Abstract
Objective
To compare the risks of postendoscopy outcomes associated with warfarin with direct oral anticoagulants (DOACs), taking into account heparin bridging and various types of endoscopic procedures.
Design
Using the Japanese Diagnosis Procedure Combination database, we identified 16 977 patients who underwent 13 types of high-risk endoscopic procedures and took preoperative warfarin or DOACs from 2014 to 2015. One-to-one propensity score matching was performed to compare postendoscopy GI bleeding and thromboembolism between the warfarin and DOAC groups.
Results
In the propensity score-matched analysis involving 5046 pairs, the warfarin group had a significantly higher proportion of GI bleeding than the DOAC group (12.0% vs 9.9%; p=0.002). No significant difference was observed in thromboembolism (5.4% vs 4.7%) or in-hospital mortality (5.4% vs 4.7%). The risks of GI bleeding and thromboembolism were greater in patients treated with warfarin plus heparin bridging or DOACs plus bridging than in patients treated with DOACs alone. Compared with percutaneous endoscopic gastrostomy, patients who underwent endoscopic submucosal dissection, endoscopic mucosal resection and haemostatic procedures including endoscopic variceal ligation or endoscopic injection sclerotherapy were at the highest risk of GI bleeding among the 13 types of endoscopic procedures, whereas those who underwent lower polypectomy endoscopic sphincterotomy or endoscopic ultrasound-guided fine needle aspiration were at moderate risk.
Conclusion
The risk of postendoscopy GI bleeding was higher in warfarin than DOAC users. Heparin bridging was associated with an increased risk of bleeding and did not prevent thromboembolism. The bleeding risk varied by the type of endoscopic procedure.
Keywords: Vitamin K antagonist (VKA), novel oral anticoagulants (NOACs), gastrointestinal hemorrhage, thromboembolism
Significance of this study.
What is already known on this subject?
Oral anticoagulant users have a higher risk of GI bleeding after therapeutic endoscopy, whereas temporal discontinuation of oral anticoagulants may increase their risk of thromboembolism.
The risk of endoscopy-related GI bleeding or thromboembolism may differ between direct oral anticoagulants (DOACs) and warfarin.
The risk of GI bleeding or thromboembolism can be affected by the use of heparin bridging or the type of endoscopic procedure in patients taking oral anticoagulants.
What are the new findings?
Warfarin users had a significantly higher proportion of GI bleeding after high-risk endoscopic procedures than did DOAC users.
Heparin bridging was associated with an increased risk of bleeding and thromboembolism.
The bleeding risk varied by the type of endoscopic procedure.
How might it impact on clinical practice in the foreseeable future?
Our results will be useful for decision making regarding switching from warfarin to DOACs before implementing high-risk endoscopic procedures.
Because bridging with unfractionated heparin does not prevent thromboembolic events and increases the risk of bleeding events, its recommendation in clinical guidelines and its current clinical use should be re-evaluated.
Risk stratification by the type of endoscopic procedure may be needed in patients taking oral anticoagulants.
Introduction
Transient interruption of anticoagulant agents before endoscopic procedures remains controversial because of difficulties in balancing the risks of GI)bleeding and thromboembolism.1–6 Among anticoagulant agents, warfarin is familiar to nearly all clinicians, and its effect can be reversed easily and rapidly.5 However, it requires complex management because of its intricate pharmacodynamic properties and narrow therapeutic range.7 8 In contrast, direct oral anticoagulants (DOACs) are prescribed at a fixed dose without the need for monitoring or dose adjustment based on their rapid onset of anticoagulant effect and short half-life,4 5 9 which allow for easier management; however, specific antidotes or reversal agents for some DOACs are lacking.1 5 Some evidence suggests that patients receiving DOACs have an increased risk of non-procedure-related GI bleeding compared with patients receiving warfarin10 11; however, the risks of procedure-related GI bleeding remain unclear.
In several previous studies, the proportion of high-risk procedure-related bleeding in patients taking anticoagulants was 38% in those who underwent gastric endoscopic submucosal dissection (ESD),1217% in those who underwent colorectal ESD,1320% in those who underwent colorectal polypectomy14 and 33% in those who underwent endoscopic ultrasound-guided fine needle aspiration (EUS-FNA).15 However, these studies involved small samples from a limited number of institutions.
Endoscopic guidelines recommend continuing warfarin and DOACs in patients undergoing low-risk endoscopic procedures and heparin bridging for warfarin users undergoing high-risk endoscopic procedures.1–3 6 In clinical practice, DOAC users may also undergo heparin bridging to prevent thromboembolism.16 However, the difference in bleeding or thromboembolic risk associated with heparin bridging between warfarin and DOAC users also remains unclear.
Because only <4% of patients who undergo high-risk endoscopic procedures receive oral anticoagulants,17 18 no single-centre study would be able to recruit a sufficient number of patients. In this study, therefore, we used a large national inpatient database in Japan to (1) compare the risks of bleeding, thromboembolism, and death between patients treated with warfarin and DOACs; (2) compare these risks among 13 types of high-risk endoscopic procedures; and (3) determine whether heparin bridging increases the incidence of adverse events.
Methods
Design, setting, participants and data sources
This retrospective cohort study was based on a national inpatient database (the Diagnosis Procedure Combination database in Japan). Data were extracted from the database for adult patients (≥20 years old) who underwent a high-risk endoscopic procedure and received an oral anticoagulant (warfarin or DOAC) prior to the endoscopic procedure from April 2014 to May 2015. Patients with atrial fibrillation, valvular disease or a history of thromboembolism are reimbursed for oral anticoagulant use by the universal health insurance system in Japan. Based on the European, American and Asian guidelines,1–3 high-risk endoscopic procedures include polypectomy, endoscopic mucosal resection (EMR), ESD, endoscopic balloon dilation of strictures, endoscopic haemostasis, endoscopic variceal ligation (EVL), endoscopic injection sclerotherapy (EIS), endoscopic sphincterotomy (EST), EUS-FNA and percutaneous endoscopic gastrostomy (PEG).
