Abstract
Background
Pain is a common and undertreated non-motor symptom in patients with Parkinson’s disease (PD). Opioids have been seldom used in PD because they could worsen cognitive and motor functions.
Objective
We aimed to assess efficacy and tolerability of tapentadol in PD patients.
Methods
We retrospectively reviewed 21 PD patients treated with tapentadol extended release (ER) for chronic pain. Patients were evaluated before treatment and at 3 and 6 months during treatment for pain intensity (current, 24-hour average, and minimum and worst) with a 0–10 Numerical Rating Scale and the painDETECT questionnaire; for motor symptom severity with the Unified PD Rating Scale part III and the Hoehn and Yahr scale; for cognitive functions with Mini-Mental Status Examination, Corsi’s Block-Tapping test, Digit Span test, Digit-Symbol Substitution test, FAS test, Rey’s Auditory Verbal Learning test, Trail-Making test A and B and the 9-Hole Peg test; for anxiety and depression with the Hospital Anxiety and Depression Scale; and for the quality of life with the Short Form-12. Data were analyzed by 1-way analysis of variance and paired t-test, and by Friedman’s and Wilcoxon’s tests. Statistical significance was taken in all cases as P<0.05.
Results
Pain intensity decreased over the course of treatment. No differences were found in PD symptom severity and dopaminergic drug dosages between pretreatment and treatment evaluations. No decrement in cognitive neuropsychological performances was found and an improvement was observed in Digit Span test, Digit-Symbol Substitution test, and FAS test. The levels of anxiety, depression, and quality of life improved. Overall, tapentadol ER was well tolerated and most patients reported no or mild and short-lived gastroenterological and neurological side effects.
Conclusion
These results indicate the potential efficacy and tolerability of medium–high doses of tapentadol ER for the treatment of pain in PD.
Keywords: Parkinson’s disease, pain, tapentadol, cognitive functions, motor functions
Introduction
Pain is one of the most common non-motor symptoms that impairs the quality of life in up to 80% of Parkinson disease (PD) patients.1–3 Isolated pain symptoms have been associated with a higher risk of developing PD and, as with other non-motor complaints, can precede PD motor symptoms by years.2,3
PD patients may present nociceptive musculoskeletal pain because of osteoarthritis of the spinal column and large joints or because of cramps, dystonia, and stiffness caused by PD itself. Furthermore, in contrast to the classical view of PD as a dopaminergic syndrome, Braak et al have proposed the concept that PD actually initiates at specific central nervous system sites and gradually evolves in distinct stages.4 In PD preclinical stages I and II, α-synuclein immunoreactive inclusions are found first in olfactory nuclei and bulbs, and then in brainstem monoaminergic nuclei of locus coeruleus and raphe, which project to the spinal dorsal horns to modulate pain processing; also, early neurodegenerative changes in PD involve nociceptive neurons in the lamina I of the spinal dorsal horns.4–6
In spite of its incidence, pain in PD is often underdiagnosed and most often treated by increasing dopaminergic drugs.7 However, not all types of pain show a clear response to dopaminergic therapy. In a recent study, the dopamine agonist rotigotine improved fluctuations related pain of the King’s PD pain scale but did not affect nocturnal, orofacial and radicular pain; rotigotine treatment was actually associated with worsening of the chronic pain domain of King’s PD pain scale.8 In PD patients treated with deep brain stimulation, no direct correlation was found between sensory/pain changes and motor improvement, suggesting that motor and non-motor symptoms of PD do not necessarily share the same mechanisms.9 Furthermore, musculoskeletal pain is the most common type of pain in PD occurring most frequently in low back, knee, and shoulder.10 These body sites often present arthritic abnormalities with advanced age; PD abnormal postures can be contributing factors to musculoskeletal pain.10
Hence, dopaminergic medications are partially effective in controlling PD pain and non-dopaminergic analgesic agents need to be investigated.7 Nonsteroidal anti-inflammatory drugs are often considered second-line treatment because of a higher risk of adverse cardiovascular, gastrointestinal, and renal events, especially in elderly patients.12 On the other hand, physicians are reluctant to prescribe opioids to PD patients because they may worsen motor and non-motor symptoms, such as constipation, hallucinations, and daytime sleepiness.7,11 However, in 2 recent (1 prospective and 1 randomized, placebo-controlled) studies, low doses of oxycodone/naloxone improved pain intensity in PD patients with no serious adverse effects.13,14 However, in the placebo-controlled study, gastroenterological side effects, such as nausea and constipation were found more frequently in the oxycodone- than in the placebo-treated group.14
Tapentadol is a relatively new opioid with reduced affinity to the µ-opioid receptor and with a serotonin/noradrenaline reuptake inhibitor activity.15 In contrast to morphine and oxycodone, tapentadol does not impair hippocampal neurogenesis, and has an improved profile of gastrointestinal and central nervous system side effects and a negligible risk of abuse.16–19 Also, because of its unique noradrenergic features, tapentadol may improve pain in PD by restoring the spinal noradrenergic inhibitory tone.15,20,21
To our knowledge, there are no studies on efficacy and tolerability of tapentadol in PD patients. Thus, the aim of this study was to report the effects of tapentadol on pain, motor symptoms, cognitive functions, and the quality of life in PD patients.
