Abstract
Background:
Substantial muscle atrophy occurs after total knee arthroplasty (TKA), resulting in decreased strength and impaired mobility. We sought to determine whether perioperative supplementation with essential amino acids (EAA) would attenuate muscle atrophy following TKA and whether the supplements were safe for ingestion in an older surgical population.
Methods:
We performed a double-blind, placebo-controlled, randomized trial of 39 adults (age range, 53 to 76 years) undergoing primary unilateral TKA who ingested 20 g of EAA (n = 19) or placebo (n = 20) twice daily for 7 days preoperatively and for 6 weeks postoperatively. At baseline and 6 weeks postoperatively, magnetic resonance imaging (MRI) scans were obtained to measure quadriceps and hamstrings muscle volume. Secondary outcomes included functional mobility and strength. Data on physical activity, diet, and patient-reported outcomes (Veterans RAND 12-Item Health Survey and Knee injury and Osteoarthritis Outcome Score) were collected. Safety was determined through blood tests evaluating blood urea nitrogen, creatinine, creatinine clearance, homocysteine, and renal and liver function. Laboratory values at baseline, on the day of surgery, and at 2 days, 2 weeks, and 6 weeks postoperatively were compared between treatment groups. Analysis of covariance models, with baseline values as covariates, were used to evaluate outcomes between treatment groups. P values were adjusted for multiple tests.
Results:
Compared with baseline, the EAA group had significantly less decrease in mean quadriceps muscle volume compared with the placebo group in the involved leg (−8.5% ± 2.5% compared with −13.4% ± 1.9%; p = 0.033) and the contralateral leg (−1.5% ± 1.6% compared with −7.2% ± 1.4%; p = 0.014). The hamstrings also demonstrated a greater muscle-volume-sparing effect for the EAA group than for the placebo group in the involved leg (−7.4% ± 2.0% compared with −12.2% ± 1.4%; p = 0.036) and contralateral leg (−2.1% ± 1.3% compared with −7.5% ± 1.5%; p = 0.005). There were no differences between the groups in terms of functional measures or strength. Blood chemistry values varied significantly between assessments periods but did not statistically differ between groups.
Conclusions:
The results of the present study suggest that EAA supplementation is safe and reduces the loss of muscle volume in older adults recovering from TKA.
Level of Evidence:
Therapeutic Level II. See Instructions for Authors for a complete description of levels of evidence.
Muscle atrophy and weakness are major barriers to returning to work and daily activities following total knee arthroplasty (TKA)1-3. Specifically, quadriceps atrophy and weakness jeopardize balance4, reduce functional mobility5,6, and increase the risk of falls7. Amino acid supplementation has been shown to increase muscle in older adult populations8. A proof-of-principle report by our group recently documented that essential amino acid (EAA) supplementation before and after TKA mitigated muscle atrophy after surgery when compared with placebo9. In the present investigation, conducted with more subjects with a broader age range and under the care of more surgeons, we sought to repeat that study.
The purpose of the present prospective clinical trial was to document changes in quadriceps and hamstrings muscle volume between groups of older patients who received either EAA or placebo before and after TKA. We hypothesized that twice-daily ingestion of 20 g of EAA between meals from 1 week before to 6 weeks after TKA would (1) mitigate muscle loss and (2) preserve functional mobility and improve patient-reported outcomes. We questioned whether or not EAA supplementation affected muscle volume and related outcomes in an older patient population undergoing a common orthopaedic procedure. The relative ease and low expense of EAA administration suggest that our protocol could be translated into clinical practice.
Materials and Methods
In the present single-center, 2-arm, double-blind, placebo-controlled, parallel-group study, patients were allocated to treatment with EAA or placebo in a 1:1 ratio. Thirty-nine subjects ranging from 53 to 76 years of age who were under the care of 4 surgeons participated between January 2015 and September 2016. The University of Oregon (UO) institutional review board (primary) and the PeaceHealth System institutional review board approved the study, which was registered on ClinicalTrials.gov (NCT02145949).
Patients between 50 and 80 years of age who were scheduled to undergo primary unilateral TKA were included. The exclusion criteria included a history of lower extremity total joint replacement surgery; uncontrolled endocrine disease; heart, kidney, liver, blood, or respiratory disease; peripheral vascular disease; active cancer; and recent treatment with anabolic steroids or oral corticosteroids for >1 week. Patients were stratified by sex and were randomly assigned, with use of a table generated with RANDOM.ORG, to ingest 20 g of EAA (n = 19) or placebo (n = 20) twice daily for 7 days preoperatively and for 6 weeks postoperatively.
