Abstract
Objective
TMEM119 is a member of transmembrane proteins family, which is abnormally expressed in human cancers and associated with tumorigenesis. In this study, we focused on the expression of TMEM119 and its role in cell invasion and migration in gastric cancer.
Methods
Real-time polymerase chain reaction, Western blotting, and immunohistochemistry were performed to examine the expression of TMEM119 in gastric cancer tissues and cell lines. After transfection with TMEM119 siRNA or recombined TMEM119-expressing vector, the invasion and migration ability of MKN45 and SGC-7901 cells was measured by transwell assay. The expression of TMEM119, p-STAT3, STAT3, VEGF, MMP2, and MMP9 proteins in SGC-7901 and MKN45 cells treated with TMEM119 siRNA, TMEM119-expressing vector, or AG490 was measured by Western blotting.
Results
We found that higher TMEM119 expression was found in gastric cancer tissues and cell lines and was associated with lower survival rate. TMEM119 knockdown inhibited SGC-7901 cell invasion and migration, along with the expression of p-STAT3, VEGF, MMP2, and MMP9. TMEM119 overexpression promoted MKN45 cell invasion and migration, along with the expression of p-STAT3, VEGF, MMP2, and MMP9. Additionally, AG490 treatment significantly corrected TMEM119-induced MKN45 cell migration and invasion and expression of p-STAT3, VEGF, MMP9, and MMP2 proteins.
Conclusion
The results indicated that TMEM119 promotes gastric cancer cell migration and invasion through activation of STAT3 signaling pathway, and TMEM119 may therefore act as a novel therapeutic target for gastric cancer.
Keywords: gastric cancer, TMEM119, migration, invasion, STAT3, VEGF, MMP2, MMP9
Introduction
Gastric cancer is one of the most common tumors and the third leading cause of death among malignant tumors in the world.1 About 70% of new cases of and death from gastric cancer occur each year in East Asia, with China accounting for 42% of such cases.2,3 In recent years, with improvement of people’s health conditions and dietary habits, the incidence of gastric cancer has declined. However, owing to the high metastasis and high recurrence rate of gastric cancer, the overall survival time of patients has changed significantly.4 The 5-year survival rate of advanced gastric cancer is still lower than 20%.5 Traditional treatment methods for gastric cancer, such as surgery, radiotherapy, and chemotherapy, failed to significantly improve the prognosis of gastric cancer patients.
Clinical epidemiology research reported that invasion and migration of cancer cells are the important reasons behind the high mortality rate of gastric cancer,6 and some genes are of great importance in the regulation of this invasion and migration.7 Although some progress has been made in the targeted therapy of these genes, because of the heterogeneity of tumor formation, interaction among multiple genes, and limitations of targeted drug carriers, the targeted therapy of gastric cancer is not effective.8,9 Therefore, it is critical to find new therapeutic targets and to provide reliable theoretical basis for further research on the mechanism of migration and invasion of gastric cancer cells.
TMEM119 is a member of transmembrane proteins (TMEMs) family that exists in the total biological membrane. Biological functions implicated that many TMEM members contributed to cancer development, including TMEM25, TMEM30B, TMEM39A, TMEM45A, TMEM127, and TMEM176.10–13 TMEM119, also known as osteoblast induction factor, has been reported to be associated with osteoblast differentiation through BMP2-RUNX214 and ATF4/RUNX2/Osterix signaling pathways.15 High TMEM119 expression was associated with poor outcomes in terms of overall survival in patients with prostate cancer.16 TMEM119 was found to be overexpressed in borderline/malignant compared with benign phyllodes tumors of the breast, suggesting that TMEM119 is associated with the progression of breast cancer.17 TMEM119 was downregulated in genetically unstable oral squamous cell carcinoma compared with normal fibroblasts.18 Moreover, TMEM119 was highly expressed in osteosarcoma, was associated with poor survival of osteosarcoma patients, and promoted osteosarcoma cell migration and invasion through TGF-β/BMP signaling.19 However, the functions of TMEM119 in gastric cancer still need to be explored.
STAT3 modulates the transcription of a wide variety of regulatory genes involved in cell apoptosis, differentiation, proliferation, migration, and other critical cellular functions. The amentoflavone analogues inhibited melanoma cell viability and induced apoptosis by suppressing the phosphorylation of STAT3.20 The benzofuran-conjugated metal complex was identified as the STAT3 inhibitor showing the antiproliferative activity in the prostate cell lines.21 AG490 that blocks activation of STAT3 by inhibiting the upstream kinase JAK2 is another STAT3 inhibitor widely used for testing the role of STAT3 in tumorigenic growth and metastasis of gastric cancer cells.22–24
In the present study, TMEM119 was overexpressed in gastric cancer tissues and cell lines and was associated with poor prognostic outcomes. Knockdown of TMEM119 inhibited gastric cancer cell migration and invasion as well as p-STAT3, VEGF, MMP2, and MMP9 protein expression. Importantly, blocking STAT3 signaling pathway inhibited TMEM119-induced cell migration and invasion of gastric cancer and corrected TMEM119-induced increased expression of VEGF, MMP2, and MMP9. Our results demonstrate an important role of TMEM119 in the regulation of gastric cancer cell migration and invasion.
