Abstract
Evolutionary changes in Astyanax mexicanus cavefish with respect to conspecific surface fish, including the regression of eyes, loss of pigmentation, and modification of the cranial skeleton, involve derivatives of the neural crest. However, the role of neural crest cells in cavefish evolution and development is poorly understood. One of the reasons is that experimental methods for neural crest analysis are not well developed in the Astyanax system. Here we describe neural crest transplantation between Astyanax surface fish and cavefish embryos. We found differences in the migration of cranial neural crest cells transplanted from the surface fish anterior hindbrain to the same region of surface fish or cavefish hosts. Cranial neural crest cells migrated extensively throughout the head, and to a lesser extent the trunk, in surface fish hosts but their migration was mostly restricted to the anterior and dorsal head regions in cavefish hosts. Cranial neural crest cells derived from the surface fish transplants invaded the degenerating eyes of cavefish hosts, resulting in increased eye size and suggesting that cavefish neural crest cells are defective in forming optic derivatives. We found that melanophores were formed in albino cavefish from grafts of surface fish trunk neural crest cells, showing that the cavefish tissue environment is conducive for pigment cell development, and implicating intrinsic changes in cavefish neural crest cells in loss of body pigmentation. It is concluded that changes in neural crest cells play key roles in the evolution of cavefish development.
Introduction
The evolution of vision and coloration were significant innovations in animals living on the earth’s surface. When surface dwelling animals invade dark habitats, their descendants tend to lose their eyes and pigmentation. The absence of eyes and pale skin are characteristics of cave adapted species (Culver and Pipan 2009; Protas and Jeffery 2012). Although scientific interest in cave dwelling animals has a long history, knowledge of the developmental and evolutionary mechanisms responsible for the loss of eyes and pigmentation is still incomplete. The teleost Astyanax mexicanus is a powerful model for investigating the evolutionary changes related to cave adaptation (Jeffery 2001; Gross et al. 2015). This species consists of two forms: an eyed and pigmented surface fish and multiple populations of cavefish with reduced eyes and pigmentation.
Cavefish initially form eye primordia with a lens and optic cup, although both structures are smaller than their surface fish counterparts (Jeffery 2009). The first sign of eye degeneration is apoptosis of the lens, which is followed by localized cell death in the retina, disorganization of the retinal layers, and arrest of optic growth. In addition to the lens and retina, other optic tissues fail to develop or are modified in cavefish. The cornea, iris, and ciliary body do not differentiate, probably due to lack of induction by the dysfunctional lens (Yamamoto and Jeffery 2000; Strickler et al. 2001). Although cavefish form a cartilaginous sclera, it differs from the surface fish sclera in not completing ossification (Yamamoto et al. 2003; O'Quin et al. 2015). The arrest of optic growth causes the degenerating cavefish eyes to sink into the orbits and become internalized during larval development.
The loss of melanin pigmentation in cavefish occurs by a process that is genetically distinct from eye degeneration (Jeffery et al. 2016). Some cavefish populations develop reduced numbers of lightly pigmented melanophores (Gross et al. 2009), whereas others lose all melanin pigmentation (Protas et al. 2006). Mutations in the mc1r gene are responsible for decreased coloration and reduction of melanophores in some cavefish populations (Gross et al. 2009), whereas mutations in the oca2 gene, which controls the first step of melanin synthesis, is responsible for albinism (Protas et al. 2006). In contrast to eyes, cell death is apparently not responsible for the loss of melanophore precursors, which remain detectable throughout development by their ability to convert exogenous L-DOPA into melanin (McCauley et al. 2004).
Astyanax cavefish also show constructive changes related to cave adaptation (Jeffery 2001). They have large oral jaws with more taste buds and teeth (Yamamoto et al. 2003, 2009; Atukorala et al. 2013), enhanced numbers of larger cranial neuromasts (Yoshizawa et al. 2010, 2012), and more catecholamine-producing cells in their adrenal kidneys than surface fish (Bilandžija et al. 2013). A connecting thread is revealed by comparing the different cell, tissue, and organ types showing constructive and regressive changes in cavefish: most of them either are, or include, derivatives of the neural crest.
The neural crest is a multipotent group of ectodermal stem and progenitor cells, which ingress in an anterior to posterior progression from the developing neural tube, migrate extensively through distinct pathways in the embryo, and differentiate into diverse derivatives, including body pigment cells, the cranial and pharyngeal skeleton, parts of the cornea, iris, choroid, and sclera of the eyes, and the adrenal glands (Le Douarin and Kalcheim 1999). Both intrinsic programming of cell fates and extrinsic instructions from the surrounding tissue environment are important in regulating neural crest cell differentiation (Kelsh and Erickson 2013). Despite the predicted roles of neural crest cells in constructive and regressive changes, only a single previous study has been conducted on the cavefish neural crest. McCauley et al. (2004) showed that migratory trunk neural crest cells give rise to the precursors of melanophores in cavefish, although they do not undergo final differentiation. Thus, cavefish could be defective in intrinsic neural crest fate determination and/or in the establishment of a supportive tissue environment for neural crest cell migration and differentiation.
In this investigation we have developed a transplantation method to address the role of neural crest cells in cavefish development. We describe the successful transplantation of cranial and trunk neural crest between the two forms of Astyanax. Although the migration of cranial neural crest cells was more limited in cavefish than in surface fish hosts, they invaded the degenerate eyes, resulting in an increase in eye size and implicating the cavefish neural crest in the regressive optic phenotype. We also found that trunk neural crest cells derived from surface fish donors can differentiate into melanophores in cavefish hosts, revealing an extrinsic tissue environment conducive for their maturation and indicating that defective pigmentation must be caused by a modification in the intrinsic programming of cavefish neural crest cells. We conclude that neural crest cells have important roles in regulating evolutionary changes in cavefish development.
