Abstract
Background
Interest in the use of faecal microbiota transplantation (FMT) in inflammatory bowel disease (IBD) has increased following outcomes in patients with Clostridioides difficile infection (CDI). While research exploring clinician awareness and attitude towards the use of FMT in CDI has been carried out, data for IBD are currently lacking.
Objective
To assess the perceptions of gastroenterologists and current practice relating to FMT as a treatment for IBD in the UK.
Design
A web-based survey (Snap Survey software) was distributed through the British Society of Gastroenterology (BSG) and British Society of Paediatric Gastroenterology, Hepatology and Nutrition e-newsletters, and at the BSG Conference in June 2017.
Results
61 respondents completed the survey including presubspecialty trainees, gastroenterology specialists, associate specialists and consultants. Most (95%; n=58) respondents stated that they had heard of FMT being used as a treatment for IBD prior to participating in the survey. Based on current evidence, 34% (n=21) of respondents would consider using FMT in patients with IBD, 26% (n=16) would not and 39% (n=24) were undecided. When asked to rank routes of delivery in terms of preference, nasogastric tube was the least preferred route (39%; n=24) and oral capsule was the most preferred route (34%; n=21).
Conclusions
A clear majority of UK gastroenterologists recognise FMT as a potential treatment for IBD; however, uptake is limited. A proportion of clinicians would consider FMT in IBD and the majority would consider entering patients into clinical trials. Future work should explore the utility and efficacy of oral FMT capsules in IBD.
Keywords: ulcerative colitis, IBD, crohn’s disease
Introduction
Faecal microbiota transplantation (FMT) is a medical treatment that involves the transfer of stool into the intestinal tract of a recipient. Various routes have been trialled ranging from colonoscopy to enteric coated capsules1; however, the optimal delivery method is currently unknown. The most common form of FMT involves the transfer of faecal material from a healthy donor into a patient. It should however be noted that FMT can also be autologous, where a faecal microbiota is banked by an individual for reinstatement at a later date.2 3
FMT is an effective treatment for recurrent Clostridioides difficile (previously Clostridium difficile) infection4 (CDI) with several randomised controlled trials (RCT) reporting a primary cure rate of 85%–95% in patients where antimicrobial chemotherapy has failed.5 Serious adverse events have been reported in the literature; however, these are uncommon.5 The majority of adverse events are mild, self-limiting and gastrointestinal in nature. These results prompted the National Institute for Health and Care Excellence (NICE) to issue full guidance in 2014 stating that the evidence on the safety and efficacy of FMT is adequate to support the use of FMT.1
The expanding body of data demonstrating FMT’s efficacy in CDI has prompted researchers to investigate its efficacy in inflammatory bowel disease (IBD). To date, there have been four RCTs published on FMT in ulcerative colitis (UC), three of which have been published in full6–8 and one of which has only been published in abstract.9 In these four early RCTs, FMT induced clinical remission in 28% of patients compared with 9% of patients in the placebo groups.10 There have been no RCTs published to date in Crohn’s disease (CD); however, scattered case reports and cohort studies show promise.11
Clinician perception towards FMT within the UK has been previously investigated for CDI,12 13 however, there has been no formal survey investigating the opinions of clinicians on the use of FMT in IBD. This, coupled with the documented surge in patient and clinician interest,14 led to the creation of this survey.
Methods
The survey was developed following a literature review of research and opinion articles concerning FMT and IBD and agreed by consensus of all authors. The survey was administered through Snap Survey software (https://www.snapsurveys.com/) and was targeted at members of the British Society of Gastroenterology (BSG) and British Society of Paediatric Gastroenterology, Hepatology and Nutrition through May and June newsletters and attendance at the BSG annual meeting held in Manchester in June 2017. The data were downloaded by the University of Aberdeen IT department prior to analysis by the authors.
Results
Sixty-one respondents completed the survey including presubspecialty trainees (n=4), gastroenterology specialists (n=13), associate specialists (n=3) and consultants (n=41). Eighty-two per cent (n=50) were adult gastroenterologists and 18% (n=11) were paediatric gastroenterologists. The participants were asked to provide the first three characters of the postcode of the hospital they were based at in order to illustrate the distribution of respondents across the UK. There was a wide variety of geographical representation by postcode in survey respondents (figure 1).
