Abstract
Purpose
The aim of this study was to compare diffusion tensor imaging (DTI) isotropic map (p-map) with current radiographically (T2/T2-FLAIR) methods based on abnormal hyper-signal size and location of glioblastoma tumor using a semi-automatic approach.
Materials and methods
Twenty-five patients with biopsy-proved diagnosis of glioblastoma participated in this study. T2, T2-FLAIR images and diffusion tensor imaging (DTI) were acquired 1 week before radiotherapy. Hyper-signal regions on T2, T2-FLAIR and DTI p-map were segmented by means of semi-automated segmentation. Manual segmentation was used as ground truth. Dice Scores (DS) were calculated for validation of semiautomatic method. Discordance Index (DI) and area difference percentage between the three above regions from the three modalities were calculated for each patient.
Results
Area of abnormality in the p-map was smaller than the corresponding areas in the T2 and T2-FLAIR images in 17 patients; with mean difference percentage of 30 ± 0.15 and 35 ± 0.15, respectively. Abnormal region in the p-map was larger than the corresponding areas in the T2-FLAIR and T2 images in 4 patients; with mean difference percentage of 26 ± 0.17 and 29 ± 0.28, respectively. This region in the p-map was larger than the one in the T2 image and smaller than the one in the T2-FLAIR image in 3 patients; with mean difference percentage of 34 ± 0.08 and 27 ± 0.06, respectively. Lack of concordance was observed ranged from 0.214–0.772 for T2-FLAIR/p-map (average: 0.462 ± 0.18), 0.266–0.794 for T2 /p-map (average: 0.468 ± 0.13) and 0.123–0.776 for T2/ T2-FLAIR (average: 0.423 ± 0.2). These regions on three modalities were segmented using a semi-automatic segmentation method with over 86% sensitivity, 90% specificity and 89% dice score for three modalities.
Conclusion
It is noted that T2, T2-FLAIR and DTI p-maps represent different but complementary information for delineation of glioblastoma tumor margins. Therefore, this study suggests DTI p-map modality as a candidate to improve target volume delineation based on conventional modalities, which needs further investigations with follow-up data to be confirmed.
Keywords: DTI, Glioblastoma, Isotropic map, Treatment planning
Introduction
Glioblastoma is the most aggressive brain tumor in adults. The current standard of care for patients with glioblastoma is maximal safe surgical de-bulking, followed by adjuvant radiotherapy with concurrent and adjuvant Temozolomide chemotherapy [1].
Diffuse and infiltrative growth of this tumor is a major determinant of poor prognosis. Inherent heterogeneity, unclear boundary and escaped invasive tumor cells are prominent aspects of glioblastoma, making accurate delineation of tumor boundary impossible using conventional MRI (cMRI). However, tumor delineation using cMRI is a necessary prerequisite step in diagnostic and therapeutic (monitoring treatment response) radiology in glioblastoma.
At present, treatment planning for glioblastoma tends to include the contrast-enhancing tumor on CT/T1-weighted MRI plus a 2 cm margin, or the T2-FLAIR/T2-weighted abnormality on the postoperative MRI scan plus a 1 cm margin [1]. Identifying the extension of abnormal region has been improved with recent evolutionary developments in MRI techniques [2].
Diffusion tensor imaging (DTI) is an advanced MRI method which is sensitive to infiltrated and disrupted white matter by tumor cells. Parametric DTI-maps can reveal peri-tumoral abnormalities that are not apparent on cMRI [3]. Price et al. have shown that isotropic (p) and anisotropic (q) components of water diffusion tensor are altered in peri-tumoral and gross tumor regions, respectively [3–5]. Tendency of glioblastoma to infiltrate along white matter tracts often leads to disease extension into peri-tumoral edema. Changes in white matter and edema architectures, as well as changes in cellularity cause an increase in isotropic diffusivity (essentially p parameter). Although detection of hyper–signal regions based on T2/T2-FLAIR images is the best marker for subclinical spread of the tumor, but it is not specific to the changes due to tumor infiltration [6–8]. Considering aforementioned issues, present study attempts to gain some insight into the spatial extension of postoperative hyper-signal region of glioblastoma on the three MRI modalities; T2, T2-FLAIR and DTI p-map using a semi-automatic segmentation method. Main aim of this study is to compare the three abovementioned segmented regions in size and location.
