Abstract
Evolutionary biologists have been interested in the negative interactions among life history traits for nearly a century, but the mechanisms that would create this negative interaction remain poorly understood. One variable that has emerged as a likely link between reproductive effort and longevity is oxidative stress. Specifically, it has been proposed that reproduction generates free radicals that cause oxidative stress and, in turn, oxidative stress damages cellular components and accelerates senescence. We propose that there is limited support for the hypothesis because reactive oxygen species (ROS), the free radicals implicated in oxidative damage, are not consistently harmful. With this review, we define the hormetic response of mitochondria to ROS, termed mitochondrial hormesis, and describe how to test for a mitohormetic response. We interpret existing data using our model and propose that experimental manipulations will further improve our knowledge of this response. Finally, we postulate how the mitohormetic response curve applies to variation in animal performance and longevity.
Introduction
A long-standing dogma in evolutionary biology is that current reproduction comes at a cost to future reproduction and longevity. While there is growing understanding of the ecological factors that underlie such tradeoffs (Stearns 1976; Martin 1995; Sæther and Bakke 2000; Ricklefs 2008), the physiological mechanism(s) that would underlie a cost of reproduction has proven elusive (Ricklefs and Cadena 2007; Xu et al. 2012; Speakman and Garratt 2014). One variable that has been widely proposed as a central element in the cost of reproduction is oxidative stress (Sies and Cadenas 1985). Following the assumption that mitochondrial reactive oxygen species (ROS) emission increases as a consequence of increased ATP production, the oxidative cost of reproductive hypothesis links the high-energy demand of reproduction with the accumulation of damage from ROS (i.e., oxidative damage) that contributes to a reduction in future performance and longevity (Monaghan et al. 2009; Costantini 2014). Oxidative damage accumulates when high levels of ROS overwhelm the capacity of the mitochondrion and cell to quench these highly reactive molecules with antioxidants. The result is a condition known as oxidative stress. An accumulation of oxidative damage to proteins, lipids, and DNA has been shown to directly and indirectly damage intracellular structures (e.g., mitochondria). As a consequence, an animal’s capacity to support the energetic demands of future reproduction and processes that maintain longevity is reduced (Halliwell and Gutteridge 2015).
To test this hypothesis, investigators have most commonly measured oxidative damage in select tissue(s) and asked if oxidative damage accumulates during reproduction. The results of these studies have provided limited support for this hypothesis (Selman et al. 2012; Speakman and Garratt 2014; Blount et al. 2016). Weak support for the hypothesis is perhaps not surprising given that a key assumption of the hypothesis—that increased mitochondrial ROS emission is tied to increased mitochondrial ATP production—is incorrect (Murphy 2009; Speakman and Garratt 2014). Further, this approach is problematic because, in many cases, data do not tie an energetic or a fitness consequence with the observed oxidative damage. Not including an energetic or fitness consequence turns out to be a critical omission given that the cellular performance and longevity appear to display a hormetic, rather than linear response, to ROS emission. While the relationship between ROS production and ATP production has been addressed previously (Powers and Jackson 2008; Murphy 2009; Speakman and Garratt 2014), this essay will focus on the significance and potential consequences of the hormetic response to ROS.
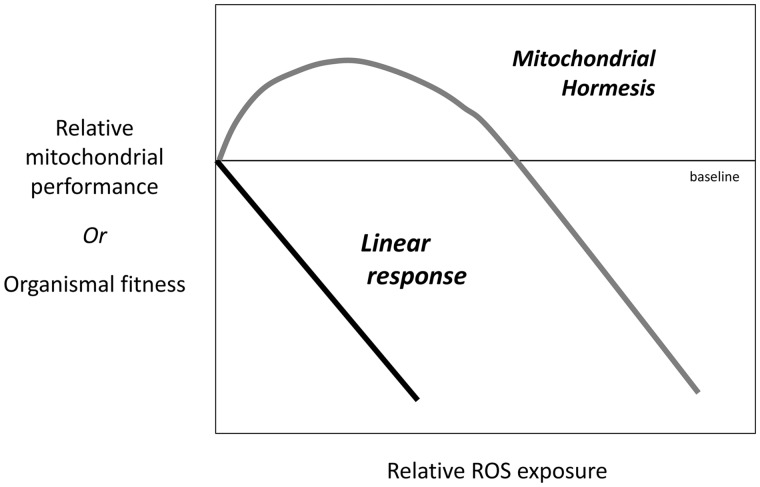
Mitochondrial hormesis (i.e., mitohormesis) describes an increase in mitochondrial performance in response to low levels of ROS and a reduction in the same variables in response to high levels of ROS (Tapia 2006; Ristow and Schmeisser 2014; Yun and Finkel 2014; Fig. 1). Building on the work of Costantini et al., who suggested a role for hormesis in the organismal response to oxidative stress (Costantini et al. 2010, 2014; Costantini 2014), we refine this model and suggest considering life history variables in a mitochondrial hormetic framework will be more effective and offer new insights into processes underlying the changing bioenergetic capacity that animals display throughout their lives.
Fig. 1.
The predicted response to ROS exposure under a linear response and mitohormetic response. The response to ROS can be indicated by its impact on mitochondrial performance or on an animal’s fitness. Under a linear response (black line), ROS becomes increasing damaging with relative exposure. Under a mitohormetic response, modest levels of ROS benefits performance while high levels negatively impact metabolism and fitness (adapted from Zhang and Hood 2016).
With this paper, we describe the characteristics required to identify a variable as hormetic. We describe the cellular responses that underlie a specific form of hormesis termed mitochondrial hormesis and recommend how to identify a mitohormetic response in cells and organisms. Finally, we will describe how mitochondrial hormesis applies to existing data, how experimental manipulation of ROS may help us to further understand how organisms respond to ROS, and briefly, postulate how the mitohormetic response could be responsible for life history variation among species. Much of this paper focuses on the potential positive effects that reproduction can have on future performance via mitochondrial hormesis. We also explain how ROS could benefit cellular performance while simultaneously contributing to an accumulation of damage that has a delayed impact on mitochondrial function that could reduce future performance and hasten senescence. Our examples focus on females for the sake of simplicity and because the energetic demand of reproduction is typically higher for females than males. As a consequence, selection on the physiological mechanisms that support reproduction is thought to be stronger in females than males (Hayssen and Orr 2017).