The database includes admission/discharge abstracts and administrative claims of approximately 7000 000 inpatients per year from more than 1000 hospitals throughout Japan.17–19 The database includes the following data: patient characteristics; main diagnoses, comorbidities that were present at admission and complications after admission coded with the International Classification of Diseases and Related Health Problems Tenth Revision codes (ICD-10)20 and text data in Japanese; drugs and procedures coded with Japanese original codes; discharge status; and length of stay.17–19 Because of the anonymous nature of the data, informed consent was waived when this study was approved by the Institutional Review Board at the University of Tokyo.
Outcomes and variables
The main clinical outcomes included therapeutic endoscopy-related GI bleeding within 30 days of endoscopy, thromboembolism within 30 days of endoscopy and death during the hospital stay. GI bleeding included overt GI bleeding after the initial high-risk endoscopic procedures that required endoscopic haemostasis and/or blood transfusion. When the initial endoscopic procedure was haemostasis, postendoscopy GI bleeding was defined as recurrent overt GI bleeding that required endoscopic haemostasis and/or blood transfusion. We defined thromboembolism as the occurrence of cardiovascular events, cerebrovascular events, pulmonary embolism, deep vein thrombosis and other types of arterial thrombosis. Cardiovascular events were identified by recorded diagnoses of ischaemic heart diseases after admission (ICD-10 codes I20–22) or performance of percutaneous coronary intervention. Cerebrovascular events were identified by recorded diagnoses of stroke after admission (ICD-10 codes I61–63) or treatment with tissue plasminogen activator. Complications that occurred after admission were used to identify pulmonary embolism (ICD-10 code I26), deep vein thrombosis (I82) and other types of arterial thrombosis (I74).
We evaluated data on age, sex, body mass index (BMI), comorbidities at admission, drugs used, heparin bridging and type of endoscopic procedures. BMI was classified into four categories (<18.5, 18.5–24.9, 25.0–29.9 and >30.0 kg/m2) in accordance with the WHO BMI Classification.21 We evaluated 13 comorbidities at admission based on the components of the Charlson Comorbidity Index: congestive heart failure, peripheral vascular disease, myocardial infarction, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatoid disease, peptic ulcer, diabetes with and without chronic complications, hemiplegia or paraplegia, renal disease, mild-to-severe liver disease and malignancy or metastatic cancer.17–19 We assessed the use of low-dose aspirin, thienopyridines, other antiplatelet drugs, non-steroidal anti-inflammatory drugs, corticosteroids, acetaminophen and proton pump inhibitors. Anticoagulants included warfarin and DOACs (rivaroxaban, apixaban, dabigatran and edoxaban). Patients undergoing heparin bridging received a prophylactic intravenous infusion of unfractionated heparin. Only unfractionated heparin is used in Japan because low-molecular-weight heparin is not covered by the public health insurance system. The most common bridging technique using unfractionated heparin in Japan involves replacing oral anticoagulants with heparin (10 000–20 000 units/day infused intravenously or 10 000–15 000 IU injected subcutaneously every 12 hours) 3 to 5 days before the endoscopic procedure after admission while adjusting the dose to attain the required activated partial thromboplastin time (APTT).3 After haemostasis has been confirmed, heparin is resumed and the anticoagulant restarted at the prewithdrawal dose.3 Heparin is discontinued when the prothrombin time–international normalised ratio (INR) has returned to the therapeutic range.3
We also assessed the annual hospital volume for high-risk therapeutic endoscopy procedures in each hospital and categorised this volume into quartiles: very low (0–691 cases/year), low (692–1089 cases/year), high (1090–1552 cases/year) and very high (>1552 cases/year).
Statistics
We performed a one-to-one propensity score matching analysis between the warfarin and DOAC groups based on the estimated propensity scores of each patient.22 To estimate the propensity score, we fitted a logistic regression model for the receipt of DOACs as a function of the following patient demographic and hospital factors: age category, sex, BMI category, 13 comorbidities, annual hospital volume for therapeutic endoscopy, 7 types of drugs used and 13 types of endoscopic procedures. We calculated the C-statistic to evaluate the goodness of fit. Each patient who received DOACs was matched with a patient who received warfarin with the closest estimated propensity on the logit scale within a specified range (≤0.2 of the pooled SD of estimated logits).
After propensity score matching, we compared the proportions of postendoscopy adverse outcomes (GI bleeding, thromboembolism and in-hospital death) between the warfarin and DOAC groups. Comparison of categorical data between the groups was performed with the χ2 test or Fisher’s exact test as appropriate. Continuous data were compared with Wilcoxon’s rank-sum test. A multivariable logistic regression was performed to estimate the ORs and 95% CIs for postendoscopy adverse outcomes in the warfarin group with reference to the DOAC group, adjusting for 13 high-risk endoscopic procedures. Because heparin bridging may affect adverse outcomes, we additionally divided the patients into the following subgroups based on the oral anticoagulant agent with and without heparin bridging: DOACs alone, warfarin alone, bridging DOACs with heparin and bridging warfarin with heparin. The ORs for adverse outcomes in these subgroups were estimated with another multivariable logistic regression model with adjustment for the 13 high-risk endoscopic procedures. The threshold for significance was p<0.05. All statistical analyses were conducted using IBM SPSS V. 23.0 (IBM SPSS).
Results
We identified a total of 16 977 patients who underwent high-risk endoscopic procedures and received oral anticoagulants prior to the endoscopic procedures in 1004 hospitals from April 2014 to May 2015. Among them, 11 896 patients received warfarin and 5081 received DOACs. By one-to-one propensity score matching, we selected 5046 pairs of the warfarin users and DOAC users, including users of rivaroxaban (n=2149), apixaban (n=1751), dabigatran (n=805) and edoxaban (n=341). The C-statistic for goodness of fit was 0.639 in the propensity score model. Before the propensity score matching, the distribution of age, BMI, hospital volume and some endoscopic procedures were significantly different between the warfarin and DOAC groups (table 1). The warfarin group showed higher proportions of peripheral vascular disease, myocardial infarction, rheumatoid disease, peptic ulcer disease, diabetes with chronic complications, chronic renal disease, liver disease, use of low-dose aspirin, use of antiplatelet drugs, use of corticosteroids, upper GI endoscopic haemostasis, lower GI EMR or polypectomy, EIS, EVL and upper GI EMR/polypectomy (table 1). The DOAC group showed higher proportions of cerebrovascular disease, dementia, hemiplegia, malignancy, use of nonsteroidal anti-inflammatory drugs, PEG and lower GI ESD (table 1). After propensity score matching, the patient distributions were closely balanced between the warfarin and DOAC groups (table 1).