Figure 1.
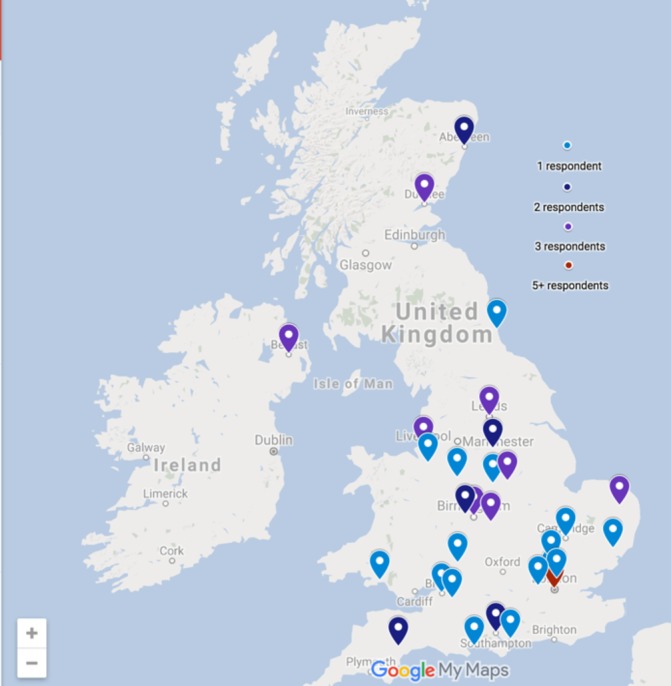
Geographical representation of respondents to the survey. Survey participants were asked to input the first three letters and numbers of postcode of the hospital that they work in. This image was generated using Google My Maps.
All respondents had heard of FMT prior to participating in the survey and 38% (n=23) had performed FMT for a patient previously with CDI. The number of FMT procedures performed by each clinician ranged from 1 to 200 with a mean of 15 procedures.
Almost all (95%; n=58) respondents had heard of FMT being used as a treatment for IBD. Based on current evidence, 34% (n=21) would consider using FMT in these patients. Twenty-six per cent (n=16) would not and 39% (n=24) were undecided. Of those who would consider using FMT in IBD, 62% (n=13) would consider implementing multidonor FMT into their practice.
With regard to whether to implement IBD in patients during a period of relapse, remission or both, 45% (n=9) of respondents chose relapse and 48% (n=10) chose both.
Of the respondents who would not consider using FMT in patients with IBD, 50% selected ‘Randomised control trial demonstrating short term safety and a statistically significant difference in remission rate when FMT is compared with placebo’ as the level of evidence required for them to implement FMT into the IBD care management pathway. Thirty-one per cent selected ‘NICE guidelines supporting the use of FMT in patients with IBD’.
Out of the 61 respondents, 25% (n=15) stated they routinely see patients under 16 years of age. These respondents were asked additional questions exploring their views on the use of FMT in paediatric patients. Based on current evidence, 40% (n=6) would consider using FMT in paediatric patients while 20% (n=3) were undecided.
Regarding the most suitable placebo for FMT trials, 48% of respondents (n=29) chose autologous FMT and 44% (n=27) selected water enema. The remaining respondents selected ‘other’.
A significant proportion of respondents (82%; n=50) stated that cost savings would influence their decision to add FMT to their routine clinical practice. Forty-three per cent of respondents said that a patient had expressed interest in FMT and a small proportion (10%; n=6) said that they were aware of a patient who has undertaken FMT on their own without medical supervision. A total of 12% (n=7) of respondents stated that they have performed FMT for a patient with IBD within the last 5 years. Only 5% (n=3) of respondents said that they have performed FMT for a patient with IBD within the last year however.