Materials and methods
Biological materials
The Astyanax surface fish and Pachón cavefish used in this investigation were derived from laboratory stocks collected at Nacimiento del Rio Choy, San Luis Potosi, Mexico or Balmorhea Springs State Park, Texas and Cueva de Pachón, Tamaulipas, Mexico, respectively. Adult fish were maintained in a running water system at 23°C on a 14-hr light and 10-hr dark photoperiod (Jeffery et al. 2000). Surface fish and cavefish embryos spawned naturally at different times during the dark cycle of the photoperiod. To synchronize their development, embryos were raised at different temperatures, 23˚C for surface fish and 25°C for cavefish, until they reached the same developmental stage, and afterward were cultured at the same temperature (21˚C). Larvae were fed brine shrimp beginning at 7 days post-fertilization (dpf), and adults were fed TetraMin Pro flakes (Tetra Holding Inc, Blacksburg, VA, USA). Methods for fish handling conformed to National Institutes of Health guidelines and were approved by the University of Maryland Animal Care and Use Committee.
Neural crest transplantation
For cranial neural crest transplantation, larvae at ∼2 dpf were washed for 30 min in calcium-free zebrafish ringer (CFZFR: 116 mM NaCl, 2.9 mM KCl, 10 mM 4-(2-hydroxylethyl)-1-piperazineethanesulfonic acid, pH 7.2) containing 0.2% ethylenediaminetetraacetic acid to loosen tissue connections, rinsed in CFZFR (40˚C), and embedded in 1.2% agar in CFZFR (40˚C) (Yamamoto and Jeffery 2000, 2002). After cooling to room temperature, the agar was cut into blocks containing individual larvae. The operations (Fig. 1A) were done with sharp tungsten needles on agar blocks arranged side by side in CFZFR. The donor tissue was removed from a region above the dorsal hindbrain facing the diamond-shaped fourth ventricle and labeled by three 30-s rinses with 40 µg/mL DiI (1, 1′-dioctadecyl-3, 3, 3′, 3′-tetramethylindocarbocyanine, Thermo Fisher Scientific, Waltham, MA, USA) dissolved in 92% dimethyl sulfoxide (Sigma Aldrich, St. Louis, MO, USA). The stained donor tissue (∼50 µm in diameter) was then inserted into a slot made with a sharp tungsten needle in approximately the same region of the hindbrain in a donor embryo. Sham-operated controls were done by making a slot above the host hindbrain in the same region as the transplant but without inserting a piece of tissue. The transplant containing and sham-operated larvae were removed from the agar blocks, allowed to recover in zebrafish ringer solution for 1 h at 22˚C, and then reared in standard fish conditioned water (pH 7.0, conductivity 600 µS/cm, and 21˚C).
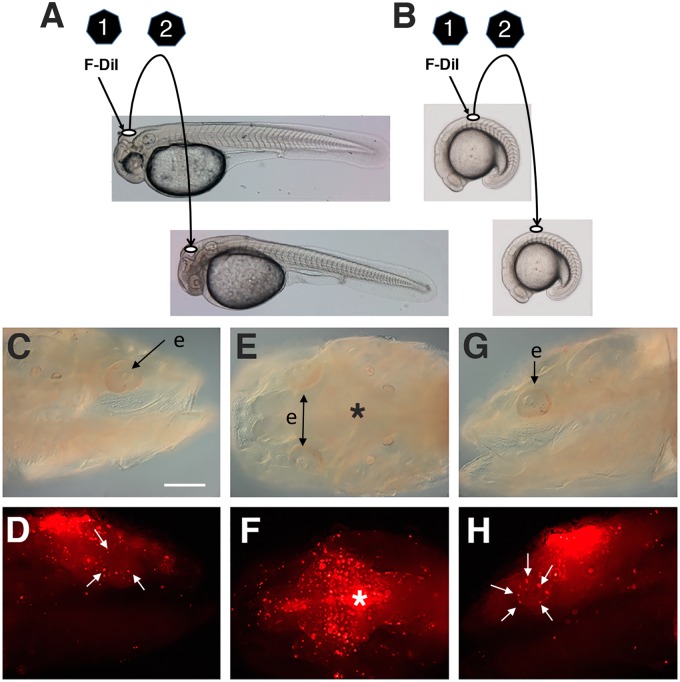
Fig. 1.
Neural crest transplantation. The two-step process of transplanting cranial (A) or trunk (B) neural crest from donor to host embryos. Step 1: Removal of a piece of the dorsal hindbrain at ∼2 dpf (A) or the neural tube above somites 3–5 in 15–20 somite stage embryos (B) and vital staining with DiI. Step 2: Grafting of the Dil labeled explants into similar regions in hosts at the same stage of development (C–H). Bright field (C–G) and fluorescence (D–H) images showing an example of results obtained when cranial transplantation was done at 2 dpf and the distribution of Dil labeled cells was determined at 6 dpf. Lateral views showing the distribution of DiI labeled cells on the right (D) and left (H) sides of the same cavefish host. White arrows: accumulation of DiI labeled cells in and around the eyes. (E, F) Dorsal views showing the graft site at the dorsal midline (asterisks) and bilateral distribution of Dil labeled cells. F-Dil, fluorescent DiI; e, eye. Scale bar: 200 µm.
