Abstract
Responsive CEST MRI biosensors offer good sensitivity and excellent specificity for detection of biomarkers with great potential for clinical translation. We report the application of fosfosal, a phosphorylated form of salicylic acid, for the detection of alkaline phosphatase (AP) enzyme. We detected conversion of fosfosal to salicylic acid in the presence of the enzyme by CEST MRI. Importantly the technique was able to detect AP enzyme expressed in cells in the presence of other cell components, which improves specificity. Various isoforms of the enzyme showed different Michaelis–Menten kinetics and yet these kinetics studies indicated very efficient catalytic rates. Our results with the fosfosal biosensor encourage further in vivo studies.
Keywords: CEST MRI, CATALYCEST, alkaline phosphatase, molecular imaging, Michaelis–Menten kinetics
Graphical Abstract
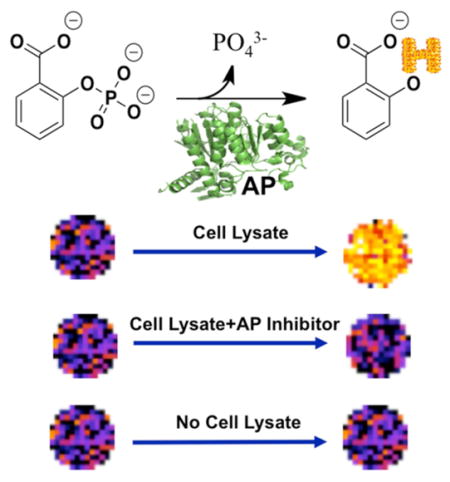
Alkaline phosphatases (AP) are a large family of enzymes that remove the phosphate group from various types of biomolecules including proteins, carbohydrates, and nucleic acids. This class of enzymes shows optimized catalytic efficiency in basic conditions. The enzyme is routinely used for biological applications such as antibody conjugates and gene expression. Furthermore, unregulated activities of subtypes of the enzyme have been reported in various disease states including osteogenic sarcoma, primary sclerosing cholangitis, and metabolic syndrome.1
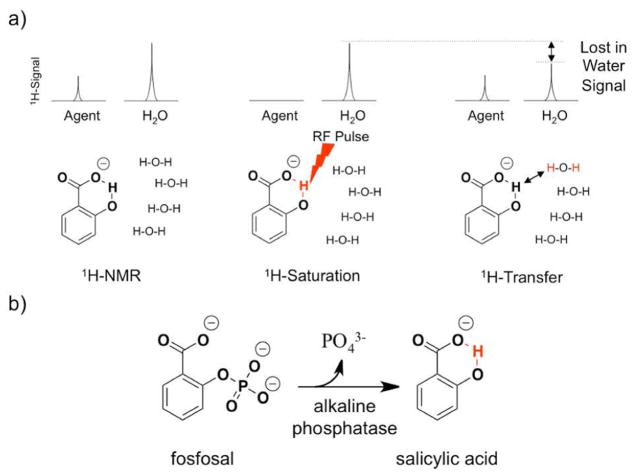
Chemical exchange saturation transfer (CEST) MRI is an imaging method that can detect biomarkers with good specificity while retaining the excellent spatial resolution and depth of view offered by MRI.2 In this technique, selective radiofrequency saturation pulses are applied at the MR frequency of a proton on the agent, which creates a pool of saturated protons. The exchange of saturated protons between the contrast agent and water molecules surrounding the contrast agent can transfer saturation to the water molecules, which results in the loss of a portion of MR signals from the bulk water (Figure 1). This loss of the bulk water signal caused by CEST is typically demonstrated by plotting the MR signal amplitude of bulk water versus a range of saturation frequencies, known as a CEST spectrum (Figure 2).3
Figure 1.
(a) CEST mechanism (b) Alkaline phosphatase hydrolyzes the phosphoester bond of fosfosal to release the phosphate group and salicylic acid. A hydroxyl proton (in red) of the salicylic acid can generate a CEST signal.
