 2009;21(2):166–176. doi: 10.1016/j.ceb.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 23.Thiery JP. Epithelial–mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 24.Miyazono K, Ehata S, Koinuma D. Tumor-promoting functions of transforming growth factor-beta in progression of cancer. Ups J Med Sci. 2012;117(2):143–152. doi: 10.3109/03009734.2011.638729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lukong KE. Understanding breast cancer—the long and winding road. BBA Clin. 2017;7:64–77. doi: 10.1016/j.bbacli.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data supporting our findings can be found in the “Results” section of the paper. Please contact authors for data request.