Materials and method
Patients selection and MRI data acquisition
Twenty-five patients (range 26 to 65 years) with a biopsy-proved diagnosis of glioblastoma were recruited after referral to our radiotherapy center for MR imaging. The institutional review board approved this study, and written informed consent was obtained from all subjects. Patients’ information is presented in Table 1.
Table 1.
Patients Characteristics
| Patient No. | Gender | Ages(Year) | Tumor Site |
|---|---|---|---|
| 1 | Male | 30 | Lt Frontal |
| 2 | Male | 62 | Rt Temporal |
| 3 | Male | 30 | Lt Temporal |
| 4 | Female | 28 | Rt Frontal |
| 5 | Female | 54 | Lt Frontal |
| 6 | Female | 54 | Lt Fronto-parietal |
| 7 | Female | 26 | Rt Frontal |
| 8 | Female | 55 | Lt Fronto-parietal |
| 9 | Male | 54 | Lt Frontal |
| 10 | Male | 60 | Rt Parietal |
| 11 | Male | 65 | Rt Parietal |
| 12 | Male | 37 | Rt Frontal |
| 13 | Male | 53 | Lt Temporal |
| 14 | Male | 50 | Rt Parietal |
| 15 | Female | 53 | Rt Parietal |
| 16 | Male | 50 | Lt Temporo-parietal |
| 17 | Male | 54 | Rt Parietal |
| 18 | Male | 45 | Rt Occipital |
| 19 | Female | 36 | Rt Frontal |
| 20 | Female | 45 | Rt Occipital |
| 21 | Female | 50 | Rt Occipital |
| 22 | Female | 40 | Rt Frontal |
| 23 | Male | 52 | Lt Parietal |
| 24 | Male | 37 | Rt Parietal |
| 25 | Female | 36 | Rt Parietal |
MRI data acquisition was performed on a Siemens 1.5 T Avanto scanner (Siemens Healthcare) with a standard head coil. Conventional MRI protocols were as follows: T2-weighted fast spin-echo images (TR/TE = 3000/106 ms, FOV = 230 mm × 230 mm, Voxel size = 0.7 × 0.7 × 5.0 mm, slice thickness = 5 mm), T2-FLAIR images (TR/TE = 7000/93 ms, FOV = 230 mm × 230 mm, Voxel size = 0.9 × 0.9 × 5.0 mm, slice thickness = 5 mm), T1-weighted sequence (TR/TE = 1940/3 ms, FOV = 250 mm × 250 mm, Voxel size = 1 × 1 × 1 mm, slice thickness = 1 mm), and DTI (single-shot SE EPI sequence and diffusion gradients with two b-values (0, 1000 s/mm2) and 12 directions of gradient (TR/TE = 4500/101 ms, FOV = 240 mm × 240 mm, Voxel size = 1.8 × 1.8 × 3.0 mm, slice thickness = 3 mm, number of slices = 30)).
DTI processing and registration
Block diagram for the entire procedure is shown in Fig. 1. DTI images were processed using Explore DTI (Version 4.8) software. After brain extraction, motion, eddy current and EPI corrections [9], three eigenvalues (ƛ1, ƛ2, ƛ3) and mean diffusivity (MD or D) were computed and used to calculate the isotropic component p () as previously described [5, 10]. After the p-map was obtained, p-map and T2 images were registered to the T2-FLAIR image as the reference image for each patient using a standard three dimensional (3D) cubic B-spline transformation with normalized mutual information cost function (SPM 12 software) [11] . Image enhancement was then applied on p-map for edge sharpening to improve results of the segmentation [12]. In addition, greyscale images were normalized to grey level values ranging from 0 to 1.