Materials and methods
Clinical samples
Tumor tissues and corresponding non-tumorous normal gastric tissues were harvested from 78 patients (17 females and 61 males) with gastric cancer treated at Zhejiang Hospital, aged between 28 and 78 years (median, 53 years), who were in stage I (n = 14), II (n = 36), IIIa (n = 24), and IIIb (n = 4) of the disease according to the criteria of the TNM classification system of malignant tumors (UICC, 1987).42 All tissues were stored at −80°C until being analyzed. The study was approved by the ethics committee of Zhejiang Hospital. Written informed consent was obtained from all patients according to the guidelines of ethics committee.
Bioinformatics analysis
Survival rate data were downloaded from GEO (access id: GSE26253). Overall survival in relation to TMEM119 expression was evaluated by the Kaplan–Meier survival curve and the log-rank nonparametric test.
Cell culture
The human gastric cancer cell lines, including AGS, SGC-7901, and MKN45, and GES-1 gastric epithelial cell lines were obtained from American Type Culture Collection (ATCC; Manassas, VA, USA), grown in RMPI-1640 (Hyclone, Logan, UT, USA) containing 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% fetal bovine serum separately, and incubated at 37°C in a humidified chamber with 5% CO2.
Cell transfection and AG490 treatment
siRNA targeting human TMEM119 mRNA (5′-UGGGA UAGUGGACUUCUUC-3′) and a nonspecific scramble siRNA sequence (control siRNA; 5′-UUCUCCGAACG UGUCACGU-3′) were synthesized and transiently trans-fected into SGC-7901 cells using Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instruction. TMEM119 coding sequence was cloned into the pLVX-Puro lentiviral vector. Briefly, 293T cells were grown in six-well plate and transfected with pLVX-Puro-TMEM119 or blank pLVX-Puro vector at 37°C for 5 h using Lipofectamine reagent (Thermo Fisher Scientific) according to the manufacturer’s instruction. After 48 h of transfection, recombined lentivirus vectors were collected and used for infecting MKN45 cells. MKN45 cells transfected or not transfected with pLVX-Puro-TMEM119 were treated with 30 μM AG490 for 48 h.
Transwell assay
After TMEM119 siRNA or pLVX-Puro-TMEM119 transfection, cells were serum-starved in serum-free RPMI-1640 medium for 24 h, and cell suspension (5×104 cells/well) was plated in the transwell chamber coated with (invasion assay) or without Matrigel (migration assay). The lower chamber was filled with 700 μL of RPMI-1640 medium supplemented with 10% fetal bovine serum. After 48 h of incubation, cells were fixed, stained, photographed, and counted under a ×200 light microscope (Olympus Corporation, Tokyo, Japan).
Real-time polymerase chain reaction (PCR)
Total RNA was collected from gastric cancer cell lines and fetal gastric epithelial cell line by using Trizol reagent (Thermo Fisher Scientific). cDNA was synthesized using a PrimeScript reagent kit (DRR037A; TaKaRa, Otsu, Japan) according to the manufacturer’s instructions. Real-time quantitative PCR using SYBR Green (Takara) was performed using the GeneAmp PCR Systems 2700 (Applied Biosystems). The primers used in the present study were as follows: TMEM119-F, 5′-GAGGCACTCTACGGAAAC-3′ and TMEM119-R, 5′-CGGGAGAATCGCTTGAAC-3′; GAPDH-F, 5′-CACCCACTCCTCCACCTTTG-3′ and GAPDH-R, 5′-CCACCACCCTGTTGCTGTAG-3′. GAPDH level was used as internal control. The relative quantification was calculated using 2−∆∆Ct cycle threshold method.
Western blot analysis
Cell total protein was extracted using a total protein extraction buffer (Beyotime Biotechnology, Shanghai, People’s Republic of China). Fifteen microliters of protein was electrophoretically separated on a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (EMD Millipore, Burlington, MA, USA). The blots were incubated with antibodies to TMEM119, p-STAT3, STAT3, VEGF, MMP2, MMP9, and GAPDH overnight and subsequently incubated with secondary antibody. The blots were used to visualize the proteins using enhanced chemiluminescence reagents (Thermo Fisher Scientific).
Immunohistochemistry (IHC)
Tumor tissues from gastric cancer patients were extracted and prepared for immunohistochemical studies as described in a previous study.25 Following incubation with goat anti-mouse horseradish peroxidase-conjugated IgG, the sections were stained with anti-TMEM119 primary antibody for 1 h at room temperature. For negative controls, Tris-buffered saline was used instead of primary antibody. Slides were stained with DAB (Shanghai Long Island Biotec. Co. Ltd, Shanghai, People’s Republic of China) and hematoxylin (Sigma-Aldrich Co., St Louis, MO, USA). The fields from each slide were analyzed under a microscope (×400), and pictures were taken using an optical microscope with camera (Olympus BX-50; Olympus Optical, Tokyo, Japan).
Statistical analyses
All the results are presented as mean ± standard deviation, and each test was repeated at least three times. All statistical analyses were carried out with the GraphPad Prism software using one-way analysis of variance followed by Tukey’s post hoc test. A P-value of <0.05 was considered to show a significant difference between two groups.