Preoperative blood chemistry, magnetic resonance imaging (MRI), strength and functional mobility, and patient-reported outcomes data were collected at baseline (2 to 6 weeks preoperatively). Blood chemistry was monitored on the day of surgery, 2 days postoperatively, and 2 and 6 weeks postoperatively. Functional measures were repeated at 2 and 6 weeks postoperatively. MRI assessment and evaluation of patient-reported outcomes were performed at 6 weeks postoperatively. Patients recorded physical activity via accelerometry and caloric intake with 3-day food diaries.
Although we examined patient-reported outcomes, functional mobility, and muscle strength, the present study was designed with sufficient statistical power to detect, primarily, differences in the more-reliable physiological measurements of muscle volume.
Investigational Supplementation
Placebo or EAA supplementation was initiated 1 week before TKA, was discontinued on the day of surgery, was resumed on the first postoperative day, and was continued for 6 weeks, for a total of 49 days (Table I). The supplement could be mixed into any liquid or food and was ingested twice daily between meals. During hospitalization, the supplement was taken 1 hour after both morning and afternoon physical therapy. After discharge, patients continued this regimen to maximize the anabolic/anticatabolic effect of EAA10,11. Ingestion was recorded and verified by research personnel. Supplements were prepared by Northwest Compounders with use of amino acids purchased from Ajinomoto U.S.A. Amino acids were added in predefined proportions based on previous work9 to achieve a master mix weighing 400 g. The master mix was vigorously shaken for several minutes and then was aliquoted into 20-g vials and dispensed in blinded vials by study personnel.
TABLE I.
Supplement Composition*
| Percentage of Total | Grams | |
| EAA | ||
| Histidine | 11% | 2.2 |
| Isoleucine | 10% | 2.0 |
| Leucine | 18% | 3.6 |
| Lysine | 16% | 3.2 |
| Methionine | 3% | 0.6 |
| Phenylalanine | 16% | 3.2 |
| Threonine | 14% | 2.8 |
| Valine | 12% | 2.4 |
| Total | 100% | 20 |
| Placebo | ||
| Alanine | 100% | 20 |
20 g of supplement (EAA or placebo) was ingested twice daily, at 10 a.m. and 2 p.m., for 7 days prior to TKA. Patients then fasted overnight, and surgery was performed the following day. Supplementation resumed on the first postoperative day. Supplementation continued for 42 days after TKA. Each patient was issued a log book in which to record the time of supplement ingestion. Log book recordings were verified by research and nursing staff during the inpatient stay and by research staff at each visit.
Muscle Volume (Primary End Point)
MRI of both lower extremities was performed with use of a Siemens 3T Skyra scanner (Siemens) at the UO Lewis Center for Neuroimaging. One researcher analyzed blinded MRI data (intraclass correlation coefficient of repeat analyses, 0.96).
Secondary End Points
Medical Image Processing, Analysis, and Visualization (MIPAV) software (v. 7.4.0, https://mipav.cit.nih.gov)12 was used to calculate mid-thigh muscle volume after excluding bone and isolating each muscle group. Magnetic eddy currents were corrected with a nonparametric, nonuniform intensity normalization algorithm13 followed by fuzzy c-means adjustment and were reported in arbitrary units (AU).
Functional mobility measurements included handgrip strength, Short Physical Performance Battery (SPPB) balance (4-m gait speed and chair stand), timed up-and-go (TUG) test, 4-m walk, timed stair ascent and timed stair descent, leg strength, and 6-minute walk. The test order was the same for all subjects.
Patients wore an accelerometer for 21 consecutive days from 1 week preoperatively through 2 weeks postoperatively, as well as at 5 and 6 weeks postoperatively. Three-day food diaries on standardized days were completed before surgery, 1 and 2 days postoperatively, and 1, 2, and 5 weeks postoperatively. Food Processor Nutrition Analysis Software (ESHA Research) was used to calculate micronutrients and macronutrients.
At baseline and 6 weeks postoperatively, patients completed the Knee injury and Osteoarthritis Outcome Score (KOOS) survey, which has adequate construct validity14 and demonstrated responsiveness to change15, and the validated Veterans RAND 12-Item Health Survey (VR-12)16,17, which yielded Physical Component Summary (PCS12) and Mental Component Summary (MCS12) scores reflecting health-related quality of life. The previously validated Patient Health Questionnaire depression module (PHQ-9)18,19 measured depression symptom severity.
The study was conducted according to U.S. Food and Drug Administration Investigational New Drug (IND) requirements (IND#121601). Accordingly, 15 blood tests were used to monitor blood urea nitrogen, creatinine, creatinine clearance, homocysteine, and renal and liver function. Values at baseline, on the day of surgery, 2 days postoperatively, and 2 and 6 weeks postoperatively are reported for both treatment groups. An independent, National Institutes of Health-appointed Data and Safety Monitoring Board reviewed adverse events and laboratory assessments. Nuclear Magnetic Resonance (NMR) at Pacific Northwest National Laboratories randomly evaluated the EAA supplement composition.