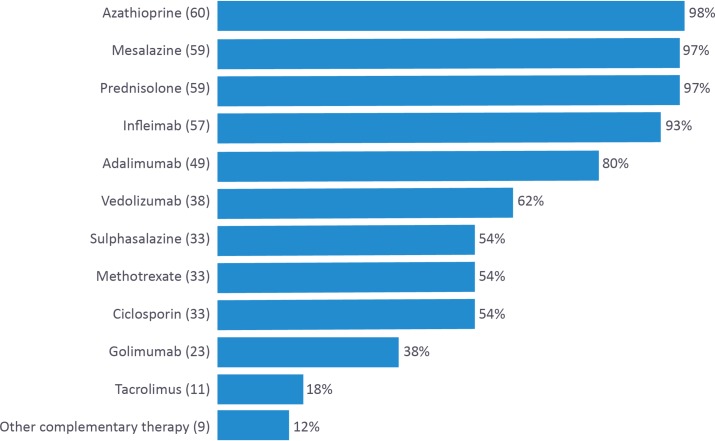
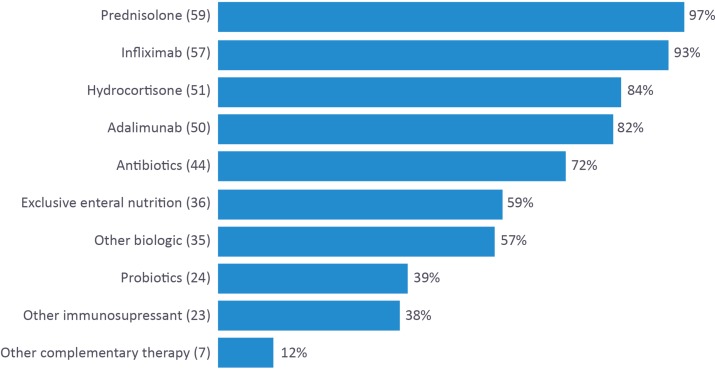
Participants were then asked to list treatments that they had personally recommended to patients with UC (figure 2) and CD, respectively (figure 3).
Figure 2.
A list of treatments currently recommended for patients with ulcerative colitis by UK-based gastroenterology trainees and consultants.
Figure 3.
A list of treatments currently recommended for patients with Crohn’s disease by UK-based gastroenterology trainees and consultants.
Regarding FMT delivery methods, a total of 21 respondents (34%) selected oral capsule as their preferred method and 24 respondents (39%) selected nasogastric tube as their least preferred method (figure 4). Respondents were then asked to rank their concerns regarding the use of FMT in patients with IBD in order or importance (figure 5). Lack of efficacy was the most important factor for 22 (36%) respondents and transfer of phenotype and trigger of relapse were the most commonly cited least concerning factors for 11 clinicians (18%).
Figure 4.
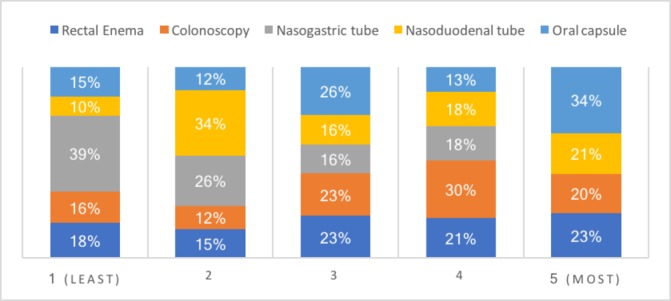
An overview of faecal microbiota transplantation (FMT) delivery method preferences where 1 is the least preferred and 5 is the most preferred.
Figure 5.
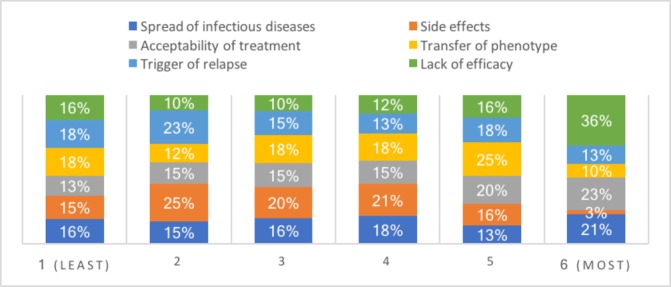
An overview of concerns regarding the use of faecal microbiota transplantation (FMT) in patients with inflammatory bowel disease (IBD) where 1 is ‘least concerning’ and 6 is ‘most concerning’.