Results
Higher TMEM119 expression is observed in gastric cancer tissues and associated with poor prognosis
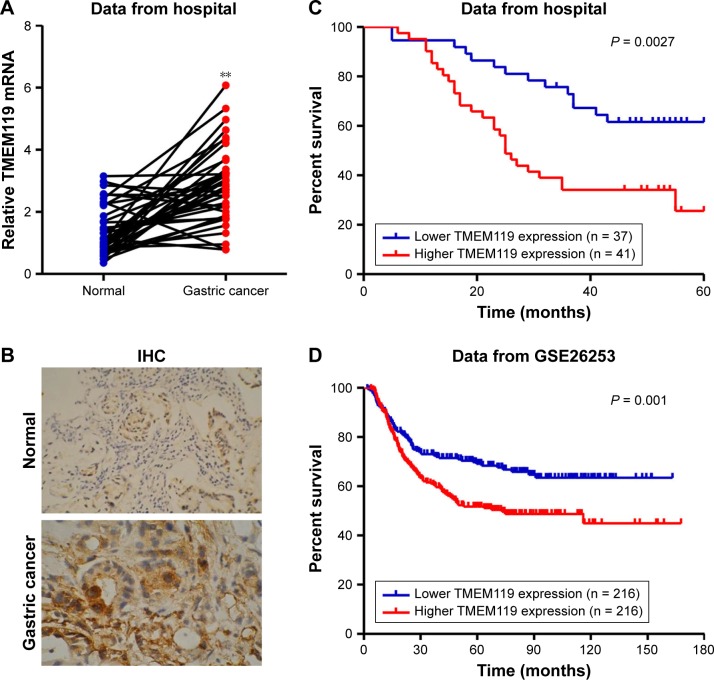
To investigate the expression of TMEM119 in gastric cancer tissues, 37 samples of tumor tissues and their paired non-tumorous normal gastric tissues were included randomly in the study. As shown in Figure 1A and B, TMEM119 expression was upregulated in gastric cancer tissues compared with that in corresponding non-tumorous normal gastric tissues as shown by real-time PCR and IHC analyses. To determine the prognostic value of TMEM119 in gastric cancer, the relationship between TMEM119 expression and survival time was analyzed in 78 patients with gastric cancer. As shown in Figure 1C, survival analysis using Kaplan–Meier and log-rank tests revealed poorer overall survival in gastric cancer patients with higher TMEM119 expression compared with patients with lower TMEM119 expression. Similar outcome was also observed in GSE26253 database (Figure 1D). These results demonstrated that TMEM119 is upregulated in gastric cancer, and higher TMEM119 expression is associated with poor prognosis of gastric cancer patients.
Figure 1.
Higher TMEM119 expression was observed in gastric cancer tissues and indicated a poor survival outcome. The expression of TMEM119 in gastric cancer tissues was measured by real-time PCR (A) and IHC (B). The overall survival time of patients with gastric cancer from our hospital (C) and GSE26253 (D) dataset was analyzed. **P<0.001 compared with normal.
Abbreviations: PCR, polymerase chain reaction; IHC, immunohistochemistry.
TMEM119 is highly expressed in gastric cancer cell lines
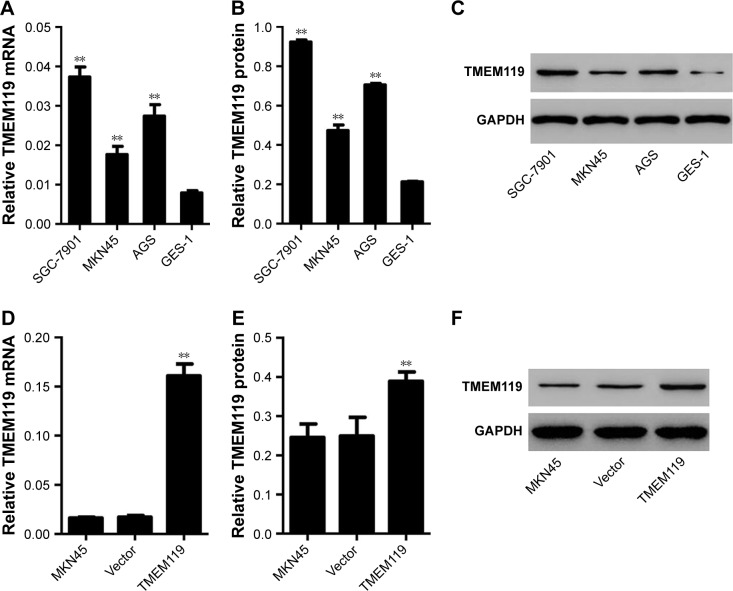
Firstly, real-time PCR and Western blot analyses were performed to investigate the TMEM119 expression in gastric cancer cell lines. The results showed that TMEM119 mRNA and protein expression was higher in three gastric cancer cell lines, including MKN45, SGC-7901, and AGS, compared with GES-1, a gastric epithelial cell line (Figure 2A–C). The protein level of TMEM119 in SGC-7901, AGS, and MKN45 cells was significantly increased by 3.4-fold, 2.3-fold, and 1.2-fold, respectively. Subsequently, recombined pLVX-Puro-TMEM119 vector was transfected into the MKN45 cells to upregulate TMEM119 expression. Figure 2D–F shows that TMEM119 mRNA and protein expression was significantly increased by 8.5-fold and 56.2%, respectively, in MKN45 cells with pLVX-Puro-TMEM119 compared with that in SGC-7901 cells with blank pLVX-Puro vector transfection.