Statistical Analyses
Separate analysis models were used to compare the EAA and placebo groups with regard to outcome variables at 2 and 6 weeks while controlling baseline values. The Benjamini-Hochberg procedure was used to adjust p values for multiple comparisons. All values are expressed as the mean percentage or value and the standard error. Analyses were performed with use of SPSS (v. 19.0, 2010; SPSS).
Results
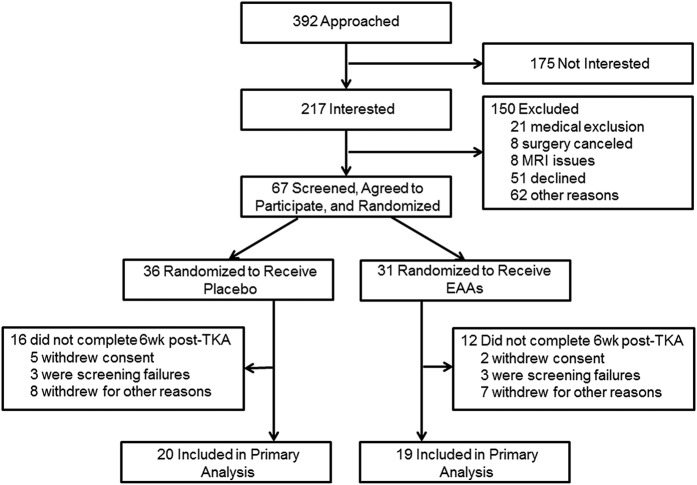
The present study included 39 patients who were randomized to treatment with EAA (19 patients) or placebo (20 patients) (Fig. 1 and Table II). The sample was 64% female and 95% non-Hispanic white, with a mean age (and standard error) of 64.41 ± 0.94 years, a baseline body mass index of 29.78 ± 1.20 kg/m2, a tourniquet time of 56.18 ± 3.74 minutes, and an operative time of 94.74 ± 3.18 minutes.
Fig. 1.
CONSORT flow diagram showing the numbers of patients who were randomly assigned to each treatment group, who were excluded or withdrew from the study, and who were included in the analysis.
TABLE II.
Patient Characteristics
| Variable | Value* |
| Participant characteristics | |
| % female | 64.1% |
| Age (yr) | 64.41 ± 0.94 |
| Height (m) | 1.68 ± 0.01 |
| Weight (kg) | 86.92 ± 3.03 |
| Body mass index (kg/m2) | 29.78 ± 1.20 |
| Tourniquet time (min) | 56.18 ± 3.74 |
| Operative time (min) | 94.74 ± 3.18 |
| Physical activity (kcal/day) | 361.60 ± 37.12 |
| Nutrients | |
| Baseline | |
| Fat intake (g/day) | 74.28 ± 4.17 |
| Carbohydrate intake (g/day) | 229.76 ± 12.33 |
| Protein intake (g/day) | 100.25 ± 9.24 |
| Protein intake (g/kg body weight/day) | 1.17 ± 0.12 |
| Calorie intake (kcal/day) | 2,012.80 ± 90.37 |
| Calorie intake (kcal/kg body weight/day) | 23.35 ± 1.12 |
| 6 weeks postop. | |
| Fat intake (g/day) | 72.37 ± 6.07 |
| Carbohydrate intake (g/day) | 216.53 ± 14.52 |
| Protein intake (g/day) | 81.18 ± 5.92 |
| Protein intake (g/kg body weight/day) | 0.93 ± 0.07 |
| Calorie intake (kcal/day) | 1,840.26 ± 124.55 |
| Calorie intake (kcal/kg body weight/day) | 21.06 ± 1.40 |
| Baseline medications† | |
| Acetaminophen | 74.4% |
| Anticoagulant | 100% |
| Anti-diarrheal | 0% |
| Baby aspirin | 25.6% |
| Blood pressure | 41.0% |
| Calcium | 33.3% |
| Cardiac glycoside | 0% |
| COPD | 0% |
| Diabetes | 7.7% |
| GI (ulcer/GERD) | 33.3% |
| Glucosamine | 12.8% |
| Estrogen | 7.7% |
| Muscle relaxant | 69.2% |
| NSAID | 100% |
| Osteoporosis | 2.6% |
| Pain reliever | 89.7% |
| Statin | 28.2% |
| SNRI | 0% |
| Non-SNRI antidepressant | 30.8% |
| Thyroid | 20.5% |
| Vitamin D | 41.0% |
The values are given as the mean and the standard error or as the percentage of the total (n = 39).