Figure 2.
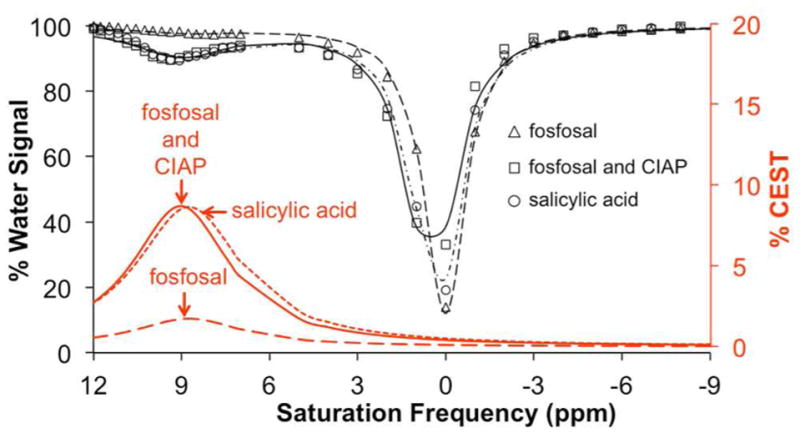
CEST spectra of fosfosal before and after catalysis with calf intestinal alkaline phosphatase (CIAP). Spectra are shown for samples of 20 mM salicylic acid (circle, short dashed red line), 20 mM of fosfosal incubated with CIAP (square, solid red line), and 20 mM of fosfosal with no enzyme (triangle, long dashed red line) for 30 min at 37 °C, pH = 7.4 ± 0.05, saturation power = 3.5 μT, and saturation time = 4 s.
Diamagnetic contrast agents are promising biosensors for the detection of biological events with CEST MRI as shown by many recent examples.4 A large chemical shift facilitates the selective detection of CEST biosensors relative to detecting CEST signals from endogenous biomolecules. Salicylic acid (SA) and its derivatives are a promising class of diamagnetic CEST biosensors that have a hydroxyl proton “trapped” by hydrogen bonding in a 6-member ring state, which allows SA to produce a detectable CEST signal relative to image noise (Figure 1b).5 The ring current places the hydroxyl proton in a deshielding region that induces a large chemical shift of 7–12 ppm (relative to water that is defined to be 0 ppm during MRI studies). SA and its derivatives have a good toxicity profile even in concentrations as high as 500 mM, which makes these biosensors superb candidates for clinical translation.6 Cells tolerate relatively high doses of SA, which increase the level of sensitivity of the technique.
CatalyCEST MRI is a unique variation of CEST MRI methodology that detects an appearance or disappearance of a CEST signal after an enzyme catalyzes a change in the chemical structure of a CEST biosensor. One or more functional groups, such amines, amides, and/or hydroxyl groups, are often incorporated into CEST biosensors, which can be chemically modified via enzyme catalysis.7 Novel catalyCEST biosensors based on SA derivatives have been reported recently that detect sulfatase, esterase, and cathepsin B enzymes.8
We sought to detect AP activity using catalyCEST MRI with a biosensor that is potentially translatable to clinical applications. Previous reports showed that nitrophenyl phosphate and other phosphorylated phenyl-based compounds are fluorogenic or bioluminogenic substrates for AP enzymes.9 Our search for a SA based compound with a phosphate group suggested fosfosal as a new catalyCEST biosensor for detecting AP enzymes. Fosfosal was introduced in the early 1980s as an analgesic that showed improved pharmacokinetic properties and biocompatibility at 1500 mg/kg (approximately 6.8 mM in vivo), suggesting potential for eventual clinical translation.10 We hypothesized that a CEST signal would not be observed from fosfosal, as there is no exchangeable proton on this molecule that has an appropriate chemical exchange rate to generate a strong CEST signal. In contrast, we hypothesized that the product of the reaction of fosfosal and AP enzyme should produce a CEST signal with a chemical shift of approximately 9 ppm as previously reported for SA (Figure 1b).