Fig. 1.
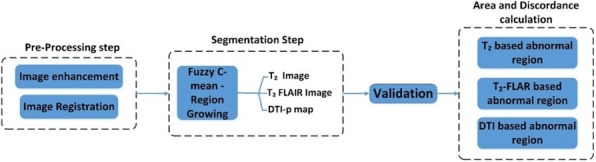
Block diagram of methodology
Image segmentation
For each patient three MRI modalities were used for segmentation: T2, T2-FLAIR and p-map. A fuzzy C-means (FCM) clustering approach was implemented for segmentation of the images [13, 14]. FCM assumes that each pixel belongs to a cluster with constant intensities which is various in different tissue. Segmentation algorithm, based on fuzzy knowledge and region growing, separated the brain region into four classes in three MR modalities (T2, T2-FLAIR and p-map). T2 images were classified into four clusters: tissues with hyper-intensity values (necrosis, tumor hemorrhages and cysts), tissues with intermediate intensity (cerebrospinal fluid (CSF) and edema), tissues with hypo-intensity values (normal white and gray matter, scalp), and tissue with very low or zero intensity (skull, background). T2-FLAIR images also were classified into four classes: tissues with hyper-intensity values (necrosis, tumor hemorrhages and cysts), tissues with intermediate intensity (edema), tissues with hypo-intensity values (scalp, normal white and gray matter) and tissue with zero intensity (CSF, skull, background). Similarly, p-maps were classified into four clusters: tissues with high hyper-intensity values (tumor hemorrhages, cysts and CSF), tissues with intermediate intensity (edema), tissues with hypo-intensity values (normal white and gray matter), and regions with zero intensity (background). We used region growing method to group pixels together according to the rate of change of their intensities over a region. An arbitrary seed pixel was chosen and similar regions from seed point gradually coalesced into expanding regions. This whole process was continued until all pixels were grouped to one region.
The semiautomatic segmentation method was applied to each patient’s data. Segmentation results were validated based on manual expert’s segmentation. Hyper-signal abnormal regions on T2, T2-FLAIR images, and obviously increased isotropy on the p-maps were manually segmented by a radiologist with 10 years of experience in neuro-oncology. They were visually evaluated and revised by another radiologist to obtain an accurate contour. Sensitivity, specificity and dice-score [15–17] were then calculated for alignment of the hyper-signal regions between each semi-automated and manual segmentation for each patient.
Calculation of area of segmented region and discordance index
For the sake of comparison, the area of abnormal masks as segmented on T2, T2-FLAIR images and p-maps were calculated in square centimeter by multiplying all pixels’ sizes with the number of pixels. Three segmented regions were defined as follows: AT (T2 derived abnormal region), AF (T2-FLAIR derived abnormal region), and AP (p-map derived abnormal region). In addition, discordance index (DI), a measure of similarity in location was used for assessing agreement of locations of the three abnormal regions. This index was defined as the ratio of union of the two regions minus the intersection of the same two regions to the union of two regions, and as follows:
DIFP: discordance index between segmented regions on T2-FLAIR image and p-map,
DITP: discordance index between segmented regions on T2 image and p-map,
DITF: discordance index between segmented regions on T2 and T2-FLAIR images,
DI yields values between 0 (one region is perfectly similar or in agreement with another region) and 1 (two regions are completely apart). Higher score of DI means worse concordance between the two considered regions; low scores of DI mean better concordance.