To transplant trunk neural crest cells, neural tubes were isolated from surface fish embryos during the somite stages (Fig. 1B). The embryos were dechorionated manually and dissociated into parts by treatment with 0.25% pancreatin (Sigma-Aldrich), as described by McCauley et al. (2004). Using tungsten needles, the top quarter of the neural tube from an area above the yolk mass was excised and cut into pieces. The pieces were labeled with DiI as described above, and grafted into a slot cut with a tungsten needle in the dorsal midline between somites 3 and 5 of dechorionated cavefish host embryos of similar age to the donors. During the grafting step the cavefish host embryos were embedded in agar blocks and otherwise treated as described above.
Microscopy, measurements of eye size, and sectioning
Larval fish containing neural crest transplants and sham operated controls were anesthetized with 2 µg/mL tricaine (Western Chemical Inc., Ferndale, WA, USA) and viewed with an Axio Zoom.V16 microscope equipped with an AxioCam HRc digital camera (Carl Zeiss Microscopy GmbH, Jena, Germany). The eyeball and lens diameters were measured on digital images using Zeiss AxioVision software (Carl Zeiss Microscopy GmbH, Jena, Germany) and standardized by measuring lengths from the anterior tip of the rostrum to the posterior tip of the larval tail. For sectioning, specimens were fixed with 4% paraformaldehyde in PBS overnight at 4˚C, embedded in Tissue-Tek O.C.T. Compound (VWR International, Radnor, PA, USA), and stored frozen (−20°C). Sections were prepared at 15 µm thickness using a Leica CM series cryostat (Leica Biosystems, Buffalo Grove, IL, USA). DiI signals were imaged as described above.
Statistical analysis
Statistical analyses using the Mann–Whitney U test and correlation analyses were performed with IBM SPSS 24.0.0.0 software (IBM, North Castle, NY, USA).
Endogenous neural crest cell tracking
Endogenous neural crest cells were tracked by DiI labeling. For DiI injection, surface fish or cavefish larvae were collected at 5–6 dpf and embedded in 1.2% agar in fish conditioned water (40˚C, pH 7.0, 700 µS/cm). After cooling to room temperature, ∼50 nL of 40 µg/mL DiI dissolved in 92% dimethyl sulfoxide was injected into the left dorsal neural tube at the level of lower rhombic lip to label neural crest cells. Control embryos were prepared by injecting DiI into the dorsal skin at the same axial level. Experimental and control fish were raised until 7–9 dpf and then anesthetized in ice-cold 67 µg/mL tricaine in conditioned water. Incorporated DiI was visualized with a fluorescence microscope (BX61WI Olympus microscope) with ×5 MPlanFL N lens, a rhodamine filter set, and an ORCA-Flash4.0 digital camera.
Results and discussion
Neural crest transplantation
Figure 1A,B illustrates the methods used to transplant pre-migratory cranial and trunk neural crest between surface fish and cavefish embryos. Transplantation of cranial neural crest cells from the anterior hindbrain region of surface fish donors to the same region of cavefish hosts resulted in detection of DiI labeled cells on both sides of the dorsal midline at 6 dpf (Fig. 1C–H). Transplantation of trunk neural crest from the anterior somite region of surface fish donors into the same region of cavefish hosts resulted in detection of Dil labeled cells in the lateral trunk and yolk epithelium at 2–3 dpf (see Fig. 4F–I). The dispersal of labeled cells from the grafts indicates that donor pre-migratory neural crest cells continued to develop and became migratory in both kinds of transplantation experiments. The role of neural crest cells in cavefish development is understudied because appropriate methods have not been previously available for experimental analysis. The development of transplantation methods will help fill this gap by providing a procedure in which neural crest cells can be exchanged between surface fish and cavefish embryos.
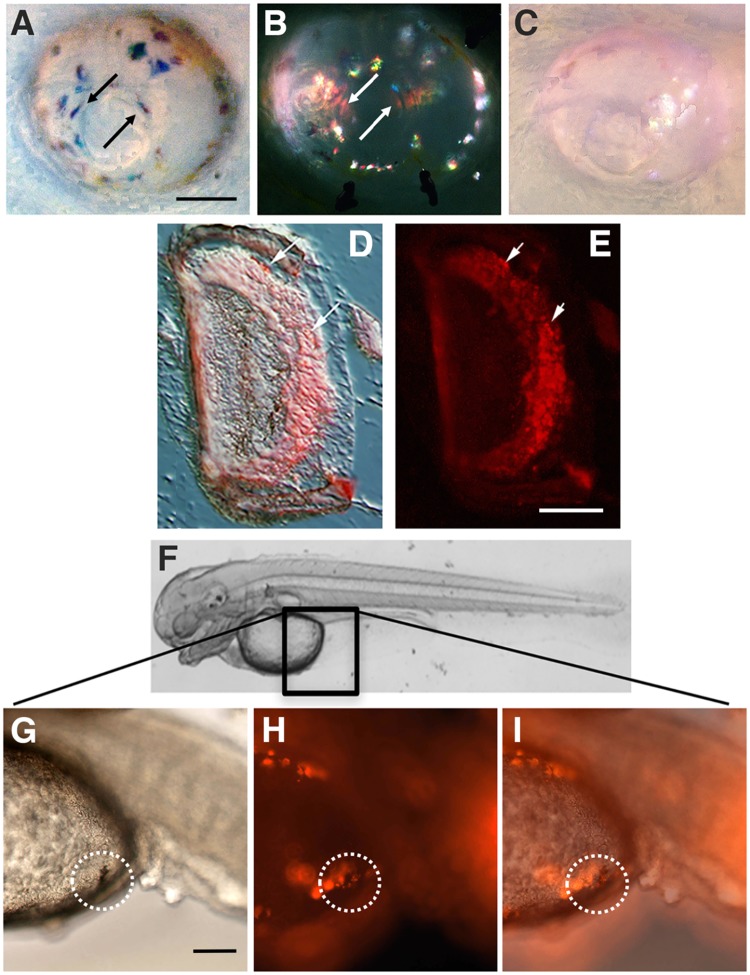
Fig. 4.
