Abstract
This study aimed to evaluate the efficacy and safety of dexmedetomidine versus any other treatment without dexmedetomidine in patients who have undergone cardiac surgery. Electronic databases including PubMed, Embase, and Cochrane Library were systematically searched without limitations of language and publication time. Randomized controlled trials (RCTs) aiming to evaluate the efficacy and safety of dexmedetomidine versus any other treatment without dexmedetomidine in patients that have undergone cardiac surgery were selected. Endpoints such as hemodynamic indexes and adverse events in eligible studies were extracted by two researchers, independently. The data was analyzed using RevMan 5.3 and Stata 11.0 software. A total of 18 RCTs met the inclusion criteria, involving 1730 patients. Compared to control (any treatment without dexmedetomidine), dexmedetomidine showed a pooled mean difference (MD) of -14.46 [95% confidence interval(CI): -24.69, -4.23; p<0.01] for systolic arterial pressure, a standardized mean difference (SMD) of -1.74 for mean arterial blood pressure (95% CI: -2.80, -0.68; P < 0.01), -2.12 (95%CI: -3.23, -1.00; p<0.01) for heart rate, and combined odds ratio (OR) of 0.22 (95%CI: 0.11, 0.44; p<0.01) for tachycardia, 3.44 (95%CI: 1.95, 5.96; p<0.01) for bradycardia, 0.74 (95%CI: 0.49, 1.12; p>0.05) for atrial fibrillation, and 0.99 (95%CI: 0.51, 1.90; p>0.05) for hypotension. In addition, dexmedetomidine could reduce time of surgery and stay in intensive care units, improve delirium with good safety. Our study shows clinical application of dexmedetomidine in cardiac surgery patients can reduce risks of abnormal hemodynamics with good safety.
Introduction
More than 2 million cardiac surgeries are performed in the world annually [1]. While cardiac surgery is often used to treat complications of ischemic heart disease, correct congenital heart disease, or treat valvular heart disease from various causes, including endocarditis, rheumatic heart disease, and atherosclerosis, these procedures have several disadvantages [2–4]. Cardiac surgery is suggested to be associated with high risks of cardiovascular complications and other adverse events when performing operations, which are often resulted in increased hospital stay and even mortality [5–10]. Although great improvements in equipment, techniques and medical care have been achieved, and decreased the incidences of major complication rates and mortality [11–16], effective and safe perioperative medication is required to further reduce these negative events [8,11,17,18].
Dexmedetomidine is an anxiety reducing sedative, and pain medication [19]. It is notable for its ability to provide sedation without any risk of respiratory depression and can provide cooperative or semi-arousable sedation [20]. Several studies suggested that dexmedetomidine may be useful for the treatment of the negative cardiovascular effects of cardiac surgery [21–26]. However, the utilization of this medication was limited in clinical practice as its common side effects such as hypotension and bradycardia, and higher economic costs [11,27].
In this study, we explored the efficacy and safety of dexmedetomidine versus other medications in cardiac surgery patients. After systematic research, the effects of dexmedetomidine on hemodynamics in patients undergoing cardiac surgery compared with other drugs were noted. Patients undergoing cardiac surgery and even other drugs were found and selected. Data was analyzed by RevMan 5.3 and Stata 11.0 software to make evidence supporting the efficacy and safety of dexmedetomidine in patients undergoing cardiac surgery.
Methods
This meta-analysis was performed by following the guidance of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)(S1 Checklist).
Search strategy
Electronic databases such as PubMed, Embase, and the Cochrane Library were systematically searched for clinical RCTs comparing the efficacy and safety of dexmedetomidine versus other medications in treating patients undergoing cardiac surgery. The following Mesh terms and free texts were used in various combinations for selecting eligible studies: "dexmedetomidine", "cardiac surgery", "cardio protection", "myocardial", "cardiopulmonary bypass", "coronary artery bypass grafting ", "heart surgery", and "heart valve". Language restriction was not introduced in this study. Unpublished articles or outcomes were also searched by contacting relevant researchers of this topic. To move more towards a perfect search, the references of similar reviews, and major cardiac surgical scientific meetings’ abstracts were also selected for further evaluation. If necessary, manual retrieval of references would be applied.
Inclusion and exclusion criteria
According to the participants, interventions, comparisons, outcomes, and study design (PICOS) protocol, the following criteria were used. Patients: who were diagnosed with complications of ischemic heart disease, or needed to treat congenital heart disease, valvular heart disease from various causes, including endocarditis, rheumatic heart disease, and atherosclerosis, and received cardiac surgery. Intervention and comparison: each study contained two comparison groups, one received dexmedetomidine, and the other group received control (treatment without dexmedetomidine). Outcomes: hemodynamics indexes including mean arterial pressure, mean pulmonary arterial pressure, pulmonary capillary wedge pressure, systemic vascular resistance index, pulmonary vascular resistance index, incidence of tachycardia/hypotension/bradycardia, heart rate and blood pressure. Other indicators such as pain score, satisfaction scores, and postoperative complications were also included. Study design: only randomized controlled trials were included to ensure the quality of pooled results. Retrospective study, observational study, reviews, and animal studies were excluded. If articles failed to provide sufficient information or data, they were also excluded.
Data extraction
Two reviewers extracted the baseline characteristics and eligible endpoint data from included studies, independently. The basal information included first author, year of publication, number of patients in each group, mean age, sex, disease diagnostic criteria, cardiac surgery types, and application information of dexmedetomidine. Outcomes included mean systolic arterial pressure, mean arterial blood pressure, central venous pressure, mean pulmonary arterial pressure and heart rate, incidence of bradycardia or tachycardia, cardiac index, and duration of ICU and surgery. Other indicators such as postoperative complications were also extracted. If controversies about the recorded data existed, a third reviewer was introduced to solve it.
Quality assessment
RCTs that met the inclusion criteria and passed the following assessment of quality were included for the final analysis. The quality assessment section of the Cochrane Handbook for Systematic Reviews of Interventions was used to evaluate the overall quality by two investigators, independently [28]. Quality items such as bias of selection, bias of blinding, bias of incomplete outcome data, bias of selective reporting, and other bias within each included study were assessed. If there were any disagreements, a third investigator was involved to solve the problem. According to the handbook mentioned above, the final quality was defined as low risk, moderate risk or high risk of bias, based on the results of the overall quality assessment.