The hormetic response and mitochondria
A variable that exhibits hormesis displays a biphasic response to a stressor, where lower levels of exposure to the stressor are beneficial and high levels are damaging. A response can be defined as hormetic when a benefit is detected. These benefits include, but are not limited to, enhanced growth rate, increased reproductive output, and increased longevity; the mechanisms that underlie these benefits include those that protect against cellular damage or enhance metabolism (Stebbing 1982; Calabrese and Baldwin 2003). Hormetic responses include innate dose–response effects and the priming effects of repeated exposure to a stressor (Stebbing 1982; Calabrese and Baldwin 2003; Costantini et al. 2010; Costantini 2014).
Mitochondria and cells are constantly exposed to ROS and, to a lesser extent, reactive nitrogen species (RNS), and most ROS/RNS are produced as a byproduct of electron loss from the electron transport system (ETS) during oxidative phosphorylation. ROS are formed when electrons react with oxygen and RNS are formed when electrons react with nitrogen (Balaban et al. 2005), and for simplicity the term ROS is used in this review. The occurrence of ROS in the mitochondrial matrix, intermembrane space, and the cytosol is commonly associated with two rapid responses: (1) readily available antioxidants quench ROS and prevent or reduce its capacity to cause further damage, or (2) ROS oxidize existing proteins, lipids, and DNA causing oxidative damage (Sies and Cadenas 1985; Monaghan et al. 2009; Murphy 2009). A third, non-mutually exclusive response is also possible. Cells and mitochondria detect a change in levels of ROS (i.e., change in the redox environment) and the change stimulates signaling event(s) that result in a beneficial intracellular response (Dröge 2002; Ray et al. 2012; Schieber and Chandel 2014). Increases in ROS production have been shown to affect a number of signaling pathways (e.g., increase endogenous antioxidants, upregulate enzymatic processes responsible for repairing damage, and elevate mitochondrial volume within cells [Morimoto and Santoro 1998; D’Autréaux and Toledano 2007; Sano and Fukuda 2008]). When ROS levels are modest, these processes not only compensate for potential negative effects, but they can result in changes to mitochondrial and cellular function to better match the current environment. For instance, sustained positive effects of ROS have been consistently observed with regular exercise and modest caloric restriction (Sohal and Weindruch 1996; Gredilla and Barja 2005; Ristow and Schmeisser 2011; Merry and Ristow 2016). The physiological responses to increased ROS are thought to be responsible for the enhanced longevity commonly associated with each of these conditions (Radak et al. 2005). These observations led Tapia (2006) to define the response of cells to ROS exposure as a specialized form of hormesis that he called mitochondrial hormesis. The significance of this cellular and organismal response has been championed by Ristow, Yan, and others (Tapia 2006; Ristow and Zarse 2010; Ristow 2014; Yun and Finkel 2014).
Hormetic processes are likely to underlie organismal capacity to respond to numerous exogenous and endogenous variables, ultimately affecting survival and probability and timing of reproduction (Costantini et al. 2010; Costantini 2014). Previous work by Costantini et al. focused on the protective effects of hormesis against oxidative damage (Costantini et al. 2006, Costantini and Moller 2009; Costantini 2014). We take this one step further to suggest that hormetic processes may alter the respiratory performance of mitochondria, providing a direct link to the capacity of an individual to allocate energy to life history events.
How to identify mitochondrial hormesis
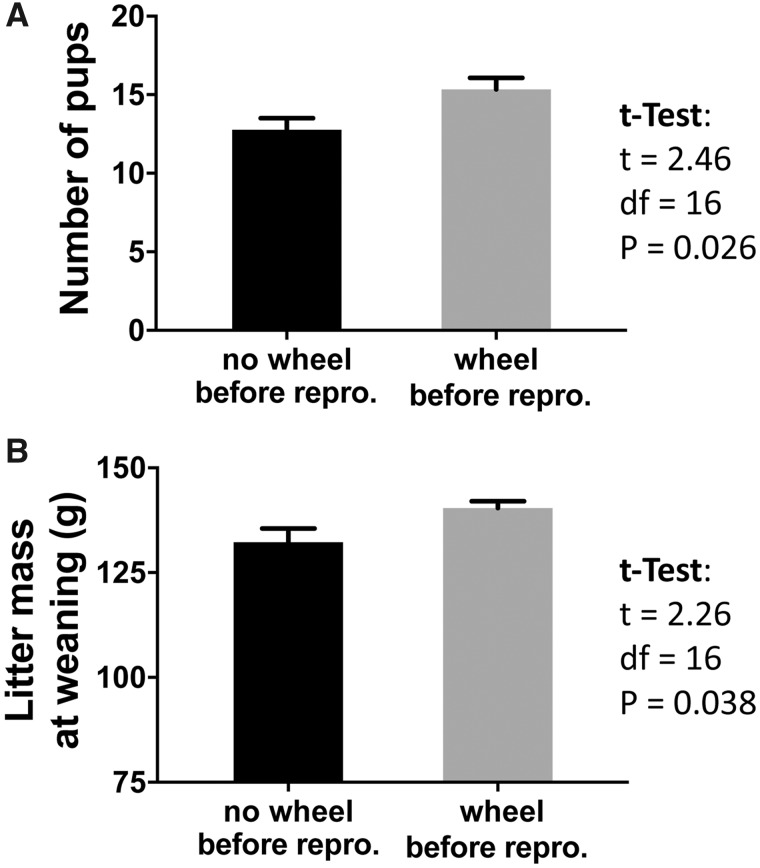
The most convincing demonstration of a hormetic response includes documentation of both the fitness benefit and mechanism that underlies the benefit. For example, Zhang et al. (2018a) compare two groups of laboratory mice, one that had access to a running wheel for 1 month before reproduction, and one that did not have access to a running wheel. A subsample from each group was collected. As predicted, the skeletal muscle mitochondria displayed higher mitochondrial respiratory capacity (respiratory control ratio, as described below) and the liver, skeletal muscle, and heart had higher mitochondrial density in mice that ran compared with mice that did not run (Zhang et al. 2018a). Then, the remaining females were bred. Females that had a wheel before reproduction gave birth to a larger litter that was heavier at weaning relative to the litters born to mice that did not have access to a wheel (Zhang et al. 2018a; Fig. 2). This improved bioenergetic capacity before reproduction and the greater allocation of energy to subsequent reproduction (both based on offspring number and mass) is highly suggestive of a mitohormetic response. The authors of this study concluded that a known stimulus of ROS production (i.e., exercise) was responsible for improved reproductive fitness.
Fig. 2.
Litter size at birth (A) and cumulative mass of the litter at weaning (B) for females with and without access to a running wheel before reproduction. Adapted from Zhang et al (2018a).