Patients and methods
Patients
We retrospectively analyzed PD patients who were treated with tapentadol for pain from June 2016 to June 2017, and who met the inclusion and exclusion criteria. The PD patient data were part of a longitudinal clinical dataset of the Pain Clinic of Padua University (Italy). Approval of the ethics committee was not required for the study because the Italian legislation that pertains to clinical research studies does not provide statements on observational studies on routinely collected, anonymous data. All patients signed an approved consent (DL17-09, Padua Hospital Company, Padua, Italy), which allows the anonymous use of their clinical data for research purposes.
Patients were included in the analysis if: 1) they met diagnosis of idiopathic PD according to UK Brain Bank criteria; 2) they presented pain lasting ≥3 months and with an average 24-hour score ≥4 measured on a 0–10 Numerical Rating Scale (NRS), despite optimal dopaminergic therapy; 3) they presented contraindications and/or lack of efficacy to non-steroidal anti-inflammatory drugs and paracetamol; 4) they were on stable L-Dopa dosage in the last month.22 Patients were not included if: 1) they had already undergone an opioid therapy in the last 6 months; 2) they presented uncontrolled psychiatric disorders requiring recent hospitalization; 3) they were on monoamine oxidase inhibitors therapy.
Patients were treated in accordance with drug-approved indications and local standard guidelines for treating chronic pain. Tapentadol extended release (ER) was titrated with the aim of finding, for each patient, the dose that would provide meaningful pain relief with acceptable side effects. Patients were started on tapentadol ER 25 mg twice a day for 7 days, then 50 mg twice a day for 7 days. Then, doses could be incremented by 50 mg every week. In the case of intolerable side effects, tapentadol was down-titrated by 50 mg per week.
Pain intensity, vital signs (i.e., pulse rate, diastolic and systolic blood pressure, body temperature, and respiratory rate), body weight, drug dosages, and side effects were determined at pretreatment baseline, weekly during dose titration, and then monthly when on a stable dose. Motor symptom severity, cognitive functions, levels of anxiety and depression, and quality of life were determined at pretreatment baseline and 3 and 6 months of treatment.
Pain
At each visit, patients were asked to rate their current pain, and their average, minimum, and worst pain in the last 24 hours, and their average and worst pain in the last month on a 0–10 NRS (0 = no pain, 10 = extreme pain), and were asked to respond to the painDETECT questionnaire, a screening measure for neuropathic pain.23 The painDETECT score ranges from −1 to 38, with scores ≥19 suggesting a high likelihood of neuropathic pain.23 At each treatment visit, the patients were also asked to rate their pain relief on a 0%–100% rating scale.
Parkinson’s disease
PD motor status and stage were assessed using the Unified Parkinson’s Disease Rating Scale (UPDRS) part III and the modified Hoehn and Yahr scale.24,25
Cognitive functions, mood and anxiety, and quality of life assessment
Cognitive status, mood level, and quality of life of PD patients were assessed using a battery of neuropsychological and psychiatric tests.
The Mini-Mental Status Examination (MMSE) is a validated instrument to assess global cognitive function.26 MMSE ranges from 0 to 30, with lower scores indicating greater cognitive impairment.26
The Corsi’s Block-Tapping (CBT) test evaluates a short-term visuospatial working memory.27 The patient is required to repeat sequences of increasing lengths of blocks, tapped by the examiner. CBT score ranges from 0 to 9, with higher scores corresponding to a better performance.27
The Digit Span (DS) test is a component of the Wechsler Intelligence Scale and assesses attention and verbal working memory.28 The patient is asked to repeat a series of numbers of increasing lengths, in both forward (direct) and backward (reverse) order.28 Scores range from 0 to 14 in each phase, with higher scores meaning better performance.28
The Digit-Symbol Substitution test (DSST) is a subscale of the Wechsler Adult Intelligence Scale, and is a measure of mental processing speed, sustained attention, and visual–spatial abilities.28 The patient is presented with numbers from 0 to 9 and is instructed to draw under each number the corresponding symbol using a key at the top of the page. DSST score ranges from 0 to 93, with a higher score indicating a better performance.28
The FAS test is a part of the Neurosensory Center Comprehensive Examination for Aphasia and assesses verbal fluency. The patient is requested to name as many words as possible that begin with the letters F, A, and S, within 1 minute.29 The score is the total number of words; higher scores indicate better performances.29
The Rey’s Auditory Verbal Learning test (RAVLT) is a neuropsychological test designed to evaluate short-term and long-term verbal memory; RAVLT requires encoding, storing, and retrieval of verbal material.30 RAVLT consists in 5 consecutive free recall trials and a 20-minute delayed recall trial, after an interference list. The RAVLT immediate recall score is the sum of words the patient recalled in the first 5 trials; RAVLT delayed recall score is the number of words recalled after a 20 minute delay. Higher scores mean better performances.30
The Trail-Making test A and B (TMTA and TMTB) are timed tests of complex visual scanning, motor speed, and mental flexibility consisting of 25 circles distributed on a sheet of paper. In TMTA, the circles contain numbers (1–25), and in TMTB, numbers (1–13) and letters (A–L). In TMTA, the patient is asked to draw a line connecting circles numbered in ascending order, 1–25. In TMTB, the patient is asked to draw lines connecting circles in ascending order, but alternating between numbers and letters, from 1 to A, then A to 2, 2 to B, and lastly from 12 to L.31 TMTA and TMTB scores are the time taken to complete the tasks, with lower time scores indicating better performances.31
The 9-Hole Peg test (9HPT) is a quantitative timed test of upper extremity function.32 The patient is instructed to pick up the 9 pegs, 1 at a time, as fast as possible and put them in the 9 holes of a wood block and, once they are all in the holes, to remove them again as fast as possible, 1 at a time, and place them back into the container.32 The score is the total time taken to place in, and remove, pegs from the block, with lower time scores indicating better performances.
