Abstract
Several hypotheses are used to explain species richness patterns. Some of them (e.g. species-area, species-energy, environment-energy, water-energy, terrestrial primary productivity, environmental spatial heterogeneity, and climatic heterogeneity) are known to explain species richness patterns of terrestrial organisms, especially when they are combined. For aquatic organisms, however, it is unclear if these hypotheses can be useful to explain for these purposes. Therefore, we used a selection model approach to assess the predictive capacity of such hypotheses, and to determine which of them (combined or not) would be the most appropriate to explain the fish species distribution in small Brazilian streams. We perform the Akaike’s information criteria for models selections and the eigenvector analysis to control the special autocorrelation. The spatial structure was equal to 0.453, Moran’s I, and require 11 spatial filters. All models were significant and had adjustments ranging from 0.370 to 0.416 with strong spatial component (ranging from 0.226 to 0.369) and low adjustments for environmental data (ranging from 0.001 to 0.119) We obtained two groups of hypothesis are able to explain the richness pattern (1) water-energy, temporal productivity-heterogeneity (AIC = 4498.800) and (2) water-energy, temporal productivity-heterogeneity and area (AIC = 4500.400). We conclude that the fish richness patterns in small Brazilian streams are better explained by a combination of Water-Energy + Productivity + Temporal Heterogeneity hypotheses and not by just one.
Introduction
Studies on the distribution pattern of species richness have been of great interest in environmental, biogeographical, and paleontological research programs since the early 19th century [1–3]. However, there is no clear or unique answer to this topic [2,4]. Different biological groups, such as plants [4,5], insects [6,7], amphibians [8], reptiles [9,10], birds [11], and fishes [2,7,12–15], have already been tested for richness patterns. In most cases, the influence of the latitudinal gradient in species richness is the main aspect examined [16], which is considered the oldest manner to explain richness distribution [17]. The pattern usually observed is the increasing of species richness toward the equator, which can be explained by various hypotheses [2,17,18]. However, the decreasing of species richness toward the poles is dependent on the scale and the organism studied [17].
Various hypotheses are used to explain richness distribution, the species-area and species-energy hypotheses being the ones that have stood out [13]. The species-area relationship [19] predicts that species richness increases as a function of the increase in the area, which is a power function. On the other hand, the species-energy hypothesis [20] predicts that species richness is a function of the amount of energy available in the system. A third hypothesis used to explain the richness gradient is environment-energy [21], which is a derivation of the energy-species hypothesis and predicts that there is a direct relationship between the temperature of the environment and species richness. The water-energy hypothesis [22] predicts the species richness as a function of the amount of water and evapotranspiration available in the system. By this way, richness would exhibit greater relationship with the amount of water available in the system at low latitudes, since energy would not be a limiting factor. On the other hand, this relationship would be reversed at high latitudes, with the energy being the limiting factor [1,4,6]. The hypothesis of terrestrial primary productivity [23] predicts that species richness would be limited by terrestrial primary productivity. However, for aquatic environments, this relationship is not so simple, since the aquatic primary productivity tends to be 90 times smaller than terrestrial productivity, and temperate continental aquatic environments are ten times less productive than continental tropical waters [13,14,24,25]. The hypothesis of environmental spatial heterogeneity [26] predicts that areas with greater variation in physical environmental characteristics (greater number of habitats potentially available) would also have greater species richness, since these areas could support more species in one place. Finally, the temporal climatic heterogeneity hypothesis [27] predicts that areas with greater variation of climatic characteristics would have greater richness, because they would support more species over time.
When we assess spatially structured data, such as species richness [28–32], we increase the likelihood of occurrence of type I error, i.e., discarding the null hypothesis when it is actually true [33–35]. In addition, the spatial structuring of data may modify the relationship between the dependent and independent variables, exhibiting a negative effect when it is positive, positive when it is negative, or even a null effect when in fact it is not null [30,32]. Still, many studies that explain the richness gradient of the ichthyofauna are not concerned with this effect. Numerous studies do not use any form of control or integration of spatial structure in the analyses [2,13,36,37].
There are studies that have described the richness gradient in rivers as a function of terrestrial primary productivity of the area drained by the basin and flow at the mouth of a river [2,13]. Other studies relate fish species richness in streams to physical variables, such as width, depth, and flow [36,37]. As a theoretical structure for the relationships found, the following hypotheses are listed: (i) energy—represented by the terrestrial primary productivity [2,12,13]; (ii) species-area—represented by the area drained by the basin [12]; (iii) passive dispersal—represented by the flow [36]; (iv) environmental spatial heterogeneity—represented by width and depth; and (v) climatic heterogeneity—represented by temperature variation in the environment [37]. However, none of these hypotheses is able to explain richness patterns if they are used isolated, thus requiring the integration of two or more hypotheses [13]. Furthermore, and the way a combination of hypotheses influence the patterns of ichthyofaunal richness is uncertain, since there are no studies that confront systematically the hypotheses and the different combinations of hypotheses with the gradient of fish species richness. Therefore, we sought to select models to assess the predictive capacity of the hypotheses, trying to find which hypotheses or combinations of hypotheses would be the most appropriate to explain the species richness distribution in small Brazilian streams. We tested the following combination of hypotheses: (i) energy—fish communities of streams are related to evapotranspiration rates; (ii) water-energy—species richness is structured on the basis of evapotranspiration and the average annual rainfall; (iii) terrestrial primary productivity—species richness is dependent on the terrestrial primary productivity; (iv) temporal heterogeneity—the variation of temperature and annual rainfall is used as predictor variables to determine the species richness of streams; (v) area—species richness is related to the amount of available area in the basin; and (vi) neutral—the geographical distances between the collection sites determinate the species richness of streams.
Materials and methods
Database
Databases obtained in field collections carried out by the authors were used for testing the hypotheses. These collections should meet the following criteria to be entered in the database: (i) collection carried out in first-to third-order streams; (ii) georeferenced streams; (iii) sampling of ichthyofauna carried out with electric fishing, trawl net, and/or hand net; (iv) a minimum of 50-meter stretch of stream sampled by site; (v) a single sample site by stream; (vi) species identified by experienced researchers in accordance with the taxonomic literature [14,38–52]; (vii) list of species per sampled site; and (viii) collections carried out in locations with the lowest possible anthropic impact. Scientific articles published in journals, monographs, dissertations, and theses were compiled to supplement the database. CAPES journals website (https://goo.gl/D2gE54) and the keywords ‘peixe’, ‘fish’, ‘riacho’, ‘stream’, ‘lista’, and ‘checklist’ were used for the literature search. In all cases, only the studies that met the criteria previously determined were included in the database. These criteria were chosen in order to ensure comparability between samples and decrease the heterogeneity of the database. At the end of the search, 18 studies that addressed 89 streams were included in the database (S1 Table). The streams from the literature were initially compiled in the database of the collaborators with 570 streams, totaling 659 streams (Fig 1 and S2 Table). Fish species richness was determined for each stream of the database.