Figure 2.
TMEM119 was highly expressed in gastric cancer cell lines. TMEM119 mRNA and protein expression in gastric cancer cell lines, including SGC-7901, AGS, and MKN45, and gastric epithelial cell line GES-1 was measured by real-time PCR (A) and Western blotting (B and C), respectively. TMEM119 mRNA and protein expression in MKN45 cells with recombined pLVX-Puro-TMEM119 vector transfection was measured by real-time PCR (D) and Western blotting (E and F). **P<0.01 compared with GES-1 cells or vector.
Abbreviation: PCR, polymerase chain reaction.
TMEM119 silencing inhibits cell migration and invasion of gastric cancer
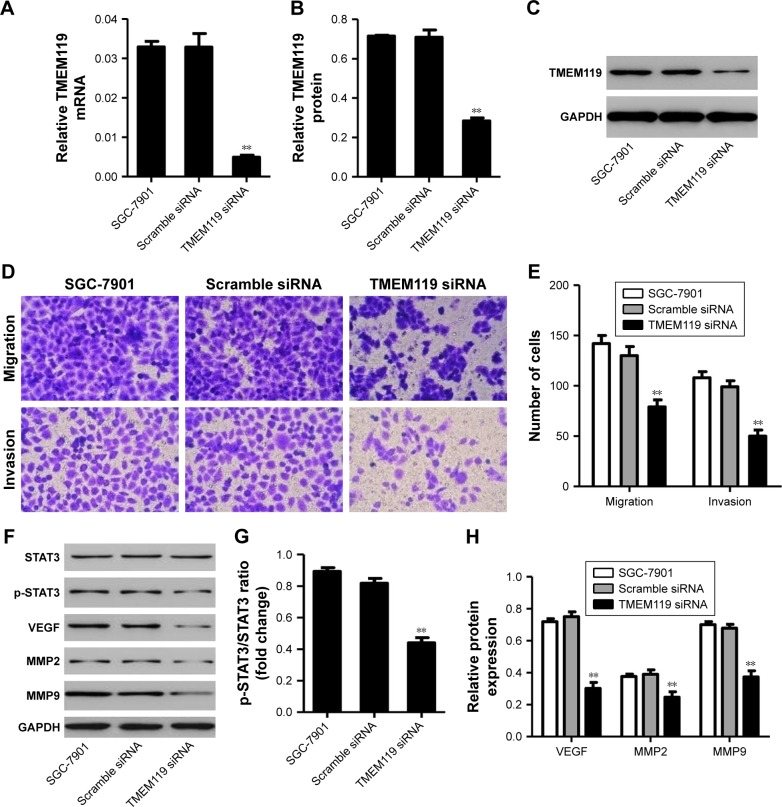
To further examine the function of TMEM119 in gastric cancer tumorigenesis, TMEM119 siRNA or scramble siRNA (negative control) was transfected into the SGC-7901 cells to knock down TMEM119 expression. The cell invasion and migration were measured by transwell analysis, and the expression of STAT3 and p-STAT3 as well as its downstream signaling proteins such as VEGF, MMP2, and MMP9 was measured by Western blot analysis. Figure 3A–C demonstrates that TMEM119 mRNA and protein expression was significantly decreased by 84.9% and 60.1%, respectively, in SGC-7901 cells with TMEM119 siRNA transfection compared with that in SGC-7901 cells with scramble siRNA transfection. TMEM119 knockdown significantly inhibited SGC-7901 cell migration and invasion by 64.6% and 98.0%, respectively, compared with scramble siRNA transfection (Figure 3D and E). Furthermore, TMEM119 knockdown significantly inhibited the protein expression of p-STAT3, VEGF, MMP2, and MMP9, but had no effect on the STAT3 expression, when compared with scramble siRNA transfection (Figure 3F–H).
Figure 3.
TMEM119 knockdown inhibited SGC-7901 cell migration and invasion. TMEM119 mRNA and protein expression in SGC-7901 cells with TMEM119 siRNA transfection was measured by real-time PCR (A) and Western blotting (B and C). Cell migration and invasion (D and E), and protein expression of STAT3, p-STAT3, VEGF, MMP2, and MMP9 (F–H) in SGC-7901 cells after TMEM119 siRNA transfection was measured by transwell and Western blot analysis, respectively. **P<0.01 compared with scramble siRNA.
Abbreviation: PCR, polymerase chain reaction.
