Abstract
Background
Monocyte chemoattractant protein-1 (MCP-1), a marker of inflammation and monocyte recruitment to atherosclerotic plaques, is associated with cardiovascular outcomes in patients with acute coronary syndrome. Although plasma levels are elevated in CKD, associations with reduced kidney function or outcomes in CKD have not been explored.
Methods
In this population-based, probability-sampled, longitudinal cohort of 3,257 participants, including 286 (8.8%) with CKD, we studied the association of plasma MCP-1 with eGFR, albuminuria, death, and intermediate and hard cardiovascular outcomes in CKD and non-CKD individuals. Cox proportional hazards regression assessed associations of baseline MCP-1 with all-cause death and atherosclerotic events.
Results
MCP-1 was higher in CKD than non-CKD participants (P<0.001), and negatively associated with eGFR (r=−0.23, P<0.0001) but not albuminuria in CKD. MCP-1 was associated with pulse wave velocity and coronary artery calcification in non-CKD but not CKD individuals. At 13.5 years, there were 230 (7.7%) deaths and 168 (6.4%) atherosclerotic events in the non-CKD vs. 97 (34.0%) and 62 (27.9%) in the CKD group (P<0.001 for each). MCP-1 was associated with death (hazard ratio 2.0 [1.4, 2.9] per log-unit increase) and atherosclerotic events (1.7 [1.0, 2.9]) in CKD individuals. The hazard ratio for death in CKD remained significant (1.6 [1.1, 2.3]) after adjusting for cardiovascular risk factors.
Conclusions
Although plasma MCP-1 increased with decreased eGFR, it remained an independent risk factor for death in CKD. MCP-1 did not correlate with intermediate CV outcomes, implicating pathways other than atherosclerosis in the association of MCP-1 with death in CKD.
Keywords: CKD, MCP-1, inflammation, death, cardiovascular, outcomes, albuminuria, biomarkers
INTRODUCTION
Persons with chronic kidney disease (CKD) are at a disproportionately higher risk of cardiovascular (CV) disease and death than non-CKD individuals, and are substantially more likely to die, primarily of CV disease, than to reach dialysis-dependence [1,2]. Prognostic models based on traditional CV risk factors do not predict outcomes as accurately in people with CKD vs. those without CKD [3,4]. This highlights the need to identify non-traditional factors to improve risk prediction in the growing CKD population.
Monocyte chemoattractant protein-1 (MCP-1) is a chemokine produced by macrophages, vascular smooth muscle cells, and endothelial cells in response to hypercholesterolemia and other causes of arterial injury, that is thought to play a role in early monocyte recruitment to atherosclerotic plaques and areas of inflammation [5,6]. Plasma MCP-1 levels are higher in advanced CKD than in those with normal kidney function [7]. It is unclear whether increased levels are driven by lower renal clearance or upregulated MCP-1 production in the setting of systemic inflammation associated with CKD [8–10]. Uremic toxins, such as p-cresol and indoxyl sulfate, upregulate MCP-1 production by vascular smooth muscle and endothelial cells in vitro [11,12]. Corresponding correlations between these factors and MCP-1 were shown in hemodialysis patients [13], indicating that decreased renal clearance is likely not the only factor underlying MCP-1 elevation in CKD.
Elevated plasma MCP-1 is associated with CV mortality in overweight asymptomatic outpatients and individuals with known coronary artery disease (CAD) or acute coronary syndrome (ACS) [14–17]. However, the few studies that reported associations between MCP-1 and CV outcomes in CKD or hemodialysis patients were limited by small samples, too few events to allow for adjustment, inclusion of only those with ACS or CAD, or lack of hard CV outcome events [8,18,19].
We sought to test the hypothesis that elevated plasma MCP-1 level is associated with death and CV events using a large, multi-ethnic cohort where both intermediate and hard CV outcomes were measured. The first aim was to investigate associations of plasma MCP-1 with lower estimated glomerular filtration rate (eGFR), increased albuminuria, and intermediate CV outcomes, such as coronary artery calcification (CAC), left ventricular hypertrophy (LVH), and aortic pulse wave velocity (PWV), in order to explore mechanisms by which MCP-1 may lead to poor outcomes. The second aim was to investigate whether plasma MCP-1 was independently associated with all-cause death or atherosclerotic CV disease (ASCVD) events and whether CKD presence would modify this association, after controlling for traditional CV risk factors and eGFR.
MATERIALS AND METHODS
Study participants
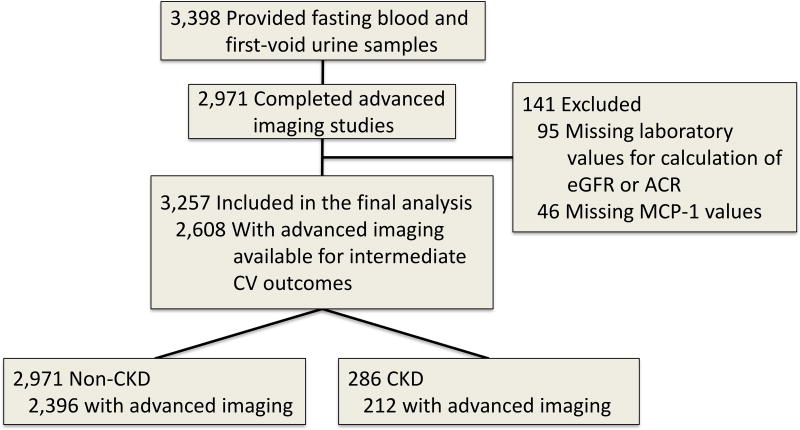
The Dallas Heart Study (DHS) is a community-based multi-ethnic longitudinal cohort study of residents of Dallas County, Texas [20], approved by the University of Texas Southwestern Institutional Review Board, and adherent to the Declaration of Helsinki. A total of 6,101 persons provided informed consent and completed a visit to collect health-related data. Of those, 3,398 participants provided fasting blood and first-void urine samples, and 2,971 underwent advanced imaging studies for measurement of intermediate CV phenotypes. Our primary analysis included 3,257 with measurement of plasma MCP-1 level, urinary albumin-to-creatinine ratio (ACR), and information available for calculation of eGFR (Figure 1). Participants were followed for a median of 13.5 years.
Figure 1. Flow chart of the study population.
MCP-1 measurement
Fasting venous blood samples were stored in EDTA tubes for ≤4 hours at 4°C, then centrifuged at 1,430 g for 15 minutes. Plasma was separated and frozen at −80°C until assays were performed in duplicate at Biosite Inc. (now Alere, San Diego, CA) in 384-well microtiter plates on a high-throughput robotic platform (TECAN Genesis RSP 200/8). The binding of alkaline phosphatase-conjugated antibody quantified the plasma concentration of MCP-1, with a reportable range of 40 to 2,000 pg/mL [14,19].