Table 1.
Baseline characteristics of unmatched and propensity score-matched patients treated with warfarin and DOACs
| Unmatched | Propensity score matched | |||||
| Warfarin (n=11 896) |
DOACs (n=5081) |
Standardised difference (%) | Warfarin (n=5046) |
DOACs (n=5046) |
Standardised difference (%) | |
| Age, years | ||||||
| <60 | 678 (5.7) | 183 (3.6) | 10.0 | 185 (3.7) | 183 (3.6) | 0.5 |
| 60–69 | 2080 (17.5) | 756 (14.9) | 7.1 | 746 (14.8) | 755 (15.0) | 0.6 |
| 70–79 | 4619 (38.8) | 1952 (38.4) | 0.8 | 1931 (38.3) | 1940 (38.4) | 0.2 |
| ≥80 | 4519 (38.0) | 2190 (43.1) | 10.4 | 2184 (43.3) | 2168 (43.0) | 0.6 |
| Sex (male) | 7707 (64.8) | 3334 (65.6) | 1.7 | 3325 (65.9) | 3310 (65.6) | 0.6 |
| Body mass index, kg/m2 | ||||||
| <18.5 | 1734 (15.5) | 823 (17.4) | 5.1 | 779 (16.5) | 815 (17.3) | 2.1 |
| 18.5–24.9 | 6709 (59.9) | 2769 (58.5) | 2.8 | 2797 (59.3) | 2757 (58.6) | 1.4 |
| 25.0–29.9 | 2340 (20.9) | 965 (20.4) | 1.2 | 959 (20.3) | 957 (20.4) | 0.2 |
| ≥30.0 | 415 (3.7) | 177 (3.7) | 0.0 | 181 (3.8) | 172 (3.7) | 0.5 |
| Comorbidities | ||||||
| Congestive heart failure | 2561 (21.5) | 1083 (21.3) | 0.5 | 1069 (21.2) | 1079 (21.4) | 0.5 |
| Peripheral vascular disease | 482 (4.1) | 123 (2.4) | 9.6 | 115 (2.3) | 123 (2.4) | 0.7 |
| Myocardial infarction | 472 (4.0) | 125 (2.5) | 8.5 | 108 (2.1) | 125 (2.5) | 2.7 |
| Cerebrovascular disease | 2200 (18.5) | 1365 (26.9) | 20.2 | 1288 (25.5) | 1337 (26.5) | 2.3 |
| Dementia | 484 (4.1) | 266 (5.2) | 5.2 | 229 (4.5) | 263 (5.2) | 3.3 |
| Chronic pulmonary disease | 367 (3.1) | 185 (3.6) | 2.8 | 176 (3.5) | 182 (3.6) | 0.5 |
| Rheumatoid disease | 199 (1.7) | 54 (1.1) | 5.1 | 53 (1.1) | 54 (1.1) | 0 |
| Peptic ulcer disease | 1509 (12.7) | 583 (11.5) | 3.7 | 586 (11.6) | 583 (11.6) | 0 |
| Diabetes without chronic complications | 1888 (15.9) | 850 (16.7) | 2.2 | 833 (16.5) | 845 (16.7) | 0.5 |
| Diabetes with chronic complications | 542 (4.6) | 172 (3.4) | 6.1 | 178 (3.5) | 172 (3.4) | 0.5 |
| Hemiplegia or paraplegia | 147 (1.2) | 140 (2.8) | 11.4 | 118 (2.3) | 126 (2.5) | 1.3 |
| Renal disease | 1057 (9.7) | 83 (1.6) | 35.6 | 81 (1.6) | 83 (1.6) | 0 |
| Mild liver disease | 573 (4.8) | 202 (4.0) | 3.9 | 228 (4.5) | 202 (4.0) | 2.5 |
| Moderate or severe liver disease | 314 (2.6) | 67 (1.3) | 9.4 | 81 (1.6) | 67 (1.3) | 2.5 |
| Malignancy | 2171 (18.2) | 1009 (19.9) | 4.3 | 1002 (19.9) | 1005 (19.9) | 0 |
| Metastatic cancer | 223 (1.9) | 75 (1.5) | 3.1 | 79 (1.6) | 75 (1.5) | 0.8 |
| Hospital annual procedure volume | ||||||
| Very low (0–691) | 2801 (23.5) | 1443 (28.4) | 11.2 | 1442 (28.6) | 1422 (28.2) | 0.9 |
| Low (692–1089) | 2941 (24.7) | 1320 (26.0) | 3.0 | 1289 (25.5) | 1312 (26.0) | 1.1 |
| High (1090–1552) | 3178 (26.7) | 1260 (24.8) | 4.3 | 1242 (24.6) | 1255 (24.9) | 0.7 |
| Very high (>1552) | 2976 (25.0) | 1058 (20.8) | 10.0 | 1073 (21.3) | 1057 (20.9) | 1.0 |
| Drugs use | ||||||
| Low-dose aspirin | 2300 (19.3) | 662 (13.0) | 17.2 | 606 (12.0) | 662 (13.1) | 3.3 |
| Thienopyridines | 848 (7.1) | 391 (7.7) | 2.3 | 342 (6.8) | 387 (7.7) | 3.5 |
| Other antiplatelet drugs | 913 (7.7) | 342 (6.7) | 3.9 | 334 (6.6) | 332 (6.6) | 0 |
| Non-steroidal anti-inflammatory drugs | 2516 (21.1) | 1293 (25.4) | 10.2 | 1289 (25.5) | 1273 (25.2) | 0.7 |
| Corticosteroids | 1758 (14.8) | 662 (13.0) | 5.2 | 636 (12.6) | 662 (13.1) | 1.5 |
| Proton pump inhibitors | 8107 (68.1) | 3478 (68.5) | 0.9 | 3477 (68.9) | 3461 (68.6) | 0.6 |
| Endoscopic procedures | ||||||
| Upper GI endoscopic haemostasis | 2465 (20.7) | 902 (17.8) | 7.4 | 915 (18.1) | 902 (17.9) | 0.5 |
| PEG | 2322 (19.5) | 1484 (29.2) | 22.7 | 1426 (28.3) | 1452 (28.8) | 1.1 |
| EST | 1623 (13.6) | 706 (13.9) | 0.9 | 696 (13.8) | 706 (14.0) | 0.6 |
| Lower GI EMR | 2234 (18.8) | 699 (13.8) | 13.6 | 730 (14.5) | 698 (13.8) | 2.0 |
| Lower GI polypectomy | 684 (5.7) | 227 (4.5) | 5.5 | 225 (4.5) | 227 (4.5) | 0 |
| Lower GI ESD | 206 (1.7) | 121 (2.4) | 4.9 | 111 (2.2) | 121 (2.4) | 1.3 |
| Lower GI haemostasis | 795 (6.7) | 322 (6.3) | 1.6 | 313 (6.2) | 321 (6.4) | 0.8 |
| EUS-FNA | 218 (1.8) | 111 (2.2) | 2.9 | 105 (2.1) | 111 (2.2) | 0.7 |
| EIS | 117 (1.0) | 24 (0.5) | 5.8 | 28 (0.5) | 24 (0.5) | 0 |
| EVL | 218 (1.8) | 52 (1.0) | 6.8 | 54 (1.1) | 52 (1.0) | 1.0 |
| Endoscopic balloon dilatation | 143 (1.2) | 77 (1.5) | 2.6 | 74 (1.5) | 76 (1.5) | 0 |
| Upper GI EMR/polypectomy | 259 (2.2) | 81 (1.6) | 4.4 | 68 (1.3) | 81 (1.6) | 2.5 |
| Upper GI ESD | 612 (5.1) | 275 (5.4) | 1.3 | 301 (6.0) | 275 (5.4) | 2.6 |
Data are presented as n (%) with the exception of the standardised difference.