Statistical analysis
Meta-analysis was performed using the RevMan 5.3 software. To present the dichotomous data, the odds ratio (OR) with a 95% confidence interval (CI) was used to represent the effects of intervention on interest indicators over that of control. To present the continuous variables, the mean ± standard deviation was used. The mean difference (MD) was applied if the measurement unit of continuous variables was in accordance with each other. Otherwise the standardized mean difference (SMD) was used. The heterogeneity test was evaluated by Q test and I2 coefficient. In addition, the method for calculating standard deviation (SD), provided by the Handbook, was used to get indirect SD [28]. If the I2%> 75% suggested that there was obvious heterogeneity between the studies; if the I2%<40%, the study could be considered homogeneous; if the I2% was between 30% to 60%, a moderate heterogeneity was considered [28]. According to the results of the heterogeneity detection, the random effect model was used if there was significant heterogeneity; otherwise, the fixed effect model was used. When indicating significant heterogeneity, the subgroup analysis of the target data was introduced. The results of meta-analysis were presented in forms of forest plots. To detect publication bias, the funnel plot, Begg' test and Egger test was used. All significance testing was two-sided, and if P < 0.05 was considered as statistically significant.
Results
Search results
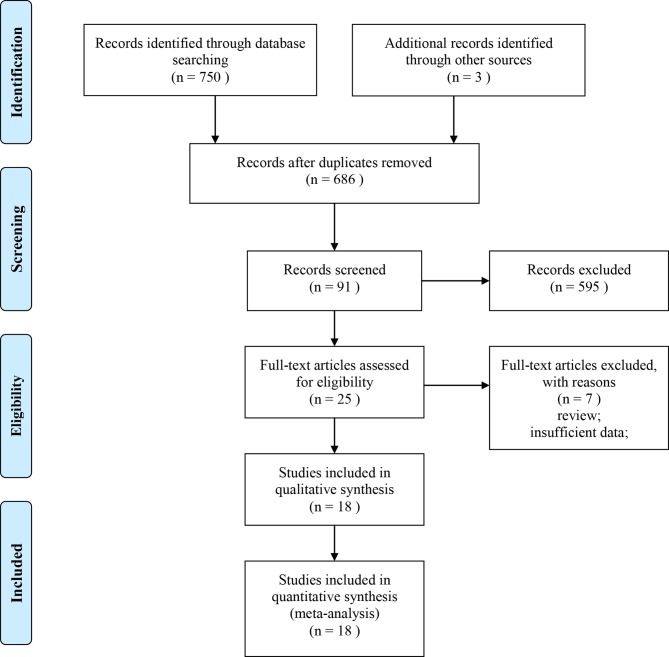
A total of 753 literatures were retrieved, and the literatures were screened strictly according to the inclusion and exclusion criteria by two reviewers, independently. 595 of these references were discarded after the first screen process. Further review of the abstract and full text of the remaining 91 articles was followed. At last, the final 18 RCTs [2,20–22,24,29–41] were included in this systematic review. Total of 1730 patients undergoing cardiac surgery were involved in these studies, including 865 cases in the dexmedetomidine group, and 865 cases in the control group (any treatment without dexmedetomidine). The baseline characteristics of the included studies are shown in Table 1. The detailed information of the study selection process is presented in Fig 1.
Table 1. Baseline characteristics of included studies.
| First author | Year | Number of cases | Dex dose/administration | Surgery | |
|---|---|---|---|---|---|
| Dex | Con | ||||
| Herr | 2003 | 148 | 147 | 1.0 μg/kg loading; 0.2 μg/kg/h infusion | Cardiac surgeries |
| Corbett | 2005 | 43 | 46 | 1 μg/kg loading; 0.4 μg/kg continuous infusion | Coronary artery bypass grafting |
| Shehabi | 2009 | 152 | 147 | 0.1 μg/kg/h | Cardiac surgeries |
| Rabie | 2016 | 75 | 75 | 1μg/kg loading, maintained as an infusion of 0.3μg/kg/h | Cardiac surgeries |
| Ren | 2013 | 81 | 81 | 0.2 μg/kg/h | Coronary artery bypass grafting |
| Tosun | 2013 | 18 | 20 | 0.5 μg/kg loading; 0.5 μg/kg/min continuous infusion | Coronary artery bypass grafting |
| Aziz | 2011 | 14 | 14 | 0.12 ± 0.03 ug/kg/h | CABG/septal repair/valvular repair |
| Balkanay | 2015 | 30 | 30 | 8μg/cc | CABG with CPB |
| Jalonen | 1997 | 40 | 40 | 50ng/kg/min for 30 min and followed by 7ng/kg/min | CABG with CPB |
| Maldonado | 2009 | 40 | 38 | loading dose:0.4ug/kg, followed by 0.2–0.7uk/kg/h | cardiac valve surgery |
| Chi | 2016 | 34 | 33 | 1 μg/kg loading; 0.6 μg/kg continuous infusion | Off-pump coronary artery bypass grafting surgery |
| Liu | 2016 | 29 | 32 | 1.5 μg/kg/h continuous infusion | Cardiac surgeries |
| Khalil | 2016 | 25 | 25 | 1 μg/kg loading; 0.5 μg/kg/h continuous infusion | TAVI |
| Priye | 2015 | 32 | 32 | 0.4μg/kg/h continuous infusion | elective cardiac surgery |
| Karaman | 2015 | 31 | 33 | 0.2 μg/kg/h–1.0 μg/ kg/h | CABG with CPB |
| Eremenko | 2014 | 28 | 27 | 0.2–0.7 μg/kg/h | cardiac surgery |
| Sulaiman | 2012 | 30 | 30 | 0.5 μg/kg | cardiac surgery |
| Menda | 2010 | 15 | 15 | 1 μg/kg | CABG |
Abbreviation: Dex, dexmedetomidine; Con, control; M, male; F, female; NA, not available; CABG, coronary artery bypass grafting; TAVI, Transcatheter aortic valve implantation; CPB, cardiopulmonary bypass
Fig 1. Flow diagram following the PRISMA guideline.