Finally, respondents were asked if they would consider entering patients with IBD into clinical FMT trials. A total of 95% reported yes.
Discussion
Research shows that patients are receptive to FMT.15 However, there have been no data published on perceptions and practice regarding FMT and IBD to date. This survey offers the first data on clinician’s views of FMT and IBD in the UK and provides information relating to current practice, as well as perceptions and opinions relating to this emerging medical treatment.
Although there was wide geographical representation among respondents, as with all surveys, this research suffers from a risk of selection bias. Nevertheless, the term FMT now appears to be ubiquitous in the field of gastroenterology, as evidenced by 100% of the respondents having heard of the treatment prior to participating and 95% having heard of it being used as a treatment for IBD. An observation that warrants discussion is that the majority of respondents were consultant clinicians (67%). This may have impacted the results of the survey as the implementation and delivery of the procedure would likely be consultant led. Future research could investigate the differences in opinions and perceptions towards FMT in IBD between consultants and less senior clinicians in greater detail.
Interestingly, half of those who were undecided or were not in favour of implementing FMT into the IBD care pathway based on the currently available evidence specified that they required RCT evidence in order to consider the treatment, despite there being three RCTs published in full form to date.6–9 This perhaps suggests that the available evidence has not reached a ‘trigger-point’ for widespread adoption in terms of achievable efficacy, not yet penetrated sufficiently deeply into the gastroenterology community to change practice, or met a mismatch of available logistics and expertise to the adoption of widespread FMT. In support of this, 48% of respondents who stated they would use FMT in patients with IBD said they would do so in both remission and relapse. However, there hasbeen no controlled evidence to date investigating the efficacy of FMT in patients in remission, where it might arguably be better suited to altering the host microbiome.
Although 34% of responders would consider using FMT in IBD, only 7% had performed FMT in these patients. This may, at least in part, explain why 10% of respondents were aware of a patient who has undertaken FMT on their own. Lack of FMT uptake among clinicians could be due to the costs and logistical factors associated with finding donors and screening stool samples, which has been widely reported. Another reason may be that current methods of administration are invasive and are therefore not well suited for repeat FMT. The use of orally delivered encapsulated FMT produced from a regulated facility might circumnavigate these issues and indeed this was the most popular route of delivery in this survey. However, clearly the efficacy of such a route would need to be established too. In keeping with the apparent unmet need, there are organisations moving this concept forward both in the UK and the USA.16 17
There was no consensus among respondents as to what the optimal choice of placebo for FMT trials is. Surprisingly, 48% of respondents selected autologous FMT as their preferred choice. Autologous FMT is not biologically inert and has a known physiological effect.10 18 In support of this, a total of 20% of respondents who received the placebo in the RCT conducted by Rossen et al reached the primary end-point of the study, which is remarkably high.6 A total of 44% of patients selected water enema as the most suitable placebo. However, clearly this approach is not suited to a double-blind clinical trial. The optimal placebo for FMT trials is not yet established and requires further considered research.
A further result that warrants consideration was that 62% of respondents stated that they would consider implementing multidonor FMT into their practice. While this approach was implemented in the positive RCTs conducted by Costello et al 9 and Paramsothy et al,8 this approach potentially increases the risk of transmitted infections between the donors and the recipient. Furthermore, in the case of a suspected adverse event relating to a transmitted pathogen, the mixed donor approach would make identifying the source of the pathogen logistically challenging. FMT is regulated as a medicinal product in the UK and mixing donations could significantly increase the regulatory burden on the provider. In addition, this approach also makes identifying microbial signals of efficacy and engraftment more challenging. While FMT may not yet deliver striking efficacy in patients with IBD, when combined with robust microbial analysis of donors and patients, it can serve as a highly powerful research tool that might inform the design of rationally selected cocktails of bacteria that could ultimately replace FMT. Finally, during the use of mixed donations, inadvertent ‘friendly fire’ might occur between the combined donor microbial ecosystems.