The collection of longitudinal data from the same individuals provides the ideal condition for identifying mitohormetic effects, but longitudinal studies are logistically challenging because obtaining tissue samples often necessitates sacrificing the experimental animals. Nevertheless, investigators may present data suggestive of mitohormesis. For example, in longitudinal studies investigators may find improved fitness following the detection of improvement in a physiological variable that is positively correlated with mitochondrial performance. In finite studies, investigators may find improved mitochondria performance that has the potential to benefit future fitness. For example, in the running study described above, Zhang et al. (2018a) showed that liver mitochondria of post-lactating females that had access to a running wheel displayed a higher respiratory control ratio (RCR; described below) and higher mitochondrial density. These mice also had increased lipid peroxidation, as indicated by levels of a peroxidation byproduct 4-hydroxynonenal (4HNE) (Zhang et al. 2018a). Likewise, Mowry et al. (2016) found that the liver of multiparous-year-old, wild-derived house mice displayed higher lipid peroxidation (4HNE) and a trend toward greater maximal mitochondrial phosphorylation rate (state 3 respiration, P = 0.06) relative to nulliparous mice. Despite the increase in oxidative damage observed in both studies the enhanced mitochondrial measures suggest the females had greater potential to allocate more energy to liver function at the time of sacrifice.
A reduction in mitochondrial performance commonly accompanies aging, both in the presence and absence of age-related disease (Ferguson et al. 2005; Navarro and Boveris 2007). Perhaps the most interesting question that could not be addressed by the studies described above is would the females with enhanced mitochondrial performance and greater oxidative damage have a enhanced or reduced lifespan? Several studies have shown fitness consequences of increased oxidative damage (Noguera et al. 2012; Barreto and Burton 2013; Pap et al. 2018), but this is not consistent in all species or conditions. In some instances, measures of oxidative damage in individual organs are not correlated with a reduction in fitness, indicating that the costs of carrying that damage are minimal. For example, the naked mole-rat carries 2–10× more oxidative damage in its urine and several organs relative to laboratory mice matched for physiological age (Andziak et al. 2006). Despite this high level of oxidative damage, mole-rats display high protein stability, high resistance to H2O2 induced cell death, and an exceptionally long lifespan (Perez et al. 2009). In another study, oxidative damage has been shown to be a relatively trivial player in the accumulation of mitochondrial DNA mutations in old individuals (Itsara et al. 2014; Pinto and Moraes 2015). These studies suggest that caution should be used in considering the accumulation of oxidative damage as the sole indicator of future fitness.
Methods for quantifying mitochondrial performance
Identification of mitochondrial measures that could contribute to mitohormesis should include a measure of ROS production and/or oxidative damage, and measures of mitochondrial performance. Measures of antioxidants levels can also provide valuable information for understanding the oxidative stress response. ROS production can be detected by using amplex red (Armstrong and Whiteman 2007). To detect a change in metabolic performance of mitochondria, RCR and ETS complex activity (Spinazzi et al. 2012) can be measured. RCR is a measure of the relative amount of oxygen used by mitochondria when they are working at their maximum capacity (state 3) divided by the amount of oxygen used when ADP is not available (state 4). The RCR is particularly valuable because a negative change in almost any aspect of oxidative phosphorylation will lower RCR (Brand and Nicholls 2011). Other measures, such as the P/O ratio (i.e., a measure of phosphorylation efficiency) and the enzymatic activity of ETS complexes (i.e., an index of electron transport capacity), provide valuable information. In addition, the relative number of mitochondria present is also a key variable that responds to relative ROS levels and influences ATP production by any given organ. Relative citrate synthase activity and mitochondrial copy number are common measures of relative mitochondrial density (Leek et al. 2001; Clay Montier et al. 2009). Markers of oxidative damage to lipids (e.g., 4HNE, F2-isoprostanes, malondialdehyde), proteins (e.g., protein carbonyls, protein thiol oxidation), and DNA (e.g., 8-hydroxy-2′-deoxyguanosine; comet assay) can be used to compare oxidative damage between treatment groups. Numerous assays and antibodies are also available for measuring the activity and protein levels of antioxidants. The relative accuracy and precision of each assay should be carefully considered (Powers et al. 2010).
Many investigators have measured oxidative damage and antioxidant levels in the blood as a relative indicator of oxidative stress. Blood can be collected from most vertebrates without sacrificing the animal and it, therefore, affords the investigator the opportunity to evaluate oxidative stress and collect data on the fitness of the same individual over time. However, not all biomarkers of oxidative damage and antioxidants in blood accurately reflect processes occurring in individual organs. For example, Argüelles et al. (2004) found that only serum lipid hydroperoxides (but not thiobarbituric acid reactive substances, protein carbonyls, and total antioxidant activity) were an adequate marker for oxidative damage in the liver, spleen, heart, and kidney following exposure to cytotoxic diets with varying levels of iron and manganese. In contrast, Veskoukis et al. (2009) reported that two markers not supported in the aforementioned study by Arguelles (i.e., thiobarbituric acid reactive substances and protein carbonyls), and glutathione disulfide and catalase can reflect the redox status in skeletal muscle, heart, and/or liver. Furthermore, recently described methods for evaluating the respiratory function of nucleated red blood cell mitochondria, such as in birds, are intriguing (Stier et al. 2017) but additional work is needed to understand the role that red blood cell mitochondria play in animal physiology.