Quality of the included studies
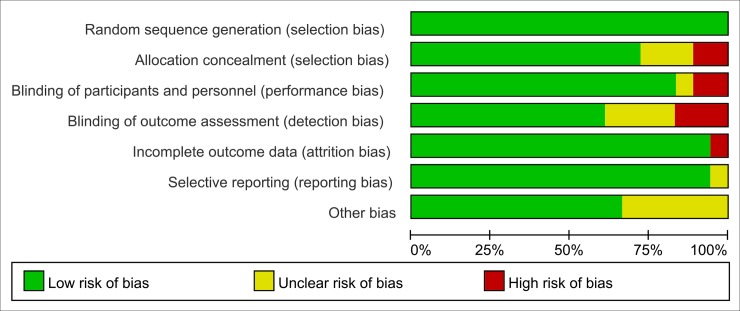
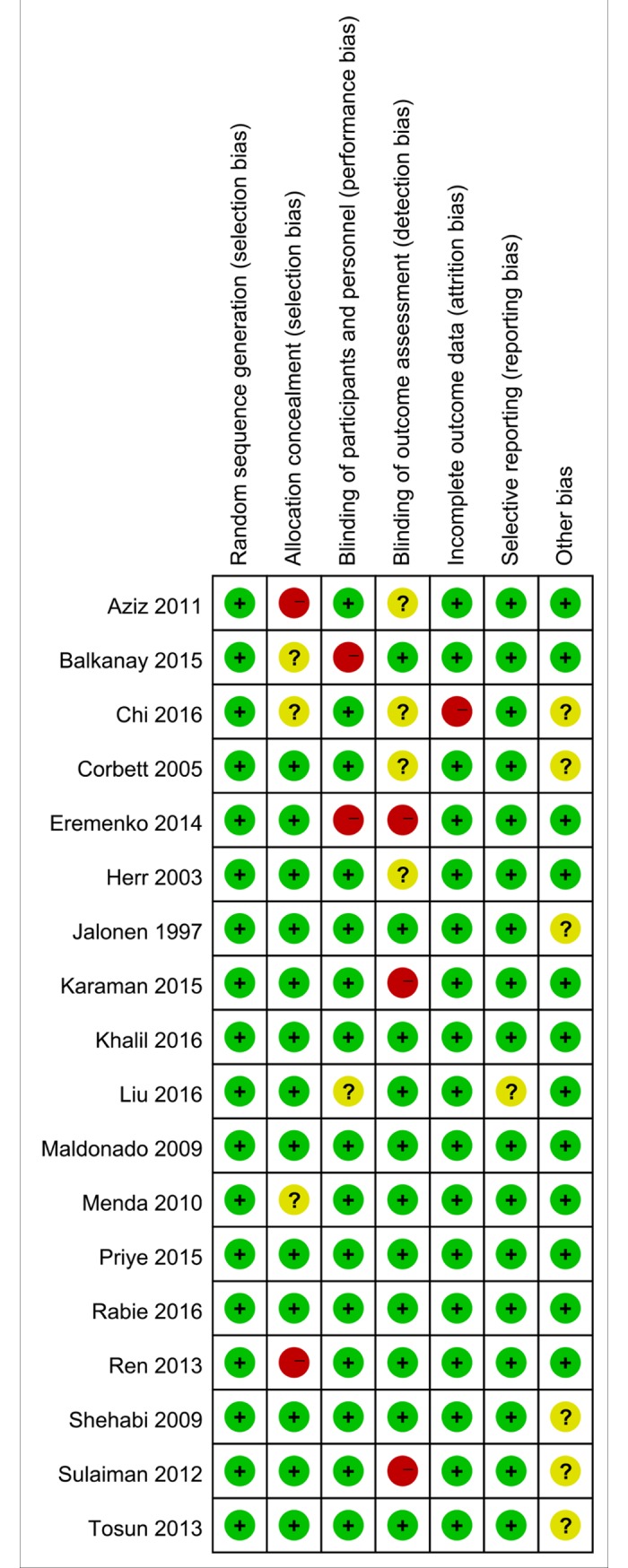
Based on the quality assessment items of the Cochrane Handbook [28] for systematic review, all the included articles were evaluated for risk of biases, such as selection bias, selective reporting bias, incomplete reporting bias, and publication bias. The quality of the literatures was defined as low, moderate, and high risk of bias according to the Handbook mentioned above. Nearly all the included RCTs were evaluated as low risk of bias, indicating good qualities (Figs 2 and 3).
Fig 2. Risk of bias graph: Review authors' judgements about each risk of bias item presented as percentages across all included studies.
Fig 3. Risk of bias summary: Review authors' judgements about each risk of bias item for each included study.
Hemodynamic indexes
Systolic arterial pressure
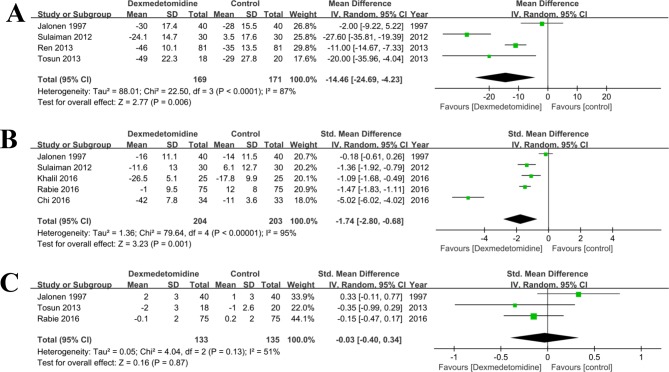
A meta-analysis of four studies [32–34,41] on dexmedetomidine versus control (any treatment without dexmedetomidine) found a significant difference in decreasing the systolic arterial pressure (MD = -14.46, 95% CI: -24.69, -4.23; P<0.0001, Fig 4A). As there was significant heterogeneity across these studies (I2% = 87%), the random effect model was used. The sensitivity analysis was used to detect the source of heterogeneity, and the result showed that the study of Tosun [32] might be responsible for it.
Fig 4.
Forest plot of comparison: 1 Dexmedetomidine VS. Control (any treatment without dexmedetomidine) for patients undergoing cardiac surgery, outcome: A, Systolic arterial pressure; B, mean arterial blood pressure; C, Central venous pressure.
Mean arterial blood pressure
Five studies [2,20,22,34,41] provided data on mean arterial blood pressure. There was statistical heterogeneity among these studies (P<0.01, I2% = 95%). Combined results from the five RCTs of dexmedetomidine versus control (any treatment without dexmedetomidine) also found a significant difference in decreasing the mean arterial blood pressure (SMD = -1.74, 95% CI: -2.80, -0.68; P < 0.01, Fig 4B). The sensitivity analysis was used to detect the source of heterogeneity, and the result showed that the study of Chi [22] could be responsible for it.
Central venous pressure
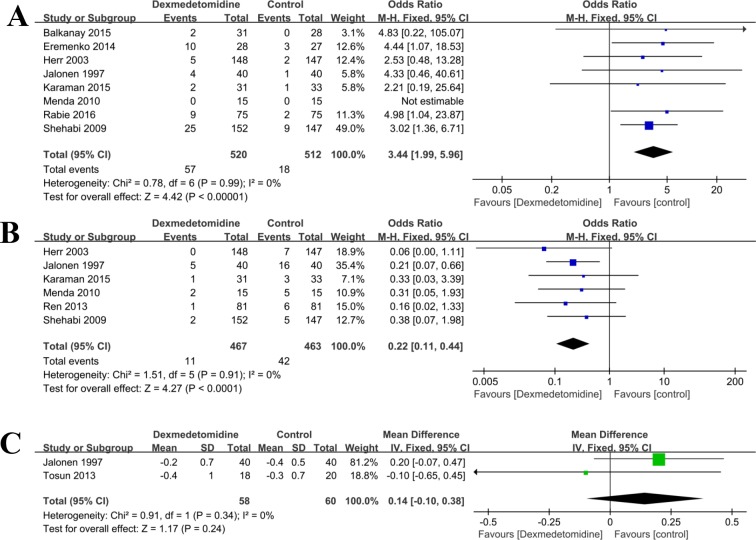
There were three RCTs [2,32,41] compared the data of central venous pressure in patients undergoing cardiac surgery. There was a moderate heterogeneity among these studies (P = 0.13, I2 = 51%), and the random effect model was used. The combined results suggested that the central venous pressure in the dexmedetomidine group was similar to that in the control (any treatment without dexmedetomidine) group (SMD = -0.03, 95% CI: -0.40, 0.34; P = 0.87, Fig 4C). The sensitivity analysis was used to detect the source of heterogeneity, but did not discover the source.