Results
Evaluation of semiautomatic segmentation
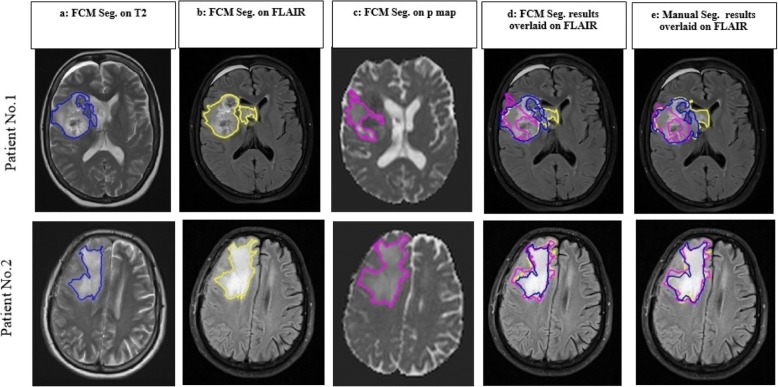
Examples of the results of FCM– RG method and manual segmentation are presented for two patients in Fig. 2. Columns a, b and c demonstrate the results of FCM segmentation on T2, T2-FLAIR images and p-maps. Extracted hyper-signal abnormal regions from FCM and manual segmentation were overlaid on T2-FLAIR images that are shown in columns d and e, respectively. This figure shows that semi-automatic segmented regions visually correspond closely to the expert’s segmented ones. Three metrics as sensitivity, specificity and dice score were calculated for each patient and each modality. Average value of each metric over all patients are summarized in Table 2, showing that mean value of sensitivity and specificity are above 0.85 for each modality. Mean value for dice score over all patients between manual and semiautomatic contouring was 0.89 ± 0.08, 0.91 ± 0.05 and 0.92 ± 0.04 for segmented regions on T2, T2-FLAIR images and p-map, respectively. These values indicate that semi-automatic segmentation is matched well with the expert’s segmented regions.
Fig. 2.
Results of segmentation on two patient’s data with glioblastoma who had undergone partial resection. Columns (a-c): FCM segmentation on (a) T2 (blue), (b) T2-FLAIR (yellow), (c) p maps (pink). (d) results of FCM segmentation overlaid on T2-FLAIR images, (e) Manual segmented region on three images overlaid on T2-FLAIR. First row represents the less agreement in location between T2/T2-FLAIR and p-map (DITF = 0.26, DITP = 0.57, DIFP = 0.65). Second row indicates the close similarity between three modalities (DITF = 0.18, DITP = 0.23, DIFP = 0.19)
Table 2.
Evaluation of semiautomatic segmentation for each modality
| Sensitivity Mean(±SD) |
Specificity Mean(±SD) |
Dice Score Mean(±SD) |
|
|---|---|---|---|
| T2 | 0.86(±0.08) | 0.92(±0.001) | 0.89(±0.08) |
| T2-FLAIR | 0.88(±0.07) | 0.94(±0.04) | 0.91 (±0.05) |
| DTI-p map | 0.87(±0.05) | 0.93(±0.01) | 0.92 ± (0.04) |
Abnormal regions comparison in relation to size and discordance index
The area of segmented abnormal regions from T2, T2-FLAIR images and p-map are presented in Table 3.
-
A)
Comparison of abnormality area in the p-map with the corresponding areas in the T2 and T2-FLAIR images:
In 17 out of 25 patients, the abnormality area in the p-map was smaller than in the corresponding areas in the T2 and T2-FLAIR images (AP < AT2, AP < AF) with mean difference percentage of 30 ± 0.15 (min: 7%, max: 61%) and 35 ± 0.15 (min:13%, max:63%), respectively.
In 4 out of 25 patients, the abnormality area in the p-map was larger than in the corresponding areas in the T2-FLAIR and T2 images (AP > AF, AP > AT2) with mean difference percentage of 26 ± 0.17 and 29 ± 0.28, respectively
In 3 out of 25 patients, the abnormality area in the p-map was larger than the one in the T2 image and smaller than the one in the T2-FLAIR image (AT2 < AP <AF) with mean difference percentage of 34 ± 0.08 and 27 ± 0.06, respectively.