TMEM119 overexpression promotes cell invasion and migration of gastric cancer
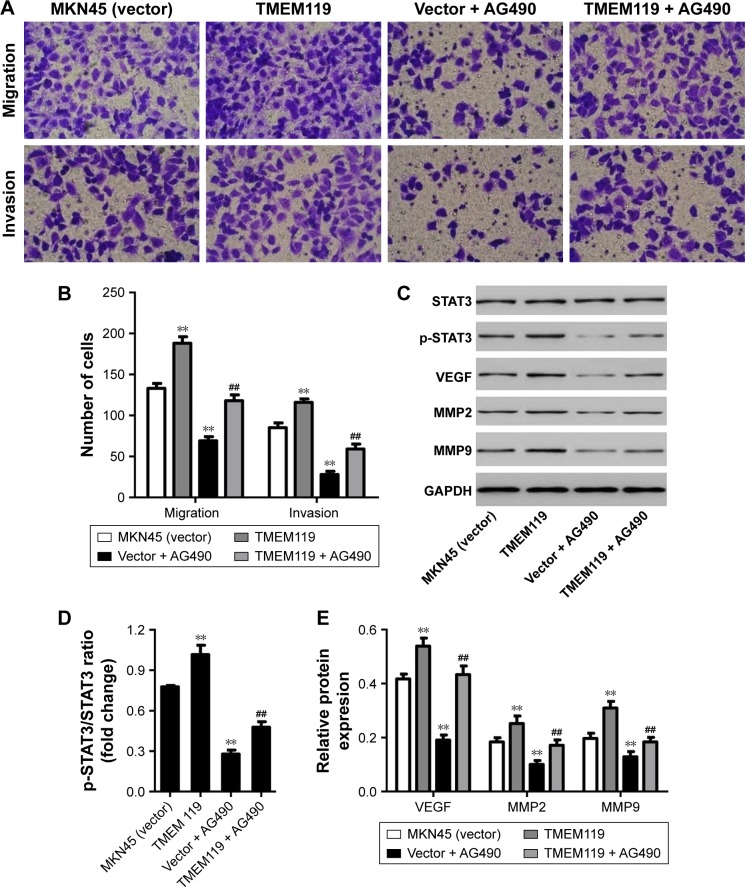
Our results demonstrated that TMEM119 overexpression significantly promoted MKN45 cell migration and invasion by 41.4% and 36.5%, respectively, compared with blank vector transfection (Figure 4A and B). Furthermore, TMEM119 overexpression significantly increased the protein expression of p-STAT3, VEGF, MMP9, and MMP2, but had no effect on the STAT3 expression, when compared with blank vector transfection (Figure 4C–E). Taken together, our results suggest that TMEM119 may regulate migration and invasion of gastric cancer cells through STAT3 signaling pathway.
Figure 4.
AG490 treatment inhibited TMEM119-induced MKN45 cell migration and invasion. Cell migration and invasion (A and B), and protein expression of STAT3, p-STAT3, VEGF, MMP2, and MMP9 (C–E) in MKN45 cells with recombined pLVX-Puro-TMEM119 vector transfection and/or AG490 (30 μM) treatment was measured by transwell and Western blot analysis, respectively. **P<0.01 compared with vector. ##P<0.01 compared with TMEM119.
Blocking STAT3 signaling inhibits TMEM119-induced gastric cell migration and invasion
To confirm the hypothesis that STAT3 signaling pathway is implicated in TMEM119-induced migration and invasion of gastric cancer cells, a JAK2 inhibitor, AG490, was used in MKN45 cells. As shown in Figure 4A and B, 30 μM AG490 treatment significantly inhibited MKN45 cell migration and invasion by 48.1% and 67.1%, respectively, compared with blank vector transfection. Furthermore, 30 μM AG490 treatment significantly inhibited p-STAT3, VEGF, MMP2, and MMP9 protein expression, but had no effect on the STAT3 expression, when compared with blank vector transfection (Figure 4C–E).
Importantly, 30 μM AG490 treatment significantly inhibited TMEM119 overexpression-induced migration and invasion of MKN45 cells by 37.2% and 49.1%, respectively (Figure 4A and B). Furthermore, 30 μM AG490 treatment also significantly inhibited TMEM119 overexpression-induced increases in the p-STAT3, VEGF, MMP2, and MMP9 protein expression, but had no effect on the STAT3 expression (Figure 4C–E). Taken together, these findings indicate that TMEM119 enhances the invasion and migration of gastric cancer cells through activating STAT3 signaling pathway.
Discussion
Gastric cancer is one of the most common malignant tumors of the digestive tract, and its development is associated with oncogene activation or overexpression. TMEM is a type of integral membrane protein that functions as a gateway to permit the transport of specific substances across the biological membrane. Increasing evidence has shown its role in tumorigenesis. For example, EMMPRIN is a highly glycosylated cell surface TMEM upregulated in gastric cancer patients, and its upregulation results in the motility and growth of gastric cancer.26 TMEM106A overexpression was found in gastric cancer, and inhibited cell growth and induced apoptosis of gastric cancer.27 In the present study, higher TMEM119 expression was found in gastric cancer tissues and correlated with lower survival rate. TMEM119 was upregulated in gastric cancer cell lines compared with normal gastric epithelial cell line, suggesting an oncogenic role of TMEM119 in gastric cancer. In line with our findings, some studies showed that TMEM119 was overexpressed in osteosarcoma, benign phyllodes tumors of the breast, and prostate cancer.16,17,19 However, downregulated TMEM119 expression was observed in oral squamous cell carcinoma.18 These results suggest that the expression pattern is different in different cancers.