Pulmonary artery mean pressure
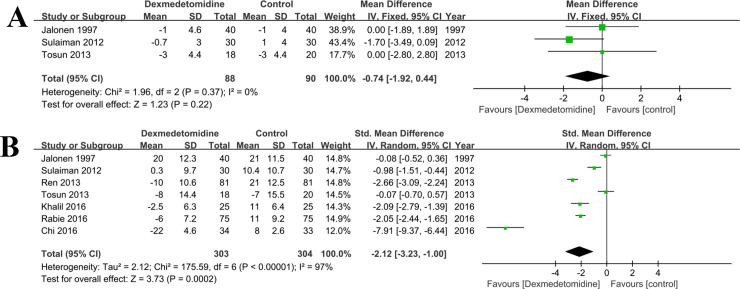
There were three RCTs [32,34,41] compared the data of pulmonary artery mean pressure in patients undergoing cardiac surgery. There was no statistical heterogeneity among these studies (P = 0.37, I2 = 0%), and the fixed effect model was used. The combined results suggested that the pulmonary artery mean pressure in the dexmedetomidine group was not significantly lower than that in the control (any treatment without dexmedetomidine) group (MD = -0.74, 95% CI: -1.92, 0.44; P = 0.22, Fig 5A).
Fig 5.
Forest plot of comparison: 1 Dexmedetomidine VS. Control (any treatment without dexmedetomidine) for patients undergoing cardiac surgery, outcome: A, Pulmonary artery mean pressure; B, Heart rate.
Other indicators
Heart rate
Seven RCTs [2,20,22,32–34,41] reported postoperative heart rates (Fig 5B). As there was significant heterogeneity (I2% = 97%), the random effect model was used. The results of meta-analysis showed that heart rates were lower in the dexmedetomidine group than in the control (any treatment without dexmedetomidine) group (MD = -2.12, 95% CI: -3.23, -1.00, P < 0.001). The sensitivity analysis was used to detect the source of heterogeneity, and the result showed that the study of Chi [22] could be responsible for it.
Bradycardia
There were eight studies [2,20–22,36,37,40,41] compared the incidences of bradycardia. There was no statistical heterogeneity (P = 0.99, I2 = 0%), and the fixed effect model was used. The results showed that significant difference in incidence of bradycardia was found between the dexmedetomidine group and the control (any treatment without dexmedetomidine) group (OR = 3.44, 95% CI: 1.99, 5.96; P<0.001, Fig 6A).
Fig 6.
Forest plot of comparison: 1 Dexmedetomidine VS. Control (any treatment without dexmedetomidine) for patients undergoing cardiac surgery, outcome: A, Bradycardia; B, Tachycardia; C, Cardiac index.
Tachycardia
Six studies [29,33,36,37,40,41] compared the rates of tachycardia of dexmedetomidine versus control (any treatment without dexmedetomidine) during surgery. There was no statistical heterogeneity (P = 0.91, I2 = 0%) in each study, and a fixed effect model was used. The results showed significant reduction in the prevalence of tachycardia in the dexmedetomidine group than that in the control (any treatment without dexmedetomidine) group (OR = 0.22, 95% CI: 0.11, 0.44; P <0.001, Fig 6B).
Cardiac index
Two studies [32,41] compared the cardiac index in the cardiac surgery perioperative period. There was no statistical heterogeneity (P = 0.34, I2 = 0%) in each study, and a fixed effect model was used. The results showed no significant difference between the two groups (OR = 0.14, 95% CI: -0.10, 0.38; P = 0.24, Fig 6C).
Duration of ICU stay and surgery
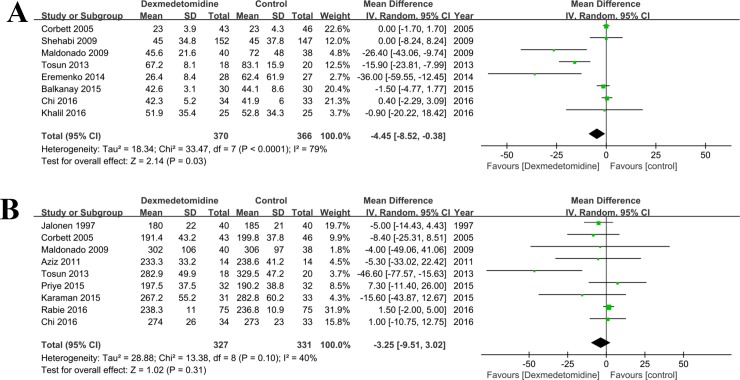
Eight [20,22,30–32,37–39] and nine [2,22,24,29,32,35,38,39,41] literatures compared the duration of ICU stay and surgery in patients treated with dexmedetomidine versus control group (any treatment without dexmedetomidine) during and after surgery (Fig 7A and 7B). There was significant heterogeneity among these studies (P<0.05), so the random effect model was introduced. Compared with the control group, the ICU stay (MD = -4.45, 95% CI: -8.52, -0.38; P = 0.03) was significantly decreased in dexmedetomidine than that in the control (any treatment without dexmedetomidine) group, but not the surgery time (MD = -3.25, 95% CI: -9.51, 3.02; P = 0.31).
Fig 7.
Forest plot of comparison: 1 Dexmedetomidine VS. Control (any treatment without dexmedetomidine) for patients undergoing cardiac surgery, outcome: A, ICU stay; B, Duration of surgery.
Postoperative complications
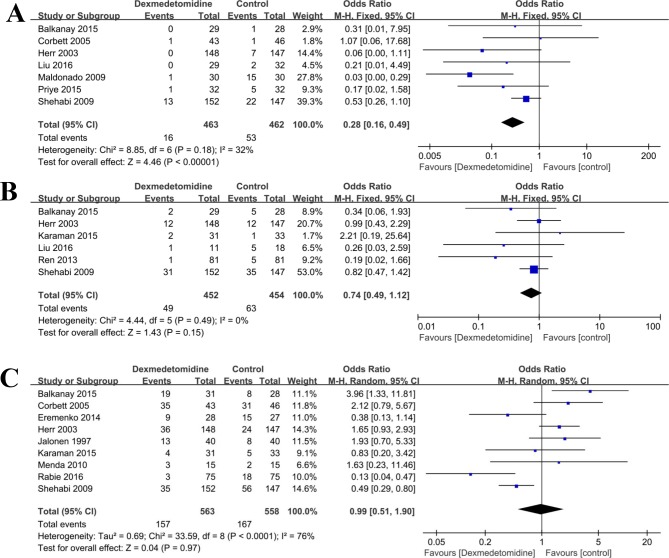
The incidences of postoperative complications including delirium, atrial fibrillation, hypotension, and other adverse events such as renal failure, pulmonary edema, myocardial ischemia, and mortality of dexmedetomidine versus control (any treatment without dexmedetomidine) group in patients who received cardiac surgery were compared (Table 2). In brief, six studies [21,29,30,33,37,40] compared the incidence of atrial fibrillation in the cardiac surgery perioperative period. There was no statistical heterogeneity (P = 0.49, I2 = 0%) in any study, and a fixed effect model was used. The results showed no significant difference between the two groups (OR = 0.74, 95% CI: 0.49, 1.12; P = 0.15, Fig 8B).