COPD = chronic obstructive pulmonary disease, GI = gastrointestinal, GERD = gastroesophageal reflux disease, NSAID = nonsteroidal anti-inflammatory drug, and SNRI = serotonin and norepinephrine reuptake inhibitor.
Muscle Volume
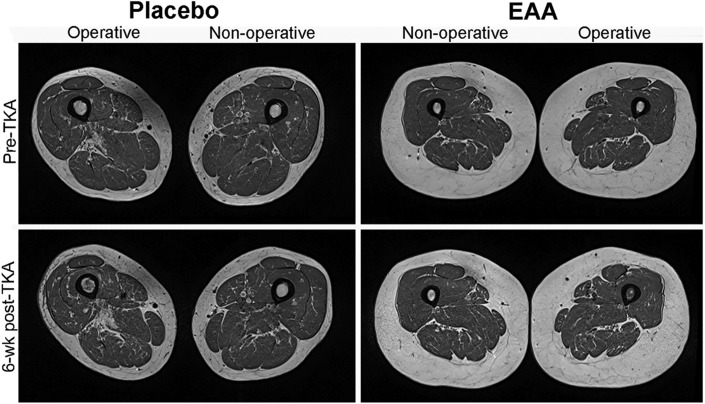
Repeated-measures analyses of variance demonstrated significant changes in mean muscle volume for both the quadriceps and the hamstrings from baseline to 6 weeks postoperatively, regardless of treatment group (p < 0.05 for all). Quadriceps muscle atrophy was significantly greater in the placebo group than in the EAA group in both the involved leg (−13.4% ± 1.9% compared with −8.5% ± 2.5%; p = 0.033) and the contralateral leg (−7.2% ± 1.4% compared with −1.5% ± 1.6%; p = 0.014) (Table III). Similarly, hamstrings muscle atrophy was significantly greater in the placebo group than in the EAA group in both the involved leg (−12.2% ± 1.4% compared with −7.4% ± 2.0%; p = 0.036) and the contralateral leg (−7.5% ± 1.5% compared with −2.1% ± 1.3%; p = 0.005). Representative MRI scans illustrating muscle atrophy are presented in Figure 2.
Fig. 2.
Example T1-weighted MRI scans of the mid-thigh region, showing representative muscle atrophy of the involved at baseline (Pre-TKA) and 6 weeks postoperatively (Post-TKA) for a 77-year-old man from the placebo group and a 66-year-old woman from the EAA group.
TABLE III.
Treatment Effects for MRI-Measured Quadriceps and Hamstrings Muscle Outcomes at 6 Weeks After TKA
| Placebo Group* (N = 20) |
EAA Group* (N = 19) |
||||
| Variable | Baseline | 6 Weeks Postop. | Baseline | 6 Weeks Postop. | P Value† |
| Quadriceps | |||||
| Involved leg | 44.81 ± 3.09 | 38.31 ± 2.39 | 48.66 ± 3.45 | 43.87 ± 2.93 | 0.03 (0.04) |
| Contralateral leg | 49.98 ± 3.05 | 46.44 ± 2.94 | 55.49 ± 3.87 | 54.41 ± 3.65 | 0.01 (0.02) |
| Hamstrings | |||||
| Involved leg | 63.86 ± 3.33 | 55.93 ± 2.91 | 69.32 ± 4.05 | 63.76 ± 3.41 | 0.04 (0.04) |
| Contralateral leg | 63.64 ± 3.33 | 58.75 ± 3.05 | 69.37 ± 4.19 | 67.50 ± 3.75 | 0.01 (0.02) |
The values are reported in arbitrary units (AU) and are expressed as the mean and the standard error.
The p values are from separate analyses of covariance, with the 6-week postoperative value as the dependent variable, treatment group as the independent variable, and the baseline value as the covariate. Observed (unadjusted) mean p values are presented first; the p values in parentheses have been adjusted for multiple tests with use of the Benjamini-Hochberg procedure.
Muscle Strength, Functional Mobility, and PROMs
Patients in both groups reported significant declines from baseline functional status at 2 weeks postoperatively (p < 0.05 for all); functional measures and patient-reported outcome measures (PROMs) did not change significantly from baseline to 6 weeks postoperatively after correction for multiple tests. Secondary analyses focused on quadriceps and hamstrings strength, functional mobility, and PROMs demonstrated no significant treatment effects in these measures at 2 or 6 weeks postoperatively (Table IV).
TABLE IV.