In 1 out of 25 patients, the regions on the three modalities were approximately equal in size (AP≃ AF ≃ AT2) with mean difference value less than 5%.
-
B)
Comparison of the abnormality in the T2 images with the corresponding area in the T2-FLAIR images:
In 22 out of 25 patients, the abnormality area in the T2-FLAIR image was larger than the corresponding area in the T2 image with mean difference percentage of 27 ± 0. 29.
In 3 out of 25 patients the abnormality area in the T2 image was larger than the corresponding area in the T2-FLAIR image with mean difference percentage of 15 ± 0.2.
Table 3.
Obtained area and discordance indices between pathological region extracted from segmentation
| Patient No. | Area(cm2) | Discordance Index | ||||
|---|---|---|---|---|---|---|
| (PT2) | (PF) | (PP) | DITP | DIFP | DITF | |
| 1 | 15.07 | 18.04 | 12.35 | 0.609 | 0.481 | 0.672 |
| 2 | 7.18 | 12.67 | 8.89 | 0.411 | 0.304 | 0.457 |
| 3 | 13.95 | 14.43 | 13.99 | 0.296 | 0.340 | 0.187 |
| 4 | 12.32 | 14.23 | 17.06 | 0.472 | 0.506 | 0.598 |
| 5 | 7.24 | 14.98 | 10.12 | 0.577 | 0.472 | 0.429 |
| 6 | 32.52 | 34.58 | 17.98 | 0.572 | 0.583 | 0.393 |
| 7 | 35.97 | 33.19 | 24.19 | 0.476 | 0.591 | 0.367 |
| 8 | 18.02 | 19.27 | 22.15 | 0.258 | 0.321 | 0.225 |
| 9 | 6.84 | 8.85 | 3.95 | 0.329 | 0.379 | 0.347 |
| 10 | 5.16 | 6.10 | 2.95 | 0.589 | 0.640 | 0.228 |
| 11 | 23.05 | 25.87 | 21.76 | 0.266 | 0.215 | 0.128 |
| 12 | 11.84 | 13.67 | 7.50 | 0.508 | 0.575 | 0.395 |
| 13 | 13.00 | 13.61 | 10.02 | 0.457 | 0.686 | 0.453 |
| 14 | 4.75 | 7.97 | 2.98 | 0.480 | 0.761 | 0.569 |
| 15 | 8.21 | 9.52 | 3.14 | 0.794 | 0.772 | 0.731 |
| 16 | 29.09 | 23.94 | 18.84 | 0.433 | 0.230 | 0.336 |
| 17 | 8.39 | 8.52 | 9.85 | 0.300 | 0.254 | 0.240 |
| 18 | 12.25 | 12.01 | 9.12 | 0.315 | 0.214 | 0.178 |
| 19 | 14.85 | 9.59 | 7.32 | 0.495 | 0.615 | 0.658 |
| 20 | 6.12 | 6.18 | 4.72 | 0.512 | 0.248 | 0.123 |
| 21 | 3.08 | 2.52 | 4.26 | 0.375 | 0.411 | 0.261 |
| 22 | 5.19 | 7.35 | 4.45 | 0.482 | 0.522 | 0.714 |
| 23 | 9.62 | 9.88 | 8.14 | 0.430 | 0.472 | 0.430 |
| 24 | 8.59 | 11.05 | 12.5 | 0.721 | 0.239 | 0.689 |
| 25 | 10.13 | 12.25 | 6.62 | 0.549 | 0.738 | 0.776 |
Abbreviation: PT T2 derived pathological region, PF T2 -FLAIR derived pathological region, PP DTI-p derived pathological region, DTFP Discordance Index between T2-FLAIR and p-map, DTTP Discordance Index between T2 and p-map, DITF Discordance Index between T2-FLAIR and T2
In addition to calculation of area, there was a need to determine the degree of similarity in location of AP, AF and AT2. So, discordance indices (DITP, DIFP, DITF) were defined as written in method section. As reported in Table 3, there was a large range of discordance index between the three regions; DITP, DIFP and DITF ranged from 0.266–0.794 (average: 0.468 ± 0.13), 0.214–0.772 (average: 0.462 ± 018) and 0.123–0.776 (average: 0.423 ± 0.2), respectively (Fig. 3).