Fig 1. Spatial location of the streams assessed (black dots) in Brazil, South America.
Macroecological variables
For testing our hypotheses, we used the variables that were originally considered by the authors as determinant for the richness gradient. We used the following variables set to test the isolated or combined hypothesis: January (AETJan) and June evapotranspiration (AETJune), with the both months representing respectively the warmest and coldest period along the year; primary productivity (PP); annual temperature variation (TempVar); annual average rainfall (AveRF); annual rainfall variation (ARV); and flow accumulation (FAC) used as a proxy for the area drained within the basin (Table 1; S1 Fig). The use of FAC allows performing faster and easier analyses and, since it has high correlation with the drained area, there is no information loss.
Table 1. Descriptive statistics of the variables used in the models.
The i-v numbers following each variable indicates in which hypothesis the variable was used: i. Energy, ii. energy-water, iii. terrestrial primary productivity, iv. temporal heterogeneity, v. area. All data are available on the S2 Table.
| Variable | Code | Mean | Standard Deviation |
|---|---|---|---|
| Species richness | SR | 11.517 | 9.347 |
| January evapotranspiration (mm day-1)i, ii | AETJan | 100.028 | 41.490 |
| June evapotranspiration (mm day-1)i, ii | AETJun | 66.735 | 39.275 |
| Primary productivity (cal. m2 day)iii | PP | 8086.156 | 3313.487 |
| Annual temperature variation (°C * 10)iv | TempVar | 1023.135 | 783.507 |
| Annual average rainfall (mm)ii, iv | AveRF | 1866.077 | 563.649 |
| Annual rainfall variation (mm)iv | ARV | 57.131 | 22.580 |
| Flow accumulationv | FAC | 532.294 | 601.729 |
The AET and PP are items of MODIS (Moderate Resolution Imaging Spectroradiometer) satellite images with information available from 2000 to 2012 on the website of the Laboratory for Image Processing and Geoprocessing of the Federal University of Goiás—LAPIG UFG (https://goo.gl/F1iWvy). The average rates of these variables were used for the analyses. For the calculation of these values, we obtained the images of AET (available monthly) occurred in January and June, and PP (available annually), both from 2000 to 2012. The images of the different years were added and divided by 12, composing a new image that represents 12-year average value of the variable. This procedure was repeated for AETJan, AETJune, and PP data. TempVar, AveRF, and ARV were retrieved from the IPCC climate scenario: A1, available at WORLDCLIM (http://www.worldclim.org). These variables result from interpolation models built with data collected from 1950 to 2000 by the Global Historical Climate Network Dataset (GHCN).
FAC data were retrieved from Hydro-1k digital elevation global model (http://edcdaac.usgs.gov/gtopo30/hydro/). FAC are products from digital elevation models of the GTOPO30 project developed by US Geological Survey-EROS. Since the resolution (pixel size) of all the images were originally 1 x 1 km, they were adjusted to 15 x 15 km. This way, the information for each site was an average value, product of 225 pixels, and not just the value of one pixel.
Statistical analyses
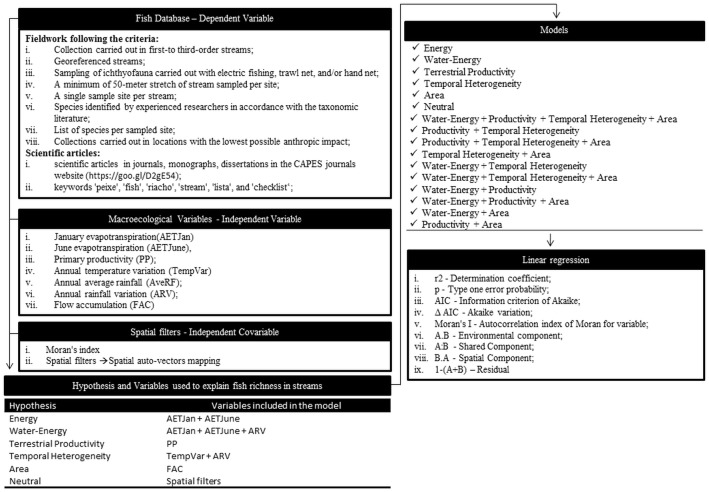
In all analyses we perform General Linear Model as statistical framework. For testing the energy hypothesis, a model between SR and AETJan and AETJune was built in order to include the energy input during the warmest and the coldest periods of the year. For testing the water-energy hypothesis, a model between SR and AETJan, AETJune, and AveRF was built, since the evapotranspiration represent the energy input into the system and the AveRF represents the annual water availability. For the productivity hypothesis, the SR was related to the PP, which represents the annual terrestrial primary productivity of the site. The hypothesis of temporal heterogeneity was tested using the ARV and TempVar variables, because these variables represent the annual rainfall and temperature variation. For the area hypothesis, species richness was related to the FAC. Finally, a model between SR and the geographical distance of the sites was built for testing the neutral hypothesis. All the models considered (Table 2) were least squares linear models and the geographical distance used was the spatial eigenvectors mapping [53].
Table 2. Variables used to explain fish species richness in streams.
| Hypothesis | Variables included in the model |
|---|---|
| Energy | AETJan + AETJune |
| Water-Energy | AETJan + AETJune + ARV |
| Terrestrial Productivity | PP |
| Temporal Heterogeneity | TempVar + ARV |
| Area | FAC |
| Neutral | Spatial filters |
ETJan: January evapotranspiration; ETJune: June evapotranspiration; PP: primary productivity; TempVar: annual temperature variation; AveRF: annual average rainfall; ARV: annual rainfall variation; and FAC: flow accumulation
We used Moran’s index (Moran’s I) to identify the autocorrelation in the species richness. Spatial auto-vectors mapping (spatial filters) was used to control the spatial structure [53], considered the best way to control the spatial structure [32]. The spatial structure of fish species richness was equal to 0.453 according to Moran’s I, requiring 11 spatial filters to control the autocorrelation effect (S2 Fig). The first spatial filter (SF1) shows the highest values for the streams located in the upper portion of the Amazon region. The second filter (SF2) shows the highest values located in the northern portion of the Paraná region. The fourth filter (SF4) shows high values for the streams of the southernmost portion of the Amazon region and the western Paraná region. The other filters do not show a clear pattern (S2 Fig). All the spatial filters were incorporated into the models as covariates (Table 2), except in the neutral hypothesis, in which the filters were used as predictor variables. Moran’s I value and Akaike’s information criterion (AIC) were presented for all models, which were compared through the AIC variation. Since some studies have already found that a single hypothesis cannot explain variation in fish species richness [12,13], we also tested the combinations between the water-energy, terrestrial productivity, temporal heterogeneity, and area models. To avoid repetition of variables in the tests, the energy and neutral models were removed from the combinations, since the variables that represent the energy model are already included in the water-energy model, and the neutral model is already represented by spatial filters in the other models. In this way, the hypotheses combined were two, three, and four at a time. Spatial auto-vectors mapping and regression analyses were carried out using the SAM software—Spatial Analysis in Macroecology [54,55]. Collinearity between the variables was measured through the variance inflation factor (VIF), which quantifies the multicollinearity of predictor variables (Fig 2). This index ranges from 1 (no collinearity) to positive infinity and provides an estimate of how much the variance of a regression coefficient is increased by collinearity. VIF values less than 10 are considered acceptable. After testing the hypotheses, species richness was subjected to a multiple regression analysis, without the use of special filters and using only the significant variables. The model obtained with this analysis was spatialized, producing a map of fish species richness in streams. For testing the accuracy of the model, we extracted the values predicted for each stream present in the analyses and subtracted the value of the richness observed from the estimated value. Thereby, positive values represent sites overestimated by the model, and negative values represent those underestimated by the model. With these values, we performed a regression analysis between the predicted and observed species richness, using all sites together and by river basin (Amazon, East Atlantic, Western Northeast Atlantic/Eastern/Parnaíba, South/Southeast Atlantic, Paraguay/Paraná, São Francisco, Tocantins-Araguaia, and Uruguay). The regression model and the map were made using the R environment [56] with Vegan [57] package.