Direct oral anticoagulants include rivaroxaban, apixaban, dabigatran and edoxaban. Low-dose aspirin includes buffered and enteric-coated aspirin. Thienopyridines include ticlopidine, clopidogreland prasugrel. Other antiplatelet drugs include sarpogrelate hydrochloride, ethyl icosapentate, limaprost, dilazep, beraprost, cilostazol and dipyridamole. Non-steroidal anti-inflammatory drugs include mefenamic acid, indomethacin farnesil, etodolac, ibuprofen, celecoxib, naproxen, zaltoprofen, diclofenac sodium, loxoprofen, meloxicam and lornoxicam. Proton pump inhibitors include omeprazole, esomeprazole, lansoprazole, rabeprazole and vonoprazan.
DOACs, direct oral anticoagulants; EIS, endoscopic injection sclerotherapy; EMR, endoscopic mucosal resection; ESD, endoscopic submucosal dissection; EST, endoscopic sphincterotomy; EUS-FNA, endoscopic ultrasound-guided fine needle aspiration; EVL, endoscopic variceal ligation; PEG, percutaneous endoscopic gastrostomy.
The warfarin group had a significantly higher proportion of GI bleeding than the DOAC group (12.0% vs 9.9%, respectively; p=0.002). No significant difference was observed in the proportion of thromboembolism (5.4% vs 4.7%) or in-hospital mortality (5.4% vs 4.7%) (table 2). In the subanalysis of DOAC types, the warfarin group had a significantly higher proportion of GI bleeding than the rivaroxaban group and a significantly higher proportion of thromboembolism than the rivaroxaban and dabigatran groups (table 2). No significant difference in in-hospital mortality was observed between warfarin and any type of DOACs (table 2).
Table 2.
Postendoscopy GI bleeding, thromboembolism and death in propensity score-matched patients treated with warfarin and DOACs (n=10 092)
| Postendoscopy GI bleeding | p Value | Postendoscopy thromboembolism* | p Value | Postendoscopy death | p Value | |
| Warfarin (n=5046) | 605 (12.0) | 275 (5.4) | 270 (5.4) | |||
| DOACs (n=5046) | 506 (10.0) | 0.002 | 239 (4.7) | 0.103 | 239 (4.7) | 0.172 |
| Rivaroxaban (n=2149) | 185 (8.6) | <0.001 | 90 (4.2) | 0.026 | 92 (4.3) | 0.059 |
| Apixaban (n=1751) | 183 (10.5) | 0.091 | 76 (4.3) | 0.079 | 99 (5.7) | 0.625 |
| Dabigatran (n=805) | 108 (13.4) | 0.246 | 24 (3.0) | 0.002 | 30 (3.7) | 0.058 |
| Edoxaban (n=341) | 30 (8.8) | 0.082 | 49 (14.4) | <0.001 | 18 (5.3) | 1.000 |
Data are presented as n (%).
*Thromboembolism included cardiovascular events (n=184), cerebrovascular events (n=129), pulmonary embolism (n=57) and deep vein thrombosis (n=166).
DOACs, direct oral anticoagulants.
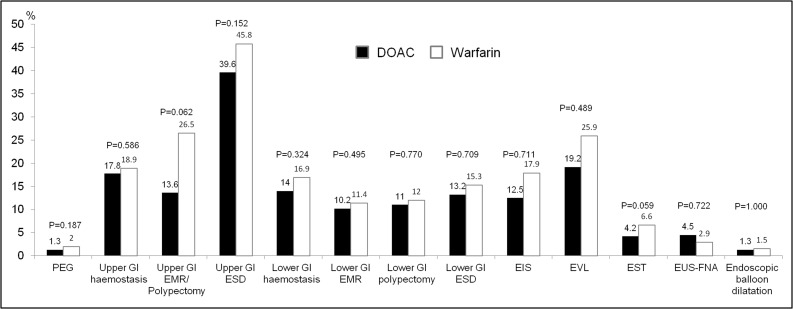
In the subanalyses of procedure types in the propensity-matched patients, the warfarin group had a higher proportion of GI bleeding than the DOAC group among patients who underwent EST (p=0.059) and upper GI EMR/polypectomy (p=0.062) (figure 1).
Figure 1.
Postendoscopy GI bleeding in patients treated with warfarin and DOACs by subgroups of 13 high-risk endoscopic procedures. DOACs, direct oral anticoagulants; EIS, endoscopic injection sclerotherapy; EMR, endoscopic mucosal resection; ESD, endoscopic submucosal dissection; EST, endoscopic sphincterotomy; EUS-FNA, endoscopic ultrasound-guided fine needle aspiration; EVL, endoscopic variceal ligation; PEG, percutaneous endoscopic gastrostomy.