Tumor metastasis is the most common cause of death in cancer patients. Although primary tumors are very dangerous, they cause death in only 10% of patients, while about 90% of patients die from metastasis. Tumor metastasis is a complex and multistage process, in which tumor cells in situ are disseminated to distant organs through a series of cascade reactions.28 TMEM45A knockdown inhibited ovarian cancer cell adhesion and invasion, which involved focal adhesion signaling pathway and TGF-β signaling pathway.29 TMEM45A silencing also inhibited glioma cell invasion and migration and decreased the MMP2 and MMP9 protein expression.30 IFN-induced TMEM1 was overexpressed in head and neck squamous cell carcinoma and promoted cell invasion and the expression of MMP13 and MMP12.31 TMEM45B knockdown inhibited invasion and migration of lung cancer cells as well as the expression of MMP9.32 TMEM49 knockdown inhibited adhesion and invasion of ovarian cancer cells as well as the expression of MMP2.33 However, evidence on the function of TMEM119 in tumorigenesis, especially cell migration and invasion, was extremely limited. Only Jiang et al19 showed that TMEM119 silencing contributed to decreased migration and invasion of osteosarcoma cells and expression of metastasis-related proteins (MMP2, N-cadherin, E-cadherin, Vimentin, and Twist1), which in part agree with our findings that TMEM119 enhanced invasion and migration of gastric cancer cells as well as the MMP2 and MMP9 expression.
STAT signaling pathway plays an important role in many physiological processes such as cell growth and differentiation.34 In physiological conditions, STAT is temporarily activated. However, the abnormal activation of STAT, especially the continuous activation, is closely related to the occurrence of many tumors. Previous study demonstrated abnormal activation of STAT3 in gastric cancer patients and strong correlation between STAT3 activation and the invasion, metastasis, and prognosis of gastric cancer.35,36 The important mechanism in the metastasis and invasion of cancer cells is the reconstruction of the basement membrane and the degradation of extracellular matrix, which requires the activation and expression of matrix metalloproteinases. Recently, MMP2 and MMP9, the most important metallo-proteinases, have been shown to play a critical role in tumor invasion, angiogenesis, metastasis, and recurrence through degrading various components of extracellular matrix.37 In the present study, TMEM119 overexpression promoted STAT3 activation, and its downstream signaling proteins MMP2 and MMP9 expression, in gastric cancer cells, which was corrected by AG490, suggesting that STAT3 contributes to TMEM119-mediated migration and invasion of gastric cancer cells. Similarly, knockdown of STAT3 was shown to inhibit motility and invasion of gastric cancer cells, as well as the activity of MMP2 and MMP9.38
New blood vessel formation or blood vessel regeneration is required for the process of tumor growth and metabolism. VEGF is the most direct factor to stimulate the growth of tumor vessels and accelerate the growth of tumor lymphatic endothelial cells, thereby playing a key role in the process of tumor invasion and metastasis.39 A previous study demonstrated that gastric cancer cells expressing high levels of STAT3 also expressed high levels of VEGF, indicating a positive correlation between the expression levels of STAT3 and VEGF.40 This is consistent with our findings that blocking STAT3 signal significantly inhibited TMEM119-induced VEGF expression in gastric cancer cells, which resulted in decreased cell migration and invasion. Moreover, blocking the activation of STAT3 in gastric cancer cells resulted in corresponding decrease in VEGF protein expression, which in turn resulted in decreased metastasis and invasion of gastric cancer cells.41
Conclusion
Our results suggest that TMEM119 is upregulated in gastric cancer tissues and cell lines and regulates cell migration and invasion through STAT3 signaling pathway. STAT3 may be considered an important factor in the diagnosis and prognosis of gastric cancer in the future. However, its regulatory mechanism remains to be further explored.