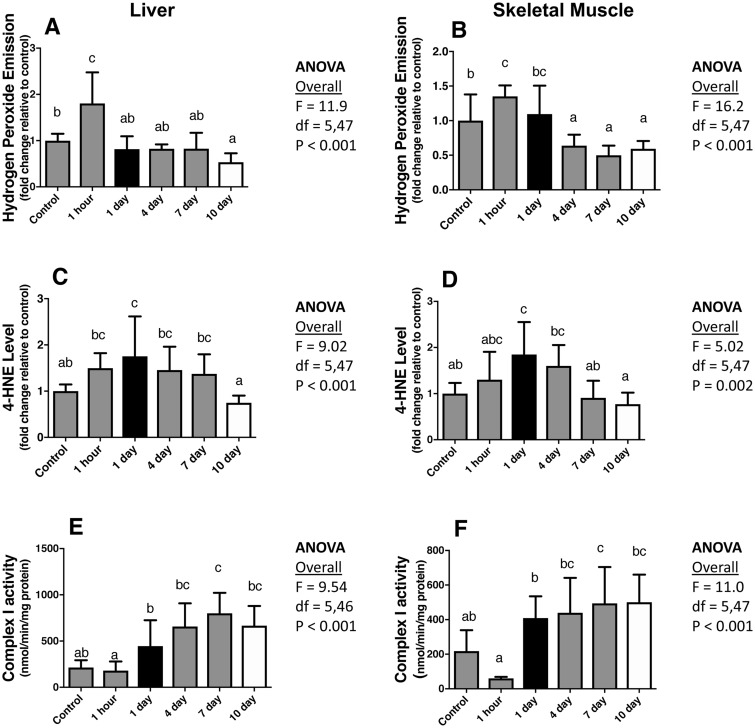
To detect whether a response to a change in ROS production is beneficial or harmful to mitochondrial function, it is also critically important to consider the timing of sample collection. Zhang et al. (2018b) recently described the temporal response to an acute increase in ROS production. X-irradiation was used as a pro-oxidant because it increases ROS while exposing the animal to few extraneous side-effects; in addition, X-irradiation evenly impacts all exposed areas (in this case, the whole animal) (Szumiel 2012; Koch and Hill 2017; Zhang et al. 2018b). In contrast, toxins such as paraquat and diquat differentially impact various organ systems and likely do so in a manner that is not similar to environmental or physiological pro-oxidants (Koch and Hill 2017). ROS emission, oxidative damage to lipids (4HNE) and proteins (protein carbonyls), and mitochondrial respiratory function of liver and skeletal muscle tissue, among other variables, were evaluated at several points following irradiation, and each were compared with non-irradiated control mice. In the liver and skeletal muscle, ROS production and oxidative damage to lipids and proteins increased in the first 1 h–1 day following irradiation (Fig. 3A–D). However, liver and skeletal muscle oxidative damage to lipids and proteins returned to baseline after 10 days, while mitochondrial ROS production decreased below the non-irradiated controls (Fig. 3A–D). Ten days after irradiation, the enzymatic activity of the liver mitochondrial complexes I, III, and IV and the enzymatic activity of skeletal muscle mitochondrial complex 1 was higher compared with the non-irradiated control mice (Fig. 3E, F). In addition, the liver mitochondrial RCR returned to control levels after a decrease that was observed at day 1 (Zhang et al. 2018b). In sum, 10 days after irradiation, the mitochondria in the liver and muscle (albeit not as pronounced) of irradiated mice outperformed control mice. Two important take-home messages of this study are that if responses to oxidative stress were quantified only in one tissue and one time point (i.e., 1 day or only 10 days post-exposure) (Fig. 3) the conclusions would be dramatically different, and it takes a considerable period of time for cells to respond to an acute oxidative event.
Fig. 3.
Change in the mitochondrial ROS production (A, B), lipid peroxidation (4HNE) (C, D), and complex 1 activity (E, F) in the liver (A, C, E) and skeletal muscle (B, D, F) of virgin wild-derived mice exposed to 500 rad x-radiation. Note that if data had only been collected 1 day post-exposure (black bars) or 10 days post-exposure (white bars), the interpretation of the response would have been very different. Different letters above the bars indicate statistical significance. Adapted from Zhang et al (2018b).
While this example represents the response to an acute pro-oxidant, the response to a lower level, chronic, or repeated increase in ROS may be quite different. Most studies have investigated the cost of reproduction during a reproductive event, but the example above highlights just how ephemeral changes in oxidative status and mitochondrial respiration can be. To evaluate changes following reproduction, we compiled data from Hyatt et al. (2018a, 2018b) and asked if RCR, ROS production, oxidative damage, or antioxidants changed between peak lactation (i.e., 14 days post-partum) and 1-week post weaning (i.e., 28 days post-partum) in Sprague-Dawley rats. We found that when compared with peak lactation, at 1-week post weaning liver mitochondrial ROS production and skeletal muscle oxidative damage (both to lipid and proteins) decreased and several liver antioxidants and skeletal muscle SOD2 increased (P < 0.05, Supplementary Materials). Any of a number of stressors including atypical temperatures, pathogen, low food availability, social stress, and aging, among others, could stimulate deviations from the baseline level of ROS being produced by the mitochondria (Costantini 2010). Thus, the challenge for researchers is selecting an appropriate time to collect data. If the goal of a study is to evaluate the impact of an oxidative event on future performance, whether associated with reproduction or otherwise, it is likely more appropriate to collect samples after organs have returned to a stable state (e.g., post weaning) rather than risking collection at time when changes are short-lived (e.g., peak lactation) and unlikely to have persistent impacts on performance (Zhang and Hood 2016).
Current models of mitochondrial hormesis and its application to life history
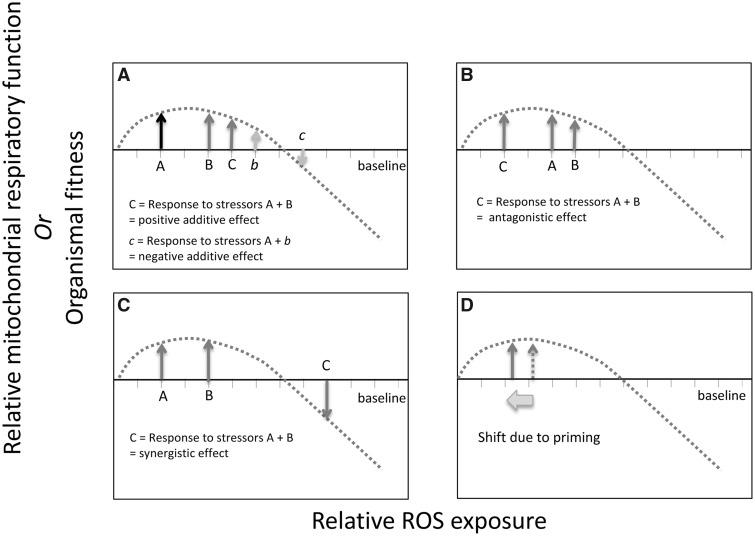
In applying mitochondrial hormesis to a life history framework, dose–response and priming effects should be expected. Dose–response effects are those that determine the immediate response to a novel stressor. Exposure to a stressor may have a positive or negative impact on cellular performance. The simultaneous response to multiple stressors, for instance if an animal is exposed to a pathogen during reproduction, can have an additive (Fig. 4A), synergistic (Fig. 4B), or antagonistic (Fig. 4C) effect (Stebbing 1982; Calabrese and Baldwin 2003). The relative impact that individual stressors have on oxidative stress and the type of interaction that occurs between multiple stressors will ultimately determine whether their interaction benefits performance or hinders it.
Fig. 4.
Effect of interacting stressors on change in ROS levels on cellular performance and fitness. Figure include examples of (A) positive and negative additive effects, synergistic effects (B), antagonistic effects (C), and priming effects (D).