Fig. 3.
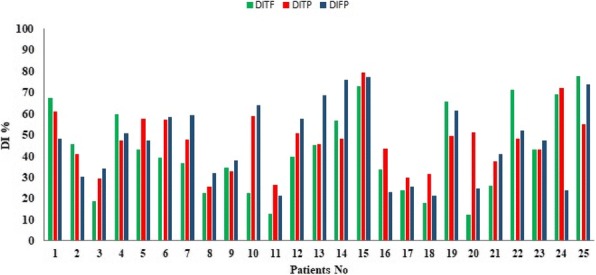
A graph showing the calculated DI% of abnormal regions extracted from three modalities. Green, Red and Blue indicate the of DITF, DITP, DIFP for twenty-five patients
Despite acquiring the results of differences in tumor extension and location (Table 3), intriguing findings were observed in some patients as follows:
In patient #21, a small hyper-signal abnormal region was seen in left temporal lobe on T2-FLAIR image and p-map that appeared normal on T2 images, while the tumoral region was detected in right occipital lobe.
In patient #11, a hyper-signal region was seen at the center of ventricles on T2-FLAIR image and p-map that was not detected on T2 image, while whole abnormal hyper-intense region was detected in right parietal lobe as listed in the Table 1.
Discussion
Glioblastoma tumor predominantly infiltrates along white matter tracts and invades to surrounding edematous region [6, 18]. Previous studies on the behavior of glioblastoma suggest that DTI-derived tensor metrics can detect the integrity of white matter structures as a valid method without missing infiltrated brain areas [3, 5]. Hence, by calculating the isotropic (p) and anisotropic (q) metrics of diffusion tensor proposed by Pena et al. [10], it is possible to probe diseased brain parenchyma in the study of complex tumor such as glioblastoma. Price et al. have compared DTI-defined invasive and noninvasive regions using perfusion and magnetic resonance spectroscopy (MRS) [7]. They contoured p and q abnormalities to identify the invasive margin and then drew three regions of interest (ROIs) on p-invasive region (area of increased p and outside the area of reduced q), p-noninvasive region (outside of p abnormal region, in an area similar to the invasive ROI according to T2 image) and contralateral normal brain. Their results showed that there are significant differences in perfusion and MRS parameters between defined invasive and non-invasive regions based on the p-map. This study has clearly demonstrated that defined invasive and non-invasive regions based on p/q-maps look similar in appearance on T2 image but different in information content on the local environment. Furthermore, Price et al. in other studies have shown that increased DTI-isotropic component (p) around gross tumor indicates the infiltrating tumor margin [3, 5, 19]. These zones extend beyond abnormal areas on both enhanced T1- and T2-weighted MRI images. Four regions were selected on the abovementioned study; tumor, possible tumor infiltration near the tumor margin, edema and normal appearing contralateral white matter. From their spatial distribution of four regions in the p: q space, it can be observed that the healthy white matter has low p value and high q value with high variance. Tumor has high p value and very low q value. Edema has high p value and slightly lower q value than white matter and tumor margin with possible tumor infiltration demonstrate high p and low q values. As shown in these studies, it is proved that affected white matter tracts by tumor can be identified on DTI in four patterns categorized on the basis of isotropy and anisotropy (p, q) components. Accordingly, results of these studies demonstrate that the hyper-signal abnormal region on the p-map is appeared due to presence of either tumor or infiltrated white matter or edema.