Urinary and kidney function measurements
Because the alkaline picrate assay was used to determine serum creatinine concentrations, the 4-variable Modification of Diet in Renal Disease (MDRD) study formula was used to calculate eGFR [21,22]. ACR was calculated in mg/g from spot urinary albumin and creatinine measured from a first-void urine sample. CKD was defined as either eGFR <60 mL/min/1.73m2 or elevated ACR (≥17 mg/g in men or ≥25 mg/g in women) [23,24]. CKD was staged as follows: stage 1, elevated ACR with eGFR ≥90; stage 2, elevated ACR with eGFR 60–89; stage 3, eGFR 30–59; stage 4, eGFR 15–29; and stage 5, eGFR <15 [23], not receiving chronic dialysis.
Outcomes
The pre-specified primary outcome was all-cause death, ascertained using the National Death Index. The pre-specified secondary outcome was ASCVD events, defined as the composite of myocardial infarction, stroke, coronary revascularization, and CV death. Tertiary outcomes were each component of the secondary outcome and intermediate CV phenotypes, including CAC, LVH, and PWV. As intermediate CV outcomes were not measured in all participants, these analyses were limited to individuals with available data (N=2,608, including 2,396 non-CKD and 212 CKD participants, Figure 1). CV events were captured by searching hospital records, queries, and final adjudication by DHS investigators. Analyses of CV events included 2,831 individuals who had been adjudicated for CV outcomes through 2012. Deaths were considered CV deaths if they were coded with International Statistical Classification of Diseases 10 codes I00-I99 [25].
Intermediate CV phenotype measures
Analyses of intermediate CV phenotypes were conducted for the 2,608 participants who underwent advanced imaging studies. A total of 2,549 participants underwent cardiac Computed Tomography (CT), 2,575 cardiac Magnetic Resonance Imaging (MRI), and 2,077 aortic MRI. CAC was measured with electron-beam CT on a single scanner (Imatron 150 XP, Imatron, Inc., San Francisco, CA) at 80% of the R-R interval with 30 cm field of view, 512 matrix with sharp kernel reconstruction [26]. The final score was the mean of two consecutive measurements or a single measurement when only one scan was performed [27]. CAC ≥100 Agatston units was pre-specified as clinically relevant, which corresponds to moderate to high 10-year CV event risk [28].
MRI was performed with a Phillips Medical Systems (Best, The Netherlands) 1.5 Tesla Intera magnet. Left ventricular (LV) mass was measured in g/m2 indexed to body surface area (BSA) [29]. LVH was defined as LV mass/BSA >97.5th sex-based percentile, which was >89 g/m2 in men and >112 g/m2 in women in a healthy subpopulation of the DHS [29]. The ascending and descending thoracic aorta were imaged using prospective electrocardiogram gating and a breath-hold, velocity-encoded, phase-contrast gradient echo sequence as previously described [30,31]. Aortic PWV was calculated in meters/second (m/s) by dividing arch distance by transit time, with higher velocity indicative of a stiffer aorta. Transit time was calculated as the time difference between ascending and descending upstroke velocities at half-maximum [30].
Statistical analysis
The analysis was stratified into CKD and non-CKD groups to address the question of whether CKD presence modifies the association of MCP-1 with intermediary and hard CV outcomes. Clinical variables and outcome measures were compared between individuals with no CKD, stages 1–2 CKD, and stages 3–5 CKD. P values for trends were derived using Cochran-Armitage tests for categorical and Jonckheere-Terpstra tests for continuous variables. Correlations of MCP-1 with eGFR and ACR were tested using Spearman correlations. Continuous variables with a non-Gaussian distribution (plasma MCP-1, eGFR, ACR, aortic PWV, and LV mass/BSA) were log-transformed prior to comparisons.
Time to event was estimated using Kaplan-Meier survival analysis and compared among quartiles of MCP-1 using log-rank tests. Associations of log-transformed MCP-1 with each outcome were determined using Cox proportional hazards regression. Models controlled for traditional CV risk factors: age, sex, race, diabetes mellitus, hypertension, current smoking, total and HDL cholesterol. Additional models also controlled for eGFR to account for the effect of renal clearance on plasma MCP-1 levels, and high sensitivity C-reactive protein (hsCRP) to account for generalized inflammation. Effect modification of CKD was also tested and considered statistically significant if the CKD × MCP-1 interaction P value was <0.1. Sensitivity analyses of those without prior CV disease (self-reported history of myocardial infarction, revascularization, heart failure, or stroke) included 3,017 participants (236 with CKD and 2,781 without CKD). Statistical analyses were performed with SAS 9.4 and SAS Enterprise Guide 7.1 (SAS Institute, Cary, NC).
RESULTS
Baseline characteristics
There were no significant differences between participants that were excluded due to missing MCP-1 values as compared to those that were included, except for a higher proportion of smokers (Supplemental Table 1). Of the 3,257 participants included, 56% were female, 52% Black, 29% Caucasian, 17% Hispanic, and 2% of other races. The total number with CKD was 286 (8.8%). There were 217 participants with CKD stages 1–2 and 69 with stages 3–5. There were significant trends in the prevalence of most traditional CV risk factors across the three groups, including smoking, hypertension, diabetes, and prior CV disease (Table 1), with a higher prevalence among those with CKD stages 1–2 than no CKD, and among those with CKD stages 3–5 as compared with stages 1–2. Age also increased across groups.
Table 1.