The Hospital Anxiety and Depression Scale (HADS) is a self-report questionnaire consisting of 14 items, 7 items measure anxiety and 7 depression, weighted on a 4-point (0–3) severity Likert scale.33 The maximal score for each subscale is 21, with a higher score indicating worse condition and scores >11 indicating a clinically significant anxiety and/or depression.33
The 12-item Short Form Health Survey (SF-12) is an abbreviated version of SF-36 consisting of 12 items selected from the SF-36. The SF-12 questionnaire was developed to reproduce the 2 physical and mental component summary scores (SF-12 PCS and SF-12 MCS, respectively) and provides an overall health-related quality of life. Higher scores mean better quality of life.34
Tolerability
Patients were instructed on the potential side effects of tapentadol ER and, at each visit, they were asked whether they had experienced any gastrointestinal side effects such as nausea, vomiting, and constipation, and/or central nervous system side effects, such as dizziness, sedation, and mental confusion, or any new symptom. Constipation was evaluated also with the Bowel Function Index (BFI). BFI is based on 3 variables (i.e., ease of defecation, feeling of incomplete bowel evacuation, and personal judgment of constipation), which were assessed on a 0–100 NRS and then averaged. Then, information on use of prescribed and over-the-counter laxatives and stool softeners was requested in order to provide a behavioral measure of constipation.
Statistics
Data are presented as means ± SDs, 95% CI, and numbers (and percentages) of patients. Normality was assessed with the Kolmogorov–Smirnov test. Normally distributed data were analyzed by 1-way repeated-measures analysis of variance (ANOVA) and non-normally distributed data were analyzed with Friedman’s test (with a Bonferroni correction to adjust for multiple comparisons). When ANOVA was significant, pairwise comparisons were performed with the paired t-test. When Friedman’s test was significant, pairwise comparisons were performed using Wilcoxon’s signed rank test (with a Bonferroni correction). Categorical variables were assessed with a χ-square test. All statistical tests were performed in SPSS 17.0 (SPSS, Chicago, IL, USA). Differences were considered statistically significant for values of P<0.05.
Results
Demographics and drug dosages
Twenty-four PD patients (age 74.0±11.6 years; 11 males, 10 females; body mass index 26.2±3.1; education 8.9±4.8 years; PD duration 5.6±2.3 years) were treated with tapentadol ER between June 2016 and June 2017 (three patients withdrew). Two patients who discontinued treatment in the first week because of intolerable side effects and 1 patient who discontinued treatment in the second month because of lack of efficacy, were not included in the final analysis.
Comorbidities were type II diabetes (5 patients, 23%), hypertension (47%), depression (23%); active medical therapies were beta-blockers (2 patients, 10%), ACE-inhibitors (24%), Ca++−antagonists (19%), and antidepressants (19%).
The vital signs (i.e., pulse rate, systolic blood pressure, diastolic blood pressure, and respiratory rate) and body weight index presented minor, not significant changes during the 6-month treatment with tapentadol ER (data not shown).
All PD patients were on L-Dopa, 8 patients were also on pramipexol, and 1 patient on ropirinol. The mean L-Dopa dose at baseline was 467.3±245.8 mg/day and remained stable in 17 of 21 patients during treatment with tapentadol ER; at month 6 of treatment, the mean final dose of L-Dopa was 502.5±219.4 mg/day. The dose of tapetandol ER was increased during titration and adjusted during maintenance; mean doses of tapentadol ER were 71.3±26.2 and 87.5±25.6 mg/day at week 2 and month 1 of treatment, and 191.3±69.5 and 206.3±102.7 at months 3 and 6 of treatment.
Effects of tapentadol on pain
The most frequent pain sites were “limbs” (13 patients, 62%), “low back” (52%), and “neck” (14%). Pain was musculoskeletal nociceptive (7 patients, 33%), dystonic (14%), nocturnal (25%), and radicular (25%).