To test our hypothesis, calf intestinal alkaline phosphatase (CIAP), an AP enzyme commonly used in research studies,1 was mixed with a sample of fosfosal at a concentration of 20 mM in AP buffer solution (Figure 2; this concentration was selected to ensure strong CEST signals during initial studies). A sample containing the same concentration of fosfosal without the enzyme, and a sample of 20 mM SA were prepared as controls. After adjusting the pH to reach a value of 7.4, the samples were incubated at 37 °C for 30 min and then were analyzed with catalyCEST MRI. The sample containing the CIAP enzyme generated a strong 8.7% CEST signal at 8.75 ppm, which closely matched the previously reported CEST signal amplitude of SA. In contrast, the fosfosal sample without the enzyme showed very low CEST signal of 1.7%, which was considered to be negligible relative to typical contrast-to-noise generated during CEST MRI studies. This result confirmed that catalyCEST MRI can detect AP enzyme activity using fosfosal.
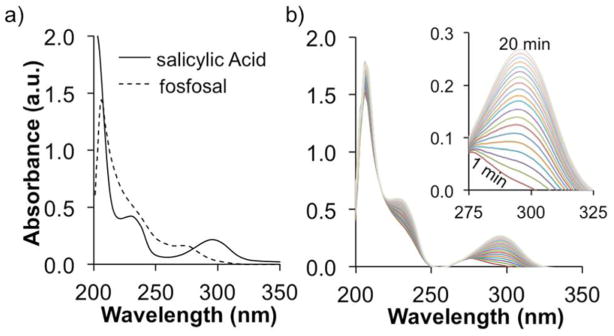
We performed UV–vis spectroscopy studies to confirm that the AP enzyme caused the conversion of fosfosal to SA (Figure 3). UV–vis absorption spectra of SA and fosfosal showed a peak at 296 and 270 nm, respectively (Figure 3a). Therefore, the appearance of a peak at 296 nm can be used to indicate the conversion of fosfosal to SA. Monitoring changes in absorbance at 296 nm in a solution of fosfosal and CIAP clearly showed an increase in SA concentration over time (Figure 3b). Similar results were obtained with human liver alkaline phosphatase (HLAP; Figure S1-a). To confirm that the conversion was solely due to the presence of the enzyme, UV–vis spectra of a fosfosal solution without AP enzyme was investigated under the same conditions (Figure S1-b). No changes in the absorbance at 296 nm over time confirmed that fosfosal was converted to SA only in the presence of the enzyme. Therefore, the UV–vis experiment confirmed that the signal observed in the CEST MRI experiments originated from the conversion of fosfosal to SA in the presence of AP.
Figure 3.
UV–vis absorption studies. (a) UV–vis absorption spectra of 100 μM salicylic acid or fosfosal in AP buffer at pH 7.40 ± 0.05. (b) Progress of the enzyme reaction with 0.05 units of calf intestinal alkaline phosphatase.
The saturation power and saturation time were adjusted to optimize CEST signal amplitude (Figure S2).11 The CEST signal amplitude increased nonlinearly with increasing saturation power or time, for concentrations of the biosensor ranging from 2.5 mM to 30 mM. However, high saturation powers may result in sample heating, and long saturation times can slow throughput in radiological clinics, each of which can limit translation of CEST MRI to clinical applications. Therefore, we performed all subsequent catalyCEST MRI studies with a 6 μT saturation power and a 4 s saturation time that still generated strong CEST signals. We evaluated the effect of pH on the CEST signal from SA because a correlation between pH and CEST signal has been reported.12 The CEST signal decreased significantly when the pH was lower than 6.6, and were relatively invariant between pH 6.6 and 7.96 (Figure S3). Thus, the CEST signal can be detected from this biosensor within a physiologically suitable pH-range.