Baseline characteristics of the cohort by CKD status
| Variable | Non-CKD N=2,971 |
CKD stages 1–2 N=217 |
CKD stages 3–5 N=69 |
P value for trend |
|---|---|---|---|---|
| Age, years, median (IQR) | 43.0 (36.0, 51.0) | 47.0 (38.0, 54.0) | 53.0 (47.0, 58.0) | <0.0001 |
| Female sex, N (%) | 1684 (56.7) | 100 (46.1) | 39 (56.5) | 0.05 |
| Race, N (%) | ||||
| Black | 1503 (50.6) | 151 (69.6) | 44 (63.7) | |
| White | 889 (29.9) | 28 (12.9) | 19 (27.5) | |
| Hispanic | 516 (17.4) | 36 (16.6) | 3 (4.4) | |
| Other | 63 (2.1) | 2 (0.9) | 3 (4.4) | |
| Current smoker, N (%) | 1323 (44.6) | 112 (51.6) | 50 (58.0) | 0.004 |
| Hypertension, N (%) | 985 (33.2) | 134 (61.8) | 56 (81.2) | <0.0001 |
| Diabetes mellitus, N (%) | 283 (9.5) | 84 (38.7) | 22 (31.9) | <0.0001 |
| Hyperlipidemia, N (%) | 376 (12.7) | 30 (13.9) | 15 (21.7) | 0.05 |
| Prior CV disease, N (%)a | 190 (6.4) | 30 (13.8) | 20 (29.0) | <0.0001 |
| Blood pressure, mmHg | ||||
| Systolic, median (IQR) | 123.6 (114.3, 134.2) | 135.9 (124.3, 153.1) | 133.9 (120.0, 154.7) | <0.0001 |
| Diastolic, median (IQR) | 77.6 (72.3, 83.6) | 83.1 (76.8, 91.7) | 79.9 (74.9, 85.9) | <0.0001 |
| BMI, kg/m2, median (IQR) | 28.3 (24.5, 33.1) | 30.7 (26.3, 35.0) | 30.1 (25.4, 35.7) | <0.0001 |
| Total cholesterol, mg/dL, median (IQR) | 177.0 (154.0, 203.0) | 174.0 (150.0, 202.0) | 182.0 (157.0, 196.0) | 0.60 |
| HDL cholesterol, mg/dL, median (IQR) | 47.0 (40.0, 57.0) | 47.0 (39.0, 54.0) | 47.0 (38.0, 59.0) | 0.29 |
| ACR, mg/g, median (IQR) | 2.7 (1.8, 4.6) | 48.7 (29.3, 107.8) | 16.2 (3.2, 105.3) | <0.0001 |
| ACR, mg/g, mean (SD) | 3.9 (3.6) | 172.4 (426.1) | 308.9 (993.4) | <0.0001 |
| Estimated GFR, mL/min/1.73 m2, median (IQR) | 98.4 (85.8, 113.2) | 99.8 (83.1, 117.5) | 49.1 (38.2, 55.9) | <0.0001 |
| MCP-1, pg/mL, median (IQR) | 164.7 (120.2, 221.0) | 183.0 (138.9, 246.0) | 233.8 (177.5, 325.8) | <0.0001 |
ACR, urine albumin-to-creatinine ratio; BMI, body mass index; CKD, chronic kidney disease; CV, cardiovascular; GFR, glomerular filtration rate; HDL, high density lipoprotein; IQR, interquartile range; MCP-1, monocyte chemoattractant protein-1
Prior cardiovascular disease was defined as self-reported history of prior myocardial infarction, revascularization, heart failure, or stroke.
Correlations of plasma MCP-1 with kidney function and albuminuria
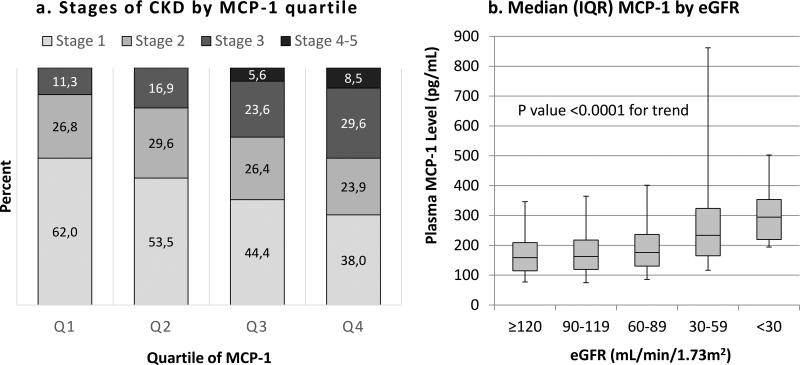
MCP-1 levels were higher in CKD than in non-CKD participants (median [IQR] 192.2 [143.6, 269.8] vs. 164.7 [120.3, 220.9] pg/mL, P<0.001), and increased with lower eGFR and across advancing CKD stages, P<0.001 (Table 1, Figure 2). In both the CKD and non-CKD groups, eGFR was lower across higher MCP-1 quartiles, but no association was seen with ACR (Supplemental Tables 2–3). In the entire cohort, a similar relationship between eGFR and MCP-1 quartiles was seen, and there was a difference in ACR between the first quartile of MCP-1 and the third and fourth quartiles (Supplemental Tables 2–3). MCP-1 correlated negatively with eGFR, Spearman rho −0.12 in the entire cohort, −0.10 in non-CKD, and −0.23 in CKD, which remained significant after adjusting for age (Supplemental Table 4, P<0.001 for all correlations). There was also a modest but significant association between albuminuria and MCP-1 in the entire cohort (rho =0.07, P<0.0001), but no association was seen in those with CKD (CKD rho=−0.02, P=0.68, Supplemental Table 4).
Figure 2. Relationship between plasma MCP-1 and kidney function.
(a) Distribution of stages of CKD by quartile of plasma MCP-1. (b) Median plasma MCP-1 levels by eGFR. Boxes represent the median values and interquartile ranges (IQR). Error bars represent the 5th and 95th percentiles. CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; MCP-1, monocyte chemoattractant protein-1.
Associations of MCP-1 with Intermediate CV Phenotypes
Participants with CKD exhibited higher CAC, PWV, LV mass/BSA, and LVH prevalence compared to those without CKD (Table 2). PWV, LV mass/BSA, and the prevalence of CAC ≥100 were higher with more advanced CKD stages, but there was no difference in the proportion with LVH (Table 2). In the non-CKD group, the prevalence of CAC ≥100 increased from 4.8% in the first MCP-1 quartile to 10.9% in the fourth quartile (P<0.001 for trend, Table 3). There were also associations between higher MCP-1 quartiles and higher aortic PWV (P=0.001 for trend), but not with LV mass/BSA or LVH in the non-CKD group. In the CKD group, no associations were observed between MCP-1 quartiles and intermediate CV phenotypes (Table 3).
Table 2.
Intermediate CV Outcomes by CKD status
| Variable | Non-CKD N=2,396 |
Any CKD N=212 |
P value | CKD 1–2 N=165 |
CKD 3–5 N=47 |
P value |
|---|---|---|---|---|---|---|
| CAC ≥100 Agatston units, N (%) | 200 (8.4) | 46 (21.8) | <0.001 | 27 (16.6) | 18 (40.0) | 0.0007 |
| LV mass/BSA, g/m2, median (IQR) | 79.6 (69.8, 92.1) | 91.9 (78.5, 110.9) | <0.001 | 93.2 (81.1, 112.1) | 83.6 (70.9, 101.9) | 0.02 |
| LVH, N (%) | 225 (9.4) | 69 (32.6) | <0.001 | 57 (34.6) | 12.(26.7) | 0.32 |
| Aortic PWV, m/s, median (IQR) | 4.2 (3.3, 5.6) | 5.4 (4.0, 7.0) | <0.001 | 5.3 (3.9, 6.7) | 6.6 (4.4, 8.6) | 0.03 |
Note: Includes 2,608 participants who underwent testing for intermediate CV outcomes
BSA, body surface area; CAC, coronary artery calcification; CKD, chronic kidney disease; IQR, interquartile range; LV, left ventricular; LVH, left ventricular hypertrophy; PWV, pulse wave velocity.
Table 3.