Treatment Effects for Functional, Strength, and Quality-of-Life Outcomes
| Placebo Group* (N = 20) |
EAA Group* (N = 19) |
||||
| Variable | Baseline | Postop. | Baseline | Postop. | P Value† |
| Functional outcomes | |||||
| 2 weeks postop. | |||||
| Physical activity (kcal/day) | 319.71 ± 56.97 | 128.03 ± 19.56 | 408.15 ± 45.37 | 124.08 ± 13.12 | 0.83 |
| Handgrip strength (kg) | 28.35 ± 1.89 | 30.00 ± 1.90 | 28.95 ± 2.39 | 30.47 ± 2.32 | 0.79 |
| Short Physical Performance Battery total score (0-11) | 5.40 ± 0.23 | 3.95 ± 0.39 | 6.11 ± 0.27 | 5.05 ± 0.29 | 0.10 |
| Timed up-and-go (sec) | 11.40 ± 0.68 | 16.02 ± 1.98 | 9.88 ± 0.46 | 12.90 ± 0.77 | 0.31 |
| 4-m walk (sec) | 3.55 ± 0.14 | 5.00 ± 0.33 | 3.33 ± 0.13 | 4.08 ± 0.19 | 0.03 |
| Stair climb up (sec) | 9.45 ± 1.10 | 16.54 ± 1.77 | 8.09 ± 0.55 | 12.73 ± 0.78 | 0.06 |
| Stair climb down (sec) | 10.70 ± 1.06 | 18.33 ± 1.78 | 8.03 ± 0.67 | 13.36 ± 1.16 | 0.18 |
| 6-min walk distance (m) | 488.30 ± 22.80 | 339.09 ± 24.22 | 511.10 ± 17.73 | 398.09 ± 19.87 | 0.06 |
| 6 weeks postop. | |||||
| Physical activity (kcal/day) | 319.71 ± 56.97 | 196.54 ± 18.43 | 408.15 ± 45.37 | 265.67 ± 35.69 | 0.12 |
| Handgrip strength (kg) | 28.35 ± 1.89 | 28.03 ± 1.87 | 28.95 ± 2.39 | 30.74 ± 2.43 | 0.03 |
| Short Physical Performance Battery total score (0-11) | 5.40 ± 0.23 | 5.90 ± 0.25 | 6.11 ± 0.27 | 6.32 ± 0.24 | 0.71 |
| Timed up-and-go (sec) | 11.40 ± 0.68 | 10.90 ± 0.85 | 9.88 ± 0.46 | 9.87 ± 0.44 | 0.76 |
| 4-m walk (sec) | 3.55 ± 0.14 | 3.56 ± 0.17 | 3.33 ± 0.13 | 3.29 ± 0.16 | 0.40 |
| Stair climb up (sec) | 9.45 ± 1.10 | 8.64 ± 0.76 | 8.09 ± 0.55 | 7.95 ± 0.64 | 0.97 |
| Stair climb down (sec) | 10.70 ± 1.06 | 9.53 ± 1.02 | 8.03 ± 0.67 | 8.12 ± 0.74 | 0.89 |
| Leg strength (maximum voluntary contraction) (N) | |||||
| Quadriceps | |||||
| Involved leg | 79.95 ± 13.68 | 81.80 ± 10.54 | 102.71 ± 9.50 | 93.63 ± 9.29 | 0.88 |
| Contralateral leg | 108.29 ± 12.08 | 101.89 ± 11.80 | 113.32 ± 11.16 | 121.56 ± 11.93 | 0.03 |
| Hamstrings | |||||
| Involved leg | 24.67 ± 2.21 | 21.95 ± 2.05 | 28.96 ± 1.96 | 28.06 ± 1.58 | 0.08 |
| Contralateral leg | 28.33 ± 1.91 | 28.09 ± 2.42 | 31.56 ± 2.26 | 30.96 ± 1.85 | 0.79 |
| 6-min walk distance (m) | 488.30 ± 22.80 | 477.02 ± 0.02 | 511.10 ± 17.73 | 464.56 ± 32.00 | 0.48 |
| Quality-of-life outcomes (6 weeks postop.) | |||||
| KOOS (0-100) | |||||
| Symptoms | 50.00 ± 3.65 | 57.00 ± 3.60 | 54.13 ± 4.35 | 68.80 ± 3.84 | 0.02 |
| Pain | 53.06 ± 3.26 | 64.77 ± 3.41 | 51.18 ± 3.61 | 68.71 ± 2.06 | 0.29 |
| Function daily living | 60.67 ± 3.58 | 76.16 ± 2.78 | 66.01 ± 3.44 | 81.41 ± 2.41 | 0.33 |
| Function sports/rec. | 30.00 ± 5.89 | 37.90 ± 5.25 | 27.90 ± 4.69 | 44.21 ± 6.36 | 0.30 |
| Knee-related quality of life | 24.01 ± 4.01 | 50.33 ± 3.75 | 30.26 ± 3.14 | 51.74 ± 4.19 | 0.98 |
| PHQ-9 score (0-27) | 4.10 ± 0.85 | 3.68 ± 0.63 | 3.42 ± 1.05 | 4.68 ± 0.85 | 0.18 |
| PCS12 score (0-100) | 33.41 ± 2.03 | 35.48 ± 1.76 | 33.17 ± 2.39 | 37.55 ± 2.08 | 0.52 |
| MCS12 score (0-100) | 57.23 ± 1.67 | 54.76 ± 2.81 | 55.48 ± 2.65 | 52.11 ± 2.60 | 0.81 |
The values are given as the mean and the standard error (n = 39).