After adjusting for high-risk endoscopic procedures, the warfarin group had an increased risk of GI bleeding (OR, 1.22; 95% CI, 1.07 to 1.39; p=0.003) among the propensity-matched patients (table 3). The increased risk of thromboembolism and death in the warfarin group was not statistically significant (table 3).
Table 3.
ORs for postendoscopy GI bleeding, thromboembolism and death in the warfarin group with reference to the DOAC group, adjusting for high-risk endoscopic procedures (n=10 092)
| Postendoscopy GI bleeding | Postendoscopy thromboembolism | Postendoscopy death | ||||
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Anticoagulants | ||||||
| DOACs | Reference | Reference | Reference | |||
| Warfarin | 1.22 (1.07 to 1.39) | 0.003 | 1.16 (0.97 to 1.39) | 0.099 | 1.15 (0.96 to 1.38) | 0.126 |
| Endoscopic procedures | ||||||
| PEG | Reference | Reference | Reference | |||
| Upper GI haemostasis | 13.6 (9.93 to 18.5) | <0.001 | 0.98 (0.76 to 1.26) | 0.871 | 0.68 (0.54 to 0.84) | <0.001 |
| Lower GI EMR | 7.27 (5.21 to 10.1) | <0.001 | 0.59 (0.43 to 0.81) | 0.001 | 0.06 (0.03 to 0.12) | <0.001 |
| EST | 3.45 (2.39 to 5.00) | <0.001 | 0.86 (0.65 to 1.14) | 0.285 | 0.18 (0.12 to 0.26) | <0.001 |
| Lower GI haemostasis | 11.0 (7.70 to 15.8) | <0.001 | 0.98 (0.68 to 1.42) | 0.920 | 0.43 (0.29 to 0.64) | <0.001 |
| Upper GI ESD | 45.2 (32.4 to 62.7) | <0.001 | 0.72 (0.47 to 1.10) | 0.126 | 0.08 (0.034 to 0.20) | <0.001 |
| Lower GI polypectomy | 7.83 (5.21 to 11.8) | <0.001 | 0.51 (0.29 to 0.88) | 0.016 | 0.02 (0.003 to 0.154) | <0.001 |
| Lower GI ESD | 10.0 (6.29 to 16.0) | <0.001 | 0.64 (0.32 to 1.27) | 0.202 | NA* | NA* |
| EUS-FNA | 2.32 (1.08 to 4.98) | 0.030 | 1.10 (0.63 to 1.93) | 0.743 | 0.80 (0.48 to 1.33) | 0.383 |
| Upper GI EMR/polypectomy | 14.69 (8.93 to 24.2) | <0.001 | 0.44 (0.16 to 1.20) | 0.109 | 0.06 (0.01 to 0.45) | 0.006 |
| Endoscopic balloon dilatation | 0.40 (0.06 to 2.95) | 0.372 | 0.66 (0.29 to 1.52) | 0.327 | 0.60 (0.30 to 1.18) | 0.137 |
| EVL | 17.62 (10.3 to 30.2) | <0.001 | 0.15 (0.02 to 1.08) | 0.060 | 0.97 (0.50 to 1.88) | 0.924 |
| EIS | 10.87 (4.85 to 24.3) | <0.001 | 1.31 (0.47 to 3.68) | 0.607 | 0.18 (0.02 to 1.32) | 0.092 |
*NA: no deaths occurred in association with any procedure.
DOACs, direct oral anticoagulants; EIS, endoscopic injection sclerotherapy; EMR, endoscopic mucosal resection; ESD, endoscopic submucosal dissection; EST, endoscopic sphincterotomy; EUS-FNA, endoscopic ultrasound-guided fine needle aspiration; EVL, endoscopic variceal ligation; PEG, percutaneous endoscopic gastrostomy.
The risks of GI bleeding, thromboembolism and death were greater in patients treated with warfarin plus heparin bridging or DOACs plus bridging than in patients treated with DOACs alone after adjusting for the 13 types of high-risk endoscopic procedures (table 4).
Table 4.
ORs for postendoscopy GI bleeding, thromboembolism and death in patients treated with warfarin alone, DOACs plus heparin bridging and warfarin plus heparin bridging with reference to patients treated with DOACs alone, adjusting for high-risk endoscopic procedures (n=10 092)
| Postendoscopy GI bleeding | Postendoscopy thromboembolism | Postendoscopy death | ||||
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Anticoagulants with and without heparin bridging | ||||||
| DOACs alone | Reference | Reference | Reference | |||
| Warfarin alone | 1.14 (0.91 to 1.43) | 0.250 | 1.27 (0.89 to 1.83) | 0.192 | 1.08 (0.79 to 1.48) | 0.619 |
| DOACs plus heparin bridging | 1.52 (1.25 to 1.85) | <0.001 | 2.60 (1.95 to 3.47) | <0.001 | 1.37 (1.05 to 1.79) | 0.023 |
| Warfarin plus heparin bridging | 1.69 (1.41 to 2.02) | <0.001 | 2.46 (1.86 to 3.26) | <0.001 | 1.53 (1.18 to 1.97) | 0.001 |
| Endoscopic procedures | ||||||
| PEG | Reference | Reference | Reference | |||
| Upper GI haemostasis | 14.9 (10.9 to 20.4) | <0.001 | 1.15 (0.89 to 1.49) | 0.275 | 0.73 (0.58 to 0.91) | 0.005 |
| Lower GI EMR | 7.10 (5.09 to 9.92) | <0.001 | 0.56 (0.41 to 0.78) | <0.001 | 0.06 (0.03 to 0.11) | <0.001 |
| EST | 3.47 (2.40 to 5.03) | <0.001 | 0.87 (0.65 to 1.16) | 0.334 | 0.18 (0.12 to 0.27) | <0.001 |
| Lower GI haemostasis | 12.4 (8.64 to 17.9) | <0.001 | 1.23 (0.85 to 1.78) | 0.283 | 0.47 (0.32 to 0.71) | <0.001 |
| Upper GI ESD | 45.3 (32.5 to 63.3) | <0.001 | 0.71 (0.46 to 1.10) | 0.123 | 0.08 (0.03 to 0.20) | <0.001 |
| Lower GI polypectomy | 7.64 (5.08 to 11.5) | <0.001 | 0.48 (0.28 to 0.84) | 0.010 | 0.02 (0.003 to 0.144) | <0.001 |
| Lower GI ESD | 9.78 (6.12 to 15.6) | <0.001 | 0.61 (0.31 to 1.22) | 0.161 | NA* | NA* |
| EUS-FNA | 2.26 (1.05 to 4.84) | 0.037 | 1.05 (0.60 to 1.85) | 0.866 | 0.78 (0.47 to 1.29) | 0.332 |
| Upper GI EMR/polypectomy | 15.2 (9.21 to 25.0) | <0.001 | 0.46 (0.17 to 1.27) | 0.135 | 0.06 (0.001 to 0.46) | 0.006 |
| Endoscopic balloon dilatation | 0.42 (0.06 to 3.10) | 0.398 | 0.72 (0.31 to 1.66) | 0.445 | 0.62 (0.31 to 1.23) | 0.170 |
| EVL | 19.7 (11.4 to 33.8) | <0.001 | 0.18 (0.03 to 1.30) | 0.089 | 1.06 (0.54 to 2.05) | 0.874 |
| EIS | 11.9 (5.31 to 26.8) | <0.001 | 1.58 (0.56 to 4.46) | 0.390 | 0.19 (0.03 to 1.41) | 0.105 |
*NA: no deaths occurred in association with any procedure.