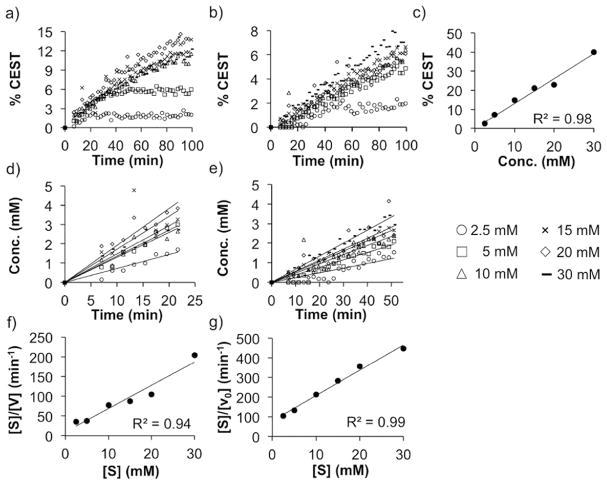
We compared the kinetics of converting fosfosal to SA with CIAP and HLAP to evaluate the utility of catalyCEST MRI for detecting AP isoforms (Figure 4). These studies were performed with 0.5–1 units of the enzymes and ≥2.5 mM of biosensor substrate to ensure pseudo-first-order kinetics at the start of the reaction. The kinetics were performed for 100 min, considering that MRI is a slow measurement technique relative to other methods such as UV–vis spectroscopy (Figure 4a, 4b). The CEST signal amplitude of the SA product was converted to concentration using a CEST-concentration calibration obtained under identical experimental conditions (Figure 4c). The linear regions of each concentration–time plot (Figure 4d,e) were used to calculate the initial velocities, which were the first 23 and 51 min of the reactions with CIAP and HLAP, respectively, based on analyses of R2 values for all possible durations for each reaction. As seen in Figures 4a–e, the error in determining the substrate concentration at each time point is non-negligible. This differs from typical Michaelis–Menten (MM) kinetics analyses where the substrate concentration can be determined with high accuracy and precision. Therefore, we used a Hanes-Woolf (HW) plot to determine MM kinetics parameters (Figure 4f, 4g), which cancels the error in the concentration of the substrate when determining the slope, leading to improved measurements of the maximum velocity, Vmax, which in turn improved the determination of the catalytic rate, kcat. The results showed that kcat was 2.7-fold faster for CIAP than for HLAP (Table 1). The Michaelis constant, KM, which approximates the dissociation constant, was 3.7-fold lower for CIAP relative to HLAP, demonstrating that the biosensor bound with more affinity to CIAP. The catalytic efficiency, kcat/KM, was an order of magnitude higher for CIAP than HLAP due to the superior binding and rate of catalysis. Yet, both enzymes had extremely efficient catalysis for the biosensor substrate, which facilitates the detection of the activity of AP isoforms with catalyCEST MRI. This kinetic efficiency has advantages for future in vivo studies, because in vivo catalyCEST MRI depends on rapid conversion of a high concentration of biosensor concentration for adequate detection. Furthermore, this biosensor should be considered to be a nonspecific substrate for detecting all alkaline phosphatases.
Figure 4.
Michaelis–Menten kinetics of fosfosal. Fosfosal incubated with (a) calf intestine alkaline phosphatase (CIAP) and (b) human liver alkaline phosphatase (HLAP). (c) CEST-concentration calibration for salicylic acid. Calibrated concentration–time graphs for (d) CIAP and (e) HLAP. Hanes-Woolf plots of (f) CIAP and (g) HLAP.
Table 1.