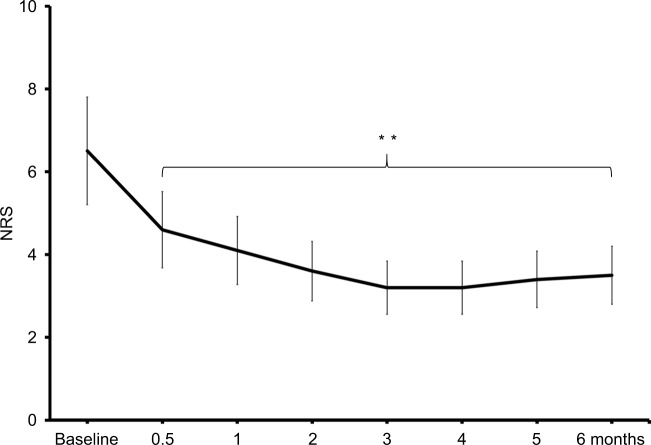
The pain intensity (i.e., current, and 24-hour average, least [not shown] and worst) measured on a 0–10 NRS decreased significantly (P<0.0001 for all measures) during treatment with tapentadol ER. The average 24-hour pain was 6.4±1.1 at pretreatment baseline (Figure 1) and its mean decreases from baseline to month 1, 3, and 6 of treatment were 2.8±1.3 (95% CI: 2.1 to 3.4, P=0.01), 3.1±2.0 (95% CI: 2.2 to 4.1, P<0.001), and 2.5±2.2 (95% CI: 1.0 to 3.4, P<0.001). At month 6 of treatment, 24-hour pain declined >50% in 10 patients, between 30% and 50% in 4 patients, and <30% in 7 patients. Similarly, the current pain NRS decreased from baseline (3.3±2.2) to month 3 of treatment (mean decrease ± SD 2.2±2.1, 95% CI: 1.2 to 3.2, P<0.001) and to month 6 of treatment (mean decrease ± SD 2.1±2.6, 95% CI: 0.5 to 2.9, P=0.021). A significant reduction was observed also in 24-hour worst pain NRS from pretreatment baseline (8.2±1.1) to treatment months 3 and 6 (mean reductions ± SD; 3.6±1.8, 95% CI: 2.7 to 4.4, P=0.002, and 3.5±2.1, 95% CI: 2.5 to 4.4, P=0.001).
Figure 1.
Mean 24-hour pain NRS in PD patients from pretreatment baseline assessment to 6-month treatment assessment; **P<0.01, different from baseline.
Abbreviations: NRS, numerical rating scale; PD, Parkinson’s disease.
The intensity of neuropathic symptoms decreased (P<0.0001) during tapentadol treatment (Table 1). The painDETECT score was 11.4±4.5 at pretreatment baseline and it declined significantly from baseline to month 3 (mean decrease ± SD 5.1±4.4, 95% CI: 3.1 to 7.1, P<0.0001) and from baseline to month 6 (5.1±4.9, 95% CI: 2.8 to 7.4, P<0.0001). At baseline, the painDETECT score was ≤12 (nociceptive pain) in 11 patients, 13–18 (mixed, nociceptive, and neuropathic pain) in 7 patients, and ≥19 (neuropathic pain) in 3 patients; at 6 months of treatment, painDETECT was <12 in 18 patients, 13–18 in 3 patients, and ≥19 (neuropathic) in no patients (P=0.006).
Table 1.
Cognitive and motor functions, anxiety and mood level, and quality of life in PD patients before and during 6 months of treatment with tapentadol
| Pretreatment | 3-month treatment | 6-month treatment | |
|---|---|---|---|
| Parkinson disease | |||
| Hoen-Yahr | 2.3±0.8 | 2.4±0.8 | 2.5±0.8 |
| UPDRS part III | 28.5±10.5 | 26.4±10.4 | 28.4±10.9 |
| Pain symptoms | |||
| 24 pain NRS | 6.4±1.1 | 3.1±2.0b | 2.4±2.2b |
| PainDETECT | 11.4±4.5 | 6.0±2.6c | 6.3±2.5c |
| Cognitive functions | |||
| MMSE | 26.8±1.8 | 26.7±1.9 | 26.3±2.2 |
| CBT | 5.0±0.9 | 5.2±1.1 | 5.2±1.2 |
| DS, forward | 5.0±1.0 | 6.3±1.1d | 6.3±1.4 |
| DS, backward | 3.0±0.9 | 3.4±1.1 | 3.6±1.0 |
| DSST | 24.4±8.8 | 26.3±8.9b | 27.3±9.9b |
| FAS test | 26.1±10.0 | 30.5±10.3c | 31.5±11.7c |
| RAVLT, immediate recall | 24.5±6.7 | 25.6±6.2c | 25.1±6.3 |
| RAVLT, delayed recall | 3.8±0.9 | 4.1±1.1 | 4.0±1.9 |
| TMTA | 60.8±25.5 | 56.0±24.1c | 54.6±26.0c |
| TMTB | 154.1±111.8 | 131.5±93.5c | 126.9±95.1c |
| 9HPT | 27.8±7.2 | 26.3±7.2 | 26.7±6.2 |
| Depression and anxiety | |||
| HADS anxiety | 6.6±3.2 | 5.3±2.8d | 5.5±3.2 |
| HADS depression | 6.5±3.3 | 4.5±2.2b | 3.7±2.3b |
| Quality of life | |||
| SF-12 PCS | 32.1±8.1 | 35.8±7.8d | 34.3±8.6 |
| SF-12 MCS | 42.3±8.8 | 46.1±7.7b | 45.6±7.9b |
Notes: Data are expressed as mean scores ± SD at pretreatment baseline and at treatment month 3 and 6. Different from pretreatment:
P<0.05,
P<0.01, 1-way ANOVA and paired t-test;
P<0.01,
P<0.05, Friedman’s test and Wilcoxon’s signed rank test.