For example, exercise, which is a classic example of mitochondrial hormesis, stimulates a priming effect, also referred to as adaptive effects, (Halliwell and Gutteridge 2015). Exercise is associated with regular fluctuations in ROS emission. It is a repeated stressor whose benefits may increase or be maintained when the activity occurs regularly but not continuously. ROS in skeletal muscle typically increases during intense activity but then drops lower than the untrained state between bouts of exercise (Powers and Jackson 2008). This effect makes it less likely that the threshold between a beneficial and detrimental effect of ROS will be crossed, and appears to do so by reducing the baseline ROS levels (Judge et al. 2005), resulting in a left shift along the x-axis in the mitohormetic response curve (Fig. 4D).
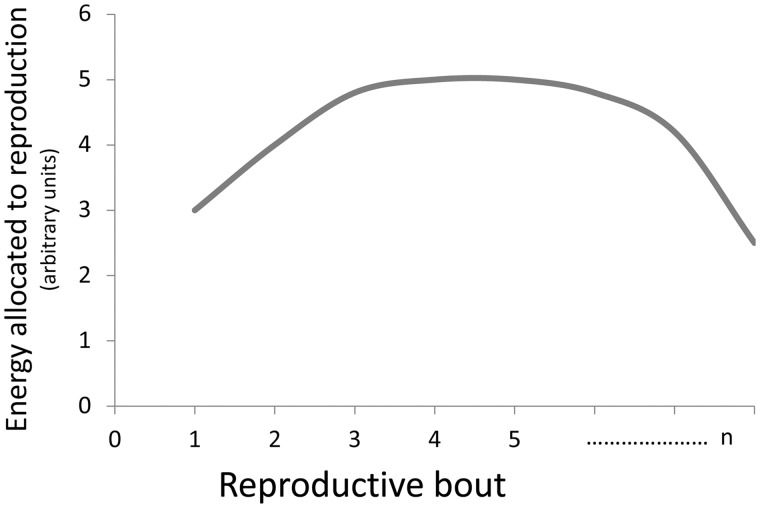
Like regular exercise, reproduction in iteroparous species is associated with numerous physiological processes that display periodicity, and thus, could allow for priming. The energy demand of the reproductive female may go up and down between reproductive events. Milk synthesis is up- and down-regulated throughout the day. The demands placed on skeletal muscle of foraging parents fluctuate with cycles of activity. The duration and intensity of each event could determine if there is an increase in ROS production during the event and whether this is ephemeral or persistent. In many species, the performance of females increases over their first few reproductive bouts (Fig. 5). While much of this change may be due to variation in experience (Lunn et al. 1994), it is also likely that some proportion of variation in performance is attributable to the priming effects of mitochondrial hormesis.
Fig. 5.
Common pattern of energy allocated to each reproductive event across the life of a female. Total energy allocated to reproduction is typically indicated by offspring number or mass but many also be indicated by direct measure of the energy content of young.
When the reproductive performance of females is evaluated throughout an animal’s life (e.g., clutch or litter size, offspring mass), reproductive performance often increases early in a female’s reproductive life, but in many species, performance also declines late in an animal’s life (Fig. 5). Again, studies of exercise physiology may provide insight into the processes that underlie how mitochondria respond to many extended and intense bouts of increasing energy expenditure. Exercise can become detrimental when bouts of activity become too long and too frequent, providing physiological systems no chance to recover (Fehrenbach and Northoff 2001). Excessive exercise can lead to DNA oxidation, strand breaks, and DNA–protein cross-links in muscle. If not adequately repaired, these changes can contribute to alterations in DNA sequence, metabolic dysfunction, and even cancer (Fehrenbach and Northoff 2001). Excessive exercise can also negatively impact organs peripheral to the skeletal muscle, as is indicated by DNA damage in circulating leukocytes, which impairs their immune function (Tuan et al. 2008). Like excessive exercise, it is feasible that repeated reproduction is associated with an accumulation of mitochondrial DNA damage. However, 70–90% of all mitochondria within an organ may have to accumulate mitochondrial DNA damage before it is possible to detect reduced mitochondrial respiratory function (Fayet et al. 2002; Trifunovic and Larsson 2008). As a consequence, the damage that accumulates in reproductive animals may not be revealed until late life. And in some cases, that threshold may never be reached; in that case, no difference in longevity between reproductive and non-reproductive animals would be revealed.
Experimental manipulation of ROS
An important caveat of the running-before-reproduction study described above (Zhang et al. 2018a) is that the relationship between RCR following running and reproductive output was merely a correlation. Numerous physiological changes underlie adaption to exercise. And thus, it is possible that the observed difference in reproductive output between groups was a consequence of a physiological process other than ROS. This could also be true of any proposed effects of reproduction on RCR. One method of confirming this effect would be to uncouple the change in ROS with other physiological variables by inducing ROS experimentally.
Experimentally induced increases or reductions in ROS can be used to confirm dose–response effects and determine if priming effects impact reproductive performance or other activities or responses. In the case of dose–response effects, different levels of ROS exposure can be used to characterize the hormetic response curve, or ROS can be altered during a specific event, such as reproduction, to determine whether the responses to ROS are additive or not. To evaluate priming effects of reproduction, ROS could be induced before the reproductive event to determine if altered ROS production changes future reproductive performance (as indicated by Zhang et al. 2018a) or ROS could be induced after reproduction to determine if reproduction impacts the ability to respond to a future ROS stressor. These approaches have been effectively applied to birds, Drosophila, and C. elgans (Wang et al. 2008; Stier et al. 2014; Yee et al. 2014; Costantini et al. 2016).
Costantini et al. (2016) increased oxidative damage in female canaries before reproduction by injecting them with DL-buthionine-(S, R)-sulfoximine. DL-buthionine-(S, R)-sulfoximine inhibits the synthesis of the antioxidant glutathione which reduces the proportion of ROS being quenched. Birds that were exposed to DL-buthionine-(S, R)-sulfoximine had higher blood oxidative damage and delayed onset of reproduction, but no difference in reproductive output relative to the controls (Costantini et al. 2016). It would have been informative to evaluate this effect across a range of DL-buthionine-(S, R)-sulfoximine doses to assess the differential effects of ROS on the onset of breeding and reproductive output. More experimental studies are needed to determine the direct role of ROS exposure on variance in reproductive performance and longevity. It would be particularly informative to confirm dose-dependent priming effects, targeted at directly revealing the positive and negative effects of ROS at different levels of exposure.
Interspecific variation in life history and the mitohormetic curve
The role that the mitohormetic response curve may play in the evolution of species-specific life-history strategies is completely unexplored and would be worthy of future investigation. Animals with longer lifespans typically display lower levels of ROS production. For example, Herrero and Barja (1998) showed that ROS production from the heart is lower in longer-lived domestic canaries (Serinus canaria) and parakeets (Melopsittacusundulatus) than shorter-lived laboratory mice (Mus musculus). In addition, Lambert et al. (2007) showed a negative correlation between longevity and heart ROS production among 10 species of mammals.