Outcomes by quartile of MCP-1 and CKD status
| Non-CKD, N=2,971 | ||||
|---|---|---|---|---|
|
| ||||
| Variable | Quartile of MCP-1 (range, pg/mL) | |||
|
| ||||
| Q1 (1.7– 120.2) |
Q2 (120.3– 164.6) |
Q3 (164.7– 220.9) |
Q4 (221.0– 2011.0) |
|
| N=743 | N=742 | N=743 | N=743 | |
| Intermediate Outcomes | ||||
| CAC ≥100 Agatston units, N (%) | 27 (4.8) | 53 (8.8) | 53 (9.1) | 65 (10.9)a |
| LV mass/BSA, g/m2, median (IQR) | 79.0 (69.8, 91.3) | 79.8 (69.8, 92.2) | 79.7 (69.6, 92.0) | 79.7 (70.1, 92.8) |
| LVH, N (%) | 59 (10.2) | 52 (8.8) | 55 (9.3) | 58 (9.6) |
| Aortic PWV, m/s, median (IQR) | 4.1 (3.3, 5.4) | 4.1 (3.3, 5.5) | 4.2 (3.3, 5.6) | 4.5 (3.4, 5.9)a |
| Hard Outcomes | ||||
| All-cause death, N (%) | 33 (5.1) | 57 (8.4) | 53 (8.0) | 87 (12.8)a |
| ASCVD events, N (%) | 43 (6.7) | 36 (5.5) | 36 (5.7) | 51 (7.9) |
| Myocardial infarction, N (%) | 14 (2.2) | 12 (1.8) | 13 (2.0) | 20 (3.1) |
| Stroke, N (%) | 14 (2.2) | 8 (1.2) | 13 (2.0) | 18 (2.8) |
| CV revascularization, N (%) | 20 (3.1) | 21 (3.2) | 23 (3.6) | 27 (4.2) |
| CV death, N (%) | 7 (1.1) | 9 (1.4) | 3 (0.5) | 14 (2.2) |
|
| ||||
| CKD, N=286 | ||||
|
| ||||
| Variable | Quartile of MCP-1 (range, pg/mL) | |||
|
| ||||
| Q1 (48.9–142.9) | Q2 (143.0–191.9) | Q3 (192.0–270.9) | Q4 (271.0–2004.0) | |
| N=71 | N=72 | N=72 | N=71 | |
|
| ||||
| Intermediate Outcomes | ||||
| CAC ≥100 Agatston units, N (%) | 8 (14.8) | 12 (25.5) | 10 (17.9) | 15 (29.4) |
| LV mass/BSA, g/m2, median (IQR) | 96.0 (84.7, 108.1) | 87.8 (77.6, 106.6) | 86.1 (74.8, 107.6) | 93.4 (83.7, 126.5) |
| LVH, N (%) | 16 (32.0) | 15 (28.3) | 16 (28.6) | 22 (43.1) |
| Aortic PWV, m/s, median (IQR) | 5.4 (4.0, 6.7) | 5.5 (3.9, 6.8) | 5.1 (3.6, 7.0) | 5.6 (4.5, 8.4) |
| Hard Outcomes | ||||
| All-cause death, N (%) | 15 (24.2) | 24 (35.8) | 23 (34.3) | 35 (53.8)a |
| ASCVD events, N (%) | 14 (25.5) | 13 (22.8) | 17 (29.3) | 18 (35.3) |
| Myocardial infarction, N (%) | 5 (9.1) | 2 (3.5) | 6 (10.3) | 4 (7.8) |
| Stroke, N (%) | 3 (5.5) | 4 (7.0) | 7 (12.1) | 4 (7.8) |
| CV revascularization, N (%) | 9 (4.1) | 4 (7.0) | 5 (8.6) | 6 (11.8) |
| CV death, N (%) | 3 (5.5) | 5 (8.8) | 7 (12.1) | 9 (17.7)a |
ASCVD, atherosclerotic cardiovascular disease; BSA, body surface area; CAC, coronary artery calcification; CKD, chronic kidney disease; CV, cardiovascular; IQR, interquartile range; LV, left ventricular; LVH, left ventricular hypertrophy; MCP-1, monocyte chemoattractant protein-1
P<0.05 for trend
Association of MCP-1 with death and CV outcomes
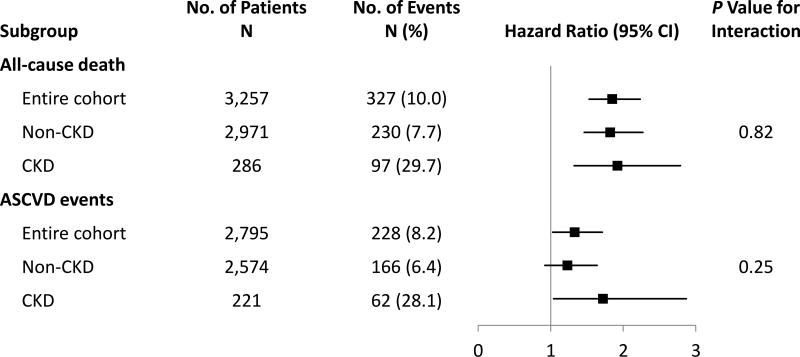
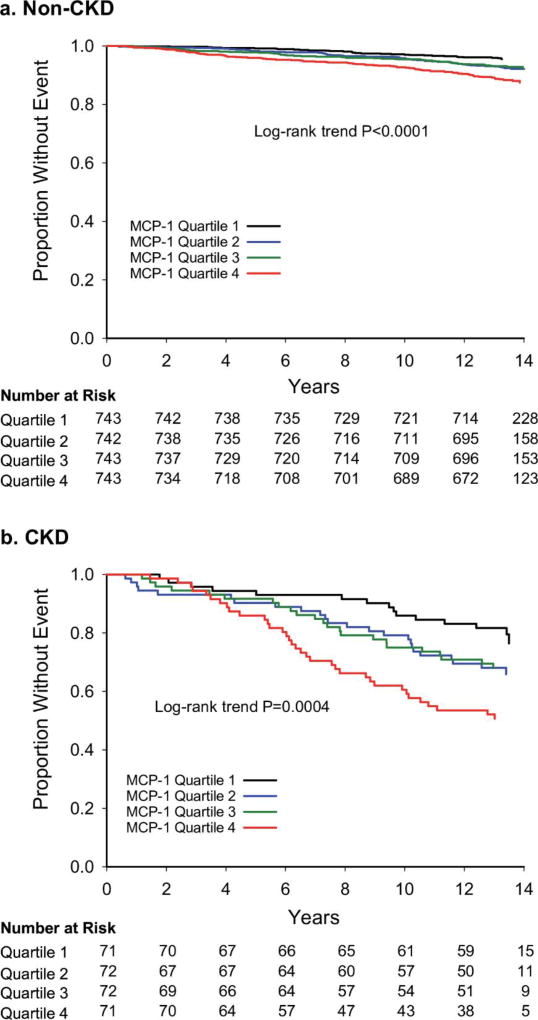
There were 327 deaths and 230 ASCVD events over median (IQR) follow-up of 161.5 (157.9, 166.7) months. Events occurred with a higher frequency in CKD than non-CKD individuals (P<0.001 for each event, Figure 3). Higher MCP-1 quartiles were associated with a higher proportion of deaths (P for trend=0.001 in CKD, <0.001 in non-CKD), but no difference was observed for ASCVD events (Table 3). In the CKD group, there was a significant trend toward increased CV death across MCP-1 quartiles (Table 3). MCP-1, taken continuously, was associated with time to all-cause death in the entire cohort and in the CKD and non-CKD subgroups (Figure 3). Higher MCP-1 quartiles were associated with death in both CKD (log-rank P=0.0004) and non-CKD (P<0.001) groups (Figure 4).