Fig 2. Flowchart representing the statistical procedure used in the article.
Results
All the models built to explain the fish species richness of streams were significant and had adjustments ranging from 0.301 to 0.416 (Tables 3 and 4). They showed strong spatial component and shared low adjustments for environmental data (Tables 3 and 4). When the models were tested individually (Table 3), the temporal heterogeneity hypothesis—according to AIC—was the best adjusted for species richness. However, when we tested the hypotheses combined (Table 4), the models built by the hypotheses "water-energy, productivity, and temporal heterogeneity" and "water-energy, productivity, temporal heterogeneity, and area" were those that best explained the fish species richness of streams.
Table 3. Regression coefficients and comparison between the five hypotheses used to explain the distribution of fish species richness in streams.
| Hypotheses | r2 | p | AIC | Δ AIC | Moran’s I | r2 for partial regression results | |||
|---|---|---|---|---|---|---|---|---|---|
| A.B | A:B | B.A | 1-(A+B) | ||||||
| Temporal Heterogeneity | 0.401 | <0.001 | 4510.500 | 0.000 | 0.093 | 0.031 | 0.054 | 0.316 | 0.599 |
| Energy | 0.376 | <0.001 | 4535.200 | 24.700 | 0.115 | 0.007 | 0.090 | 0.279 | 0.624 |
| Area | 0.301 | <0.001 | 4538.400 | 27.900 | 0.122 | 0.005 | 0.053 | 0.316 | 0.626 |
| Water-Energy | 0.376 | <0.001 | 4538.900 | 28.400 | 0.114 | 0.007 | 0.143 | 0.226 | 0.624 |
| Neutral | 0.369 | <0.001 | 4539.900 | 29.400 | 0.128 | - | - | - | 0.631 |
| Terrestrial Productivity | 0.370 | <0.001 | 4541.600 | 31.100 | 0.131 | 0.001 | 0.001 | 0.370 | 0.628 |
r2—Coefficient of determination; p—Type one error probability; AIC—Information criterion of Akaike; Δ AIC—Akaike variation; Moran’s I—Autocorrelation index of Moran for variable; A.B—Environmental component; A:B—Shared Component; B.A—Spatial Component; 1-(A+B)–Residual
Table 4. Regression coefficients and comparison between the combinations of hypotheses to explain the distribution of fish species richness in streams.
AIC: Akaike’s information criterion.
| Combination of hypotheses | r2 | p | AIC | Δ AIC | Moran’s I | r2 for partial regression results | |||
|---|---|---|---|---|---|---|---|---|---|
| A.B | A:B | B.A | 1-(A+B) | ||||||
| Water-Energy + Productivity + Temporal Heterogeneity | 0.416 | <0.001 | 4498.800 | 0.000 | 0.096 | 0.046 | 0.121 | 0.249 | 0.584 |
| Water-Energy + Productivity + Temporal Heterogeneity + Area | 0.416 | <0.001 | 4500.400 | 1.700 | 0.096 | 0.047 | 0.120 | 0.249 | 0.584 |
| Productivity + Temporal Heterogeneity | 0.407 | <0.001 | 4502.100 | 3.400 | 0.097 | 0.038 | 0.057 | 0.312 | 0.593 |
| Productivity + Temporal Heterogeneity + Area | 0.408 | <0.001 | 4503.700 | 4.900 | 0.097 | 0.038 | 0.057 | 0.312 | 0.592 |
| Temporal Heterogeneity + Area | 0.400 | <0.001 | 4510.400 | 11.600 | 0.097 | 0.030 | 0.055 | 0.315 | 0.600 |
| Water-Energy + Temporal Heterogeneity | 0.400 | <0.001 | 4514.200 | 15.400 | 0.095 | 0.031 | 0.133 | 0.237 | 0.600 |
| Water-Energy + Temporal Heterogeneity + Area | 0.401 | <0.001 | 4515.500 | 16.800 | 0.095 | 0.031 | 0.132 | 0.237 | 0.599 |
| Water-Energy + Productivity | 0.384 | <0.001 | 4530.100 | 31.300 | 0.119 | 0.014 | 0.144 | 0.226 | 0.616 |
| Water-Energy + Productivity + Area | 0.384 | <0.001 | 4531.800 | 33.000 | 0.119 | 0.014 | 0.144 | 0.226 | 0.616 |
| Water-Energy + Area | 0.376 | <0.001 | 4537.600 | 38.900 | 0.115 | 0.007 | 0.143 | 0.226 | 0.624 |