Table 2. Summarized results of included studies.
| Outcome | Studies | Participants | Statistical Method | Effect Estimate |
|---|---|---|---|---|
| Pulmonary artery mean pressure | 3 | 178 | Mean Difference (IV, Fixed, 95% CI) |
-0.74 [-1.92, 0.44] |
| Heart rate | 7 | 607 | Mean Difference (IV, Random, 95% CI) |
-15.22 [-23.50, -6.94] |
| Tachycardia | 6 | 930 | Odds Ratio (M-H, Fixed, 95% CI) |
0.22 [0.11, 0.44] |
| Hypotension | 9 | 1121 | Odds Ratio (M-H, Random, 95% CI) |
0.99 [0.51, 1.90] |
| Bradycardia | 8 | 1032 | Odds Ratio (M-H, Fixed, 95% CI) |
3.44 [1.99, 5.96] |
| Central venous pressure | 3 | 268 | Mean Difference (IV, Random, 95% CI) |
-0.06 [-1.02, 0.91] |
| Duration of surgery | 9 | 658 | Mean Difference (IV, Random, 95% CI) |
-3.25 [-9.51, 3.02] |
| Mean arterial blood pressure | 5 | 407 | Mean Difference (IV, Random, 95% CI) |
-14.54 [-25.09, -3.98] |
| Adverse events | ||||
| Renal failure | 3 | 495 | Odds Ratio (M-H, Fixed, 95% CI) |
0.67 [0.28, 1.61] |
| Pulmonary edema | 3 | 495 | Odds Ratio (M-H, Fixed, 95% CI) |
1.13 [0.43, 2.98] |
| Myocardial ischemia | 4 | 657 | Odds Ratio (M-H, Fixed, 95% CI) |
0.42 [0.22, 0.80] |
| Mortality | 4 | 588 | Odds Ratio (M-H, Fixed, 95% CI) |
0.66 [0.18, 2.35] |
| Delirium | 6 | 630 | Odds Ratio (M-H, Fixed, 95% CI) |
0.32 [0.18, 0.57] |
| ICU stay | 8 | 736 | Mean Difference (IV, Random, 95% CI) |
-4.45 [-8.52, -0.38] |
| Cardiac index | 2 | 118 | Mean Difference (IV, Fixed, 95% CI) |
0.14 [-0.10, 0.38] |
| Systolic arterial pressure | 4 | 340 | Mean Difference (IV, Random, 95% CI) |
-14.46 [-24.69, -4.23] |
| Atrial fibrillation | 6 | 906 | Odds Ratio (M-H, Fixed, 95% CI) |
0.74 [0.49, 1.12] |
Abbreviation: CI, confidence interval; ICU, intensive care unit.
Fig 8.
Forest plot of comparison: 1 Dexmedetomidine VS. Control (any treatment without dexmedetomidine) for patients undergoing cardiac surgery, outcome: A, Delirium; B, Atrial fibrillation; C, Hypotension.
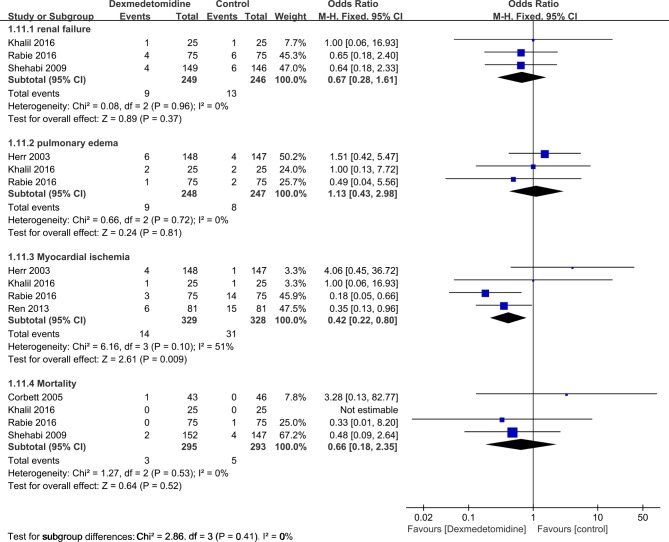
The hypotension (OR = 0.99, 95% CI: 0.51, 1.90; P = 0.97, Fig 8C), renal failure (OR = 0.67, 95% CI: 0.28, 1.61; P = 0.37, Fig 9), pulmonary edema (OR = 1.13, 95% CI: 0.43, 2.98; P = 0.81, Fig 9), and mortality (OR = 0.66, 95% CI: 0.18, 2.35; P = 0.52, Fig 9) were also similar between dexmedetomidine group and control group (any treatment without dexmedetomidine) (all p>0.05). However, the results showed that the incidences of delirium (OR = 0.32, 95% CI: 0.18, 0.57; P < 0.0001, Fig 8A), and myocardial ischemia (OR = 0.42, 95% CI: 0.22, 0.80; P = 0.009, Fig 9) were significantly lower when compared with control group (any treatment without dexmedetomidine) (Table 2).
Fig 9. Forest plot of comparison: 1 Dexmedetomidine VS. Control (any treatment without dexmedetomidine) for patients undergoing cardiac surgery, outcome: Adverse events: renal failure; pulmonary edema; myocardial ischemia; mortality.
Publication bias assessment
Using the ICU stay as an endpoint, the possible publication bias was detected from the funnel plot (S1 Fig). As indicated by the Begg's test (p = 0.174) and Egger's test (p = 0.033), there may exist some bias. Then we followed the “trim and fill” method, and it showed no publication bias there.
The funnel plots of duration of surgery (S2 Fig), heart rate (S3 Fig), delirium (S4 Fig), atrial fibrillation (S5 Fig), bradycardia (S6 Fig), tachycardia (S7 Fig), and hypotension (S8 Fig) were also conducted to detect whether publication bias existed within these endpoints, and the results showed that there was no significant bias as indicated by the symmetry of the plots. Begg's tests (p = 0.348 for duration of surgery, p = 0.764 for heart rate, p = 0.764 for delirium, p = 1.000 for atrial fibrillation, p = 0.548 for bradycardia, p = 0.452 for tachycardia, p = 0.602 for hypotension) and Egger's tests (p = 0.084 for duration of surgery, p = 0.377 for heart rate, p = 0.335 for delirium, p = 0.843 for atrial fibrillation, p = 0.367 for bradycardia, p = 0.244 for tachycardia, p = 0.963 for hypotension) also proved no significant publication bias.