Figure 3. Associations of log transformed MCP-1 with outcomes by CKD status.
Forest plot depicts the unadjusted hazard ratios and 95% confidence intervals for outcomes stratified by CKD status. ASCVD, atherosclerotic cardiovascular disease; CKD, chronic kidney disease; CV, cardiovascular; MCP-1, monocyte chemoattractant protein-1.
Figure 4. Association of MCP-1 quartiles with all-cause death by CKD status.
Kaplan-Meier curves depict the association of quartiles of MCP-1 with all-cause death in (a) non-CKD and (b) CKD groups. CKD, chronic kidney disease, MCP-1, monocyte chemoattractant protein-1.
Adjusting for traditional CV risk factors attenuated the magnitude of these associations, but MCP-1 remained independently associated with death in both groups (CKD group aHR=1.6, 95% CI 1.1, 2.3). Adjustments for eGFR and hsCRP further attenuated the association in the CKD group, but MCP-1 remained associated with death in both groups (all CKD × MCP-1 interaction P values >0.1) (Table 4). Sensitivity analyses including 3,017 individuals without prior CV disease revealed similar results (Table 4).
Table 4.
Associations of plasma MCP-1 with all-cause death and ASCVD events
| Exposure Variable | All Participants HR (95% CI) |
Non-CKD HR (95% CI) |
CKD HR (95% CI) |
|---|---|---|---|
|
| |||
| All-cause death | |||
| Total cohort | N=3,257 | N=2,971 | N=286 |
| Log MCP-1 unadjusted | 1.9 (1.5, 2.2) | 1.8 (1.5, 2.3) | 1.9 (1.3, 2.8) |
| Log MCP-1 adjusteda | 1.6 (1.3, 1.9) | 1.6 (1.3, 2.0) | 1.6 (1.1, 2.3) |
| Log MCP-1 adjusted + eGFRb | 1.6 (1.3, 1.9) | 1.6 (1.3, 2.0) | 1.5 (1.0, 2.1) |
| Log MCP1 adjusted + hsCRPc | 1.6 (1.3, 1.9) | 1.6 (1.3, 2.0) | 1.5 (1.0, 2.2) |
| Log MCP1 adjusted +ACRd | 1.6 (1.3, 1.9) | 1.6 (1.3, 2.0) | 1.5 (1.1, 2.2) |
| No prior CVD | N=3,017 | N=2,781 | N=236 |
| Log MCP-1 unadjusted | 2.0 (1.6, 2.5) | 2.0 (1.6, 2.5) | 2.1 (1.3, 3.2) |
| Log MCP-1 adjusteda | 1.8 (1.4, 2.2) | 1.8 (1.4, 2.3) | 1.7 (1.1, 2.7) |
| Log MCP-1 adjusted + eGFRb | 1.8 (1.4, 2.2) | 1.8 (1.4, 2.3) | 1.6 (1.0, 2.5) |
| Log MCP1 adjusted + hsCRPc | 1.8 (1.4, 2.2) | 1.8 (1.4, 2.3) | 1.7 (1.1, 2.6) |
| Log MCP1 adjusted + ACRd | 1.8 (1.5, 2.2) | 1.8 (1.4, 2.3) | 1.7 (1.1, 1.7) |
|
| |||
| ASCVD events | |||
|
| |||
| Total cohorte | N=2,831 | N=2,609 | N=222 |
| Log MCP-1 unadjusted | 1.3 (1.0, 1.7) | 1.2 (0.9, 1.6) | 1.7 (1.0, 2.9) |
| Log MCP-1 adjusteda | 1.0 (0.8, 1.4) | 1.0 (0.8, 1.4) | 1.2 (0.7, 2.0) |
| Log MCP-1 adjusted + eGFRb | 1.0 (0.8, 1.3) | 1.0 (0.8, 1.3) | 1.0 (0.6, 1.7) |
| Log MCP1 adjusted + hsCRPc | 1.0 (0.8, 1.3) | 1.0 (0.7, 1.3) | 1.2 (0.7, 2.0) |
| Log MCP1 adjusted + ACRd | 1.0 (0.8, 1.3) | 1.0 (0.7, 1.3) | 1.1 (0.6, 1.9) |
| No prior CVD | N=2,605 | N=2,420 | N=185 |
| Log MCP-1 unadjusted | 1.2 (0.9, 1.6) | 1.1 (0.8, 1.5) | 1.5 (0.8, 2.8) |
| Log MCP-1 adjusteda | 0.9 (0.7, 1.3) | 0.9 (0.7, 1.3) | 1.1 (0.5, 2.1) |
| Log MCP-1 adjusted + eGFRb | 0.9 (0.7, 1.2) | 0.9 (0.7, 1.3) | 0.8 (0.4, 1.7) |
| Log MCP1 adjusted + hsCRPc | 0.9 (0.7, 1.3) | 0.9 (0.7, 1.3) | 1.0 (0.5, 2.1) |
| Log MCP1 adjusted + ACRd | 0.9 (0.7, 1.3) | 0.9 (0.6, 1.2) | 1.1 (0.5, 2.2) |
ASCVD, atherosclerotic cardiovascular disease; CKD, chronic kidney disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; MCP-1, monocyte chemoattractant protein-1
Adjusted for traditional CV risk factors (age, sex, race, smoking, diabetes, hypertension, total and HDL cholesterol)
Adjusted for traditional CV risk factors and log transformed eGFR
Adjusted for traditional CV risk factors and log transformed hsCRP
Adjusted for traditional CV risk factors, log transformed log transformed ACR
Includes 2,831 individuals in which adjudication for CV outcomes occurred through 2012.
In unadjusted models, MCP-1 was associated with ASCVD events in the entire cohort and the CKD group, but not the non-CKD group (Figure 3). These associations became non-significant after adjusting for CV risk factors (Table 4). There were no significant CKD×MCP-1 interactions for ASCVD events. Sensitivity analysis in individuals without prior CVD yielded similar results (Table 4).
DISCUSSION
To our knowledge, this is the first study that reports the association of plasma MCP-1 with intermediate and hard CV outcomes using a large, multi-ethnic, longitudinal cohort based on the presence of CKD. Importantly, we show that plasma MCP-1 levels 1) are higher with lower eGFR and across higher CKD stages but do not correlate with albuminuria in CKD patients; 2) independently associate with death after adjusting for traditional CV risk factors in both CKD and non-CKD individuals; and 3) do not independently associate with intermediate CV phenotypes or ASCVD events in CKD individuals. This suggests that the association observed with death may be mediated through pathways other than atherosclerosis. Given that many other known CV risk factors perform less well in CKD than non-CKD individuals, it is notable that MCP-1 predicts death equally as well in CKD as in non-CKD individuals.