The p values are from separate analyses of covariance, with the 2-week and 6-week postoperative value as the dependent variable, treatment group as the independent variable, and the baseline value as the covariate. Observed (unadjusted) mean values are presented. All p values were >0.05 after adjusting for multiple tests with use of the Benjamini-Hochberg procedure.
Physical Activity and Caloric Intake
The placebo and EAA groups did not significantly differ in terms of average energy expended preoperatively (319.71 ± 56.97 compared with 408.15 ± 45.37 kcal/day; p = 0.24) or at 2 and 6 weeks postoperatively after adjusting for baseline values. Energy intake was moderate at baseline, averaging 1,731 and 1,949 kcal/day in the placebo and EAA groups, respectively. In both groups, caloric intake, protein intake, fat intake, and carbohydrate intake declined significantly from baseline to 2 weeks postoperatively (p < 0.05 for all), but not from baseline to 6 weeks postoperatively (p > 0.05 for all). Nutritional measures did not significantly differ between the 2 groups (p > 0.05 for all).
Compliance and Safety
Patients reported taking 99% of the EAA doses and 96% of the placebo doses. No study-related adverse events were reported. Patients who withdrew did so for non-study-related health reasons. Non-study-related adverse events did not significantly differ between the groups at the midpoint or at the end of the study according to O’Brien-Fleming-adjusted alpha thresholds. The groups did not significantly differ in terms of any of the 15 liver and kidney function or homocysteine tests (Table V). NMR analysis of 8 randomly selected EAA supplement vials revealed the following target and measured amounts: histidine (target, 2.2 g; measured, 2.94 ± 0.15 g), isoleucine (target, 2.0 g; measured, 1.97 ± 0.08 g), leucine (target, 3.6 g; measured, 3.07 ± 0.13 g), lysine (target, 3.2 g; measured, 2.64 ± 0.14 g), methionine (target, 0.6 g; measured, 0.63 ± 0.03 g), phenylalanine (target, 3.2 g; measured, 2.9 ± 0.0 g), threonine (target, 2.8 g; measured, 3.3 ± 0.10 g), and valine (target, 2.4 g; measured, 2.96 ± 0.24 g).
TABLE V.
Blood Test Values by Treatment Group
| Variable | Placebo Group* | EAA Group* | P Value† |
| Anion gap | |||
| Baseline | 8.55 ± 0.37 | 8.47 ± 0.41 | |
| Day of op. | 8.40 ± 0.40 | 8.37 ± 0.49 | 0.76 |
| 2 days postop. | 7.32 ± 0.41 | 7.88 ± 0.54 | 0.40‡ |
| 2 weeks postop. | 9.35 ± 0.38 | 8.16 ± 0.34 | 0.04 |
| 6 weeks postop. | 8.79 ± 0.31 | 9.11 ± 0.45 | 0.72 |
| Glucose (mg/dL) | |||
| Baseline | 108.70 ± 6.61 | 100.50 ± 2.47 | |
| Day of op. | 103.05 ± 3.75 | 104.53 ± 2.38 | 0.15 |
| 2 days postop. | 104.42 ± 3.08 | 112.82 ± 3.44 | 0.04 |
| 2 weeks postop. | 103.80 ± 5.25 | 101.37 ± 2.56 | 0.43 |
| 6 weeks postop. | 97.74 ± 3.75 | 98.95 ± 2.48 | 0.16 |
| Blood urea nitrogen (mg/dL) | |||
| Baseline | 17.55 ± 1.21 | 16.83 ± 1.16 | |
| Day of TKA | 20.80 ± 1.13 | 20.21 ± 1.76 | 0.93‡ |
| 2 days postop. | 15.21 ± 0.93 | 13.88 ± 1.15 | 0.67‡ |
| 2 weeks postop. | 19.35 ± 1.37 | 20.26 ± 1.69 | 0.27‡ |
| 6 weeks postop. | 17.58 ± 1.25 | 16.95 ± 1.06 | 0.78 |
| Creatinine (mg/dL) | |||
| Baseline | 0.83 ± 0.04 | 0.90 ± 0.04 | |
| Day of TKA | 0.76 ± 0.03 | 0.88 ± 0.05 | 0.07‡ |
| 2 days postop. | 0.77 ± 0.04 | 0.85 ± 0.05 | 0.83‡ |
| 2 weeks postop. | 0.77 ± 0.03 | 0.88 ± 0.05 | 0.16 |
| 6 weeks postop. | 0.76 ± 0.04 | 0.83 ± 0.05 | 0.46‡ |
| Total protein (g/dL) | |||
| Baseline | 6.86 ± 0.07 | 6.72 ± 0.07 | |
| Day of TKA | 6.88 ± 0.09 | 6.64 ± 0.08 | 0.19 |