Abbreviations: 9HPT, 9-Hole Peg test; ANOVA, analysis of variance; CBT, Corsi’s Block-Tapping test; DS, Digit Span; DSST, Digit-Symbol Substitution test; HADS, Hospital Anxiety and Depression Scale; MMSE, Mini-Mental Status Examination; NRS, Numerical Rating Scale; PD, Parkinson’s disease; RAVLT, Rey’s Auditory Verbal Learning test; SF-12 PCS, SF-12 physical component score; SF-12 MCS, SF-12 mental component score; TMTA, Trail-Making test A; TMTB, Trail-Making test B; UPDRS, Unified Parkinson’s Disease Rating Scale.
Effects of tapentadol on PD
The severity of motor symptoms and the stage of PD were not modified by tapentadol. There was no significant difference in scores of UPDRS part III and Hoen–Yahr scale from before to during tapentadol treatment (Table 1).
Effects of tapentadol on cognitive functions, mood and anxiety, and quality of life
The scores of MMSE, CBT, DS backward, RAVLT delayed recall, and 9HPT were unchanged during treatment (Table 1). The scores of DS forward, RAVLT immediate recall, HADS anxiety, and SF-12 PCS improved significantly (P<0.05) from baseline to treatment month 3 and the scores of TMTA, TMTB, DSST, FAS, HADS depression, and SF-12 MCS improved from baseline to treatment months 3 and 6 (Table 1). At the 6 months evaluation on TMTA, 16 of the 21 patients (76%) performed better, 4 worse (19%) and 1 (5%) the same as baseline; at the 6-month evaluation on DSST, 14 patients (67%) performed better, 2 (10%) worse, and 5 (23%) the same as baseline. The intensity of anxiety and depression symptoms decreased significantly (P<0.0001 for both measures) during treatment (Table 1). HADS scores of anxiety and depression were 7.3±3.4 and 6.8±3.2 at pretreatment baseline. HADS anxiety score decreased significantly from baseline to treatment month 3 (mean decrease ± SD; 2.4±2.6, 95% CI: 1.2 to 3.5, P=0.003; Table 1) and HADS depression score from baseline to treatment months 3 and 6 (mean decreases; 2.2±2.4, 95% CI: 1.1 to 3.3, P=0.002, and 2.9±3.1, 95% CI: 1.4 to 3.4, P<0.001; Table 1). Compared to the baseline, at treatment month 6, the numbers of patients with significant (HADS >11) anxiety (52% vs 14%, P=0.001) and/or depression (43% vs 14%, P=0.01) decreased significantly.
Self-rated quality of life improved during treatment with tapentadol ER (Table 1); SF-12 PCS increased from baseline to month 3 (mean increase ± SD; 4.3±4.2, 95% CI: 2.4 to 5.0, P=0.002) and the SF-12 MCS from baseline to months 3 and 6 (4.3±4.5, 95% CI: 2.0 to 5.5, P=0.001; 3.1±4.5, 95% CI: 1.0 to 4.3, P=0.01).
Tolerability
Tapentadol ER was well tolerated; adverse events led to treatment discontinuation in 2 patients and were of low to moderate intensity in the remaining 21 patients. However, at least 1 transient adverse event was reported by 10 out of 21 patients (47%); 6 patients (29%) reported nausea, 3 reported dizziness (14%), 3 sedation/somnolence (14%), and 2 patients (10%) pruritus. Tapentadol ER did not worsen PD symptoms (see aforementioned). Bowel function as assessed by BFI was not altered (P=0.12) during treatment; mean BFI was 28.2±9.4 at baseline and 29±9.1 and 30±9.4 at treatment months 3 and 6.