Acknowledgments
This study was supported by the Natural Science Foundation of Zhejiang Province (LY17H030008) and Medical and Health Science and Technology Plan of Zhejiang Province (2016ZDA001).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Liang D, Liang S, Jin J, Li D, Shi J, He Y. Gastric cancer burden of last 40 years in North China (Hebei Province): a population-based study. Medicine. 2017;96:e5887. doi: 10.1097/MD.0000000000005887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou ML, Wang L, Wang JZ, et al. Validation of the Memorial Sloan Kettering Cancer Center nomogram to predict disease-specific survival in a Chinese gastric cancer population receiving postoperative chemoradiotherapy after an R0 resection. Oncotarget. 2016;7:64757–64765. doi: 10.18632/oncotarget.11665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lv ZD, Zhao WJ, Jin LY, et al. Blocking TGF-beta1 by P17 peptides attenuates gastric cancer cell induced peritoneal fibrosis and prevents peritoneal dissemination in vitro and in vivo. Biomed Pharmacother. 2017;88:27–33. doi: 10.1016/j.biopha.2017.01.039. [DOI] [PubMed] [Google Scholar]
- 4.Yoon H, Kim N. Diagnosis and management of high risk group for gastric cancer. Gut Liver. 2015;9:5–17. doi: 10.5009/gnl14118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duo-Ji MM, Ci-Ren BS, Long ZW, Zhang XH, Luo DL. Short-term efficacy of different chemotherapy regimens in the treatment of advanced gastric cancer: a network meta-analysis. Oncotarget. 2017;8:37896–37911. doi: 10.18632/oncotarget.14664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohashi T, Komatsu S, Ichikawa D, et al. Overexpression of PBK/TOPK relates to tumour malignant potential and poor outcome of gastric carcinoma. Br J Cancer. 2017;116:218–226. doi: 10.1038/bjc.2016.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao L, Li W, Zang W, et al. JMJD2B promotes epithelial-mesenchymal transition by cooperating with beta-catenin and enhances gastric cancer metastasis. Clin Cancer Res. 2013;19:6419–6429. doi: 10.1158/1078-0432.CCR-13-0254. [DOI] [PubMed] [Google Scholar]
- 8.Fisher R, Pusztai L, Swanton C. Cancer heterogeneity: implications for targeted therapeutics. Br J Cancer. 2013;108:479–485. doi: 10.1038/bjc.2012.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bae YH, Park K. Targeted drug delivery to tumors: myths, reality and possibility. J Control Release. 2011;153:198–205. doi: 10.1016/j.jconrel.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tran Q, Park J, Lee H, et al. TMEM39A and human diseases: a brief review. Toxicol Res. 2017;33:205–209. doi: 10.5487/TR.2017.33.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flamant L, Roegiers E, Pierre M, et al. TMEM45A is essential for hypoxia-induced chemoresistance in breast and liver cancer cells. BMC Cancer. 2012;12:391. doi: 10.1186/1471-2407-12-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuajungco MP, Podevin W, Valluri VK, Bui Q, Nguyen VH, Taylor K. Abnormal accumulation of human transmembrane (TMEM)-176A and 176B proteins is associated with cancer pathology. Acta Histochem. 2012;114:705–712. doi: 10.1016/j.acthis.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hrasovec S, Hauptman N, Glavac D, Jelenc F, Ravnik-Glavac M. TMEM25 is a candidate biomarker methylated and down-regulated in colorectal cancer. Dis Markers. 2013;34:93–104. doi: 10.3233/DMA-120948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hisa I, Inoue Y, Hendy GN, et al. Parathyroid hormone-responsive Smad3-related factor, Tmem119, promotes osteoblast differentiation and interacts with the bone morphogenetic protein-Runx2 pathway. J Biol Chem. 2011;286:9787–9796. doi: 10.1074/jbc.M110.179127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka K, Kaji H, Yamaguchi T, et al. Involvement of the osteoinductive factors, Tmem119 and BMP-2, and the ER stress response PERK-eIF2alpha-ATF4 pathway in the commitment of myoblastic into osteoblastic cells. Calcif Tissue Int. 2014;94:454–464. doi: 10.1007/s00223-013-9828-1. [DOI] [PubMed] [Google Scholar]
- 16.Gasi Tandefelt D, Boormans JL, van der Korput HA, Jenster GW, Trapman J. A 36-gene signature predicts clinical progression in a subgroup of ERG-positive prostate cancers. Eur Urol. 2013;64:941–950. doi: 10.1016/j.eururo.2013.02.039. [DOI] [PubMed] [Google Scholar]
- 17.Jones AM, Mitter R, Poulsom R, et al. mRNA expression profiling of phyllodes tumours of the breast: identification of genes important in the development of borderline and malignant phyllodes tumours. J Pathol. 2008;216:408–417. doi: 10.1002/path.2439. [DOI] [PubMed] [Google Scholar]
- 18.Lim KP, Cirillo N, Hassona Y, et al. Fibroblast gene expression profile reflects the stage of tumour progression in oral squamous cell carcinoma. J Pathol. 2011;223:459–469. doi: 10.1002/path.2841. [DOI] [PubMed] [Google Scholar]