| Productivity + Area | 0.370 | <0.001 | 4540.400 | 41.600 | 0.132 | 0.001 | 0.001 | 0.369 | 0.630 |
r2—Coefficient of determination; p—Type one error probability; AIC—Information criteria of Akaike; Δ AIC—Akaike variation; Moran’s I—Autocorrelation index of Moran for variable; A.B—Environmental component; A:B—Shared Component; B.A—Spatial Component; 1-(A+B)—Residual
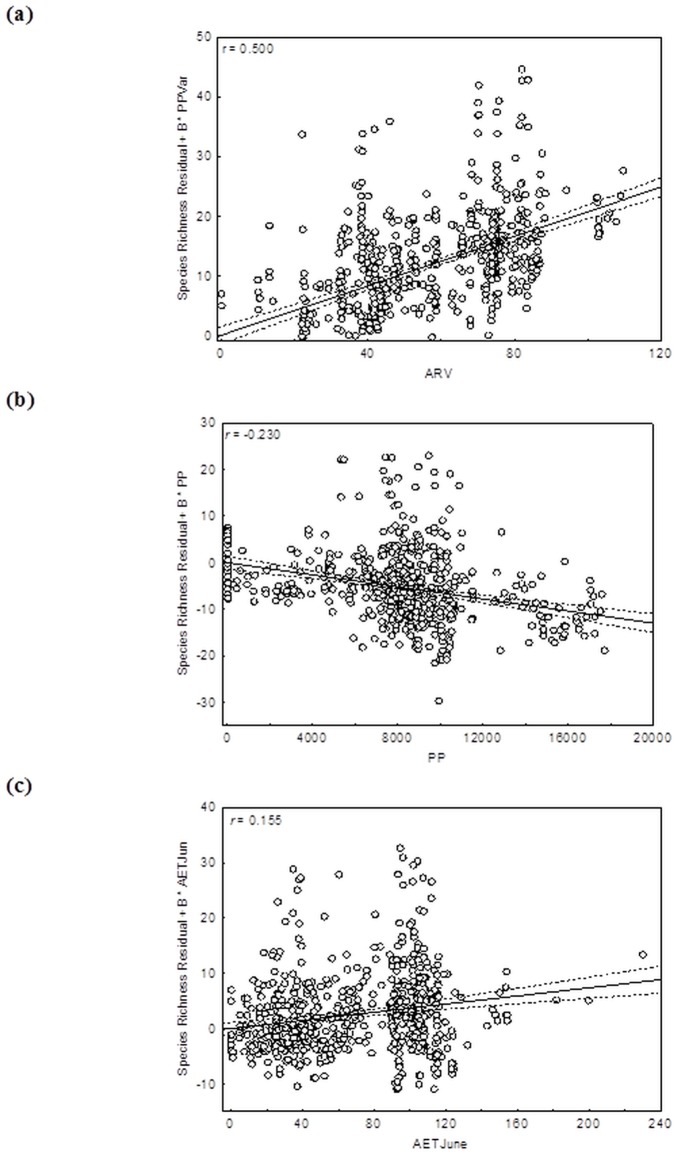
The models built with the combinations of hypotheses were better adjusted for the richness distribution than the individual models, since the Δ AIC between the best individual model (AIC = 4510.500) and the best combined model (AIC = 4498.800) was 11.700. The best combination of hypotheses was "water-energy, productivity, and temporal heterogeneity", with 0.416 adjustment and 0.096 spatial autocorrelation for the residues (Table 4), showing the fish species richness as a function of annual rainfall variation, primary productivity, and June evapotranspiration (Table 5; Fig 3). Actually, two combinations of models had the same explanation power to species richness i) Water-Energy + Productivity + Temporal Heterogeneity and ii) Water-Energy + Productivity + Temporal Heterogeneity + Area. However, the Δ AIC between these two sets was less them two, indicate the same explanation power of these. So the choice of the set with fewer variables (Water-Energy + Productivity + Temporal Heterogeneity) is more parsimonious.
Table 5. Results of the regression analyses using the best set of models (Water-Energy + Productivity + Temporal Heterogeneity) as predictors of fish species richness, and spatial filters as covariates.
| Variable | SC | VIF | t | p |
|---|---|---|---|---|
| Constant | 0.000 | 0.000 | 0.275 | 0.784 |
| AETJan | -0.069 | 2.172 | -1.560 | 0.119 |
| AETJune | 0.155 | 5.634 | 2.159 | 0.031 |
| AveRF | 0.109 | 5.539 | 1.539 | 0.124 |
| PP | -0.230 | 3.375 | -4.148 | <0.001 |
| TempVar | <0.001 | 8.739 | -0.007 | 0.995 |
| ARV | 0.500 | 7.788 | 5.934 | <0.001 |
| SF1 | 0.376 | 10.745 | 3.803 | <0.001 |
| SF2 | -0.342 | 6.007 | -4.620 | <0.001 |
| SF3 | -0.134 | 1.864 | -3.255 | 0.001 |
| SF4 | 0.328 | 1.219 | 9.832 | 0.000 |
| SF5 | 0.064 | 2.850 | 1.250 | 0.212 |
| SF6 | 0.180 | 2.053 | 4.170 | <0.001 |
| SF7 | 0.268 | 2.065 | 6.187 | <0.001 |
| SF8 | 0.295 | 1.651 | 7.597 | <0.001 |
| SF9 | 0.280 | 1.739 | 7.033 | <0.001 |
| SF11 | 0.073 | 1.241 | 2.182 | 0.029 |
| SF17 | 0.205 | 1.032 | 6.677 | <0.001 |
AETJan: January evapotranspiration; AETJune: June evapotranspiration; PP: primary productivity; TempVar: annual temperature variation; AveRF: annual average rainfall; ARV: annual rainfall variation; SF1-SF17: spatial filters; SC: standard coefficient; VIF: variance inflation factor; p: Type one error probability. Significant P values (P<0.05) are in bold.
Fig 3. Partial regression between the predictor variables and fish species richness of streams.
Species richness as a function of a) the annual rainfall variation (ARV); b) the terrestrial primary productivity (PP) and c) June evapotranspiration (AETJune).
Annual rainfall variation had greatest effect on fish species richness, with coefficient 0.500 (Table 5; Fig 3a). June evapotranspiration and primary productivity showed lower effect than Annual rainfall variation. Primary productivity showed negative effect with coefficient -0.230 (Table 5; Fig 3b), and AETJune showed positive coefficient 0.155 (Table 5; Fig 3c). None of the models was affected by the collinearity of predictor variables, since VIF was less than 10 (Table 5).