Discussion
Although advancements in cardiac surgery have remarkably reduced the incidences of mortality and serious complications, effective medication is needed to benefit patients undergoing cardiac surgery. Several studies [19,37,42,43] have reported that dexmedetomidine may have beneficial effects on clinical outcomes in patients with cardiac surgery. However, strong supportive evidence is required. By pooling data from eligible RCTs, the use of dexmedetomidine led to significantly beneficial effects on systolic arterial pressure, mean arterial blood pressure, pulmonary artery mean pressure, and heart rate. We also observed that administration of dexmedetomidine was associated with a significant reduction in the duration of ICU stay and surgery, occurrence of postoperative delirium and the incidence of tachycardia. Though above results showed that dexmedetomidine had improved outcomes, it was associated with increased risk of bradycardia.
Dexmedetomidine is suggested to have sedative, anxiolytic, and analgesic abilities in patients undergoing cardiac surgery [17]. In our study, the meta-analysis suggested that patients that were treated with dexmedetomidine had a lower heart rate, along with lower mean arterial blood pressure, systolic arterial pressure, pulmonary artery mean pressure, and reduced ICU stay than those in the control group (any treatment without dexmedetomidine). The incidences of tachycardia, hypotension, and delirium decreased in the dexmedetomidine group, when compared to the control (any treatment without dexmedetomidine) group, indicating that patients who were treated with dexmedetomidine had lower risks of getting these events. Regarding bradycardia, the rate of this event was increased by about 3.4 times than those that did not use dexmedetomidine. This suggested that the heart rates of patients using dexmedetomidine should be carefully monitored. Other adverse events including renal failure, stroke, pulmonary edema, and mortality were also compared, and the results did not suggest that there were any significant differences between dexmedetomidine and other medications or placebo, proving the safety of dexmedetomidine.
Previously, a few meta-analyses and reviews [11,17,44,45], aiming to evaluate the effect of dexmedetomidine on clinical outcomes, delirium, myocardial protective, and postoperative complications were published. Lin et al. performed a meta-analysis [11] to assess whether dexmedetomidine could serve as a safe and efficacious sedative medication in post-cardiac surgery population. By analyzing the data, extracted from a total of 11 studies, the results of their meta-analysis showed that dexmedetomidine was associated with a shorter duration of mechanical ventilation, a lower prevalence of delirium, and tachycardia, but might increase the risk of bradycardia. They also found no significant difference in ICU stay between the different medications. With respect to postoperative complications, their results suggested that dexmedetomidine may not increase the risk of atrial fibrillation, hypotension, or mortality. Most of these results are in accordance with our findings, and we find a higher risk of bradycardia and a significant shorter duration of ICU stay. This may be explained by more included samples and cases of bradycardia, and decreased time of ICU stay in the included studies. More recently, another two meta-analyses [44,45] evaluated the myocardial protective effects and possibility of decreasing risks of delirium of dexmedetomidine in patients undergoing cardiac surgery or even post cardiac surgery. Gong et al. [44] included 18 studies and found that lower heart rate, systolic blood pressure, and incidence of tachycardia were associated with dexmedetomidine treatment in both adults and pediatric patients, and also elevated the number of bradycardia. In contrast, our meta-analysis included 18 studies and all of them were RCTs in adult patients. However, most of our findings were in accordance with their myocardial protective results in adult population. Liu et al. [45] included a total of 8 RCTs, and their results revealed that a lower risk of delirium, a shorter length of intubation, but a higher incidence of bradycardia were found in dexmedetomidine group as compared to propofol. There were no statistical differences in the risks of hypotension or atrial fibrillation, or the time of ICU stay between dexmedetomidine and propofol regimens. Unlike their ICU stay result, we found a significantly shorter stay in ICU.
All the included studies were RCTs, but potential heterogeneity may still exist within and across these trials, which may limit the quality of the results. Even though well designed and performed, there are still a few limitations in the present meta-analysis. First, the lack of gray literature (such as symposium records, unpublished data, government research reports, etc.) may lead to publication bias. Second, the qualities of the included RCTs were not in accordance with each other. Third, the time, dose of usage, injection speed of dexmedetomidine and disease and surgical type were different between the included studies, which may increase the heterogeneity bias across the studies and affect the results of the analysis. This suggests that the use of dexmedetomidine is not strictly organized. When different endpoints were set or goals were adopted, it was difficult to maintain consistency among various studies, which resulted in the apparent heterogeneity. We highlight the requirement for reliable and valid pooled results to determine the ideal efficacy of dexmedetomidine. Thus, we introduce heterogeneity detection method to minimize the potential influence of inconsistency to achieve the requirement mentioned above. There may be detection bias or measurement bias in the selection and evaluation of the cognitive function evaluation scale or the blood pressure, and the reliability is limited.
Conclusion
In summary, the application of dexmedetomidine can effectively reduce the incidence of early postoperative delirium and ventricular tachycardia in patients who have undergone cardiac surgery, with tolerable adverse events. Our findings also suggest that dexmedetomidine may not increase the incidence of hemodynamic complications. Though the above conclusions are recommended, high-quality, large sample, randomized controlled clinical trials are needed to verify the effects and safety of dexmedetomidine in cardiac surgery patients.
Supporting information
(DOC)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by Grants from Zhejiang Provincial Natural Science Foundation of China (Project No. LY15H150001).