(A–E) DiI labeled cells in the eye after surface fish cranial neural crest transplantation into cavefish hosts. Bright field (A) and fluorescence (B) images of the same eye from a 14-dpf cavefish host showing DiI labeled cells (arrows). (C) Bright field image of an eye from a sham operated control. Scale bar: 40 µm. Bright field (D) and fluorescence (E) images of the same sectioned eye from a 2-month old cavefish host with transplanted surface fish cranial neural crest. Arrows show Dil-labeled cells incorporated into the retina. In A–E, N = 4 transplantations. (F–I) DiI labeled cells in the yolk epithelium (boxed region in F) after surface fish trunk neural crest transplantation into cavefish hosts. Bright field (G), fluorescence (H), and merged (I) images showing a DiI labeled melanophore (circled regions) in a 2–3 dpf cavefish host with transplanted trunk neural crest. Scale bar: 10 µm. In H–I, N = 5 transplantations.
Neural crest cells ingress and migrate along the anteroposterior axis throughout the period of neural tube formation and development. Although we have followed the fates of hindbrain and trunk neural crest cells, our method is likely to be useful in studying neural crest development along the entire anteroposterior axis. Therefore, in future experiments, we can address the involvement of different lineages of neural crest cells in constructive and regressive changes in cavefish, the effects of exchanging lineages in the two forms of Astyanax, and the rescue of modified cavefish traits by introducing neural crest cells from surface fish donors into cavefish hosts, as exemplified below for eyes and pigmentation.
Surface fish cranial neural crest cell migration in surface fish hosts
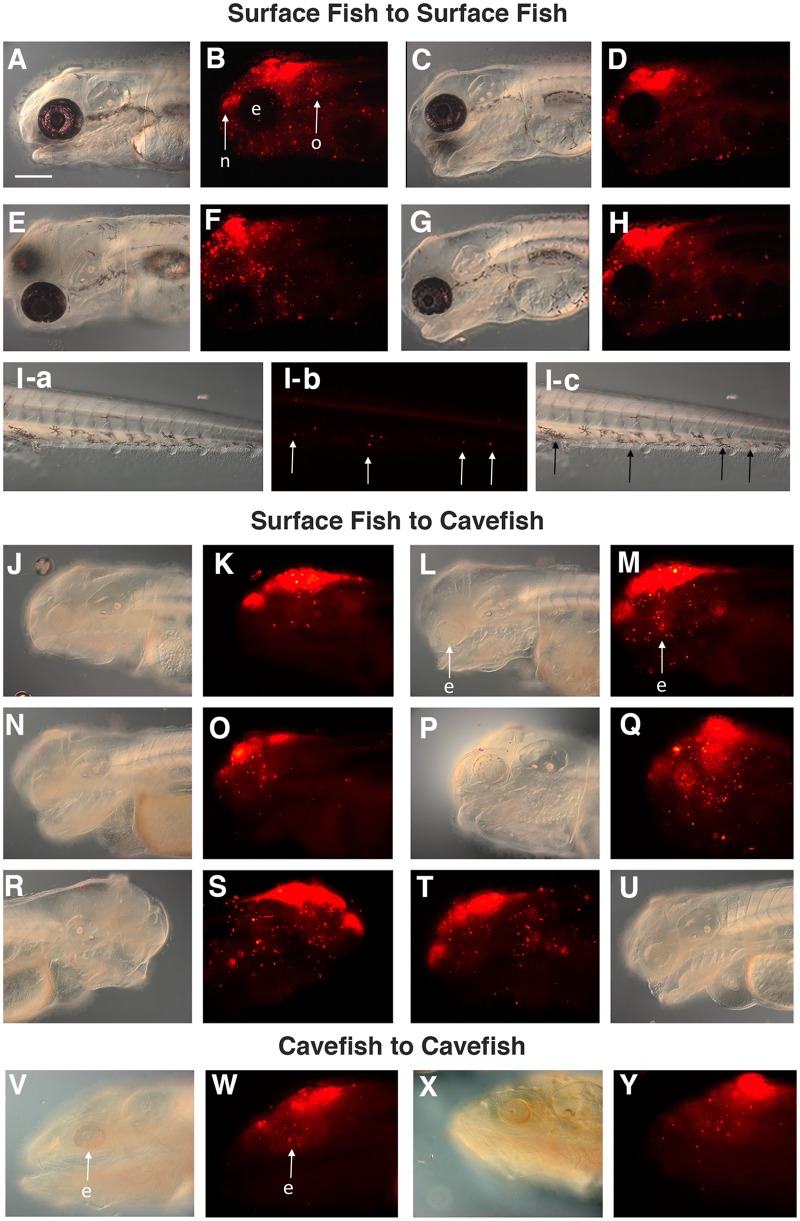
We employed transplantation (Fig. 1A) to infer the pattern of cranial neural crest cell migration in surface fish hosts. In these experiments, transplantation was carried out at ∼2 dpf and the results were determined at 4 dpf. DiI labeled neural crest cells originating from the donor grafts were detected in the head and trunk of the surface fish hosts (Fig. 2A–I). The results were similar in all surface fish hosts. One group of labeled cells was concentrated in the developing nasal area anterior to the graft (Fig. 2A–H), suggesting that cells migrated around the eyes into the region of developing olfactory primordia. A second group of labeled cells was detected posterior and ventral to the eyes in the region of the developing mouth and pharyngeal arches (Fig. 2A–H), suggesting that these cells migrated posteriorly around the eyes into ventral regions of the head. DiI labeled cells were observed immediately around the pigmented edges the eyes (Fig. 2A–H). In some transplantations, a few DiI labeled cells were also scattered within the eyes (Fig. 2B, F) but in others the eyes were devoid of labeled cells (Fig. 2D, H). The role of the developing eyes in channeling cranial neural crest cell migration has been described previously in zebrafish embryos (Langenberg et al. 2008). A third group of DiI labeled cells was detected immediately posterior to the graft with some cells accumulating near or within the developing otic vesicle (Fig. 2A–H). Lastly, in one of the hosts, a few DiI labeled cells were located at distant sites in the trunk posterior to the original graft. The latter cells were distributed along horizontal rows, suggesting an association with the developing lateral line (Fig. 2Ia–c).