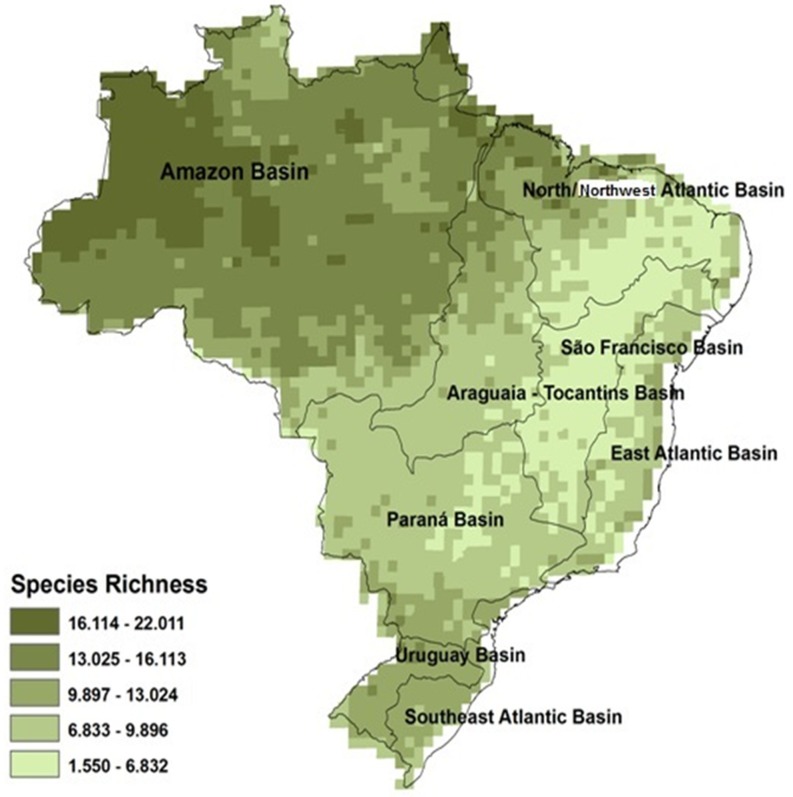
The map of fish species richness for lower than third-order streams shows the richest streams in the northwest portion of the Amazon hydrographic region, with up to 24 fish species, and the poorest streams in the northern region of the São Francisco hydrographic region, with a single species predicted for streams (Fig 4). The southern Paraná hydrographic region, the entire Uruguay hydrographic region, and middle portions of the Tocantins River and North/Northeast Atlantic showed median richness values (Fig 4). The analysis of the model accuracy as a whole shows that there were more underestimated than overestimated sites (S3 Fig). The underestimation of the model reaches up to 40 species, and the overestimation of the model does not exceed 20 species. When each basin was assessed separately, we identified that the Southeast Atlantic Basin showed overestimated sites, reaching approximately 12 species with few underestimated sites two sites showing errors of four species at the most. The Paraná Basin and the East Atlantic Basin showed overestimation of up to 10 species, and the Araguaia-Tocantins Basin showed the lowest overestimated values, with only five species. The highest underestimation values were found in the Amazon Basin, which underestimated the richness up to 40 species, followed by the Paraná Basin and Araguaia-Tocantins Basin with underestimation of 30 species. The São Francisco Basin showed underestimation of 14 species and overestimation of eight species, and the North/Northwest Atlantic Basin had overestimation of eight species and underestimation of up to 12 species (S3 Fig).
Fig 4. Fish species richness prediction for 1st to 3rd order streams in Brazil.
The map was drawn up from the regression model found.
Discussion
When we tested all hypotheses separately, the temporal heterogeneity best explains the distribution of fish species richness in streams. However, under a multiple-hypotheses approach, we observed that the water-energy + productivity + temporal heterogeneity hypotheses together best described the richness distribution. Although it could be considered an acceptable model by AIC, when we add the area (flow accumulation), the results adjustment to this model is not improved. Separately, the flow accumulation variable had no effect on fish species richness. On the other hand, when we confronted the best hypothesis isolated (temporal heterogeneity) with the best set of hypotheses (water-energy, productivity, and temporal heterogeneity), the Δ AIC value is 11.700, arguing that temporal heterogeneity is still the best explanation for the phenomena studied. Multiple hypotheses approach has already been observed in the literature. [13] used the hypotheses energy, area, and history to explain fish species richness in rivers of five continents. In the same paper [13], the authors observed that the hypotheses tested individually were not robust, so they considered them together. [2] used the same data obtained by [12] and [13] and identified the relationship between richness and area and energy (as originally described by [13] and [12]), in addition to the relationship between richness and historical factors. However, none of these studies addressed temporal heterogeneity as an important alternative.
There was a strong relationship between ichthyofauna and space with the spatial filters in all the models assessed, being responsible for more than 50% of the model adjustment. The spatial autocorrelation found in the richness component is a feature repeatedly discussed in the literature [4,6–10,15]. For aquatic organisms, it is expected that the spatial component of the analyses would be greater when organisms are highly dependent on the water, since the hydrological system is longitudinally, laterally, and vertically influenced [37,58]. For fishes, which can disperse only along their own watercourses, a strong spatial component is expected [36], as we found in our study. In spite of the great influence of spatial factors, local conditions cannot be discarded, since they influence the assemblages structure, even in a smaller degree [36,59]. The contribution of the spatial component in the fish species richness distribution is an important factor for the Brazilian ichthyofauna, since, due to historical factors, several sites of endemism that present aggregated distribution in space have been formed [60]. In addition, changes in physical, physicochemical, and chemical properties of watercourses also determine spatial patterns, with gradual changes from the headwaters to the mouth, increasing fish species richness in this same direction [25,61]. There is evidence that this gradient is also related to an increased local heterogeneity [62,63] and the stabilization of hydrological variations [25,58,64]. Other factors that can increase the autocorrelation are the relationship between the area drained, the flow [2,12,13,37,64–67], the terrestrial primary productivity [2,12,13], and the regional richness [12]. The area and the flow are regionally important factors, though of weaker influence at larger scales [2,12,37]. At larger scales, it is expected that the structure of the ichthyofauna is dependent on the colonization and extinction processes, and less influenced by the physical, chemical, and physicochemical processes [36,38,68].
The terrestrial primary productivity was related to richness, but flow accumulation was not reinforcing the idea of climate and topography as strong predictors; however, less predictive at larger scales [2,12,37]. The negative relationship observed between terrestrial primary productivity and fish species richness has also been described by [37] and [2], explaining 76 to 93% of the ichthyofaunal richness variation through the drained area of the basin. [2] and [13] concluded that the increased availability of energy would lead to accumulation of terrestrial biomass, which would be made available to the aquatic environments by being transported laterally. However, terrestrial primary productivity tends to underestimate the aquatic primary productivity by at least 10 times [13,24,25]. When open tropical environments are taken into consideration as is the case of streams of Cerrado this underestimation of the aquatic terrestrial productivity is even greater, since open environments are subject to greater energy input and consequent increase in primary productivity [69]. The input of allochthonous material may occur from the lateral portions of streams and from the upstream portions of the sampled sites, since the organic material can be moved within the body of water in the upstream-downstream direction during the rainy season [70,71]. This way, the little relationship between the terrestrial primary productivity and the ichthyofaunal richness in streams can be attributed both to the underestimation of aquatic primary productivity and the amount of organic matter drifted from upstream sites.
In addition to the terrestrial primary productivity, June evapotranspiration and rainfall variation also demonstrated a positive relationship with the ichthyofaunal richness. The influence of evapotranspiration on diversity patterns is expected only for sites at higher latitudes [1,4,6], with less evident effect at low latitudes or completely replaced by the effect of the amount of water present in the system [4]. The relationship between these two variables (evapotranspiration and water) and fish species richness suggests that the amount of energy would not be a limiting factor at low latitudes, since the available energy is abundant, contrary to the amount of water, which would be the limiting factor. This relationship would be reversed in regions at high latitudes, with the energy being the limiting factor [1,4,6]. This relationship has been found for dragonflies in Europe [6], butterflies in Europe and Africa [29,72], and fishes in this study. [29] and [72] showed that temperature is an important descriptor for Europe, while the water is more important for areas of northern Africa. The water-energy hypothesis was not related to richness. However, in the hypothesis of interaction, a positive relationship between rainfall variation and richness was observed, since this relationship exhibited a larger coefficient than evapotranspiration.