In addition, we also know that within species, ROS levels can vary among populations and these differences can correlate with multiple life history traits. Two ecotypes of the western terrestrial garter snake (Thamnophis elegans) are found in northeast California. The slow-living ecotype, which is found in grassy meadows, experiences low predation and displays low litter sizes (4.3 young) and a relatively long lifespan (8 years). In contrast, the fast-living ecotype, found at the rocky shore, experiences high predation and displays larger litter sizes (8.8 young) and relative shorter lifespans (4 years) (Bronikowski and Arnold 1999; Schwartz and Bronikowski 2011). The relatively K-selected slow-living ectomorph displays lower ROS production in the liver and has a greater efficiency of DNA repair in red blood cells compared with the relatively r-selected fast-living ectomorph (Robert and Bronikowski 2010; Schwartz and Bronikowski 2011).
It is unknown if priming or selection can change the relative shape of the mitohormetic curve, altering the threshold at which ROS becomes damaging, and the amplitude, which determines how beneficial or how detrimental a change in ROS may be. In addition, the extent that stressors of different magnitudes and frequencies influence ROS production and longevity warrants further study. Interestingly, the longest-lived rodent, the naked mole rat (Heterocephalus glaber), which displays high levels of oxidative damage but high protein stability, also displays ROS levels that fall in the center of the ROS and longevity curve (Andziak et al. 2006; Perez et al. 2008). This suggests that while their propensity to repair oxidative damage deviates from comparably sized species, the ROS levels do not (Lambert et al. 2007). This finding further emphasizes the importance of considering the type of response to ROS exhibited in life history studies.
Conclusions
Observations published in the biomedical literature provide overwhelming evidence that a change in ROS can contribute to a hormetic response. To date, comparative biologists have primarily considered the mitohormetic framework when interpreting unexpected results. We argue that the cellular and organismal response to ROS should be fundamental in forming our hypotheses and designing our studies. Taking into account the complexity of responses to ROS, it is vital to make further progress in characterizing how biogenetic processes contribute to variation in the life history patterns and fitness of individuals.
Supplementary Material
Acknowledgments
The authors would like to thank Geoff Hill and members of the Hood and Hill labs for valuable discussion and feedback on an earlier version of this manuscript. In particular, we are appreciative of the suggestions of Ashley Williams, Ryan Weaver, and Matt Powers.
Funding
This work was supported by the National Science Foundation [IOS1453784 and OIA1736150 to WRH] and the National Institutes of Health [R03 HD083654-01 to WRH and ANK]. Support for the symposium was provided by the National Science Foundation [IOS-1738378 to WRH and KS], SICB’s division of Comparative Physiology and Biochemistry and Comparative Endocrinology, the Company of Biologists, the Society of Experimental Biology, and the Canadian Society of Zoology.
Supplementary data
Supplementary data available at ICB online.
References
- Andziak B, O’Connor TP, Qi W, DeWaal EM, Pierce A, Chaudhuri AR, Van Remmen H, Buffenstein R.. 2006. High oxidative damage levels in the longest-living rodent, the naked mole-rat. Aging Cell 5:463–71. [DOI] [PubMed] [Google Scholar]
- Argüelles S, García S, Maldonado M, Machado A, Ayala A.. 2004. Do the serum oxidative stress biomarkers provide a reasonable index of the general oxidative stress status? Biochim Biophys Acta 1674:251–9. [DOI] [PubMed] [Google Scholar]
- Armstrong JS, Whiteman M.. 2007. Measurement of reactive oxygen species in cells and mitochondria. Methods Cell Biol 80:355–77. [DOI] [PubMed] [Google Scholar]
- Balaban RS, Nemoto S, Finkel T.. 2005. Mitochondria, oxidants, and aging. Cell 120:483–95. [DOI] [PubMed] [Google Scholar]
- Barreto FS, Burton RS.. 2013. Elevated oxidative damage is correlated with reduced fitness in interpopulation hybrids of a marine copepod. Proc R Soc B Biol Sci 280:20131521.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount JD, Vitikainen EIK, Stott I, Cant MA.. 2016. Oxidative shielding and the cost of reproduction. Biol Rev 91:483–97. [DOI] [PubMed] [Google Scholar]
- Brand MD, Nicholls DG.. 2011. Assessing mitochondrial dysfunction in cells. Biochem J 435:297–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronikowski AM, Arnold SJ.. 1999. The evolutionary ecology of life history variation in the garter snake Thamnophis elegans. Ecology 80:2314–25. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA.. 2003. Hormesis: the dose–response revolution. Annu Rev Pharmacol Toxicol 43:175–97. [DOI] [PubMed] [Google Scholar]
- Clay Montier LL, Deng JJ, Bai Y.. 2009. Number matters: control of mammalian mitochondrial DNA copy number. J Genet Genomics 36:125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini D. 2010. Redox physiology in animal function: the struggle of living in an oxidant environment. Curr Zool 56:687–702. [Google Scholar]
- Costantini D. 2014. Oxidative stress and hormesis in evolutionary ecology and physiology In: A marriage between mechanistic and evolutionary approaches. Berlin: Springer; p. 1-362. [Google Scholar]
- Costantini D, Casagrande S, De Filippis S, Brambilla G, Fanfani A, Tagliavini J, Dell’Omo G.. 2006. Correlates of oxidative stress in wild kestrel nestlings (Falco tinnunculus). J Comp Physiol B 176:329–37. [DOI] [PubMed] [Google Scholar]
- Costantini D, Casasole G, AbdElgawad H, Asard H, Eens M, Hopkins W.. 2016. Experimental evidence that oxidative stress influences reproductive decisions. Funct Ecol 30:1169–74. [Google Scholar]
- Costantini D, Casasole G, Eens M.. 2014. Does reproduction protect against oxidative stress? J Exp Biol 217:4237–43. [DOI] [PubMed] [Google Scholar]
- Costantini D, Metcalfe NB, Monaghan P.. 2010. Ecological processes in a hormetic framework. Ecol Lett 13:1435–47. [DOI] [PubMed] [Google Scholar]
- Costantini D, Moller AP.. 2009. Does immune response cause oxidative stress in birds? A meta-analysis. Comp Biochem Physiol A Mol Integr Physiol 153:339–44. [DOI] [PubMed] [Google Scholar]