DOACs, direct oral anticoagulants; EIS, endoscopic injection sclerotherapy; EMR, endoscopic mucosal resection; ESD, endoscopic submucosal dissection; EST, endoscopic sphincterotomy; EUS-FNA, endoscopic ultrasound-guided fine needle aspiration; EVL, endoscopic variceal ligation; PEG, percutaneous endoscopic gastrostomy.
With reference to the PEG group, a significantly higher risk of GI bleeding was associated with upper GI haemostasis, lower GI EMR, EST, lower GI haemostasis, upper GI ESD, lower GI polypectomy, lower GI ESD, EUS-FNA, upper GI EMR/polypectomy, EVL and EIS (tables 3 and 4). Compared with the PEG group, the risk of thromboembolism was significantly lower in association with lower GI EMR and lower GI polypectomy (tables 3 and 4). With reference to PEG, in-hospital mortality was significantly lower in association with upper GI haemostasis, lower GI EMR, EST, lower GI haemostasis, upper GI ESD and lower GI polypectomy (tables 3 and 4).
Discussion
In this study, we found that warfarin users had a significantly higher proportion of GI bleeding than did DOAC users in the propensity score-matched analyses. The risks of all adverse events were greater in patients treated with warfarin plus heparin bridging or DOACs plus bridging than in patients treated with DOACs alone. Compared with PEG, patients who underwent ESD, upper EMR/polypectomy and haemostatic procedures including EVL or EIS were at the highest risk of postprocedure GI bleeding among the 13 types of endoscopic procedures, whereas those who underwent lower GI EMR, lower GI polypectomy, EST or EUS-FNA were at moderate risk.
Why warfarin was associated with a higher risk of GI bleeding than were DOACs remains speculative. A possible explanation is that the slow onset/offset of the anticoagulant effect of warfarin may increase the risk of bleeding compared with DOACs, which exhibit rapid onset/offset of anticoagulation.4 5 In particular, the half-life of warfarin is approximately 40 hours with an average duration of anticoagulant activity ranging from 2 to 5 days,6 7 making it difficult for physicians to determine the optimal timing of endoscopic procedures. If the endoscopic procedure is started immediately after the temporary cessation of warfarin in the pre-endoscopic period, GI bleeding can occur. Consistent with our findings, a meta-analysis of Japanese patients with atrial fibrillation showed that patients treated with DOACs had a lower risk of GI bleeding than those treated with warfarin.23 Our results may be useful for decision making regarding switching from warfarin to DOACs before implementing high-risk endoscopic procedures.
Additionally, why warfarin plus heparin bridging showed the highest risk of thromboembolism is speculative, but it is possible that patients with a high risk of GI bleeding also have a risk of subsequent thromboembolism. In clinical practice, physicians attach weight to the bleeding risk once GI bleeding has occurred; in such cases, they stop heparin bridging or postpone the resumption of oral anticoagulants, which may cause thromboembolism. Another possible reason for this is that after warfarin has been replaced with heparin, frequent laboratory monitoring (INR or APTT) is required before and after endoscopy6 7; such monitoring may delay the resumption of warfarin, leading to a risk of thromboembolism. Our findings are consistent with those of previous studies showing that heparin bridging did not reduce thromboembolic events and led to a higher proportion of major bleeding compared with non-bridging.16 24 However, the patients in these studies mainly included those with a low-to-moderate risk of thromboembolism.16 24 A trial of heparin bridging for patients with a high risk of thromboembolism is ongoing.25
We have no data on bridging with low-molecular-weight heparin, which is widely used in Western countries. This is because only unfractionated heparin is covered by the public health insurance system in Japan and is permitted for use during endoscopic or surgical procedures.3 26 One prospective study showed no significant difference in major bleeding and thromboembolism between bridging with low-molecular-weight heparin and unfractionated heparin for patients with mechanical prosthetic heart valves undergoing long-term oral anticoagulant therapy, but the implications of applying this data to management in the periendoscopic period remain unknown.
In a subanalysis of DOAC types, we found that the warfarin group had a higher proportion of GI bleeding than the rivaroxaban group and apixaban group, but this proportion of bleeding was lower than for the dabigatran group. These results are consistent with the atrial fibrillation (AF) trial in Japanese patients,23 specifically the GI bleeding rate in the J ROCKET AF trial (warfarin, 2.3% vs rivaroxaban, 1.3%), the ARISTOTLE trial (warfarin, 3.4% vs apixaban, 1.3%) and the RE-LY trial (warfarin, 0.9% vs dabigatran, 1.8%). Conversely, in the more globally representative ROCKET trials, GI bleeding occurred less frequently in the warfarin group than in the rivaroxaban group.27 This discrepancy between Japanese patients and other patients from around the world might be attributed to ethnic differences in the GI bleeding risk or to healthcare divergence in the diagnosis of GI bleeding.