In any large-scale analysis of complex ecological processes, it is expected that the unexplained residues result mostly from the natural complexity of the system and the unmeasured variables, which, combined, can influence the results in several scales. These results suggest that the inclusion of temporal climatic heterogeneity not previously included in similar studies was an important advance of the present study. The predictive map of fish species richness in first-to third-order streams show discrepancies with other studies that presented macroecological models [2,73]. These studies show Brazil as a megadiverse country and richness values over 200 species per basin. However, the model presented here was made only for first to third order streams and not to large rivers and basins. The second caveat is related to the accuracy of the model, which overestimated places, as the Southeast Atlantic Basin, to underestimated places, as the Amazon Basin. This underestimation of richness can be the result of environmental factors that were not included in the study, such as the volume of water, which has been described in the literature as an important predictor of fish species richness in streams [25,38], or a feature of continental freshwater systems, which have non-stationary relationships between the different hydrographic units, highlighting the importance of historical factors of biomes, hydrographic areas, and ecoregions. Anyway, we can conclude that the use of multiple hypothesis approach build better models to explain the fish richness pattern in Brazilian streams.
Supporting information
(TIF)
All the spatial filters are related to positive autocorrelation. The squares are positioned over the streams assessed. The highest values are black squares and the smallest values white squares.
(TIF)
(TIF)
(XLSX)
The name and unit of variables are indicating on the Table 1.
(XLSX)
Acknowledgments
We would like to thank the staff lab of Aquatic Ecology Laboratory of the UFC.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico 457463/2012-0 Dr Jorge Iván Sánchez Botero and Dra. Danielle Sequeira Garcez; Conselho Nacional de Desenvolvimento Científico e Tecnológico 552009/2011-3 Dr Jorge Iván Sánchez Botero and Dra. Danielle Sequeira Garcez; Conselho Nacional de Desenvolvimento Científico e Tecnológico 471283/2006-1 Dr Francisco Leonardo Tejerina-Garro; Conselho Nacional de Desenvolvimento Científico e Tecnológico 304002/2014-3 Dr Paulo Santos Pompeu; Fundação de Amparo à Pesquisa do Estado de Minas Gerais 304002/2014-3 Dr Paulo Santos Pompeu; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior Dr Thiago Bernardi Vieira.
References
- 1.Gaston KJ. Global patterns in biodiversity. Nature. 2000; 10.1038/35012228 [DOI] [PubMed] [Google Scholar]
- 2.Oberdorff T, Tedesco PA, Hugueny B, Leprieur F, Beauchard O, Brosse S, et al. Global and regional patterns in riverine fish species richness: A review. Int J Ecol. 2011; 10.1155/2011/967631 [DOI] [Google Scholar]
- 3.Watkins JE, Cardelús C, Colwell RK, Moran RC. Species richness and distribution of ferns along an elevational gradient in Costa Rica. Am J Bot. 2006; 10.3732/ajb.93.1.73 [DOI] [Google Scholar]
- 4.Eiserhardt WL, Bjorholm S, Svenning JC, Rangel TF, Balslev H. Testing the water-energy theory on american palms (arecaceae) using geographically weighted regression. PLoS One. 2011; 10.1371/journal.pone.0027027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kier G, Mutke J, Dinerstein E, Ricketts TH, Küper W, Kreft H, et al. Global patterns of plant diversity and floristic knowledge. J Biogeogr. 2005; 10.1111/j.1365-2699.2005.01272.x [DOI] [Google Scholar]
- 6.Keil P, Simova I, Hawkins B a, SIMO Va IREN a. Water-energy and the geographical species richness pattern of European and North African dragonflies (Odonata). Insect Conserv Divers. 2008; 10.1111/j.1752-4598.2008.00019.x [DOI] [Google Scholar]
- 7.Novotny V, Drozd P, Miller SE, Kulfan M, Janda M, Basset Y, et al. Why are there so many species of herbivorous insects in tropical rainforests? Science (80-). 2006; 10.1126/science.1129237 [DOI] [PubMed] [Google Scholar]
- 8.Rodríguez MÁ, Belmontes JA, Hawkins BA. Energy, water and large-scale patterns of reptile and amphibian species richness in Europe. Acta Oecologica. 2005; 10.1016/j.actao.2005.02.006 [DOI] [Google Scholar]
- 9.Terribile LC, Diniz-Filho JAF, Rodríguez MÁ, Rangel TFLVB. Richness patterns, species distributions and the principle of extreme deconstruction. Glob Ecol Biogeogr. 2009; 10.1111/j.1466-8238.2008.00440.x [DOI] [Google Scholar]
- 10.Terribile LC, Olalla-Tárraga MÁ, Morales-Castilla I, Rueda M, Vidanes RM, Rodríguez MÁ, et al. Global richness patterns of venomous snakes reveal contrasting influences of ecology and history in two different clades. Oecologia. 2009; 10.1007/s00442-008-1244-2 [DOI] [PubMed] [Google Scholar]
- 11.Blackburn TM, Gaston KJ. The relationship between geographic area and the latitudinal gradient in species richness in New World birds. Evol Ecol. 1997; 10.1023/A:1018451916734 [DOI] [Google Scholar]
- 12.Oberdorff T, Guegan J-FF, Hugueny B, Oberdoff T, Guegan J-FF, Hugueny B. Global scale patterns of fish species richness in rivers. Ecography (Cop). 1995; 10.1111/j.1600-0587.1995.tb00137.x [DOI] [Google Scholar]
- 13.Guégan JF, Lek S, Oberdorff T. Energy availability and habitat heterogeneity predict global riverine fish diversity. Nature. 1998; 10.1038/34899 [DOI] [Google Scholar]
- 14.Mazzoni R, Lobón-Cerviá J. Longitudinal structure, density and production rates of a neotropical stream fish assemblage: the river Ubatiba in the Serra do Mar, southeast Brazil. Ecography (Cop). 2008; 10.1111/j.1600-0587.2000.tb00178.x [DOI] [Google Scholar]
- 15.Knouft JH. Regional analysis of body size and population density in stream fish assemblages: testing predictions of the energetic equivalence rule. Can J Fish Aquat Sci. 2002; 10.1139/f02-110 [DOI] [Google Scholar]
- 16.Pianka ER. Latitudinal Gradients in Species Diversity: A Review of Concepts. Am Nat. 1966; 10.1086/282398 [DOI] [Google Scholar]
- 17.Willig MR, Kaufman DM, Stevens RD. Latitudinal Gradients of Biodiversity: Pattern, Process, Scale, and Synthesis. Annu Rev Ecol Evol Syst. 2003; 10.1146/annurev.ecolsys.34.012103.144032 [DOI] [Google Scholar]
- 18.Hawkins BA, Diniz-Filho JAF, Bini L Mauricio, De Marco P, Blackburn TM. Red herrings revisited: Spatial autocorrelation and parameter estimation in geographical ecology. Ecography (Cop). 2007; 10.1111/j.2007.0906-7590.05117.x [DOI] [Google Scholar]
- 19.MacArthur RH, Wilson EO. The theory of island biogeography. Monographs in Population Biology. 1967. 10.2307/1796430 [DOI] [Google Scholar]
- 20.Wright DH. Species-Energy Theory: An Extension of Species-Area Theory. Oikos. 1983; 10.2307/3544109 [DOI] [Google Scholar]
- 21.Turner JRG, Gatehouse CM, Corey CA. Does Solar Energy Control Organic Diversity? Butterflies, Moths and the British Climate. Oikos. 1987; 10.2307/3565855 [DOI] [Google Scholar]
- 22.O’Brien EM. Water-energy dynamics, climate, and prediction of woody plant species richness: an interim general model. J Biogeogr. 1998; 10.1046/j.1365-2699.1998.252166.x [DOI] [Google Scholar]
- 23.Waide RB, Willig MR, Steiner CF, Mittelbach G, Gough L, Dodson SI, et al. The Relationship Between Productivity and Species Richness. Annu Rev Ecol Syst. 1999; [Google Scholar]
- 24.Davies PM, Bunn SE, Hamilton SK. Primary production in tropical streams and rivers. Tropical Stream Ecology. 2008. 10.1016/B978-012088449-0.50004-2 [DOI] [Google Scholar]
- 25.Hugueny B, Oberdorff T, Tedescco PA. Community Ecology of River Fishes: A Large-Scale Perspective. In: Jackson DA, Gido KB, editors. Community Ecology of Stream Fishes: Concepts, Approaches, and Techniques. Maryland: American Fisheries Society, Symposium 73, Bethesda; 2010.