Fig. 2.
Distribution of DiI labeled cells after cranial neural crest transplantation. (A–I) Transplantation from surface fish donors into surface fish hosts. (J–U) Transplantation from surface fish donors into cavefish hosts. (A–Q) Each pair of bright field and fluorescence images shows four different host individuals that received a neural crest graft at 2 dpf and were assayed at 4 dpf. (Ia–c) Trunk region of the individual shown in G, H with arrows indicating DiI labeled cells. Posterior is on the right in each frame. (R–U) The same cavefish host viewed from the right (R, S) and left (T, U) sides. (V–Y) Transplantation from cavefish donors into cavefish hosts. Each pair of bright field and fluorescence images shows two different host individuals that received a neural crest graft at 1 dpf and were assayed at 4 dpf. e, eye; n, nasal area; o, otic area. Scale bar: 200 µm. Bright field images: A, C, E. G, I-a, J, L, N. P, R, U, V, X. Fluorescence images: B, D, F, H, I-b, K, M, O, Q, S, T, W, Y. Merged images of I-a and I-b: I-c.
Cranial neural crest cells migrate over long distances in spatially ordered waves that eventually target stereotypic regions of the head (Kulesa et al. 2010). Fate mapping experiments in zebrafish and other vertebrates have shown that neural crest cells migrating from the hindbrain contribute primarily to the pharyngeal skeleton (Schilling and Kimmel 1994), whereas diencephalic and mesencephalic neural crest cells migrate into the more anterior parts of the cranium (Wada et al. 2005). Assuming a similar fate map in Astyanax, the first few pharyngeal arches would be expected destinations for neural crest cells derived from rhomdomeres of the anterior hindbrain. Consistent with this expectation, some of the neural crest cells migrated into the area of the developing pharyngeal arches in our transplantation experiments (Fig. 2A–H). However, the olfactory area, the otic area, and rarely the distal trunk were also populated by DiI labeled cells originating from the grafts.
The plasticity of cranial neural crest cells probably explains the broad pattern of neural crest cell distribution in surface fish hosts. The grafts are a source of additional cranial neural crest cells that amplify those already present in host embryos. In chick embryos, hindbrain-derived neural crest cells are re-routed in novel directions when they encounter physical barriers or pathways that are already saturated with endogenous neural crest cells (Kulesa and Fraser 2000). Re-routing, however, is unlikely to account for the appearance of cranial neural crest cells in distant parts of the trunk (Fig. 2Ia–c). In zebrafish, cranial neural crest derived glial cells migrate posteriorly along the lateral line nerve, a cranial placode derivative (Gilmour et al. 2002). Thus, the best candidates for DiI labeled cells in the trunk are glial cells associated with the lateral line nerve.
Surface fish cranial neural crest cell migration in cavefish hosts
We next transplanted pre-migratory cranial neural crest from the anterior hindbrain of surface fish donors into the same region of cavefish hosts. The transplantations were done at ∼2 dpf and Dil labeled cells were assayed at 4 dpf and later stages of cavefish development. Differences were seen in the distribution of surface fish neural crest cells in cavefish (Fig. 2J–Q) relative to surface fish hosts (Fig. 2A–I). First, DiI labeled cells were concentrated mainly in the dorsal regions of the cavefish head and tended to be excluded from the anterior and ventral regions, including the pharyngeal arches. Second, in most surface fish to cavefish transplantations DiI labeled cells accumulated within and around the degenerating cavefish eyes (Fig. 2M, O, Q; see also Fig. 1H). The following control experiments were performed to substantiate these results. In the first control, neural crest was transplanted from surface fish donors into cavefish hosts and the distribution of DiI labeled cells was compared on the left and right sides of the head. Both sides of the cavefish head showed that same dorsal restrictions of DiI labeled cells (Fig. 2R–U). In the second control, cranial neural crest was transplanted from cavefish hosts into the same position in cavefish donors, and dorsal restriction of DiI labeled cells originating from cavefish was also observed (Fig. 2V–Y). In cavefish to cavefish transplantations, DiI labeled cells were also seen in the degenerating eyes (Fig. 2W,Y). In the third control, the endogenous distribution of cranial neural crest cells in surface fish and cavefish embryos was determined by DiI labeling (Supplementary Fig. S1). In surface fish, cranial neural crest cells migrated throughout the dorsal part of the head and invaded the developing eyes (Supplementary Fig. 1C–H). In cavefish, cranial neural crest cells were fewer in number and showed a more dorsally restricted distribution than surface fish cranial neural crest cells, although they also invaded the eyes (Supplementary Fig. 1G–N).