We estimated the risk of each procedure with reference to PEG because PEG was the most common among the 13 procedures, and post-PEG GI bleeding was assumed to be rare either with or without anticoagulation.28 Our results indicate that risk stratification according to the type of endoscopic procedure performed may be needed in patients taking oral anticoagulants. It is possible that ESD or EMR usually results in larger mucosal defects than polypectomy or EST, which presumably increases the risk of bleeding. In particular, ESD was associated with a higher risk of bleeding than EMR in our study. In agreement with this, a previous meta-analysis of 15 studies showed that ESD was associated with a higher proportion of procedure-related bleeding than was EMR.29 Generally, haemostatic procedures are indicated for acute GI bleeding. The reported rebleeding rate in patients with acute GI bleeding who are taking anticoagulants is high at 14%,30 which is similar to the finding in our study. We found that the proportions of postendoscopy GI bleeding in patients taking oral anticoagulants were 14.0%, 17.0%, 4.4% and 3.0% in those who underwent EIS, EVL, EST and EUS-FNA, respectively. Previous studies showed relatively lower proportions of procedure-related bleeding: 4.0%, 2.4%–5.7%, 2.0%–3.2% and 1.3%–6.0% in patients who underwent EIS, EVL, EST and EUS-FNA, respectively.6
Our study has several limitations. First, although propensity score matching was used to reduce bias in causal estimates due to observed differences between the warfarin and DOAC users, unmeasured confounders may have existed in this study. We failed to match some indications for oral anticoagulant use between the two groups because of a lack of data (eg, atrial fibrillation or valve disease). Second, the database did not include information on the INR, the performance or timing of drug cessation or resumption, lesion location and specific size, lesion morphology, lesion histopathology or endoscopists. Third, the recorded diagnoses and procedures in the DPC database have been cross-validated with chart reviews. A previous study showed that the specificity of recorded diagnoses exceeded 90%, while the sensitivity was relatively low. Both the sensitivity and specificity of recorded procedures exceeded 90% in the database.31 Fourth, GI bleeding and thromboembolism were defined as events within 30 days of the endoscopic procedure, but data for death were available only for the in-hospital period. Fifth, we could not differentiate between procedure-related GI bleeding and non-procedure-related GI bleeding after the procedure. Finally, some patients who were not using oral anticoagulants may have undergone endoscopic procedures on a day-care basis without being registered in the inpatient database. We used only data on patients whose oral anticoagulant therapy was continued at admission.
In conclusion, our nationwide study using propensity-matched analysis demonstrated that warfarin was associated with a higher risk of postendoscopy GI bleeding even after adjustment for 13 types of high-risk endoscopic procedures than were DOACs. All risks of adverse events were greater in patients treated with warfarin plus heparin bridging or DOACs plus bridging than in patients treated with DOACs alone. Patients who underwent ESD, EMR or haemostatic procedures were at higher risk of postprocedure GI bleeding, whereas those who underwent polypectomy, EST or EUS-FNA were at moderate risk.
Acknowledgments
The authors thank the staff in the Department of Clinical Epidemiology and Health Economics, School of Public Health, University of Tokyo, Tokyo, Japan. None of these contributors received any financial compensation.
Footnotes
Contributors: NN designed the study; HY, HM and KF collected the data; NN, RN and HY performed the statistical analysis; and NN, KW, JA and NU prepared the draft of the manuscript. NN and HY mainly edited the revised the manuscript. All authors read and edited the manuscript and approved the submitted version of the manuscript.
Funding: This work was supported by grants from the Ministry of Health, Labour and Welfare, Japan (grant numbers: H28-Policy-Designated-009 and H27-Policy-Strategy-011), the Japan Agency for Medical Research and Development, and Grants-in-Aid for Research from the National Center for Global Health and Medicine (29-2001). The funders played no role in the study design, data collection or analysis, decision to publish or preparation of the manuscript.
Competing interests: None declared.
Ethics approval: the Institutional Review Board at the University of Tokyo.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Acosta RD, Abraham NS, Chandrasekhara V, et al. . The management of antithrombotic agents for patients undergoing GI endoscopy. Gastrointest Endosc 2016;83:3–16. 10.1016/j.gie.2015.09.035 [DOI] [PubMed] [Google Scholar]
- 2. Veitch AM, Vanbiervliet G, Gershlick AH, et al. . Endoscopy in patients on antiplatelet or anticoagulant therapy, including direct oral anticoagulants: British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guidelines. Gut 2016;65:374–89. 10.1136/gutjnl-2015-311110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fujimoto K, Fujishiro M, Kato M, et al. . Guidelines for gastroenterological endoscopy in patients undergoing antithrombotic treatment. Dig Endosc 2014;26:1–14. 10.1111/den.12183 [DOI] [PubMed] [Google Scholar]
- 4. Abraham NS, Castillo DL. Novel anticoagulants: bleeding risk and management strategies. Curr. Opin. Gastroenterol 2013;29:676–83. [DOI] [PubMed] [Google Scholar]
- 5. Baron TH, Kamath PS, McBane RD. New anticoagulant and antiplatelet agents: a primer for the gastroenterologist. Clin Gastroenterol Hepatol 2014;12:187–95. 10.1016/j.cgh.2013.05.020 [DOI] [PubMed] [Google Scholar]
- 6. Kwok A, Faigel DO. Management of anticoagulation before and after gastrointestinal endoscopy. Am J Gastroenterol 2009;104:3085–97. 10.1038/ajg.2009.469 [DOI] [PubMed] [Google Scholar]