Prior studies from the DHS reported that MCP-1 was highly correlated with age [19], which is included in the calculation of eGFR. In our study, MCP-1 remained correlated with eGFR after adjusting for age, suggesting an independent relationship between decreased kidney function and MCP-1. The only other published study demonstrating an association between MCP-1 and eGFR used data from the African American Diabetes Heart Study. Because black individuals differ in patterns of both kidney and CV disease than non-blacks, it is important that we demonstrated this finding in a large racially and ethnically diverse sample [10]. Possible explanations include decreased renal clearance of MCP-1, generalized inflammation associated with CKD, or upregulation of MCP-1 production in the setting of uremia [9,32]. In this study, only 5% of the variability in MCP-1 was accounted for by eGFR in those with CKD, suggesting that additional underlying processes in individuals with CKD that either increase MCP-1 production or decrease its breakdown may lead to elevated levels. It is possible that generalized inflammation in CKD at least partially drives increased plasma MCP-1 levels [6,9].
Although there was a modest but statistically significant association between MCP-1 and albuminuria in the entire cohort, this association became non-significant when stratifying by CKD presence. It is possible that an association between albuminuria and MCP-1 may not have been apparent in our cohort comprised largely of individuals without CKD that had very little if any albuminuria. One study reported that MCP-1 correlated with albumin excretion rate in 15 type 1 diabetics [33]. However, analyses including larger samples from the African American Diabetes Heart Study and the Framingham Offspring Cohort showed no association between plasma MCP-1 and ACR [10,34], which we confirm using a multi-ethnic cohort. This implies that higher levels of MCP-1 with advancing CKD stages may be primarily driven by factors other than albuminuria.
To our knowledge, there are no published studies investigating associations of MCP-1 with hard outcomes in CKD patients. Even in the general population, most studies showing associations of plasma MCP-1 with death and CV outcomes have included only participants with ACS, angina, or stable CAD [14–16,35]. Only two prior studies reported associations with CV outcomes in persons without ACS [17,36]. However, neither eGFR nor CKD presence were reported in these studies, so the effect of renal function on associations of MCP-1 with outcomes was not investigated.
Even fewer data exist regarding associations of MCP-1 with CV disease in CKD samples. Only one cross-sectional study reported that although plasma MCP-1 was elevated in 81 hemodialysis patients compared with controls, levels were not different in those with a history of CV events [8]. However, only 25 participants had prior CV events, and the study was likely underpowered to detect a difference. To our knowledge, no other published studies investigated the association of plasma MCP-1 with outcomes in CKD participants, despite evidence suggesting a role for MCP-1 in atherogenesis in the general population, and data suggesting an association of MCP-1 with kidney function decline.
The correlation of MCP-1 with lower eGFR implies that decreased renal clearance may account for some of the association of MCP-1 with outcomes. Indeed, adjusting for eGFR slightly attenuated the association of MCP-1 with all-cause death. Another contributing factor may be the presence of systemic inflammation in CKD, previously implicated in oxidative stress, endothelial dysfunction, atherosclerosis, malnutrition, and CKD progression [37–39], reflected by the attenuation of the association of MCP-1 with death when adjusting for hsCRP. Monocyte chemotaxis mediated through MCP-1 may account for an important element of the inflammation-associated mortality risk in CKD.
We also sought to characterize the mechanisms by which MCP-1 may lead to adverse outcomes by exploring associations of MCP-1 with CV disease intermediaries. While higher MCP-1 levels were associated with elevated CAC and PWV in non-CKD participants, no such correlations were observed in CKD individuals. However, elevated CAC and PWV were more prevalent in CKD vs. non-CKD participants, even in a sample heavily weighted toward earlier CKD stages. These findings may suggest that although MCP-1 is involved in the development of atherosclerosis in the general population, the worsening of these intermediate CV phenotypes in CKD may depend on other pathogenic mechanisms. It is known that individuals with CKD develop medial arterial calcification in addition to intimal atherosclerotic calcified plaques as seen in the general population [40], which cannot be distinguished using CAC. It is not clear whether this results from a more severe manifestation of the same underlying processes, or whether CKD upregulates different pathogenic pathways to cause this medial calcification [40]. The absence of association between MCP-1 and elevated CAC in CKD participants could suggest that MCP-1 may lead to death through mechanisms other than calcific atherosclerosis. Although there was a trend seen toward increased CV deaths across MCP-1 quartiles in the CKD group, the number of outcome events was too low to derive definitive conclusions. Alternatively, the association seen between MCP-1 and death but not ASCVD events in adjusted models may implicate traditional CV risk factors in the excess ASCVD risk, or that non-atherosclerotic disease pathways, such as infection or cancer, link monocyte chemotaxis to all-cause death in CKD individuals. We did not have non-CV causes of death available to further explore this hypothesis.
Several limitations deserve mentioning. First, the effect of time-varying measures of plasma MCP-1 on outcomes was not assessed. Given the separation in mortality between MCP-1 quartiles approximately 2–3 years after baseline MCP-1 was measured, the predictive value of repeated measures of MCP-1 deserves exploration. Second, since creatinine was measured using the alkaline picrate assay, we were unable to use the CKD-EPI equation, which estimates GFR more accurately than the MDRD equation for eGFR >60 [41]. However, a sensitivity analysis using the CKD-EPI equation showed that only 3 participants were misclassified by using the MDRD equation. Third, the cohort had relatively few persons with CKD stages 4–5, limiting generalizability to this segment of CKD. However, about 75% of the cohort was comprised of early CKD stages with preserved eGFR, where interventions would make the highest impact.
Fourth, not all participants underwent imaging for intermediate CV outcomes, slightly decreasing the sample size for these analyses. Finally, power may have limited the finding of an independent association between MCP-1 and ASCVD events in CKD participants. However, the fact that a similar association was not found in the larger non-CKD group comprised of about 3,000 individuals makes the lack of power a less likely explanation.
In conclusion, plasma MCP-1 was associated with decreased eGFR in this multi-ethnic community-based cohort. Despite this, MCP-1 was independently associated with death in both CKD and non-CKD participants, even after controlling for traditional CV risk factors. MCP-1 may mediate mortality through pathways involving inflammation. MCP-1 was also associated with ASCVD events in unadjusted models, suggesting that atherosclerotic events likely contribute to but do not fully account for the association of MCP-1 with mortality. Despite worse CV outcomes in those with CKD, associations between MCP-1 and CV disease intermediaries were non-significant in CKD individuals, further implicating pathways other than atherosclerosis in the association of MCP-1 with death in CKD. Future studies should investigate whether pathways that activate MCP-1 are altered in the uremic milieu and identify other circulating biomarkers that may clarify the pathogenesis of CV disease in CKD. The clinical utility of plasma MCP-1 for mortality prognostication should be validated in larger cohorts with advanced CKD.