- D’Autréaux B, Toledano MB.. 2007. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol cell Biol 8:813–24. [DOI] [PubMed] [Google Scholar]
- Dröge W. 2002. Free radicals in the physiological control of cell function. Physiol Rev 82:47–95. [DOI] [PubMed] [Google Scholar]
- Fayet G, Jansson M, Sternberg D, Moslemi A-R, Blondy P, Lombès A, Fardeau M, Oldfors A.. 2002. Ageing muscle: clonal expansions of mitochondrial DNA point mutations and deletions cause focal impairment of mitochondrial function. Neuromuscul Disord 12:484–93. [DOI] [PubMed] [Google Scholar]
- Fehrenbach E, Northoff H.. 2001. Free radicals, exercise, apoptosis, and heat shock proteins. Exerc Immunol Rev 7:66–89. [PubMed] [Google Scholar]
- Ferguson M, Mockett RJ, Shen Y, Orr WC, Sohal RS.. 2005. Age-associated decline in mitochondrial respiration and electron transport in Drosophila melanogaster. Biochem J 390:501–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gredilla R, Barja G.. 2005. The role of oxidative stress in relation to caloric restriction and longevity. Endocrinology 146:3713–7. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC.. 2015. Free radicals in biology and medicine. 5th ed. Oxford (UK: ): Oxford University Press. [Google Scholar]
- Hayssen VD, Orr T.. 2017. Reproduction in mammals: the female perspective. Baltimore (MD): John Hopkins University Press. [Google Scholar]
- Herrero A, Barja G.. 1998. H2O2 production of heart mitochondria and aging rate are slower in canaries and parakeets than in mice: sites of free radical generation and mechanisms involved. Mech Ageing Dev 103:133–46. [DOI] [PubMed] [Google Scholar]
- Hyatt HW, Zhang Y, Hood WR, Kavazis AN.. 2018. Physiological, mitochondrial, and oxidative stress differences in the presence or absence of lactation in rats. Reprod Biol Endocrinol 16:2–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt HW, Zhang Y, Hood WR, Kavazis AN.. 2018. Changes in metabolism, mitochondrial function, and oxidative stress between female rats under nonreproductive and 3 reproductive conditions. Reprod Sci 193371911876626 (. 10.1177/1933719118766264). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itsara LS, Kennedy SR, Fox EJ, Yu S, Hewitt JJ, Sanchez-Contreras M, Cardozo-Pelaez F, Pallanck LJ.. 2014. Oxidative stress is not a major contributor to somatic mitochondrial DNA mutations. PLoS Genet 10:e1003974.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge S, Jang YM, Smith A, Selman C, Phillips T, Speakman JR, Hagen T, Leeuwenburgh C.. 2005. Exercise by lifelong voluntary wheel running reduces subsarcolemmal and interfibrillar mitochondrial hydrogen peroxide production in the heart. Am J Physiol Integr Comp Physiol 289:R1564–72. [DOI] [PubMed] [Google Scholar]
- Koch RE, Hill GE.. 2017. An assessment of techniques to manipulate oxidative stress in animals. Funct Ecol 31:9–21. [Google Scholar]
- Lambert AJ, Boysen HM, Buckingham JA, Yang T, Podlutsky A, Austad SN, Kunz TH, Buffenstein R, Brand MD.. 2007. Low rates of hydrogen peroxide production by isolated heart mitochondria associate with long maximum lifespan in vertebrate homeotherms. Aging Cell 6:607–18. [DOI] [PubMed] [Google Scholar]
- Leek BT, Mudaliar SRD, Henry R, Mathieu-Costello O, Richardson RS.. 2001. Effect of acute exercise on citrate synthase activity in untrained and trained human skeletal muscle. Am J Physiol Integr Comp Physiol 280:R441–7. [DOI] [PubMed] [Google Scholar]
- Lunn NJ, Boyd IL, Croxall JP.. 1994. Reproductive performance of female antarctic fur seals: the influence of age, breeding experience, environmental variation and individual quality. J Anim Ecol 63:827. [Google Scholar]
- Martin TE. 1995. Avian life-history evolution in relation to nest sites, nest predation, and food. Ecol Monogr 65:101–27. [Google Scholar]
- Merry TL, Ristow M.. 2016. Mitohormesis in exercise training. Free Radic Biol Med 98:123–30. [DOI] [PubMed] [Google Scholar]
- Monaghan P, Metcalfe NB, Torres R, Dev S.. 2009. Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecol Lett 12:75–92. [DOI] [PubMed] [Google Scholar]
- Morimoto RI, Santoro MG.. 1998. Stress-inducible responses and heat shock proteins: new pharmacologic targets for cytoprotection. Nat Biotechnol 16:833–8. [DOI] [PubMed] [Google Scholar]
- Mowry AV, Kavazis AN, Sirman, AE, Potts WK, Hood WR.. 2016. Reproduction does not adversely affect liver mitochondrial respiratory function but results in lipid peroxidation and increased antioxidants in house mice. PLoS ONE 11:e0160883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MP. 2009. How mitochondria produce reactive oxygen species. Biochem J 417:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro A, Boveris A.. 2007. The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol 292:C670–86. [DOI] [PubMed] [Google Scholar]
- Noguera JC, Kim S-Y, Velando A.. 2012. Pre-fledgling oxidative damage predicts recruitment in a long-lived bird. Biol Lett 8:61–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pap PL, Vincze O, Fülöp A, Székely-Béres O, Pătraș L, Pénzes J, Vágási CI.. 2018. Oxidative physiology of reproduction in a passerine bird: a field experiment. Behav Ecol Sociobiol 72:18. [Google Scholar]
- Perez VI, Buffenstein R, Masamsetti P, Salmon AB, Mele J, Friguet B, Ward W, Richardson A, Chaudhuri A.. 2008. Maintenance of protein stability and resistance to oxidative stress during aging in the longest living rodent, the naked mole-rat; are these determinants of longevity? Free Radic Biol Med 45:S99. [Google Scholar]
- Perez VI, Buffenstein R, Masamsetti V, Leonard S, Salmon AB, Mele J, Andziak B, Yang T, Edrey Y, Friguet B, et al. 2009. Protein stability and resistance to oxidative stress are determinants of longevity in the longest-living rodent, the naked mole-rat. Proc Natl Acad Sci U S A 106:3059–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto M, Moraes CT.. 2015. Mechanisms linking mtDNA damage and aging. Free Radic Biol Med 85:250–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers SK, Jackson MJ.. 2008. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 88:1243.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers SK, Smuder AJ, Kavazis AN, Hudson MB.. 2010. Experimental guidelines for studies designed to investigate the impact of antioxidant supplementation on exercise performance. Int J Sport Nutr Exerc Metab 20:2–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radak Z, Chung HY, Goto S.. 2005. Exercise and hormesis: oxidative stress-related adaptation for successful aging. Biogerontology 6:71–5. [DOI] [PubMed] [Google Scholar]