- 7. Phillips KW, Ansell J. Outpatient management of oral vitamin K antagonist therapy: defining and measuring high-quality management. Expert Rev Cardiovasc Ther 2008;6:57–70. 10.1586/14779072.6.1.57 [DOI] [PubMed] [Google Scholar]
- 8. Humbert X, Roule V, Chequel M, et al. . Non-vitamin K oral anticoagulant treatment in elderly patients with atrial fibrillation and coronary heart disease. Int J Cardiol 2016;222:1079–83. 10.1016/j.ijcard.2016.07.212 [DOI] [PubMed] [Google Scholar]
- 9. Comerota AJ, Ramacciotti E. A Comprehensive overview of direct oral anticoagulants for the management of venous thromboembolism. Am J Med Sci 2016;352:92–106. 10.1016/j.amjms.2016.03.018 [DOI] [PubMed] [Google Scholar]
- 10. Ruff CT, Giugliano RP, Braunwald E, et al. . Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–62. 10.1016/S0140-6736(13)62343-0 [DOI] [PubMed] [Google Scholar]
- 11. Holster IL, Valkhoff VE, Kuipers EJ, et al. . New oral anticoagulants increase risk for gastrointestinal bleeding: a systematic review and meta-analysis. Gastroenterology 2013;145:105–12. 10.1053/j.gastro.2013.02.041 [DOI] [PubMed] [Google Scholar]
- 12. Yoshio T, Nishida T, Kawai N, et al. . Gastric ESD under Heparin Replacement at High-Risk Patients of Thromboembolism Is Technically Feasible but Has a High Risk of Delayed Bleeding: Osaka University ESD Study Group. Gastroenterol Res Pract 2013;2013:1–7. 10.1155/2013/365830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Terasaki M, Tanaka S, Shigita K, et al. . Risk factors for delayed bleeding after endoscopic submucosal dissection for colorectal neoplasms. Int J Colorectal Dis 2014;29:877–82. 10.1007/s00384-014-1901-3 [DOI] [PubMed] [Google Scholar]
- 14. Inoue T, Nishida T, Maekawa A, et al. . Clinical features of post-polypectomy bleeding associated with heparin bridge therapy. Dig Endosc 2014;26:243–9. 10.1111/den.12123 [DOI] [PubMed] [Google Scholar]
- 15. Kien-Fong Vu C, Chang F, Doig L, et al. . A prospective control study of the safety and cellular yield of EUS-guided FNA or Trucut biopsy in patients taking aspirin, nonsteroidal anti-inflammatory drugs, or prophylactic low molecular weight heparin. Gastrointest Endosc 2006;63:808–13. 10.1016/j.gie.2005.09.033 [DOI] [PubMed] [Google Scholar]
- 16. Beyer-Westendorf J, Gelbricht V, Förster K, et al. . Peri-interventional management of novel oral anticoagulants in daily care: results from the prospective Dresden NOAC registry. Eur Heart J 2014;35:1888–96. 10.1093/eurheartj/eht557 [DOI] [PubMed] [Google Scholar]
- 17. Niikura R, Yasunaga H, Yamada A, et al. . Factors predicting adverse events associated with therapeutic colonoscopy for colorectal neoplasia: a retrospective nationwide study in Japan. Gastrointest Endosc 2016;84:971–82. 10.1016/j.gie.2016.05.013 [DOI] [PubMed] [Google Scholar]
- 18. Hamada T, Yasunaga H, Nakai Y, et al. . Bleeding after endoscopic sphincterotomy or papillary balloon dilation among users of antithrombotic agents. Endoscopy 2015;47:997–1004. 10.1055/s-0034-1392408 [DOI] [PubMed] [Google Scholar]
- 19. Sako A, Yasunaga H, Horiguchi H, et al. . Prevalence and in-hospital mortality of gastrostomy and jejunostomy in Japan: a retrospective study with a national administrative database. Gastrointest Endosc 2014;80:88–96. 10.1016/j.gie.2013.12.006 [DOI] [PubMed] [Google Scholar]
- 20. Brämer GR. International statistical classification of diseases and related health problems. Tenth revision. World Health Stat Q 1988;41:32–6. [PubMed] [Google Scholar]
- 21. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–63. 10.1016/S0140-6736(03)15268-3 [DOI] [PubMed] [Google Scholar]
- 22. D’Agostino RB. Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat. Med 1998;17:2265–81. [DOI] [PubMed] [Google Scholar]
- 23. Senoo K, Lau YC, Dzeshka M, et al. . Efficacy and safety of non-vitamin K antagonist oral anticoagulants vs. warfarin in Japanese patients with atrial fibrillation – meta-analysis. Circ J 2015;79:339–45. 10.1253/circj.CJ-14-1042 [DOI] [PubMed] [Google Scholar]
- 24. Douketis JD, Spyropoulos AC, Kaatz S, et al. . Perioperative Bridging Anticoagulation in Patients with Atrial Fibrillation. N Engl J Med Overseas Ed 2015;373:823–33. 10.1056/NEJMoa1501035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. BRIDGE Study Investigators. Bridging anticoagulation: is it needed when warfarin is interrupted around the time of a surgery or procedure? Circulation 2012;125:e496–e498. 10.1161/CIRCULATIONAHA.111.084517 [DOI] [PubMed] [Google Scholar]
- 26. Ono K, Hidaka H, Koyama Y, et al. . Effects of heparin bridging anticoagulation on perioperative bleeding and thromboembolic risks in patients undergoing abdominal malignancy surgery. J Anesth 2016;30:723–6. 10.1007/s00540-016-2187-0 [DOI] [PubMed] [Google Scholar]
- 27. Patel MR, Mahaffey KW, Garg J, et al. . Rivaroxaban versus Warfarin in Nonvalvular Atrial Fibrillation. N Engl J Med Overseas Ed 2011;365:883–91. 10.1056/NEJMoa1009638 [DOI] [PubMed] [Google Scholar]
- 28. Ruthmann O, Seitz A, Richter S, et al. . [Percutaneous endoscopic gastrostomy. Complications with and without anticoagulation]. Chirurg 2010;81:247–54. 10.1007/s00104-009-1718-8 [DOI] [PubMed] [Google Scholar]
- 29. Cao Y, Liao C, Tan A, et al. . Meta-analysis of endoscopic submucosal dissection versus endoscopic mucosal resection for tumors of the gastrointestinal tract. Endoscopy 2009;41:751–7. 10.1055/s-0029-1215053 [DOI] [PubMed] [Google Scholar]
- 30. Sengupta N, Feuerstein JD, Patwardhan VR, et al. . The risks of thromboembolism vs. recurrent gastrointestinal bleeding after interruption of systemic anticoagulation in hospitalized inpatients with gastrointestinal bleeding: a prospective study. Am J Gastroenterol 2015;110:328–35. 10.1038/ajg.2014.398 [DOI] [PubMed] [Google Scholar]
- 31. Yamana H, Moriwaki M, Horiguchi H, et al. . Validity of diagnoses, procedures, and laboratory data in Japanese administrative data. J Epidemiol 2017. 10.1016/j.je.2016.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]