Several conclusions can be made from these experiments. First, there are differences between the migratory behaviors of endogenous and grafted cranial neural crest cells involving their tendencies to invade the eyes and their distributions in the head, including the ventral cranial regions. The possible explanations for these differences are that migratory behaviors could vary at different times of development and/or that grafted neural crest cells may be excluded from pathways that are already occupied by endogenous neural crest cells and as a consequence distribute more broadly. Second, there may be fewer cranial neural crest cells in cavefish than in surface fish, and their migration (except for into the eyes) may be more limited. The latter conclusion, which is supported by transplantation as well as endogenous neural crest tracking experiments, could be explained by differences in both intrinsic and extrinsic programs for cavefish cranial neural crest development.
Surface fish cranial neural crest cells rescue cavefish eye size
The results described above prompted further investigation into the contributions of DiI labeled cells to the degenerating cavefish eyes. At 8 dpf and 14 dpf, Dil labeled cells originating from neural crest grafts were still detectable in the dorsal part of the cavefish head and eyes (Fig. 3A–C). To determine the effects of surface fish neural crest cells on eye size, eye diameters were measured in transplant and control hosts at 14 dpf (Fig. 3C,D). The mean eye size of cavefish hosts with transplanted surface fish neural crest was significantly larger than the eye size of sham operated controls (Fig. 3E). Moreover, the number of DiI cells in the eyes of 14 dpf cavefish hosts was positively correlated with the increase in rostral-caudal and dorso-ventral eye diameters (Fig. 3F,G,I) but not with lens diameters (Fig. F,H). The results suggest that the outer layers of the eyes are enlarged in cavefish hosts as a consequence of the incorporation of neural crest cells originating from the surface fish graft.
Fig. 3.
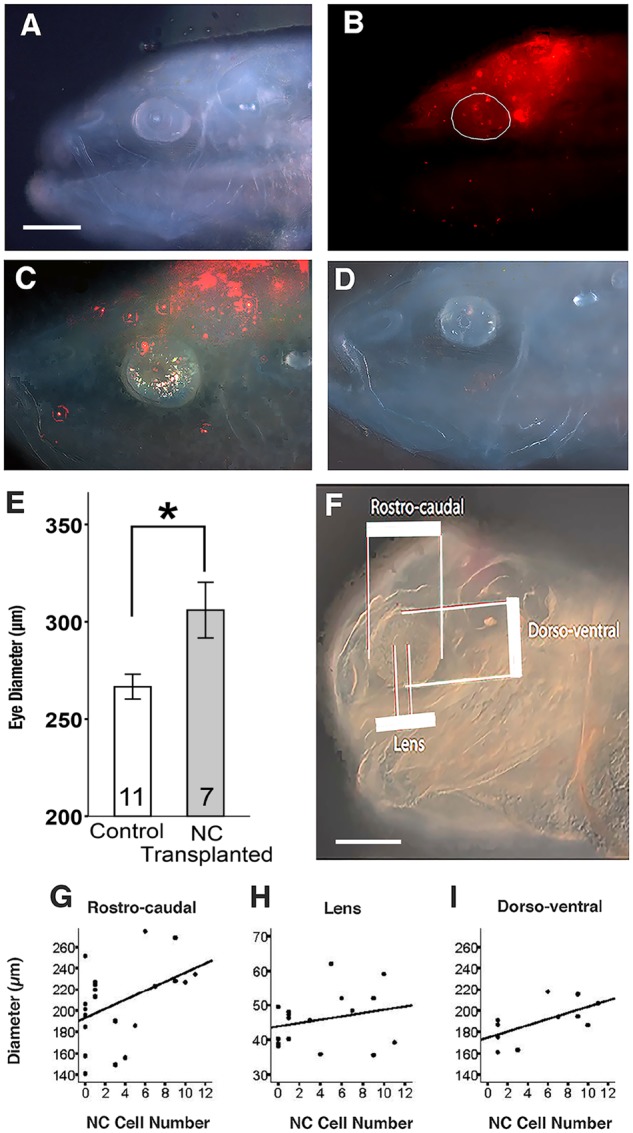
Incorporation of transplanted surface fish cranial neural crest cells into the eyes of cavefish hosts and effects on eye size. (A, B) Distribution of DiI labeled cells from a surface fish graft in the dorsal head and eyes of a cavefish host at 8 dpf. Bright field (A) and fluorescence (B) images of the same individual. The eye is circled in B. (C, D) Fluorescence images of 14 dpf cavefish host with a surface fish cranial neural crest graft (C) and a sham-operated cavefish control (D) showing the differences in eye size. Scale bar: 200 µm. In B–D, N = 8 transplantations. (E) A bar graph showing the effects of surface fish cranial neural crest transplantations on eye size in cavefish hosts at 14 dpf compared to sham operated cavefish controls. Error bars indicate standard errors of the means. The number of host or sham operated cavefish analyzed are shown at the base of the columns. *Mann–Whitney U test = 14.0; P = 0.0265. Note: the eye diameters of surface fish hosts containing surface fish cranial neural crest grafts and sham operated surface fish controls did not show any significant differences: Mann–Whitney U = 14.5; P = 0.589, N = 9 and 4, respectively. (F) Bright field image of a 14-dpf cavefish host illustrating areas measured in the regression analysis. Scale bar: 200 µm. (G–I) Regression analysis of neural crest (NC) cell number in the eyes versus rostro-caudal eye diameters (G), lens diameters (H), and dorso-ventral eye diameters (I). R2, P, and N values for G–I are 0.195, 0.045, and 21 for G (rostro-caudal diameter), 0.060, 0.342, and 17 for I (lens diameter), and 0.388, 0.041, and 11 for H (dorso-ventral diameter), respectively.