Supplementary Material
Acknowledgments
Financial Disclosure: J. A. de Lemos has received grant support and consulting income from Roche Diagnostics and Abbott Diagnostics and has served on endpoint committees for Siemen’s Health Care Diagnostics and Radiometer.
Funding: The Dallas Heart Study was supported by a grant from the Donald W. Reynolds Foundation and by USPHS GCRC grant # M01-RR00633 from NIH/NCRR-CR. This work was supported in part by UT Southwestern O’Brien Kidney Research Core Center (NIDDK, P30DK079328). Research reported in this publication was also supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001105 to the University of Texas Southwestern Medical Center, as well as grant T32DK007257 from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (L. P. Gregg).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Veterans Affairs.
Footnotes
Parts of these data were presented in abstract form on April 19, 2017 at the 2017 Spring Clinical Meeting of the National Kidney Foundation in Orlando, FL and on November 4, 2017 at the American Society of Nephrology Kidney Week Meeting in New Orleans, LA.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Dalrymple LS, Katz R, Kestenbaum B, Shlipak MG, Sarnak MJ, Stehman-Breen C, Seliger S, Siscovick D, Newman AB, Fried L. Chronic kidney disease and the risk of end-stage renal disease versus death. J Gen Intern Med. 2011;26:379–385. doi: 10.1007/s11606-010-1511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW American Heart Association Councils on Kidney in Cardiovascular Disease HBPRCC, Epidemiology, Prevention. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the american heart association councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Circulation. 2003;108:2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 4.Gregg LP, Adams-Huet B, Li X, Colbert G, Jain N, de Lemos JA, Hedayati SS. Effect modification of chronic kidney disease on the association of circulating and imaging cardiac biomarkers with outcomes. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.005235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gosling J, Slaymaker S, Gu L, Tseng S, Zlot CH, Young SG, Rollins BJ, Charo IF. Mcp-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein b. J Clin Invest. 1999;103:773–778. doi: 10.1172/JCI5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang Y, Beller DI, Frendl G, Graves DT. Monocyte chemoattractant protein-1 regulates adhesion molecule expression and cytokine production in human monocytes. J Immunol. 1992;148:2423–2428. [PubMed] [Google Scholar]
- 7.Fukami A, Yamagishi S, Adachi H, Matsui T, Yoshikawa K, Ogata K, Kasahara A, Tsukagawa E, Yokoi K, Imaizumi T. High white blood cell count and low estimated glomerular filtration rate are independently associated with serum level of monocyte chemoattractant protein-1 in a general population. Clin Cardiol. 2011;34:189–194. doi: 10.1002/clc.20834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papayianni A, Alexopoulos E, Giamalis P, Gionanlis L, Belechri AM, Koukoudis P, Memmos D. Circulating levels of icam-1, vcam-1, and mcp-1 are increased in haemodialysis patients: Association with inflammation, dyslipidaemia, and vascular events. Nephrol Dial Transplant. 2002;17:435–441. doi: 10.1093/ndt/17.3.435. [DOI] [PubMed] [Google Scholar]
- 9.Stinghen AE, Goncalves SM, Martines EG, Nakao LS, Riella MC, Aita CA, Pecoits-Filho R. Increased plasma and endothelial cell expression of chemokines and adhesion molecules in chronic kidney disease. Nephron Clin Pract. 2009;111:c117–126. doi: 10.1159/000191205. [DOI] [PubMed] [Google Scholar]
- 10.Murea M, Register TC, Divers J, Bowden DW, Carr JJ, Hightower CR, Xu J, Smith SC, Hruska KA, Langefeld CD, Freedman BI. Relationships between serum mcp-1 and subclinical kidney disease: African american-diabetes heart study. BMC Nephrol. 2012;13:148. doi: 10.1186/1471-2369-13-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maciel RA, Rempel LC, Bosquetti B, Finco AB, Pecoits-Filho R, Souza WM, Stinghen AE. P-cresol but not p-cresyl sulfate stimulate mcp-1 production via nf-kappab p65 in human vascular smooth muscle cells. J Bras Nefrol. 2016;38:153–160. doi: 10.5935/0101-2800.20160024. [DOI] [PubMed] [Google Scholar]
- 12.Masai N, Tatebe J, Yoshino G, Morita T. Indoxyl sulfate stimulates monocyte chemoattractant protein-1 expression in human umbilical vein endothelial cells by inducing oxidative stress through activation of the nadph oxidase-nuclear factor-kappab pathway. Circ J. 2010;74:2216–2224. doi: 10.1253/circj.cj-10-0117. [DOI] [PubMed] [Google Scholar]
- 13.Borges NA, Barros AF, Nakao LS, Dolenga CJ, Fouque D, Mafra D. Protein-bound uremic toxins from gut microbiota and inflammatory markers in chronic kidney disease. J Ren Nutr. 2016;26:396–400. doi: 10.1053/j.jrn.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 14.de Lemos JA, Morrow DA, Sabatine MS, Murphy SA, Gibson CM, Antman EM, McCabe CH, Cannon CP, Braunwald E. Association between plasma levels of monocyte chemoattractant protein-1 and long-term clinical outcomes in patients with acute coronary syndromes. Circulation. 2003;107:690–695. doi: 10.1161/01.cir.0000049742.68848.99. [DOI] [PubMed] [Google Scholar]
- 15.de Lemos JA, Morrow DA, Blazing MA, Jarolim P, Wiviott SD, Sabatine MS, Califf RM, Braunwald E. Serial measurement of monocyte chemoattractant protein-1 after acute coronary syndromes: Results from the a to z trial. J Am Coll Cardiol. 2007;50:2117–2124. doi: 10.1016/j.jacc.2007.06.057. [DOI] [PubMed] [Google Scholar]
- 16.Ding D, Su D, Li X, Li Z, Wang Y, Qiu J, Lin P, Zhang Y, Guo P, Xia M, Li D, Yang Y, Hu G, Ling W. Serum levels of monocyte chemoattractant protein-1 and all-cause and cardiovascular mortality among patients with coronary artery disease. PLoS One. 2015;10:e0120633. doi: 10.1371/journal.pone.0120633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piemonti L, Calori G, Lattuada G, Mercalli A, Ragogna F, Garancini MP, Ruotolo G, Luzi L, Perseghin G. Association between plasma monocyte chemoattractant protein-1 concentration and cardiovascular disease mortality in middle-aged diabetic and nondiabetic individuals. Diabetes Care. 2009;32:2105–2110. doi: 10.2337/dc09-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kusano KF, Nakamura K, Kusano H, Nishii N, Banba K, Ikeda T, Hashimoto K, Yamamoto M, Fujio H, Miura A, Ohta K, Morita H, Saito H, Emori T, Nakamura Y, Kusano I, Ohe T. Significance of the level of monocyte chemoattractant protein-1 in human atherosclerosis. Circ J. 2004;68:671–676. doi: 10.1253/circj.68.671. [DOI] [PubMed] [Google Scholar]