- Ray PD, Huang B-W, Tsuji Y.. 2012. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 24:981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklefs RE. 2008. The evolution of senescence from a comparative perspective. Funct Ecol 22:379–92. [Google Scholar]
- Ricklefs RE, Cadena CD.. 2007. Lifespan is unrelated to investment in reproduction in populations of mammals and birds in captivity. Ecol Lett 10:867–72. [DOI] [PubMed] [Google Scholar]
- Ristow M. 2014. Unraveling the truth about antioxidants: mitohormesis explains ROS-induced health benefits. Nat Med 20:709–11. [DOI] [PubMed] [Google Scholar]
- Ristow M, Schmeisser K.. 2014. Mitohormesis: promoting health and lifespan by increased levels of reactive oxygen species (ROS). Dose Response 12:288–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristow M, Schmeisser S.. 2011. Extending life span by increasing oxidative stress. Free Radic Biol Med 51:327–36. [DOI] [PubMed] [Google Scholar]
- Ristow M, Zarse K.. 2010. How increased oxidative stress promotes longevity and metabolic health: the concept of mitochondrial hormesis (mitohormesis). Exp Gerontol 45:410–8. [DOI] [PubMed] [Google Scholar]
- Robert KA, Bronikowski AM.. 2010. Evolution of senescence in nature: physiological evolution in populations of garter snake with divergent life histories. Am Nat 175:147–59. [DOI] [PubMed] [Google Scholar]
- Sæther B-E, Bakke Ø.. 2000. Avian life history variation and contribution of demographic traits to the population growth rate. Ecology 81:642–53. [Google Scholar]
- Sano M, Fukuda K.. 2008. Activation of mitochondrial biogenesis by hormesis. Circ Res 103:1191.. [DOI] [PubMed] [Google Scholar]
- Schieber M, Chandel NSS.. 2014. ROS function in redox signaling and oxidative stress. Curr Biol 24:R453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz T, Bronikowski AM. 2011. Molecular stress pathways and the evolution of life histories in reptiles. In: Flatt T, Heyland A, editors. Mechanisms of life history evolution: the genetics and physiology of life history traits and trade-offs. Oxford: Oxford University Press. p. 193-209.
- Selman C, Blount JD, Nussey DH, Speakman JR.. 2012. Oxidative damage, ageing, and life-history evolution: where now? Trends Ecol Evol 27:570–77. [DOI] [PubMed] [Google Scholar]
- Sies H, Cadenas E.. 1985. Oxidative stress: damage to intact cells and organs. Philos Trans R Soc Lond B Biol Sci 311:617–31. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R.. 1996. Oxidative stress, caloric restriction, and aging. Science 273:59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman JR, Garratt M.. 2014. Oxidative stress as a cost of reproduction: beyond the simplistic trade-off model. BioEssays 36:93–106. [DOI] [PubMed] [Google Scholar]
- Spinazzi M, Casarin A, Pertegato V, Salviati L, Angelini C.. 2012. Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat Protoc 7:1235.. [DOI] [PubMed] [Google Scholar]
- Stearns SC. 1976. Life-history tactics—review of ideas. Q Rev Biol 51:3–47. [DOI] [PubMed] [Google Scholar]
- Stebbing ARD. 1982. Hormesis—the stimulation of growth by low levels of inhibitors. Sci Total Environ 22:213–34. [DOI] [PubMed] [Google Scholar]
- Stier A, Bize P, Roussel D, Schull Q, Massemin S, Criscuolo F.. 2014. Mitochondrial uncoupling as a regulator of life-history trajectories in birds: an experimental study in the zebra finch. J Exp Biol 217:3579–89. [DOI] [PubMed] [Google Scholar]
- Stier A, Romestaing C, Schull Q, Lefol E, Robin J-P, Roussel D, Bize P.. 2017. How to measure mitochondrial function in birds using red blood cells: a case study in the king penguin and perspectives in ecology and evolution. Methods Ecol Evol 8:1172–82. [Google Scholar]
- Szumiel I. 2012. Radiation hormesis: autophagy and other cellular mechanisms. Int J Radiat Biol 88:619–28. [DOI] [PubMed] [Google Scholar]
- Tapia PC. 2006. Sublethal mitochondrial stress with an attendant stoichiometric augmentation of reactive oxygen species may precipitate many of the beneficial alterations in cellular physiology produced by caloric restriction, intermittent fasting, exercise and dietary phytonutrients. Med Hypotheses 66:832–43. [DOI] [PubMed] [Google Scholar]
- Trifunovic A, Larsson NG.. 2008. Mitochondrial dysfunction as a cause of ageing. J Intern Med 263:167–78. [DOI] [PubMed] [Google Scholar]
- Tuan T-C, Hsu T-G, Fong M-C, Hsu C-F, Tsai KKC, Lee C-Y, Kong C-W.. 2008. Deleterious effects of short-term, high-intensity exercise on immune function: evidence from leucocyte mitochondrial alterations and apoptosis. Br J Sports Med 42:11–5. [DOI] [PubMed] [Google Scholar]
- Veskoukis AS, Nikolaidis MG, Kyparos A, Kouretas D.. 2009. Blood reflects tissue oxidative stress depending on biomarker and tissue studied. Free Radic Biol Med 47:1371–4. [DOI] [PubMed] [Google Scholar]
- Wang Z, Liu R, Wang A, Du L, Deng X.. 2008. Phototoxic effect of UVR on wild type, ebony and yellow mutants of Drosophila melanogaster: life span, fertility, courtship and biochemical aspects. Sci China C Life Sci 51:885–93. [DOI] [PubMed] [Google Scholar]
- Xu YC, Yang DB, Wang DH.. 2012. No evidence for a trade-off between reproductive investment and immunity in a rodent. PLoS One 7:e37182.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee C, Yang W, Hekimi S.. 2014. The intrinsic apoptosis pathway mediates the pro-longevity response to mitochondrial ROS in C. elegans. Cell 157:897–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun J, Finkel T.. 2014. Mitohormesis. Cell Metab 19:757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Brasher AL, Park NR, Taylor HA, Kavazis AN, Hood WR.. 2018a. High activity before breeding improves reproductive performance by enhancing mitochondrial function and biogenesis. J Exp Biol 221:jeb177469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hood WR.. 2016. Current versus future reproduction and longevity: a re-evaluation of predictions and mechanisms. J Exp Biol 219:3177–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Humes F, Almond G, Kavazis AN, Hood WR.. 2018b. A mitohormetic response to pro-oxidant exposure in the house mouse. Am J Physiol Regul Integr Comp Physiol 314:R122.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.