- 19.Jiang ZH, Peng J, Yang HL, et al. Upregulation and biological function of transmembrane protein 119 in osteosarcoma. Exp Mol Med. 2017;49:e329. doi: 10.1038/emm.2017.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu KJ, Huang JM, Zhong HJ, et al. A natural product-like JAK2/STAT3 inhibitor induces apoptosis of malignant melanoma cells. PLoS One. 2017;12:e0177123. doi: 10.1371/journal.pone.0177123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang TS, Wang W, Zhong HJ, et al. An anti-prostate cancer benzofuran-conjugated iridium(III) complex as a dual inhibitor of STAT3 and NF-kappaB. Cancer Lett. 2017;396:76–84. doi: 10.1016/j.canlet.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 22.Meydan N, Grunberger T, Dadi H, et al. Inhibition of acute lymphoblastic leukaemia by a Jak-2 inhibitor. Nature. 1996;379:645–648. doi: 10.1038/379645a0. [DOI] [PubMed] [Google Scholar]
- 23.Kanda N, Seno H, Konda Y, et al. STAT3 is constitutively activated and supports cell survival in association with survivin expression in gastric cancer cells. Oncogene. 2004;23:4921–4929. doi: 10.1038/sj.onc.1207606. [DOI] [PubMed] [Google Scholar]
- 24.Luo J, Yan R, He X, He J. Constitutive activation of STAT3 and cyclin D1 overexpression contribute to proliferation, migration and invasion in gastric cancer cells. Am J Transl Res. 2017;9:5671–5677. [PMC free article] [PubMed] [Google Scholar]
- 25.Chung KY, Shia J, Kemeny NE, et al. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol. 2005;23:1803–1810. doi: 10.1200/JCO.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 26.Zheng HC, Takahashi H, Murai Y, et al. Upregulated EMMPRIN/CD147 might contribute to growth and angiogenesis of gastric carcinoma: a good marker for local invasion and prognosis. Br J Cancer. 2006;95:1371–1378. doi: 10.1038/sj.bjc.6603425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu D, Qu L, Hu J, et al. Transmembrane protein 106A is silenced by promoter region hypermethylation and suppresses gastric cancer growth by inducing apoptosis. J Cell Mol Med. 2014;18:1655–1666. doi: 10.1111/jcmm.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarwar MS, Zhang HJ, Tsang SW. Perspectives of plant natural products in inhibition of cancer invasion and metastasis by regulating multiple signaling pathways. Curr Med Chem. 2017 Sep 18; doi: 10.2174/0929867324666170918123413. Epub. [DOI] [PubMed] [Google Scholar]
- 29.Guo J, Chen L, Luo N, Yang W, Qu X, Cheng Z. Inhibition of TMEM45A suppresses proliferation, induces cell cycle arrest and reduces cell invasion in human ovarian cancer cells. Oncol Rep. 2015;33:3124–3130. doi: 10.3892/or.2015.3902. [DOI] [PubMed] [Google Scholar]
- 30.Sun W, Qiu G, Zou Y, et al. Knockdown of TMEM45A inhibits the proliferation, migration and invasion of glioma cells. Int J Clin Exp Pathol. 2015;8:12657–12667. [PMC free article] [PubMed] [Google Scholar]
- 31.Hatano H, Kudo Y, Ogawa I, et al. IFN-induced transmembrane protein 1 promotes invasion at early stage of head and neck cancer progression. Clin Cancer Res. 2008;14:6097–6105. doi: 10.1158/1078-0432.CCR-07-4761. [DOI] [PubMed] [Google Scholar]
- 32.Hu R, Hu F, Xie X, et al. TMEM45B, up-regulated in human lung cancer, enhances tumorigenicity of lung cancer cells. Tumour Biol. 2016;37:12181–12191. doi: 10.1007/s13277-016-5063-5. [DOI] [PubMed] [Google Scholar]
- 33.Zheng L, Chen L, Zhang X, Zhan J, Chen J. TMEM49-related apoptosis and metastasis in ovarian cancer and regulated cell death. Mol Cell Biochem. 2016;416:1–9. doi: 10.1007/s11010-016-2684-3. [DOI] [PubMed] [Google Scholar]
- 34.Zyuz’kov GN, Udut EV, Miroshnichenko LA, et al. Particular role of JAK/STAT3 signaling in functional control of mesenchymal progenitor cells. Bull Exp Biol Med. 2018;164(3):316–319. doi: 10.1007/s10517-018-3980-6. [DOI] [PubMed] [Google Scholar]
- 35.Ma DH, Li BS, Liu JJ, et al. miR-93-5p/IFNAR1 axis promotes gastric cancer metastasis through activating the STAT3 signaling pathway. Cancer Lett. 2017;408:23–32. doi: 10.1016/j.canlet.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 36.Zhang S, Huang S, Deng C, et al. Co-ordinated overexpression of SIRT1 and STAT3 is associated with poor survival outcome in gastric cancer patients. Oncotarget. 2017;8:18848–18860. doi: 10.18632/oncotarget.14473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grzelczyk WL, Szemraj J, Jozefowicz-Korczynska M. The matrix metalloproteinase in larynx cancer. Postepy Hig Med Dosw (Online) 2016;70:1190–1197. [PubMed] [Google Scholar]
- 38.Wei Z, Jiang X, Qiao H, et al. STAT3 interacts with Skp2/p27/p21 pathway to regulate the motility and invasion of gastric cancer cells. Cell Signal. 2013;25(4):931–938. doi: 10.1016/j.cellsig.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 39.Fossey SL, Bear MD, Kisseberth WC, Pennell M, London CA. Oncostatin M promotes STAT3 activation, VEGF production, and invasion in osteosarcoma cell lines. BMC Cancer. 2011;11:125. doi: 10.1186/1471-2407-11-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joo MK, Park JJ, Kim SH, et al. Antitumorigenic effect of plumbagin by induction of SH2-containing protein tyrosine phosphatase 1 in human gastric cancer cells. Int J Oncol. 2015;46:2380–2388. doi: 10.3892/ijo.2015.2935. [DOI] [PubMed] [Google Scholar]
- 41.Chen J, Wang J, Lin L, et al. Inhibition of STAT3 signaling pathway by nitidine chloride suppressed the angiogenesis and growth of human gastric cancer. Mol Cancer Ther. 2012;11:277–287. doi: 10.1158/1535-7163.MCT-11-0648. [DOI] [PubMed] [Google Scholar]
- 42.Hermanek P, Scheibe O, Spiessl B, Wagner G. TNM classification of malignant tumors: the new 1987 edition. Rontgenblatter. 1987;40:200. [PubMed] [Google Scholar]