- 26.Kerr JT, Packer L. Habitat heterogeneity as a determinant of mammal species richness in high-energy regions. Nature. 1997; 10.1038/385252a0 [DOI] [Google Scholar]
- 27.Currie DJ. Energy and Large-Scale Patterns of Animal- and Plant-Species Richness. Am Nat. 1991; 10.1086/285144 [DOI] [Google Scholar]
- 28.Legendre P, Legendre L. Numerical Ecology Numerical Ecology Second English Edition. 1998. [Google Scholar]
- 29.Hawkins BA, Field R, Cornell H V., Currie DJ, Guégan JF, Kaufman DM, et al. Energy, water, and broad-scale geographic patterns of species richness. Ecology. 2003. 10.1890/03-8006 [DOI] [Google Scholar]
- 30.Kühn I. Incorporating spatial autocorrelation may invert observed patterns. Divers Distrib. 2007; 10.1111/j.1472-4642.2006.00293.x [DOI] [Google Scholar]
- 31.Kissling WD, Carl G. Spatial autocorrelation and the selection of simultaneous autoregressive models. Glob Ecol Biogeogr. 2008; 10.1111/j.1466-8238.2007.00334.x [DOI] [Google Scholar]
- 32.Bini LM, Diniz-Filho JAF, Rangel TFLVB, Akre TSB, Albaladejo RG, Albuquerque FS, et al. Coefficient shifts in geographical ecology: An empirical evaluation of spatial and non-spatial regression. Ecography (Cop). 2009; 10.1111/j.1600-0587.2009.05717.x [DOI] [Google Scholar]
- 33.Legendre P. Spatial autocorrelation: trouble or new paradigm? Ecology. 1993; 10.2307/1939924 [DOI] [Google Scholar]
- 34.Legendre P, Dale MRT, Fortin M-J, Gurevitch J, Hohn M, Myers D. The consequences of spatial structure for the design and analysis of ecological field surveys. Ecography (Cop). 2002; 10.1034/j.1600-0587.2002.250508.x [DOI] [Google Scholar]
- 35.Diniz-Filho JAF, Bini LM, Hawkins BA. Spatial autocorrelation and red herrings in geographical ecology. Glob Ecol Biogeogr. 2003; 10.1046/j.1466-822X.2003.00322.x [DOI] [Google Scholar]
- 36.Beisner BE, Peres-Neto PR, Lindström ES, Barnett A, Longhi ML. The role of environmental and spatial processes in structuring lake communities from bacteria to fish. Ecology. 2006; 10.1890/0012-9658(2006)87[2985:TROEAS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 37.Das MK, Naskar M, Mondal ML, Srivastava PK, Dey S, Rej A. Influence of ecological factors on the patterns of fish species richness in tropical Indian rivers. Acta Ichthyol Piscat. 2012; 10.3750/AIP2011.42.1.06 [DOI] [Google Scholar]
- 38.Angermeier PL, Schlosser IJ. Species-Area Relationship for Stream Fishes. Ecology. 1989; 10.2307/1938204 [DOI] [Google Scholar]
- 39.Santos GM, Mérona B, Juras AA, Jégu M. Peixes do baixo rio Tocantins 20 anos depois da usina hidrelétrica Tucuruí. Brasilia DF: Eletronorte; 2004. [Google Scholar]
- 40.Malabarba LR. Peixes de Riachos da Mata Atlântica nas Unidades de Conservação do Vale do Rio Ribeira de Iguape no Estado de São Paulo. Neotrop Ichthyol. 2006;4: 377–377. 10.1590/S1679-62252006000300012 [DOI] [Google Scholar]
- 41.Baumgartner G, Pavanelli CS, Baumgartner D, Bifi AG, Debona T, Frana VA. Peixes do Baixo Rio Iguaçu [Internet]. Maringá: Eduem; 2012. http://linkinghub.elsevier.com/retrieve/pii/S0927521503801480 [Google Scholar]
- 42.Mazzoni R, Caramaschi R, Iglesias-Rios R. Usina Hidrelétrica de Serra da Mesa: 15 anos de Estudos da Ictiofauna do Alto Tocantins. Rio de Janeiro: Furnas Centrais Elétricas; 2012. [Google Scholar]
- 43.Malabarba LR, Neto PC, Bertaco VA, Carvalho TP, Santos JF, Artioli LGS. Guia de identificação dos peixes da bacia do Rio Tramandaí. Via Sapiens; 2013; 140. [Google Scholar]
- 44.Menezes NA. Systematics and evolution of the tribe Acestrorhynchini (Pisces, Characidae). Arq Zool. 1969;18: 1 doi: 10.11606/issn.2176-7793.v18i1-2p1-150 [Google Scholar]
- 45.Astorga A, Heino J, Luoto M, Muotka T. Freshwater biodiversity at regional extent: Determinants of macroinvertebrate taxonomic richness in headwater streams. Ecography (Cop). 2011; 10.1111/j.1600-0587.2010.06427.x [DOI] [Google Scholar]
- 46.Bloch CP, Higgins CL, Willig MR. Effects of large-scale disturbance on metacommunity structure of terrestrial gastropods: Temporal trends in nestedness. Oikos. 2007; 10.1111/j.0030-1299.2007.15391.x [DOI] [Google Scholar]
- 47.Carstensen DW, Lessard JP, Holt BG, Borregaard M Krabbe, Rahbek C. Introducing the biogeographic species pool. Ecography (Cop). 2013; 10.1111/j.1600-0587.2013.00329.x [DOI] [Google Scholar]
- 48.Chick JH, Mclvor CC. Patterns in the Abundance and Composition of Fishes among Beds of Different Macrophytes: Viewing a Littoral Zone as a Landscape. Can J Fish Aquat Sci. 1994; 10.1139/f94-286 [DOI] [Google Scholar]
- 49.Alves CBM, dos S Pompeu P, editors. Peixes do Rio das Velhas : Passado e Presente. 2nd ed Belo Horizonte, MG: Argvmentvm; 2010. [Google Scholar]
- 50.Britski HA, Sato Y, Rosa ABS. Manual de identificacao de peixes da regiao de Tres Marias (com chaves de identificacao para os peixes da Bacia do Sao Francisco). Brasilia DF: CODEVASF; 1984. [Google Scholar]