Kinetics Parameters for Calf Intestinal Alkaline Phosphatase and Human Liver Alkaline Phosphate Enzymes at 37°C
| enzyme | CIAP | HLAP |
|---|---|---|
| KM (M) | 1.63 × 10−3 | 6.07 × 10−3 |
| Vmax (M.s−1) | 2.83 × 10−3 | 1.29 × 10−3 |
| kcat (s−1) | 2.12 × 106 | 7.74 × 105 |
| kcat/KM (M−1.s−1) | 1.30 × 109 | 1.28 × 108 |
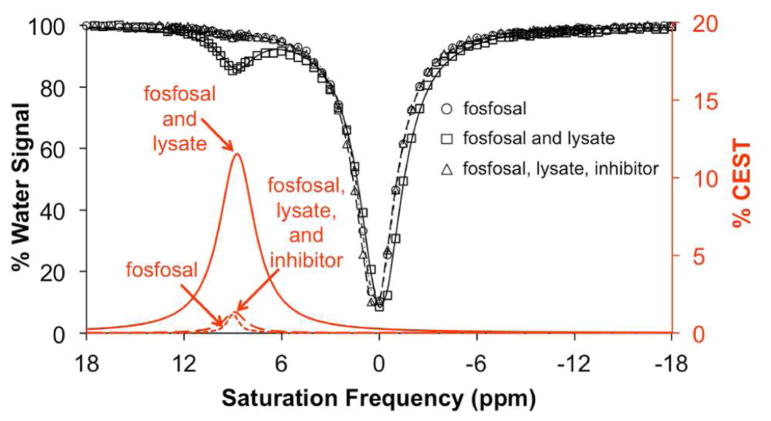
We performed studies with cell lystate to demonstrate that catalyCEST MRI with fosfosal can detect biologically relevant levels of AP enzymes.13 Confluent HEK293T cells were lysed by mammalian protein extraction reagent (mPER) lysis solution. This lysate was mixed with fosfosal solution and incubated for 3 h at 25 °C. CEST MRI showed that the mixture produced a CEST signal at 8.75 ppm, indicating the appearance of SA (Figure 5). To confirm that the signal was produced by the reaction between AP enzyme and fosfosal, a solution containing a similar concentration of fosfosal but with no lysate and a solution containing fosfosal, lysate, and a cocktail containing AP enzyme inhibitors were studied with catalyCEST MRI. Both of these additional samples showed the same negligible CEST signal amplitude, which confirmed that the observed CEST signal from fosfosal in lysate was produced by conversion of fosfosal to SA. This showed that fosfosal could be used to detect biologically relevant concentrations of AP enzymes in vitro. In particular, intracellular AP enzymes are only expected to be detected in necrotic tissues, because necrosis causes cells to release their intracellular components. Thus, catalyCEST MRI with fosfosal may be useful for detecting necrotic tumor tissues after initiating cancer treatment, and before macroscopic changes in tumor size can be observed.
Figure 5.
CEST spectra of fosfosal before and after catalysis with cell lysate. Spectra are shown for 27 mM fosfosal (triangle and short dashed red line), 27 mM fosfosal with lysate (square and solid red line), and 27 mM fosfosal with lysate pretreated with the inhibitor cocktails (circle and long dashed red line) with pH of 7.40 ± 0.05 were incubated with lysate solution for 3 h at 25 °C.
In conclusion, fosfosal can be used as a biosensor for the detection of AP enzymes with excellent specificity and in the presence of other biomolecules. Our results clearly showed that catalyCEST MRI can detect the activity of various isoforms of AP enzymes with fosfosal. High catalytic efficiency of the isoforms of AP enzymes for fosfosal facilitated the catalyCEST MRI detection of AP activity. Moreover, we were able to show that biologically relevant concentrations of AP enzyme can be detected by our method. In addition to biologically relevant experimental conditions, catalyCEST MRI parameters that were used in this experiment were within the range acceptable for clinical translation, which warrants future in vivo studies.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Indraneel Ghosh and Javier Castillo-Montoya for useful discussions. The authors also thank Dr. Elisa Tomat, Eman Akam, Ritika Gautam, and Dr. Kimberly M. Lincoln for permission to use their laboratory instruments and active involvement in collecting UV–vis results. This research was supported by the National Institutes of Health (NIH) through grant R01 CA169774 and P01 CA95060. KMJ was supported through NIH Cardiovascular Training Grant T32HL007955 and T32HL066988.