On the other hand, and according to the current standards, clinical target volume concepts are based on either T2 or T2-FLAIR images to encompass possible microscopic disease. T2 and T2-FLAIR images are helpful for assessing non-enhancing tumor and edema extent but are not specific to changes due to tumor infiltration. Therefore, various MRI sequences reflect different properties of tissue, and no single imaging metric is currently sufficient to delineate the region of non-enhancing tumor. Consequently, we concluded that there is a need for further evaluation of extension of the hyper-signal regions on DTI p-map and T2/T2-FLAIR conventional images as a preliminary study. Thus, p-maps were considered beyond segmentation method for T2, T2-FLAIR images in FCM-RG semi-automatic segmentation procedure. Results of differences between size of abnormal regions on T2 and T2-FLAIR images (≃15%) for each patient show that using only one of these two structural techniques may not be adequate for delineation of boundary of the hyper-signal abnormal regions in radiotherapy planning. However, these images cannot differentiate between pure edema and tumor-infiltrated edema. Noticeble differences were found between the size and location of hyper-signal abnormalities on the p-map in comparison with T2/T2-FLAIR images. A large range of Discordance Index (DI) between the segmented abnormal regions on the p-map/T2 image and p-map/T2-FLAIR image in Table 3 represent that hyper-signal regions on three images were different not only in size but also in location. For example, for patient #3, results show that in spite of equality in abnormality’s size between three modalities, three regions are not completely concord to each other (DITP = 0.296, DIFP = 0.34 and DITF = 0.187). T2 or T2-FLAIR images only reveal partial tissue signatures of brain-tumor microenvironments. Furthermore, DTI p-map can identify diffusion signature of tissue and subtle white matter abnormalities. Therefore, p-map may be used to assist in delineation of whole abnormal hyper-signal regions in treatment planning of glioblastoma based on cMRI.
Main limitation of this study was DTI acquisition with only 12 directions and 2 b-value. Another important limitation was the lack of a follow-up imaging data to assess recurrence site in relation to three abnormal regions. Future work in this direction can include a larger prospective study based on a more patient population with follow-up imaging to investigate recurrence site.
Conclusion
This study suggests that DTI p-map has the potential to improve target volume delineation based on T2 and T2-FLIAR modalities, but further investigation is needed to confirm it. Accurate manual segmentation of unclear boundary of abnormality on p-map is time-consuming and difficult, whilst the proposed segmentation procedure in this study results to decrease segmentation time. Therefore, this method might be a reliable way to segment hyper-signal regions on three modalities.
Acknowledgements
This research has been supported by Tehran University of Medical Sciences & Health Services grant number; 29387-30-03-94. We are extremely grateful to Ms. Aramesh Safari and Mr. Pedram Rostami for providing imaging data in Payambaran MRI center.
Funding
This research has been funded by Tehran University of Medical Sciences & Health Services grant number; 29387–30–03-94. The role of this funding body was funding the MR imaging in MRI center.
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- CSF
Cerebrospinal Fluid
- CTV
Clinical Target Volume
- DI
Discordance Index
- DS
Dice Scores
- DTI
Diffusion Tensor Imaging
- FCM
Fuzzy C-Means
- FLAIR
Fluid Attenuation Inversion Recovery
- FOV
Field of View
- MRI
Magnetic Resonance Imaging
- TE
Time Echo
- TR
Time Repetition
- VE
Vasogenic Edema
Authors’ contributions