- 51.Britski HA, Silimon KZS, Lopes BS. Peixes do pantanal: manual de identificação. Brasilia DF: Embrapa; 2007. [Google Scholar]
- 52.BUCKUP PA. The monophyly of the Characidiinae, a Neotropical group of characiform fishes (Teleostei: Ostariophysi). Zool J Linn Soc. 1993; 10.1111/j.1096-3642.1993.tb00297.x [DOI] [Google Scholar]
- 53.Dormann C F., McPherson J M., Araújo M B., Bivand R, Bolliger J, Carl G, et al. Methods to account for spatial autocorrelation in the analysis of species distributional data: A review. Ecography. 2007. 10.1111/j.2007.0906-7590.05171.x [DOI] [Google Scholar]
- 54.Rangel TF, Diniz JAF, Bini LM. rSAM: a comprehensive application for Spatial Analysis in Macroecology. Ecography (Cop). 2010; [Google Scholar]
- 55.Rangel TF, Diniz-Filho JAF, Bini LM. SAM: A comprehensive application for Spatial Analysis in Macroecology. Ecography (Cop). 2010; 10.1111/j.1600-0587.2009.06299.x [DOI] [Google Scholar]
- 56.R Development Core Team R. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2011. [Google Scholar]
- 57.Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, et al. vegan: Community Ecology Package. R package version 2.0–9. R package version. 2013. 10.4135/9781412971874.n145
- 58.Vannote RL, Minshall GW, Cummins KW, Sedell JR, Cushing CE. The River Continuum Concept. Can J Fish Aquat Sci. 1980; 10.1139/f80-017 [DOI] [Google Scholar]
- 59.Cottenie K. Integrating environmental and spatial processes in ecological community dynamics. Ecol Lett. 2005; 10.1111/j.1461-0248.2005.00820.x [DOI] [PubMed] [Google Scholar]
- 60.Hubert N, Duponchelle F, Nuñez J, Garcia-Davila C, Paugy D, Renno JF. Phylogeography of the piranha genera Serrasalmus and Pygocentrus: Implications for the diversification of the Neotropical ichthyofauna. Mol Ecol. 2007; 10.1111/j.1365-294X.2007.03267.x [DOI] [PubMed] [Google Scholar]
- 61.Grossman GD, Sabo JL. Comparing stochastic properties of stream hydrographs and the resilience of fish assemblages. In: Jackson DA, Gido KB, editors. Community ecology of stream fishes: concepts, approaches, and techniques. Maryland: American Fisheries Society, Symposium 73, Bethesda; 2010.
- 62.Schlosser IJ. A conceptual framework for fish communities in small warmwater streams. Community Evol Ecol North Am stream fishes. 1987; [Google Scholar]
- 63.McGarvey DJ, Hughes RM. Longitudinal Zonation of Pacific Northwest (U.S.A.) Fish Assemblages and the Species-Discharge Relationship. Copeia. 2008; 10.1643/CE-07-020 [DOI] [Google Scholar]
- 64.Horwitz RJ. Temporal Variability Patterns and the Distributional Patterns of Stream Fishes. Ecol Monogr. 1978; 10.2307/2937233 [DOI] [Google Scholar]
- 65.Livingstone DA, Rowland M, Bailey PE. On the size of african riverine fish faunas. Integr Comp Biol. 1982; 10.1093/icb/22.2.361 [DOI] [Google Scholar]
- 66.Hugueny B. West African rivers as biogeographic islands: species richness of fish communities. Oecologia. 1989; 10.1007/BF00388483 [DOI] [PubMed] [Google Scholar]
- 67.Tedesco PA, Oberdorff T, Lasso CA, Zapata M, Hugueny B. Evidence of history in explaining diversity patterns in tropical riverine fish. J Biogeogr. 2005; 10.1111/j.1365-2699.2005.01345.x [DOI] [Google Scholar]
- 68.Olden JD, Jackson DA, Peres-Neto PR. Spatial isolation and fish communities in drainage lakes. Oecologia. 2001; 10.1007/s004420000620 [DOI] [PubMed] [Google Scholar]
- 69.Ringler NH, Hall JD. Effects of logging on water temperature and dissolved oxygen in spawning beds. Trans Am Fish Soc. 1975; [Google Scholar]
- 70.Webster JR, Meyer JL. Organic Matter Budgets for Streams: A Synthesis. J North Am Benthol Soc. 1997; 10.2307/1468247 [DOI] [Google Scholar]
- 71.Paula De FR, Ferraz SFDB, Gerhard P, Vettorazzi CA, Ferreira A. Large woody debris input and its influence on channel structure in agricultural lands of Southeast Brazil. Environ Manage. 2011; 10.1007/s00267-011-9730-4 [DOI] [PubMed] [Google Scholar]
- 72.Hawkins BA, Porter EE. Water-energy balance and the geographic pattern of species richness of western Palearctic butterflies. Ecol Entomol. 2003; 10.1111/j.1365-2311.2003.00551.x [DOI] [Google Scholar]
- 73.Abell R, Thieme ML, Revenga C, Bryer M, Kottelat M, Bogutskaya N, et al. Freshwater Ecoregions of the World: A New Map of Biogeographic Units for Freshwater Biodiversity Conservation. Bioscience. 2008; 10.1641/B580507 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
All the spatial filters are related to positive autocorrelation. The squares are positioned over the streams assessed. The highest values are black squares and the smallest values white squares.
(TIF)
(TIF)
(XLSX)
The name and unit of variables are indicating on the Table 1.
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.