| 2 days postop. | 6.04 ± 0.12 | 6.01 ± 0.20 | 0.61‡ |
| 2 weeks postop. | 6.83 ± 0.08 | 6.69 ± 0.08 | 0.70 |
| 6 weeks postop. | 6.86 ± 0.10 | 6.72 ± 0.09 | 0.54 |
| Albumin (g/dL) | |||
| Baseline | 4.05 ± 0.06 | 4.02 ± 0.05 | |
| Day of TKA | 3.79 ± 0.05 | 3.83 ± 0.06 | 0.28‡ |
| 2 days postop. | 3.21 ± 0.06 | 3.14 ± 0.07 | 0.45‡ |
| 2 weeks postop. | 3.76 ± 0.05 | 3.73 ± 0.05 | 0.99‡ |
| 6 weeks postop. | 3.94 ± 0.06 | 3.94 ± 0.05 | 0.90 |
| Globulin (g/dL) | |||
| Baseline | 2.81 ± 0.06 | 2.68 ± 0.06 | |
| Day of TKA | 3.09 ± 0.09 | 2.81 ± 0.07 | 0.05‡ |
| 2 days postop. | 2.84 ± 0.10 | 2.69 ± 0.09 | 0.96 |
| 2 weeks postop. | 3.07 ± 0.07 | 2.97 ± 0.06 | 0.92‡ |
| 6 weeks postop. | 2.93 ± 0.08 | 2.78 ± 0.07 | 0.76‡ |
| Calcium (mg/dL) | |||
| Baseline | 9.46 ± 0.08 | 9.33 ± 0.10 | |
| Day of op. | 9.14 ± 0.07 | 9.16 ± 0.06 | 0.16‡ |
| 2 days postop. | 8.57 ± 0.07 | 8.51 ± 0.07 | 0.75‡ |
| 2 weeks postop. | 9.54 ± 0.09 | 9.45 ± 0.07 | 0.89 |
| 6 weeks postop. | 9.49 ± 0.07 | 9.49 ± 0.06 | 0.38 |
| Total bilirubin (mg/dL) | |||
| Baseline | 0.67 ± 0.04 | 0.84 ± 0.06 | |
| Day of TKA | 0.68 ± 0.04 | 0.77 ± 0.05 | 0.61 |
| 2 days postop. | 0.65 ± 0.04 | 0.81 ± 0.07 | 0.12 |
| 2 weeks postop. | 0.69 ± 0.03 | 0.65 ± 0.05 | 0.19 |
| 6 weeks postop. | 0.56 ± 0.04 | 0.59 ± 0.05 | 0.36‡ |
| Alkaline phosphatase (IU/L) | |||
| Baseline | 74.55 ± 5.69 | 70.28 ± 3.95 | |
| Day of op. | 63.75 ± 4.11 | 63.74 ± 3.33 | 0.63‡ |
| 2 days postop. | 58.06 ± 3.87 | 57.23 ± 4.32 | 0.94‡ |
| 2 weeks postop. | 80.50 ± 5.19 | 80.63 ± 4.43 | 0.65‡ |
| 6 weeks postop. | 77.47 ± 4.65 | 79.79 ± 4.27 | 0.45 |
| Alanine aminotransferase (U/L) | |||
| Baseline | 26.95 ± 3.04 | 23.94 ± 1.81 | |
| Day of op. | 24.25 ± 2.18 | 21.16 ± 1.19 | 0.39 |
| 2 days postop. | 20.82 ± 3.74 | 30.69 ± 6.76 | 0.17 |
| 2 weeks postop. | 23.20 ± 2.17 | 23.16 ± 2.06 | 0.63 |
| 6 weeks postop. | 18.16 ± 1.42 | 20.37 ± 1.26 | 0.11‡ |
| Aspartate aminotransferase (U/L) | |||
| Baseline | 26.60 ± 2.11 | 22.61 ± 1.31 | |
| Day of op. | 24.70 ± 1.38 | 21.95 ± 1.23 | 0.45 |
| 2 days postop. | 25.35 ± 3.01 | 30.31 ± 5.21 | 0.23 |
| 2 weeks postop. | 22.65 ± 1.42 | 21.16 ± 1.40 | 0.74‡ |
| 6 weeks postop. | 19.53 ± 0.87 | 19.84 ± 0.80 | 0.26‡ |
| Glomerular filtration rate§ (mL/min) | |||
| Baseline | 59.65 ± 0.26 | 59.44 ± 0.38 | |
| Day of op. | 59.65 ± 0.26 | 59.32 ± 0.68 | 0.80 |
| 2 days postop. | 59.63 ± 0.26 | 59.24 ± 0.54 | 0.55 |
| 2 weeks postop. | 60.00 ± 0.00 | 57.95 ± 1.62 | 0.21 |
| 6 weeks postop. | 62.63 ± 1.81 | 59.53 ± 0.47 | 0.13 |
| C-reactive protein# (mg/L) | |||
| Baseline | 0.80 ± 0.11 | 0.62 ± 0.06 | |
| Day of op. | 0.57 ± 0.05 | 0.51 ± 0.01 | 0.85‡ |
| 2 days postop. | 8.78 ± 0.73 | 8.43 ± 0.99 | 0.93‡ |
| 2 weeks postop. | 1.46 ± 0.36 | 1.26 ± 0.22 | 0.35‡ |
| 6 weeks postop. | 0.89 ± 0.22 | 0.54 ± 0.04 | 0.23 |
| Homocysteine (µmol/L) | |||
| Baseline | 9.16 ± 0.62 | 10.44 ± 0.51 | |
| Day of op. | 8.83 ± 0.48 | 11.00 ± 0.89 | 0.15 |
| 2 days postop. | 9.22 ± 1.07 | 9.83 ± 1.34 | 0.71 |
| 2 weeks postop. | 9.26 ± 0.62 | 11.43 ± 0.60 | 0.05 |
| 6 weeks postop. | 8.84 ± 0.64 | 10.69 ± 0.60 | 0.65 |
The values are given as the mean and the standard error (n = 39).
The p values are from an analysis of covariance model with the 6-week postoperative value as the dependent variable, treatment group as the independent variable, and the baseline value as the covariate. The p values in the table are unadjusted; all p values were >0.05 after adjusting for multiple tests with the Benjamini-Hochberg procedure.