Conclusion
This survey demonstrates that UK gastroenterologists recognise FMT as a potential treatment for IBD. The majority of clinicians would enter patients into FMT clinical trials, and approximately one in three clinicians would consider using FMT in IBD based on current evidence. Most clinicians who are currently unwilling to implement FMT require further evidence through RCTs or NICE guidance before implementing the treatment into their routine clinical practice. Orally delivered encapsulated FMT is the preferred route of administration and future work should focus on exploring the efficacy of this route and making it an easy to access option for clinicians.
Significance of this study.
What is already known on this topic
To date, four double-blind randomised controlled trials have been conducted investigating the efficacy of faecal microbiota transplantation (FMT) in patients with ulcerative colitis, three of which reported statistical superiority over the placebo arm. Previous research has explored clinician awareness and attitude towards the use of FMT in Clostridioides difficile infection, which revealed clinicians believe that the evidence base favours FMT for this indication. In contrast, data for inflammatory bowel disease (IBD) are currently lacking, with no formal survey of UK gastroenterologists being published to date.
What this study adds
The survey found that a clear majority of UK-based gastroenterologists from a range of medical career grades recognise FMT as a potential treatment for IBD. Approximately one in three clinicians would consider using FMT in IBD based on current evidence; however, uptake is limited. Worryingly, 10% of respondents were aware of a patient who has undertaken FMT on their own. The majority of clinicians would enter their patients into FMT clinical trials; however, in our survey there was no consensus as to what the optimal placebo arm should be in these studies.
How might it impact on clinical practice in the foreseeable future
This survey offers the first data on clinician’s views of FMT and IBD in the UK and provides information relating to current practice, as well as perceptions and opinions relating to this emerging medical treatment. Our results suggest that the available evidence has not reached a ‘trigger-point’ for widespread adoption in terms of achievable efficacy, not yet penetrated sufficiently deeply into the gastroenterology community to change practice, or met a mismatch of available logistics and expertise to the adoption of widespread FMT.
Acknowledgments
The authors are grateful to Dr Keith Lindley, Dr Rafeeq Muhammed and Mrs Carla Lloyd for their support in surveying the membership of BSPGHAN as well as Julie Solomon for her support in surveying the membership of BSG. The authors are also grateful to Olwynn Say and David Ritchie from the Data Management team at the University of Aberdeen for supporting data collection. The authors acknowledge the link that has been published in an abstract supplement: https://academic.oup.com/ecco-jcc/article/12/supplement_1/S443/4808310.
Footnotes
Contributors: GLH is the guarantor of the article. JRM, NN, AH, RH and GLH reviewed the literature and developed the survey. JRM and NN prepared the manuscript. All authors have revised the manuscript critically and prepared its final version. All authors approved the final draft prior to submission.
Funding: RH is funded by a Career Researcher Fellowship from NHS Research Scotland. The Glasgow Paediatric IBD team is supported by the Catherine McEwan Foundation.
Competing interests: JRM has received salary, consultancy fees and other from EnteroBiotix Limited and Biotechspert Limited during the conduct of the study. RH has received speaker’s fees, consultancy fees or travel support from Nutricia, MSD Immunology, Dr Falk and 4D Pharma. GLH has received consultancy fees or travel support from Nutricia and 4D Pharma. AH has served as consultant, advisory board member or speaker for AbbVie, Atlantic, Bristol-Myers Squibb, Celltrion, Falk, Ferring, Janssen, MSD, Napp Pharmaceuticals, Pfizer, Pharmacosmos, Shire and Takeda. She also serves on the Global Steering Committee for Genentech. NN has nothing to disclose.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Correction notice: This article has been corrected since it published Online First. The joint author statement has been added.
Presented at: The abstract was displayed in poster format at the 13th Congress of The European Crohn’s and Colitis Organisation (ECCO) in Vienna, February 2018.
References
- 1. Nice N. Faecal microbiota transplant for recurrent Clostridium difficile infection: NICE Interventional Procedure Guidance. 2014:485.