Discussion
This is, to our knowledge, the first report on efficacy and tolerability of tapentadol ER in PD. In this retrospective analysis, tapentadol was effective on pain and well tolerated in PD patients. Tapentadol provided a clinically significant and sustained pain relief in most patients. During treatment with tapentadol, cognitive, and motor functions were unchanged or improved and mood level and quality of life improved. The side effect profile of tapentadol, and especially the incidence of gastrointestinal, and central nervous system, symptoms were similar to those reported in previous trials of tapentadol for musculoskeletal pain and of oxycodone/naloxone for pain in PD.13,14,17,18
Tapentadol ER produced significant relief in 24-hour average pain already at 1 month of treatment (mean decrease from baseline ± SD, 2.8±1.3) and the analgesic relief was sustained. At months 3 and 6 of treatment, 24-hour pain declined by ≥30% in 67% and 43% of patients. In a previous observational study, oxycodone/naloxone 5/2.5 mg twice a day produced >30% pain relief after 2 months of treatment (mean pain NRS decrease 2.31±0.52).13 In a randomized, double-blind, placebo-controlled trial, although it was not superior to placebo at the primary 16 weeks outcome, oxyco-done/naloxone (mean daily dose 18.8±8.4 mg) reduced pain and yielded higher rates of responders than placebo.14 At week 16 of treatment, responders (>30% decrease from baseline) were 48% in the oxycodone/naloxone group and 34% in the placebo group.14 In our study, we used a maintenance daily dose of tapentadol of 206.3±102.7 mg that is within the dose range of tapentadol for musculoskeletal pain but is, in terms of opioid equivalent dose, substantially higher than doses of oxycodone studied in PD insofar.13,14,17,18,35 These findings suggest that some PD patients may tolerate and benefit from doses of opioids higher than previously reported.
PD and chronic pain states have both been associated with impaired cognition. The combined impact on cognition by PD, chronic pain, and chronic opioid therapy has yet to be established and is an important issue because of the reported aggravating effects of opioids on PD.7,10 Consistent with previous reports on oxycodone/naloxone, tapentadol ER did not alter measures of global cognition.13,14 In this study, tapentadol ER did not impair cognitive functions in PD in neuropsychological tests for immediate and delayed verbal memory, spatial memory (i.e., CBT, DS, RAVLT), and hand dexterity (i.e., 9HPT). Furthermore, a statistically significant improvement was observed in cognitive tests of verbal fluency (i.e., FAS), psychomotor speed and pattern recognition (i.e., DSST), and psychomotor speed and set shifting (i.e., TMTA and TMTB). These findings are in agreement with studies showing that stable doses of opioids do not have a negative impact on cognition and may actually improve cognitive performances in patients with malignant cancer pain and non-malignant musculoskeletal pain.36–39 As pain itself can significantly impair cognition in healthy subjects and patients, the cognitive improvement reported here may be due to pain relief.40,41 As this study is limited by its retrospective design and the lack of a control group, a placebo effect and/or a practice effect cannot be ruled out. Treatment with tapentadol ER was associated with improvement also of both physical and mental component scores of the SF-12. Patients rated higher in their physical and mental abilities during tapentadol treatment than at baseline. The improvement of pain symptoms was associated also to decrease of HADS anxiety and depression scores both in patients with clinical depression and in patients with minor depressive symptoms. Compared to baseline, at month 6 assessment, the numbers of patients with significant (HADS >11) anxiety (52% vs 14%) and/or depression (43% vs 14%) decreased significantly.
The retrospective open-label design, the small number of our patient group, and the lack of a control group, represent obvious and important limitations of this study. Although some studies found only a low placebo effect in PD, there is ample evidence for a large placebo effect that can be estimated in ~30% of the therapeutic response, both in chronic pain and PD patients.14,42,43 However, in the present study, the magnitude of response to tapentadol was significantly higher: 67% and 43% of PD patients reported >30% pain reduction after 3 and 6 months of treatment, respectively.
This study had exploratory aims and further studies are warranted to confirm this first evidence on the efficacy and tolerability of tapentadol ER in PD. However, these findings support the idea that PD patients may benefit in terms of analgesia and quality of life from tapentadol in a dose range effective for chronic musculoskeletal pain.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Giuffrida R, Vingerhoets FJ, Bogousslavsky J, Ghika J. Pain in Parkinson’s disease. Rev Neurol (Paris) 2005;161:407–418. doi: 10.1016/s0035-3787(05)85070-2. [DOI] [PubMed] [Google Scholar]