ABBREVIATIONS
- CEST
Chemical Exchange Saturation Transfer
- MRI
Magnetic Resonance Imaging
- AP
Alkaline Phosphatase
- CIAP
Calf Intestinal Alkaline Phosphatase
- HLAP
Human Liver Alkaline Phosphatase
- SA
Salicylic Acid
- MM
Michaelis–Menten
Footnotes
Author Contributions
I.D. and M.D.P. designed the experiments; I.D. and M.M.G. performed the studies; I.D. and K.M.J. analyzed the results; I.D. developed the manuscript and all coauthors approved the final version.
Notes
The authors declare no competing financial interest.
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acssensors.6b00203.
CEST and pH calibration graphs, UV–vis spectra, detailed procedures of experiments and the CEST protocol (PDF)
References
- 1.(a) Millán JL, editor. Phospharase Modulators. Human Press; New York: 2013. [Google Scholar]; (b) Millán JL. Alkaline phosphatasesstructure, substrate specificity and functional relatedness to other members of a large superfamily of enzymes. Purinergic Signalling. 2006;2:335–341. doi: 10.1007/s11302-005-5435-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Al Mamari S, Djordjevic J, Halliday JS, Chapman RW. Improvement of serum alkaline phosphatase to < 1.5 upper limit of normal predicts better outcome and reduced risk of cholangiocarcinoma in primary sclerosing cholangitis. J Hepatol. 2013;58:329–334. doi: 10.1016/j.jhep.2012.10.013. [DOI] [PubMed] [Google Scholar]; (d) Kaliannan K, Hamarneh SR, Economopoulos KP, Nasrin Alam S, Moaven O, Patel P, Malo NS, Ray MS, Abtahi M, Muhammad N, Raychowdhury A, Teshager A, Mohamed MMR, Moss AK, Ahmed R, Hakimian S, Narisawa S, Millán JL, Hohmann E, Warren HS, Bhan AK, Malo MS, Hodin RA. Intestinal alkaline phosphatase prevents metabolic syndrome in mice. Proc Natl Acad Sci U S A. 2013;110:7003–7008. doi: 10.1073/pnas.1220180110. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) McComb RB, Bowers GN, Posen S., Jr . Alkaline Phosphatase. Plenum Press; New York & London: 1979. [Google Scholar]
- 2.Guivel-Scharen V, Sinnwell T, Wolff SD, Balaban RS. Detection of proton chemical exchange between metabolites and water in biological tissues. J Magn Reson. 1998;133:36–45. doi: 10.1006/jmre.1998.1440. [DOI] [PubMed] [Google Scholar]
- 3.(a) Yoo B, Pagel MD. A paraCEST MRI contrast agent to detect enzyme activity. J Am Chem Soc. 2006;128:14032–14033. doi: 10.1021/ja063874f. [DOI] [PubMed] [Google Scholar]; (b) Bryant RG. The dynamics of water-protein interactions. Annu Rev Biophys Biomol Struct. 1996;25:29–53. doi: 10.1146/annurev.bb.25.060196.000333. [DOI] [PubMed] [Google Scholar]
- 4.Hingorani DV, Bernstein AS, Pagel MD. A review of responsive MRI contrast agents: 2005–2014. Contrast Media Mol Imaging. 2015;10:245–265. doi: 10.1002/cmmi.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu G, Ali MM, Yoo B, Griswold MA, Tkach JA, Pagel MD. PARACEST MRI with improved temporal resolution. Magn Reson Med. 2009;61:399–408. doi: 10.1002/mrm.21863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Yang X, Song X, Li Y, Liu G, Ray Banerjee S, Pomper MG, McMahon MT. Salicylic acid and analogues as diaCEST MRI contrast agents with highly shifted exchangeable proton frequencies. Angew Chem, Int Ed. 2013;52:8116–8119. doi: 10.1002/anie.201302764. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Bar-Shir A, Bulte JWM, Gilad AA. Molecular engineering of nonmetallic biosensors for CEST MRI. ACS Chem Biol. 2015;10:1160–1170. doi: 10.1021/cb500923v. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Noble E, Janssen L, Dierickx PJ. Comparative cytotoxicity of 5-aminosalicylic acid (mesalazine) and related compounds in different cell lines. Cell Biol Toxicol. 1997;13:445–451. doi: 10.1023/a:1007423911613. [DOI] [PubMed] [Google Scholar]