References
- 1.Mao H, Katz N, Ariyanon W, Blanca-Martos L, Adybelli Z, Giuliani A, et al. Cardiac surgery-associated acute kidney injury. Cardiorenal Med. 2013; 3: 178–199. 10.1159/000353134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soliman R, Zohry G. The myocardial protective effect of dexmedetomidine in high-risk patients undergoing aortic vascular surgery. Ann Card Anaesth. 2016; 19: 606–613. 10.4103/0971-9784.191570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bulow NM, Colpo E, Pereira RP, Correa EF, Waczuk EP, Duarte MF, et al. Dexmedetomidine decreases the inflammatory response to myocardial surgery under mini-cardiopulmonary bypass. Braz J Med Biol Res. 2016; 49: e4646 10.1590/1414-431X20154646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ueki M, Kawasaki T, Habe K, Hamada K, Kawasaki C, Sata T. The effects of dexmedetomidine on inflammatory mediators after cardiopulmonary bypass. Anaesthesia. 2014; 69: 693–700. 10.1111/anae.12636 [DOI] [PubMed] [Google Scholar]
- 5.Magapu P, Haskard D, Fisher M. A review of the peri-operative risk stratification assessment tools used for the prediction of cardiovascular complications in non-cardiac surgery. Perfusion. 2016; 31: 358–365. 10.1177/0267659115615207 [DOI] [PubMed] [Google Scholar]
- 6.Tiwari D, Jurkovitz CT, Zhang Z, Bowen J, Kolm P, Wygant G, et al. Risk factors for cardiovascular events and bleeding complications following non-cardiac surgery or procedure in patients with drug eluting stent placement. Heart Asia. 2014; 6: 69–75. 10.1136/heartasia-2013-010471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Afolabi BA, Novaro GM, Szomstein S, Rosenthal RJ, Asher CR. Cardiovascular complications of obesity surgery in patients with increased preoperative cardiac risk. Surg Obes Relat Dis. 2009; 5: 653–656. 10.1016/j.soard.2009.06.009 [DOI] [PubMed] [Google Scholar]
- 8.Wijeysundera DN, Beattie WS, Rao V, Karski J. Calcium antagonists reduce cardiovascular complications after cardiac surgery: a meta-analysis. J Am Coll Cardiol. 2003; 41: 1496–1505. [DOI] [PubMed] [Google Scholar]
- 9.Hellerstein HK, Feil H. Cardiovascular complications of cardiac and noncardiac surgery. J Am Assoc Nurse Anesth. 1949; 17: 207–223. [PubMed] [Google Scholar]
- 10.Chaudhry R, Zaki J, Wegner R, Pednekar G, Tse A, Sheinbaum R, et al. Gastrointestinal Complications After Cardiac Surgery: A Nationwide Population-Based Analysis of Morbidity and Mortality Predictors. J Cardiothorac Vasc Anesth. 2017; 31: 1268–1274. 10.1053/j.jvca.2017.04.013 [DOI] [PubMed] [Google Scholar]
- 11.Lin YY, He B, Chen J, Wang ZN. Can dexmedetomidine be a safe and efficacious sedative agent in post-cardiac surgery patients? A meta-analysis. Critical Care. 2012; 16: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lurati Buse GA, Bolliger D, Seeberger E, Kasper J, Grapow M, Koller MT, et al. Troponin T and B-type natriuretic peptide after on-pump cardiac surgery: prognostic impact on 12-month mortality and major cardiac events after adjustment for postoperative complications. Circulation. 2014; 130: 948–957. 10.1161/CIRCULATIONAHA.113.007253 [DOI] [PubMed] [Google Scholar]
- 13.Krzych LJ, Wybraniec MT, Krupka-Matuszczyk I, Skrzypek M, Bolkowska A, Wilczynski M, et al. Detailed insight into the impact of postoperative neuropsychiatric complications on mortality in a cohort of cardiac surgery subjects: a 23,000-patient-year analysis. J Cardiothorac Vasc Anesth. 2014; 28: 448–457. 10.1053/j.jvca.2013.05.005 [DOI] [PubMed] [Google Scholar]
- 14.Kamiya H, Tanzeem N, Akhyari P, Pedraza A, Kallenbach K, Lichtenberg A, et al. Impact of severe postoperative complications after cardiac surgery on mortality in patients aged over 80 years. Ann Thorac Cardiovasc Surg. 2014; 20: 383–389. [DOI] [PubMed] [Google Scholar]
- 15.Curiel-Balsera E, Mora-Ordonez JM, Castillo-Lorente E, Benitez-Parejo J, Herruzo-Aviles A, Ravina-Sanz JJ, et al. Mortality and complications in elderly patients undergoing cardiac surgery. J Crit Care. 2013; 28: 397–404. 10.1016/j.jcrc.2012.12.011 [DOI] [PubMed] [Google Scholar]
- 16.Bolsin SN, Raineri F, Lo SK, Cattigan C, Arblaster R, Colson M. Cardiac complications and mortality rates in diabetic patients following non-cardiac surgery in an Australian teaching hospital. Anaesth Intensive Care. 2009; 37: 561–567. [DOI] [PubMed] [Google Scholar]
- 17.Geng J, Qian J, Cheng H, Ji F, Liu H. The Influence of Perioperative Dexmedetomidine on Patients Undergoing Cardiac Surgery: A Meta-Analysis. PLoS One. 2016; 11: e0152829 10.1371/journal.pone.0152829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su F, Gastonguay MR, Nicolson SC, DiLiberto M, Ocampo-Pelland A, Zuppa AF. Dexmedetomidine Pharmacology in Neonates and Infants After Open Heart Surgery. Anesth Analg. 2016; 122: 1556–1566. 10.1213/ANE.0000000000000869 [DOI] [PubMed] [Google Scholar]
- 19.Mahboobi SK. Dexmedetomidine and Renal Protection after Cardiac Surgery. Journal of Clinical Anesthesia. 2017; 40: 121–122. 10.1016/j.jclinane.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 20.Khalil M, Al-Agaty A, Asaad O, Mahmoud M, Omar AS, Abdelrazik A, et al. A comparative study between propofol and dexmedetomidine as sedative agents during performing transcatheter aortic valve implantation. J Clin Anesth. 2016; 32: 242–247. 10.1016/j.jclinane.2016.03.014 [DOI] [PubMed] [Google Scholar]
- 21.Liu X, Zhang K, Wang W, Xie G, Cheng B, Wang Y, et al. Dexmedetomidine Versus Propofol Sedation Improves Sublingual Microcirculation After Cardiac Surgery: A Randomized Controlled Trial. J Cardiothorac Vasc Anesth. 2016; 30: 1509–1515. 10.1053/j.jvca.2016.05.038 [DOI] [PubMed] [Google Scholar]
- 22.Chi X, Liao M, Chen X, Zhao Y, Yang L, Luo A, et al. Dexmedetomidine Attenuates Myocardial Injury in Off-Pump Coronary Artery Bypass Graft Surgery. J Cardiothorac Vasc Anesth. 2016; 30: 44–50. 10.1053/j.jvca.2015.06.026 [DOI] [PubMed] [Google Scholar]