- 19.Deo R, Khera A, McGuire DK, Murphy SA, Meo Neto Jde P, Morrow DA, de Lemos JA. Association among plasma levels of monocyte chemoattractant protein-1, traditional cardiovascular risk factors, and subclinical atherosclerosis. J Am Coll Cardiol. 2004;44:1812–1818. doi: 10.1016/j.jacc.2004.07.047. [DOI] [PubMed] [Google Scholar]
- 20.Victor RG, Haley RW, Willett DL, Peshock RM, Vaeth PC, Leonard D, Basit M, Cooper RS, Iannacchione VG, Visscher WA, Staab JM, Hobbs HH Dallas Heart Study I. The dallas heart study: A population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol. 2004;93:1473–1480. doi: 10.1016/j.amjcard.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 21.Kramer H, Toto R, Peshock R, Cooper R, Victor R. Association between chronic kidney disease and coronary artery calcification: The dallas heart study. J Am Soc Nephrol. 2005;16:507–513. doi: 10.1681/ASN.2004070610. [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of diet in renal disease study group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 23.National Kidney F. K/doqi clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 24.Warram JH, Gearin G, Laffel L, Krolewski AS. Effect of duration of type i diabetes on the prevalence of stages of diabetic nephropathy defined by urinary albumin/creatinine ratio. J Am Soc Nephrol. 1996;7:930–937. doi: 10.1681/ASN.V76930. [DOI] [PubMed] [Google Scholar]
- 25.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J American Heart Association Statistics C, Stroke Statistics S. Executive summary: Heart disease and stroke statistics-2010 update: A report from the american heart association. Circulation. 2010;121:948–954. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 26.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 27.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: Objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 28.Baber U, de Lemos JA, Khera A, McGuire DK, Omland T, Toto RD, Hedayati SS. Non-traditional risk factors predict coronary calcification in chronic kidney disease in a population-based cohort. Kidney Int. 2008;73:615–621. doi: 10.1038/sj.ki.5002716. [DOI] [PubMed] [Google Scholar]
- 29.Drazner MH, Dries DL, Peshock RM, Cooper RS, Klassen C, Kazi F, Willett D, Victor RG. Left ventricular hypertrophy is more prevalent in blacks than whites in the general population: The dallas heart study. Hypertension. 2005;46:124–129. doi: 10.1161/01.HYP.0000169972.96201.8e. [DOI] [PubMed] [Google Scholar]
- 30.Maroules CD, Khera A, Ayers C, Goel A, Peshock RM, Abbara S, King KS. Cardiovascular outcome associations among cardiovascular magnetic resonance measures of arterial stiffness: The dallas heart study. J Cardiovasc Magn Reson. 2014;16:33. doi: 10.1186/1532-429X-16-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goel A, Maroules CD, Mitchell GF, Peshock R, Ayers C, McColl R, Vongpatanasin W, King KS. Ethnic difference in proximal aortic stiffness: An observation from the dallas heart study. JACC Cardiovasc Imaging. 2017;10:54–61. doi: 10.1016/j.jcmg.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 32.Stenvinkel P, Lindholm B, Heimburger M, Heimburger O. Elevated serum levels of soluble adhesion molecules predict death in pre-dialysis patients: Association with malnutrition, inflammation, and cardiovascular disease. Nephrol Dial Transplant. 2000;15:1624–1630. doi: 10.1093/ndt/15.10.1624. [DOI] [PubMed] [Google Scholar]
- 33.Chiarelli F, Cipollone F, Mohn A, Marini M, Iezzi A, Fazia M, Tumini S, De Cesare D, Pomilio M, Pierdomenico SD, Di Gioacchino M, Cuccurullo F, Mezzetti A. Circulating monocyte chemoattractant protein-1 and early development of nephropathy in type 1 diabetes. Diabetes Care. 2002;25:1829–1834. doi: 10.2337/diacare.25.10.1829. [DOI] [PubMed] [Google Scholar]
- 34.Upadhyay A, Larson MG, Guo CY, Vasan RS, Lipinska I, O'Donnell CJ, Kathiresan S, Meigs JB, Keaney JF, Jr, Rong J, Benjamin EJ, Fox CS. Inflammation, kidney function and albuminuria in the framingham offspring cohort. Nephrol Dial Transplant. 2011;26:920–926. doi: 10.1093/ndt/gfq471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tunon J, Blanco-Colio L, Cristobal C, Tarin N, Higueras J, Huelmos A, Alonso J, Egido J, Asensio D, Lorenzo O, Mahillo-Fernandez I, Rodriguez-Artalejo F, Farre J, Martin-Ventura JL, Lopez-Bescos L. Usefulness of a combination of monocyte chemoattractant protein-1, galectin-3, and n-terminal probrain natriuretic peptide to predict cardiovascular events in patients with coronary artery disease. Am J Cardiol. 2014;113:434–440. doi: 10.1016/j.amjcard.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 36.Piemonti L, Calori G, Mercalli A, Lattuada G, Monti P, Garancini MP, Costantino F, Ruotolo G, Luzi L, Perseghin G. Fasting plasma leptin, tumor necrosis factor-alpha receptor 2, and monocyte chemoattracting protein 1 concentration in a population of glucose-tolerant and glucose-intolerant women: Impact on cardiovascular mortality. Diabetes Care. 2003;26:2883–2889. doi: 10.2337/diacare.26.10.2883. [DOI] [PubMed] [Google Scholar]
- 37.Koeda Y, Nakamura M, Tanaka F, Onoda T, Itai K, Tanno K, Ohsawa M, Makita S, Ishibashi Y, Koyama T, Yoshida Y, Omama S, Ogasawara K, Ogawa A, Kuribayashi T, Okayama A. Serum c-reactive protein levels and death and cardiovascular events in mild to moderate chronic kidney disease. Int Heart J. 2011;52:180–184. doi: 10.1536/ihj.52.180. [DOI] [PubMed] [Google Scholar]
- 38.Betriu A, Martinez-Alonso M, Arcidiacono MV, Cannata-Andia J, Pascual J, Valdivielso JM, Fernandez E Investigators from the NS. Prevalence of subclinical atheromatosis and associated risk factors in chronic kidney disease: The nefrona study. Nephrol Dial Transplant. 2014;29:1415–1422. doi: 10.1093/ndt/gfu038. [DOI] [PubMed] [Google Scholar]
- 39.Recio-Mayoral A, Banerjee D, Streather C, Kaski JC. Endothelial dysfunction, inflammation and atherosclerosis in chronic kidney disease--a cross-sectional study of predialysis, dialysis and kidney-transplantation patients. Atherosclerosis. 2011;216:446–451. doi: 10.1016/j.atherosclerosis.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 40.Mathew RO, Bangalore S, Lavelle MP, Pellikka PA, Sidhu MS, Boden WE, Asif A. Diagnosis and management of atherosclerotic cardiovascular disease in chronic kidney disease: A review. Kidney Int. 2016;91:797–807. doi: 10.1016/j.kint.2016.09.049. [DOI] [PubMed] [Google Scholar]
- 41.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J Ckd EPI. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.