- 2. Bulrow C, Langdon A, Hink T, et al. . Impact of Amoxicillin/clavulanate and autologous fecal microbiota transplantation (fmt) on the fecal microbiome and resistome. Open Forum Infectious Diseases 2016;3:2228. [Google Scholar]
- 3. McIlroy J, Ianiro G, Mukhopadhya I, et al. . Review article: the gut microbiome in inflammatory bowel disease-avenues for microbial management. Aliment Pharmacol Ther 2018;47:1–17. 10.1111/apt.14384 [DOI] [PubMed] [Google Scholar]
- 4. Lawson PA, Citron DM, Tyrrell KL, et al. . Reclassification of clostridium difficile as clostridioides difficile (Hall and O’Toole 1935) Prévot 1938. Anaerobe 2016;40:95–9. 10.1016/j.anaerobe.2016.06.008 [DOI] [PubMed] [Google Scholar]
- 5. Quraishi MN, Widlak M, Bhala N, et al. . Systematic review with meta-analysis: the efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Aliment Pharmacol Ther 2017;46:479–93. 10.1111/apt.14201 [DOI] [PubMed] [Google Scholar]
- 6. Rossen NG, Fuentes S, van der Spek MJ, et al. . Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology 2015;149:110–8. 10.1053/j.gastro.2015.03.045 [DOI] [PubMed] [Google Scholar]
- 7. Moayyedi P, Surette MG, Kim PT, et al. . Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology 2015;149:102–9. 10.1053/j.gastro.2015.04.001 [DOI] [PubMed] [Google Scholar]
- 8. Paramsothy S, Kamm MA, Kaakoush NO, et al. . Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. The Lancet 2017;389:1218–28. 10.1016/S0140-6736(17)30182-4 [DOI] [PubMed] [Google Scholar]
- 9. Costello S, Waters O, Bryant R, et al. . OP036 Short duration, low intensity pooled faecal microbiota transplantation induces remission in patients with mild-moderately active ulcerative colitis: a randomised controlled trial. Journal of Crohn’s and Colitis 2017;11:S23 10.1093/ecco-jcc/jjx002.035 [DOI] [Google Scholar]
- 10. Costello SP, Soo W, Bryant RV, et al. . Systematic review with meta-analysis: faecal microbiota transplantation for the induction of remission for active ulcerative colitis. Aliment Pharmacol Ther 2017;46:213–24. 10.1111/apt.14173 [DOI] [PubMed] [Google Scholar]
- 11. Paramsothy S, Paramsothy R, Rubin DT, et al. . Faecal microbiota transplantation for inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis 2017;11:1180–99. 10.1093/ecco-jcc/jjx063 [DOI] [PubMed] [Google Scholar]
- 12. Porter RJ, Fogg C. Faecal microbiota transplantation for Clostridium difficile infection in the United Kingdom. Clin Microbiol Infect 2015;21:578–82. 10.1016/j.cmi.2015.01.020 [DOI] [PubMed] [Google Scholar]
- 13. Quraishi MN, Segal J, Mullish B, et al. . National survey of practice of faecal microbiota transplantation for Clostridium difficile infection in the UK. J Hosp Infect 2017;95:444–5. 10.1016/j.jhin.2016.10.023 [DOI] [PubMed] [Google Scholar]
- 14. Paramsothy S, Borody TJ, Lin E, et al. . Donor recruitment for fecal microbiota transplantation. Inflamm Bowel Dis 2015;21:1600–6. 10.1097/MIB.0000000000000405 [DOI] [PubMed] [Google Scholar]
- 15. Kahn SA, Vachon A, Rodriquez D, et al. . Patient perceptions of fecal microbiota transplantation for ulcerative colitis. Inflamm Bowel Dis 2013;19:1506–13. 10.1097/MIB.0b013e318281f520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. OpenBiome. Home. 2017. www.openbiome.org (accessed 23 Aug 2017).
- 17. EnteroBiotix. Modulating the human microbiome to address important unmet clinical needs. 2017. http://www.enterobiotix.com (accessed 23 Aug 2017).
- 18. Kelly CR, Khoruts A, Staley C, et al. . Effect of fecal microbiota transplantation on recurrence in multiply recurrent clostridium difficile infection: a randomized trial. Ann Intern Med 2016;165:609–16. 10.7326/M16-0271 [DOI] [PMC free article] [PubMed] [Google Scholar]