- 23.Rasheed MA, Punera DC, Bano M, Palaria U, Tyagi A, Sharma S. A study to compare the overall effectiveness between midazolam and dexmedetomidine during monitored anesthesia care: A randomized prospective study. Anesth Essays Res. 2015; 9: 167–172. 10.4103/0259-1162.156299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Priye S, Jagannath S, Singh D, Shivaprakash S, Reddy DP. Dexmedetomidine as an adjunct in postoperative analgesia following cardiac surgery: A randomized, double-blind study. Saudi Journal of Anaesthesia. 2015; 9: 353–358. 10.4103/1658-354X.154715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren C, Zhang X, Liu Z, Li C, Zhang Z, Qi F. Effect of Intraoperative and Postoperative Infusion of Dexmedetomidine on the Quality of Postoperative Analgesia in Highly Nicotine-Dependent Patients After Thoracic Surgery: A CONSORT-Prospective, Randomized, Controlled Trial. Medicine (Baltimore). 2015; 94: e1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kadam SV, Tailor KB, Kulkarni S, Mohanty SR, Joshi PV, Rao SG. Effect of dexmeditomidine on postoperative junctional ectopic tachycardia after complete surgical repair of tetralogy of Fallot: A prospective randomized controlled study. Ann Card Anaesth. 2015; 18: 323–328. 10.4103/0971-9784.159801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szumita PM, Baroletti SA, Anger KE, Wechsler ME. Sedation and analgesia in the intensive care unit: evaluating the role of dexmedetomidine. Am J Health Syst Pharm. 2007; 64: 37–44. 10.2146/ajhp050508 [DOI] [PubMed] [Google Scholar]
- 28.Higgins Jpt GS. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Naunyn-Schmiedebergs Archiv für experimentelle Pathologie und Pharmakologie. 2011; 5: S38. [Google Scholar]
- 29.Karaman Y, Abud B, Tekgul ZT, Cakmak M, Yildiz M, Gonullu M. Effects of dexmedetomidine and propofol on sedation in patients after coronary artery bypass graft surgery in a fast-track recovery room setting. J Anesth. 2015; 29: 522–528. 10.1007/s00540-015-1975-2 [DOI] [PubMed] [Google Scholar]
- 30.Balkanay OO, Goksedef D, Omeroglu SN, Ipek G. The dose-related effects of dexmedetomidine on renal functions and serum neutrophil gelatinase-associated lipocalin values after coronary artery bypass grafting: a randomized, triple-blind, placebo-controlled study. Interact Cardiovasc Thorac Surg. 2015; 20: 209–214. 10.1093/icvts/ivu367 [DOI] [PubMed] [Google Scholar]
- 31.Eremenko AA, Chemova EV. [Comparison of dexmedetomidine and propofol for short-term sedation in early postoperative period after cardiac surgery]. Anesteziol Reanimatol. 2014: 37–41. [PubMed] [Google Scholar]
- 32.Tosun Z, Baktir M, Kahraman HC, Baskol G, Guler G, Boyaci A. Does dexmedetomidine provide cardioprotection in coronary artery bypass grafting with cardiopulmonary bypass? A pilot study. J Cardiothorac Vasc Anesth. 2013; 27: 710–715. 10.1053/j.jvca.2012.12.013 [DOI] [PubMed] [Google Scholar]
- 33.Ren J, Zhang H, Huang L, Liu Y, Liu F, Dong Z. Protective effect of dexmedetomidine in coronary artery bypass grafting surgery. Exp Ther Med. 2013; 6: 497–502. 10.3892/etm.2013.1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sulaiman S, Karthekeyan RB, Vakamudi M, Sundar AS, Ravullapalli H, Gandham R. The effects of dexmedetomidine on attenuation of stress response to endotracheal intubation in patients undergoing elective off-pump coronary artery bypass grafting. Annals of Cardiac Anaesthesia. 2012; 15: 39–43. 10.4103/0971-9784.91480 [DOI] [PubMed] [Google Scholar]
- 35.Abd Aziz N, Chue MC, Yong CY, Hassan Y, Awaisu A, Hassan J, et al. Efficacy and safety of dexmedetomidine versus morphine in post-operative cardiac surgery patients. Int J Clin Pharm. 2011; 33: 150–154. 10.1007/s11096-011-9480-7 [DOI] [PubMed] [Google Scholar]
- 36.Menda F, Koner O, Sayin M, Ture H, Imer P, Aykac B. Dexmedetomidine as an adjunct to anesthetic induction to attenuate hemodynamic response to endotracheal intubation in patients undergoing fast-track CABG. Ann Card Anaesth. 2010; 13: 16–21. 10.4103/0971-9784.58829 [DOI] [PubMed] [Google Scholar]
- 37.Jalonen J, Hynynen M, Kuitunen A, Heikkilä H, Perttilä J, Salmenperä M, et al. Dexmedetomidine as an anesthetic adjunct in coronary artery bypass grafting. Anesthesiology. 1997; 86: 331–345. [DOI] [PubMed] [Google Scholar]
- 38.Herr DL, Sum-Ping ST, England M. ICU sedation after coronary artery bypass graft surgery: dexmedetomidine-based versus propofol-based sedation regimens. J Cardiothorac Vasc Anesth. 2003; 17: 576–584. [DOI] [PubMed] [Google Scholar]
- 39.Menda F, Köner Ö, Sayin M, Türe H, Imer P, Aykaç B. Dexmedetomidine as an adjunct to anesthetic induction to attenuate hemodynamic response to endotracheal intubation in patients undergoing fast-track CABG. Annals of Cardiac Anaesthesia. 2010; 13: 16–21. 10.4103/0971-9784.58829 [DOI] [PubMed] [Google Scholar]
- 40.Shehabi Y, Grant P, Wolfenden H, Hammond N, Bass F, Campbell M, et al. Prevalence of delirium with dexmedetomidine compared with morphine based therapy after cardiac surgery: a randomized controlled trial (DEXmedetomidine COmpared to Morphine-DEXCOM Study). Anesthesiology. 2009; 111: 1075–1084. 10.1097/ALN.0b013e3181b6a783 [DOI] [PubMed] [Google Scholar]
- 41.Corbett SM, Rebuck JA, Greene CM, Callas PW, Neale BW, Healey MA, et al. Dexmedetomidine does not improve patient satisfaction when compared with propofol during mechanical ventilation. Crit Care Med. 2005; 33: 940–945. [DOI] [PubMed] [Google Scholar]
- 42.Maldonado JR, Wysong A, van der Starre PJ, Block T, Miller C, Reitz BA. Dexmedetomidine and the reduction of postoperative delirium after cardiac surgery. Psychosomatics. 2009; 50: 206–217. 10.1176/appi.psy.50.3.206 [DOI] [PubMed] [Google Scholar]
- 43.El Amrousy DM, Elshmaa NS, El-Kashlan M, Hassan S, Elsanosy M, Hablas N, et al. Efficacy of prophylactic dexmedetomidine in preventing postoperative junctional ectopic tachycardia after pediatric cardiac surgery. Journal of the American Heart Association. 2017; 6: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suzumura EA, Ribeiro RA, Kawano-Dourado L, de Barros ESPG, Oliveira C, Figueiro MF, et al. Effects of perioperative statin use on cardiovascular complications in patients submitted to non-cardiac surgery: protocol for a systematic review, meta-analysis, and trial sequential analysis. Syst Rev. 2017; 6: 116 10.1186/s13643-017-0500-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gong Z, Ma L, Zhong YL, Li J, Lv J, Xie YB. Myocardial protective effects of dexmedetomidine in patients undergoing cardiac surgery: A meta-analysis and systematic review. Exp Ther Med. 2017; 13: 2355–2361. 10.3892/etm.2017.4227 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.