MB carried out the diffusion Tensor studies and participated in design, analysis of MR images and draft the manuscript. MS carried out the MR image analysis. AA and MT participated in the manual segmentation of MR images and referring the patients. MSM participated in the MRI data acquisition in MRI center and determination of image protocols. AA helped to manage the project. HS was study’s manager and participated in its coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Our institutional consent forms were used for each patient.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Whitfield GA, et al. Imaging and target volume delineation in glioma. Clin Oncol. 2014;26(7):364–376. doi: 10.1016/j.clon.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 2.Price S, Gillard J. Imaging biomarkers of brain tumour margin and tumour invasion. The British journal of radiology. 2011;84(special_issue_2):S159–S167. doi: 10.1259/bjr/26838774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Price S, et al. Improved delineation of glioma margins and regions of infiltration with the use of diffusion tensor imaging: an image-guided biopsy study. Am J Neuroradiol. 2006;27(9):1969–1974. [PMC free article] [PubMed] [Google Scholar]
- 4.Price SJ, et al. Predicting patterns of glioma recurrence using diffusion tensor imaging. Eur Radiol. 2007;17(7):1675–1684. doi: 10.1007/s00330-006-0561-2. [DOI] [PubMed] [Google Scholar]
- 5.Price SJ, et al. Tissue signature characterisation of diffusion tensor abnormalities in cerebral gliomas. Eur Radiol. 2004;14(10):1909–1917. doi: 10.1007/s00330-004-2381-6. [DOI] [PubMed] [Google Scholar]
- 6.Sternberg E, Lipton M, Burns J. Utility of diffusion tensor imaging in evaluation of the peritumoral region in patients with primary and metastatic brain tumors. Am J Neuroradiol. 2014;35(3):439–444. doi: 10.3174/ajnr.A3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price SJ, et al. Multimodal MRI can identify perfusion and metabolic changes in the invasive margin of glioblastomas. J Magn Reson Imaging. 2016;43(2):487–494. doi: 10.1002/jmri.24996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan J-L, et al. Extent of resection of peritumoral diffusion tensor imaging–detected abnormality as a predictor of survival in adult glioblastoma patients. J Neurosurg. 2017;126(1):234–241. doi: 10.3171/2016.1.JNS152153. [DOI] [PubMed] [Google Scholar]
- 9.Leemans A, Jones DK. The B-matrix must be rotated when correcting for subject motion in DTI data. Magn Reson Med. 2009;61(6):1336–1349. doi: 10.1002/mrm.21890. [DOI] [PubMed] [Google Scholar]
- 10.Pena A, et al. Enhanced visualization and quantification of magnetic resonance diffusion tensor imaging using the p: q tensor decomposition. Br J Radiol. 2006;79(938):101–109. doi: 10.1259/bjr/24908512. [DOI] [PubMed] [Google Scholar]
- 11.Maes F, et al. Multimodality image registration by maximization of mutual information. IEEE Trans Med Imaging. 1997;16(2):187–198. doi: 10.1109/42.563664. [DOI] [PubMed] [Google Scholar]
- 12.Carmona Salazar, O.D. And J. Calderon Gonzalez, Image enhancement with Matlab algorithms. 2015, Universitat Politècnica de Catalunya.
- 13.Lin G-C, et al. Multispectral MR images segmentation based on fuzzy knowledge and modified seeded region growing. Magn Reson Imaging. 2012;30(2):230–246. doi: 10.1016/j.mri.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Kazerooni AF, et al. Multi-parametric (ADC/PWI/T2-w) image fusion approach for accurate semi-automatic segmentation of tumorous regions in glioblastoma multiforme. MAGMA. 2015;28(1):13–22. doi: 10.1007/s10334-014-0442-7. [DOI] [PubMed] [Google Scholar]
- 15.Menze, B.H., et al. A generative model for brain tumor segmentation in multi-modal images. In International Conference on Medical Image Computing and Computer-Assisted Intervention. 2010. Springer. [DOI] [PMC free article] [PubMed]
- 16.Zou KH, et al. Statistical validation of image segmentation quality based on a spatial overlap index1: scientific reports. Acad Radiol. 2004;11(2):178–189. doi: 10.1016/S1076-6332(03)00671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strickland, R.N., Image-processing techniques for tumor detection. 2002: CRC Press.
- 18.Lu S, et al. Peritumoral diffusion tensor imaging of high-grade gliomas and metastatic brain tumors. Am J Neuroradiol. 2003;24(5):937–941. [PMC free article] [PubMed] [Google Scholar]
- 19.Price S, Gillard J. Imaging biomarkers of brain tumour margin and tumour invasion. Br J Radiol. 2014. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.