- 2.Lin CH, Wu RM, Chang HY, Chiang YT, Lin HH. Preceding pain symptoms and Parkinson’s disease: a nationwide populationbased cohort study. Eur J Neurol. 2013;20(10):1398–1404. doi: 10.1111/ene.12197. [DOI] [PubMed] [Google Scholar]
- 3.Schrag A, Horsfall L, Walters K, Noyce A, Petersen I. Prediagnostic presentations of Parkinson’s disease in primary care: a case-control study. Lancet Neurol. 2015;14(1):57–64. doi: 10.1016/S1474-4422(14)70287-X. [DOI] [PubMed] [Google Scholar]
- 4.Braak H, Sastre M, Bohl JR, de Vos RA, Del Tredici K. Parkinson’s disease: lesions in dorsal horn layer I, involvement of parasympathetic and sympathetic pre- and postganglionic neurons. Acta Neuropathol. 2007;113(4):421–429. doi: 10.1007/s00401-007-0193-x. [DOI] [PubMed] [Google Scholar]
- 5.Scherder E, Wolters E, Polman C, Sergeant J, Swaab D. Pain in Parkinson’s disease and multiple sclerosis: its relation to the medial and lateral pain systems. Neurosci Biobehav Rev. 2005;29(7):1047–1056. doi: 10.1016/j.neubiorev.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Del Tredici K, Braak H. Idiopathic Parkinson’s disease: staging an alphasynucleinopathy with a predictable pathoanatomy. In: Kahle P, Haass C, editors. Molecular Mechanisms in Parkinson’s Disease. Georgetown, TX, USA: Landes Bioscience; 2004. pp. 1–32. [Google Scholar]
- 7.Brefel-Courbon C, Grolleau S, Thalamas C, et al. Comparison of chronic analgesic drugs prevalence in Parkinson’s disease, other chronic diseases and the general population. Pain. 2009;141(1–2):14–18. doi: 10.1016/j.pain.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 8.Rascol O, Zesiewicz T, Chaudhuri KR, et al. Randomized controlled exploratory pilot study to evaluate the effect of rotigotine transdermal patch on Parkinson’s disease-associated chronic pain. J Clin Pharmacol. 2016;56(7):852–861. doi: 10.1002/jcph.678. [DOI] [PubMed] [Google Scholar]
- 9.Cury RG, Galhardoni R, Teixeira MJ, et al. Subthalamic deep brain stimulation modulates conscious perception of sensory function in Parkinson’s disease. Pain. 2016;157(12):2758–2765. doi: 10.1097/j.pain.0000000000000697. [DOI] [PubMed] [Google Scholar]
- 10.Lien WH, Lien WC, Kuan TS, Wu ST, Chen YT, Chiu CJ. Parkinson disease and musculoskeletal pain: an 8-year population-based cohort study. Pain. 2017;158(7):1234–1240. doi: 10.1097/j.pain.0000000000000904. [DOI] [PubMed] [Google Scholar]
- 11.Karras B, McKee N, Regier L, Stone S. Opioids for chronic noncancer pain in the elderly: an osteoarthritis case. Can Fam Physician. 2011;57(8):907–911. [PMC free article] [PubMed] [Google Scholar]
- 12.Berg D, Becker G, Reiners K. Reduction of dyskinesia and induction of akinesia induced by morphine in two parkinsonian patients with severe sciatica. J Neural Transm (Vienna) 1999;106(7–8):725–728. doi: 10.1007/s007020050192. [DOI] [PubMed] [Google Scholar]
- 13.Madeo G, Schirinzi T, Natoli S, et al. Efficacy and safety profile of prolonged release oxycodone in combination with naloxone (OXN PR) in Parkinson’s disease patients with chronic pain. J Neurol. 2015;262(9):2164–2170. doi: 10.1007/s00415-015-7823-3. [DOI] [PubMed] [Google Scholar]
- 14.Trenkwalder C, Chaudhuri KR, Martinez-Martin P, et al. PANDA study group Prolonged-release oxycodone-naloxone for treatment of severe pain in patients with Parkinson’s disease (PANDA): a double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2015;14(12):1161–1170. doi: 10.1016/S1474-4422(15)00243-4. [DOI] [PubMed] [Google Scholar]
- 15.Tzschentke TM, Christoph T, Kögel B, et al. (−)-(1R,2R)-3-(3-dimethylamino-1-ethyl-2-methyl-propyl)-phenol hydrochloride (tapentadol HCl): a novel mu-opioid receptor agonist/norepinephrine reuptake inhibitor with broad-spectrum analgesic properties. J Pharmacol Exp Ther. 2007;323(1):265–276. doi: 10.1124/jpet.107.126052. [DOI] [PubMed] [Google Scholar]
- 16.West NA, Severtson SG, Green JL, Dart RC. Trends in abuse and misuse of prescription opioids among older adults. Drug Alcohol Depend. 2015;149:117–121. doi: 10.1016/j.drugalcdep.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 17.Baron R, Jansen JP, Binder A, et al. Tolerability, safety, and quality of life with tapentadol prolonged release (PR) compared with oxycodone/naloxone PR in patients with severe chronic low back pain with a neuropathic component: a randomized, controlled, open-label, phase 3b/4 Trial. Pain Pract. 2016;16(5):600–619. doi: 10.1111/papr.12361. [DOI] [PubMed] [Google Scholar]
- 18.Baron R, Eberhart L, Kern KU, et al. Tapentadol prolonged release for chronic pain: a review of clinical trials and 5 years of routine clinical practice data. Pain Pract. 2017;17(5):678–700. doi: 10.1111/papr.12515. [DOI] [PubMed] [Google Scholar]