At 2-months post-fertilization, DiI labeled cells were still seen in the eyes of cavefish hosts containing surface fish neural crest transplants (Fig. 4A–E). Some of the Dil labeled cells were probably iridophores, based on their reflection of light in bright field microscopy, but others appeared to be incorporated into deeper layers of the eye, exclusive of the lens (Fig. 4A,B). The 2-month old cavefish hosts were sectioned through the eyes to determine the location of the Dil labeled cells. The results showed that DiI labeled cells were incorporated in the regions surrounding the eyes, presumably corresponding to the choroid and sclera, and also into the retinal layers (Fig. 4D,E). None of the neural or glial cells forming the retinal layers are known to be neural crest derivatives. An intriguing possibility, however, is that the invasive neural crest cells may be retinal pericytes (or their precursors), which normally line the choroid and retinal blood vessels of the eye and are the only known neural crest derivative in the retina (Trost et al. 2013).
The eyes and body grow in concert during the larval and adult life of teleosts (Fernald 1990). However, the increase in cavefish body size greatly exceeds the growth of the eyes, which arrest only a few days after fertilization (Jeffery 2009) and lack, or have extensively modified, the neural crest derived cornea, iris, and sclera. In addition to forming many of the tissues of the vertebrate head, cranial neural crest cells are known to be strong inducers of head structures produced by other germ layers (Schneider and Helms 2003; Le Douarin et al. 2012). Thus, it is probably not only the invasion of neural crest cells from the graft, but also the ability of these cells to induce the activity of other cells, that increases eye size. Our results are consistent with the hypothesis that alterations in cranial neural crest cells are important factors in cavefish eye loss.
Surface fish trunk neural crest produces body pigmentation in cavefish hosts
A procedure similar to cranial neural crest transplantation was used for transplanting trunk neural cells from surface fish donors to cavefish hosts (Fig. 1B). In 3-dpf cavefish hosts, Dil labeled cells were distributed throughout the mid-portion of the trunk, and were concentrated in the yolk epithelium (Fig. 4F–I), a site in which endogenous trunk neural crest cells have been tracked in surface fish and cavefish embryos by DiI labeling and where body melanophores appear during surface fish development (McCauley et al. 2004). In addition, the trunk neural crest transplantations revealed that melanophores labeled with DiI are present in the yolk epithelium of the cavefish hosts (Fig. 4G–I). Melanin containing pigment cells are normally not found in the body (or eyes) of Pachón cavefish, which are albinos (Protas et al. 2006; Jeffery et al. 2016). Therefore, DiI labeling of body melanophores in cavefish hosts implies that trunk neural crest cells arising from the surface fish graft were able to differentiate in the cavefish tissue environment.
The results of trunk neural crest transplantations are pertinent to whether the cavefish tissue environment in which neural crest cells migrate and differentiate is normal or defective. The appearance of DiI-labeled melanophores in the yolk epithelium suggests that the cavefish tissue environment is indeed conducive to the migration and differentiation of melanophores, and that the loss of melanin pigmentation must be due to changes in intrinsic differentiation/maturation mechanisms of cavefish melanogenic trunk neural crest cells. This conclusion is consistent with the identification of oca2, which is expressed in migratory neural crest cells (Bilandžija et al. 2013), as the mutated gene responsible for cavefish albinism (Protas et al. 2006).
Conclusions and prospectus
We have developed an effective means for exchanging neural crest cells between surface fish and cavefish embryos and used these methods to demonstrate that surface fish neural crest transplantation can affect cavefish development, thus implicating neural crest cells as key agents in cavefish eye and pigment regression. The use of transplantation will facilitate further studies to elucidate the functions of the neural crest in generating the evolutionary changes in cavefish development.
Supplementary Material
Acknowledgment
We thank Amy Parkhurst for technical assistance.
Funding
This research was supported by National Institutes of Health grant EY024941 to W.R.J. This symposium was generously sponsored by the Company of Biologists (http://www.biologists.com), the Paleontological Association (PA-GA201707), the American Microscopical Society, the Crustacean Society, and the SICB divisions DEDB, DEE, DIZ, DNB, and DPCB.
Supplementary data
Supplementary data available at ICB online.