- 7.Daryaei I, Pagel MD. Double agents and secret agents: the emerging fields of exogenous chemical exchnage saturation transfer and T2-exchange magnetic resonance. Res Rep Nucl Med. 2015;5:19–32. doi: 10.2147/RRNM.S81742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Fernandez-Cuervo G, Sinharay S, Pagel MD. A catalyCEST MRI contrast agent that can simultaneously detect two enzyme activities. ChemBioChem. 2016;17:383–387. doi: 10.1002/cbic.201500586. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sinharay S, Fernández-Cuervo G, Acfalle JP, Pagel MD. Detection of sulfatase enzyme activity with a catalyCEST MRI contrast agent. Chem - Eur J. 2016;22:6491–6495. doi: 10.1002/chem.201600685. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Hingorani DH, Montano LA, Randtke EA, Lee YS, Cárdenas-Rodríguez J, Pagel MD. A single diamagnetic catalyCEST MRI contrast agent that detects cathepsin B enzyme activity by using a ratio of two CEST signals. Contrast Media Mol Imaging. 2016;11:130–138. doi: 10.1002/cmmi.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Levine MN, Raines RT. Sensitive fluorogenic substrate for alkaline phosphatase. Anal Biochem. 2011;418:247–252. doi: 10.1016/j.ab.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhou W, Andrews C, Liu J, Shultz JW, Valley MP, Cali JJ, Hawkins EM, Klaubert DH, Bulleit RF, Wood KV. Self-cleavable bioluminogenic luciferin phosphatase as alkaline phosphatase reporters. ChemBioChem. 2008;9:714–715. doi: 10.1002/cbic.200700644. [DOI] [PubMed] [Google Scholar]
- 10.(a) Ramis J, Torrent J, Mis R, Barbanoj M, Abadias M, Jane F, Forn J. Pharmacokinetic of fosfosal after single and multiple oral doses in man. Int J Clin Pharmacol Ther Toxicol. 1988;26:421–427. [PubMed] [Google Scholar]; (b) Ramis J, Gich I, Torrent J, Mis R, Jané F, Forn J. Bioavailability study of fosfosal and codeine administered alone or in combination. Int J Clin Pharmacol Ther Toxicol. 1989;27:352–357. [PubMed] [Google Scholar]
- 11.McMahon MT, Gilad AA, Zhou J, Sun PZ, Bulte JWM, van Zijl PCM. Quantifying exchange rates in chemical exchange saturation transfer agents using the saturation time and saturation power dependencies of the magnetization transfer effect on the magnetic resonance imaging. Magn Reson Med. 2006;55:836–847. doi: 10.1002/mrm.20818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ward KM, Balaban RS. Determination of pH using water protons and chemical exchange dependent saturation transfer (CEST) Magn Reson Med. 2000;44:799–802. doi: 10.1002/1522-2594(200011)44:5<799::aid-mrm18>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 13.(a) Domar U, Nilsson B, Baranov V, Gerdes U, Stigbrand T. Expression of intestinal alkaline phosphatase in human organs. Histochemistry. 1992;98:359–364. doi: 10.1007/BF00271071. [DOI] [PubMed] [Google Scholar]; (b) Benham FJ, Fogh J, Harris H. Alkaline phosphatase expression in human cell lines derived from various malignancies. Int J Cancer. 1981;27:637–644. doi: 10.1002/ijc.2910270510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.