- 19.Bortolotto V, Grilli M. Opiate analgesics as negative modulators of adult hippocampal neurogenesis: potential implications in clinical practice. Front Pharmacol. 2017;8:254. doi: 10.3389/fphar.2017.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rickli A, Liakoni E, Hoener MC, Liechti ME. Opioid-induced inhibition of the human 5-HTand noradrenaline transporters in vitro: link to clinical reports of serotonin syndrome. Br J Pharmacol. 2018;175(3):532–543. doi: 10.1111/bph.14105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao LF, Peng XY, Huang Y, et al. Restoring spinal noradrenergic inhibitory tone attenuates pain hypersensitivity in a rat model of Parkinson’s disease. Neural Plast. 2016;2016:6383240. doi: 10.1155/2016/6383240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinicopathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freynhagen R, Baron R, Gockel U, Tölle TR. PainDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22(10):1911–1912. doi: 10.1185/030079906X132488. [DOI] [PubMed] [Google Scholar]
- 24.Fahn S, Elton RL. Members of the UPDRS Development Committee. Unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Calne D, Holstein N, editors. Recent Developments in Parkinson’s Disease. Vol. 2. Macmillian Healthcare Information; Plorhan Park, NJ, USA: 1987. pp. 153–163. [Google Scholar]
- 25.Goetz CG, Poewe W, Rascol O, et al. Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease Movement disorder society task force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord. 2004;19(9):1020–1028. doi: 10.1002/mds.20213. [DOI] [PubMed] [Google Scholar]
- 26.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 27.Corsi PM. Human memory and the medial temporal region of the brain. Diss Abstr Int. 1972;34:891B. [Google Scholar]
- 28.Wechsler D. Manual for the Wechsler Adult Intelligence Scale, Revised. New York, NY, USA: Psychological Corporation; 1981. [Google Scholar]
- 29.Borkowski JG, Benton AL, Spreen O. Word fluency and brain damage. Neuropsychologia. 1967;5:135–140. [Google Scholar]
- 30.Bean J. Rey Auditory Verbal Learning Test, Rey AVLT. In: Kreutzer JS, DeLuca J, Caplan B, editors. Encyclopedia of Clinical Neuropsychology. Springer; New York, NY, USA: 2011. [Google Scholar]
- 31.Lezak MD. Neuropsychological Assessment. 2nd Edition. New York, NY, USA: Oxford University Press; 1983. [Google Scholar]
- 32.Earhart GM, Cavanaugh JT, Ellis T, Ford MP, Foreman KB, Dibble L. The 9-hole PEG test of upper extremity function: average values, testretest reliability, and factors contributing to performance in people with Parkinson disease. J Neurol Phys Ther. 2011;35(4):157–163. doi: 10.1097/NPT.0b013e318235da08. [DOI] [PubMed] [Google Scholar]
- 33.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 34.Kodraliu G, Mosconi P, Groth N, et al. Subjective health status assessment: evaluation of the Italian version of the SF-12 health survey. Results from the MiOS project. J Epidemiol Biostat. 2001;6(3):305–316. doi: 10.1080/135952201317080715. [DOI] [PubMed] [Google Scholar]
- 35.Santos J, Alarcão J, Fareleira F, Vaz-Carneiro A, Costa J. Tapentadol for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2015;5:CD009923. doi: 10.1002/14651858.CD009923.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Menefee LA, Frank ED, Crerand C, et al. The effects of transdermal fentanyl on driving, cognitive performance, and balance in patients with chronic nonmalignant pain conditions. Pain Med. 2004;5(1):42–49. doi: 10.1111/j.1526-4637.2004.04005.x. [DOI] [PubMed] [Google Scholar]
- 37.Panjabi SS, Panjabi RS, Shepherd MD, Lawson KA, Johnsrud M, Barner J. Extended-release, once-daily morphine (Avinza) for the treatment of chronic nonmalignant pain: effect on pain, depressive symptoms, and cognition. Pain Med. 2008;9(8):985–993. doi: 10.1111/j.1526-4637.2008.00483.x. [DOI] [PubMed] [Google Scholar]
- 38.Jamison RN, Schein JR, Vallow S, Ascher S, Vorsanger GJ, Katz NP. Neuropsychological effects of long-term opioid use in chronic pain patients. J Pain Symptom Manage. 2003;26(4):913–921. doi: 10.1016/s0885-3924(03)00310-5. [DOI] [PubMed] [Google Scholar]
- 39.Rowbotham MC, Twilling L, Davies PS, Reisner L, Taylor K, Mohr D. Oral opioid therapy for chronic peripheral and central neuropathic pain. N Engl J Med. 2003;348(13):1223–1232. doi: 10.1056/NEJMoa021420. [DOI] [PubMed] [Google Scholar]
- 40.Lorenz J, Bromm B. Event-related potential correlates of interference between cognitive performance and tonic experimental pain. Psychophysiology. 1997;34(4):436–445. doi: 10.1111/j.1469-8986.1997.tb02387.x. [DOI] [PubMed] [Google Scholar]
- 41.Mazza S, Frot M, Rey AE. A comprehensive literature review of chronic pain and memory. Prog Neuropsychopharmacol Biol Psychiatry. 2017 Aug 8; doi: 10.1016/j.pnpbp.2017.08.006. Epub. [DOI] [PubMed] [Google Scholar]
- 42.Safety and efficacy of pramipexole in early Parkinson’s disease A randomized dose-ranging study. Parkinson Study Group. JAMA. 1997;278(2):125–130. doi: 10.1001/jama.1997.03550020057038. [DOI] [PubMed] [Google Scholar]
- 43.de la Fuente-Fernández R, Stoessl AJ. The placebo effect in Parkinson’s disease. Trends Neurosci. 2002;25(6):302–306. doi: 10.1016/s0166-2236(02)02181-1. [DOI] [PubMed] [Google Scholar]