References
- Atukorala AD, Hammer C, Dufton M, Franz-Odendaal TA.. 2013. Adaptive evolution of the lower jaw dentition in Mexican tetra (Astyanax mexicanus). Evodevo 4:28.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilandžija H, Ma L, Parkhurst A, Jeffery WR.. 2013. A potential benefit of albinism in Astyanax cavefish: downregulation of the oca2 gene increases L-tyrosine and catecholamine levels as an alternative to melanin synthesis. PLoS One 8:e80823.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culver DC, Pipan T.. 2009. The biology of caves and other subterranean habitats. Oxford (UK: ): Oxford University Press. [Google Scholar]
- Fernald RD. 1990. Teleost vision: seeing while growing. J Exp Zool 5:167–80. [DOI] [PubMed] [Google Scholar]
- Gilmour DT, Maischein H-M, Nüsslein-Volhard C.. 2002. Migration and function of a glial subtype in the vertebrate peripheral nervous system. Neuron 34:577–88. [DOI] [PubMed] [Google Scholar]
- Gross JB, Meyer B, Perkins M.. 2015. The rise of Astyanax cavefish. Dev Dyn 244:1031–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JB, Borowsky R, Tabin CJ.. 2009. A novel role for Mc1r in the parallel evolution of depigmentation in independent populations of the cavefish Astyanax mexicanus. PLoS Genet 5:e1000326.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery WR. 2001. Cavefish as a model system in evolutionary developmental biology. Dev Biol 231:1–12. [DOI] [PubMed] [Google Scholar]
- Jeffery WR. 2009. Evolution and development in the cavefish Astyanax. Curr Top Dev Biol 86:191–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery WR, Strickler AG, Guiney S, Heyser DG, Tomarev SI.. 2000. Prox 1 in eye degeneration and sensory organ compensation during development and evolution of the cavefish Astyanax. Dev Genes Evol 210:223–30. [DOI] [PubMed] [Google Scholar]
- Jeffery WR, Ma L, Parkhurst A, Bilandžija H.. 2016. Pigment regression and albinism in Astyanax cavefish In: Keene A, Yoshizawa M, McGaugh S, editors. Biology and evolution of the Mexican cavefish. New York: Elsevier; p. 155–73. [Google Scholar]
- Kelsh RN, Erickson CA.. 2013. Neural crest: origin, migration and differentiation In: Encyclopedia of life sciences. Chichester: John Wiley & Sons Ltd; http://www.els.net (doi: 10.1002/9780470015902.a0000786.pub2). [Google Scholar]
- Kulesa PM, Fraser SE.. 2000. In ovo time-lapse analysis of chick hindbrain neural crest cell migration shows cell interactions during migration to the branchial arches. Development 127:1161–72. [DOI] [PubMed] [Google Scholar]
- Kulesa PM, Bailey CM, Kasemeier-Kulesa JC, McLennan R.. 2010. Cranial neural crest migration: new routes for an old road. Dev Biol 344:543–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenberg T, Kahana A, Wszalek JA, Halloran MC.. 2008. The eye organizes neural crest cell migration. Dev Dyn 237:1645–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin N, Kalcheim C.. 1999. The neural crest. Cambridge(UK: ): Cambridge University Press. [Google Scholar]
- Le Douarin NM, Couly G, Creuzet SE.. 2012. The neural crest is a powerful regulator of pre-otic brain development. Dev Biol 366:74–82. [DOI] [PubMed] [Google Scholar]
- McCauley DW, Hixon E, Jeffery WR.. 2004. Evolution of pigment cell regression in the cavefish Astyanax: a late step in melanogenesis. Evol Dev 6:209–18. [DOI] [PubMed] [Google Scholar]
- O'Quin KE, Doshi P, Lyon A, Hoenemeyer E, Yoshizawa M, Jeffery WR.. 2015. Complex evolutionary and genetic patterns characterize the loss of scleral ossification in the blind cavefish Astyanax mexicanus. PLoS One 10:e0142208.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protas ME, Hersey C, Kochanek D, Zhou Y, Wilkens H, Jeffery WR, Zon LI, Borowsky R, Tabin CJ.. 2006. Genetic analysis of cavefish reveals molecular convergence in the evolution of albinism. Nat Genet 38:107–11. [DOI] [PubMed] [Google Scholar]
- Protas M, Jeffery WR.. 2012. Evolution and development of cave animals: from fish to crustaceans. Wiley Interdiscip Rev Dev Biol 1:823–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling TF, Kimmel CB.. 1994. Segment and cell type lineage restrictions during pharyngeal arch development in the zebrafish embryo. Development 120:483–94. [DOI] [PubMed] [Google Scholar]
- Schneider RA, Helms JA.. 2003. The cellular and molecular origins of beak morphology. Science 299:565–8. [DOI] [PubMed] [Google Scholar]
- Strickler AG, Yamamoto Y, Jeffery WR.. 2001. Early and late changes in Pax 6 expression accompany eye degeneration during cavefish development. Dev Genes Evol 211:138–44. [DOI] [PubMed] [Google Scholar]
- Trost A, Schroedl F, Lange S, Rivera FJ, Tempfer H, Korntner S, Stolt CC, Wegner M, Bogner B, Kaser-Eichberger A, et al. 2013. Neural crest origins of retinal and choroidal pericytes. Invest Ophthalmol Vis Sci 54:7910–21. [DOI] [PubMed] [Google Scholar]
- Wada H, Javidan Y, Nelson S, Carney TJ, Kelsh RN, Schilling TW.. 2005. Hedgehog signaling is required for cranial neural crest morphogenesis and chondrogenesis at the midline in the zebrafish skull. Development 132:3977–88. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Jeffery WR.. 2000. Central role for the lens in cavefish eye degeneration. Science 289:631–3. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Jeffery WR.. 2002. Probing vertebrate eye development by lens transplantation. Methods 28:420–6. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Espinasa L, Stock DW, Jeffery WR.. 2003. Development and evolution of craniofacial patterning is mediated by eye-dependent and -independent processes in the cavefish Astyanax. Evol Dev 5:435–46. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Byerly MS, Jackman WR, Jeffery WR.. 2009. Pleiotropic functions of embryonic sonic hedgehog expression link jaw and taste bud amplification with eye loss during cavefish evolution. Dev Biol 330:200–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa M, Goricki S, Soares D, Jeffery WR.. 2010. Evolution of a behavioral shift mediated by superficial neuromasts helps cavefish find food in darkness. Curr Biol 20:1631–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa M, Yamamoto Y, O'Quin KE, Jeffery WR.. 2012. Evolution of an adaptive behavior and its sensory receptors promotes eye regression in blind cavefish. BMC Biol 10:108. doi: 10.1186/1741-7007-10-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.