Significant change from baseline regardless of treatment condition after adjusting for multiple tests with use of the Benjamini-Hochberg procedure.
Values of >60 were recoded to 60.
Values of <0.5 were recoded to 0.5.
Discussion
The present study confirms our proof-of-principle findings from 2013 showing preservation of muscle following TKA in older adults receiving EAA treatment compared with those receiving placebo9. That study showed that twice-daily ingestion of 20 g of EAA for 1 week before through 6 weeks after TKA had muscle-sparing effects in the quadriceps and hamstrings on the involved and contralateral sides at 6 weeks postoperatively. In the present study, the EAA group lost <5% in quadriceps and hamstrings muscle volume at 6 weeks across both legs, replicating the previous study. Specifically, the patients who received EAA treatment lost an average 4.9% in muscle volume in the present study, compared with 4.6% in the previous study. Among patients who received placebo treatment, however, those in the current study lost less muscle overall (10.1%) than those in the previous study (13.6%). As a result, the difference in atrophy rates between the 2 treatment groups in the present study was less (5% difference) than that in the previous study (9% difference). Despite the differences between the studies, the results favoring EAA supplementation are promising.
The present study, unlike the previous study, did not demonstrate significant differences in functional mobility or strength outcomes between treatment groups. Given that the results related to muscle atrophy and functional mobility were more similar between the 2 treatment groups in the present study than in the previous study, the lack of significant between-group differences in functional mobility measures might be expected. Measures of functional mobility are less reliable than physiological measures such as muscle volume; idiosyncratic patient factors (e.g., common cold) may have introduced noise into the functional results, making functional differences harder to detect, but would not have affected muscle volume results. The present study was designed primarily to detect differences in highly reliable physiological measures and may have lacked statistical power to detect functional measures. However, the absence of replication with these measures is not necessarily a contradiction. The likelihood of conflicting results at the p = 0.05 level from 2 studies with power of 0.80 is “32% or about 1 chance in 3.”20
Differences in results between the 2 studies may reflect differences in hospital protocols. Postoperative recovery expectations were higher for all patients in the present study, including those in the placebo group, than for those in the previous study. Between studies, the participating hospitals implemented a fast-track program, which has been associated with reduced pain21, earlier achievement of 90° of flexion, reduced reliance on walking aids22, and lower rates of subsequent joint manipulation23. The recent standardized hospital program with earlier hospital discharge may have decreased differences between conditions on functional outcomes during the weeks immediately following surgery.
EAA supplement compounding practices changed between the studies, which may have affected treatment outcomes. The EAA mixing and dispensing practices used in the present study resulted in inconsistent amino acid compositions across all vials. Most notably, the leucine concentration fell consistently short of the 3.6 g/vial target by an average 0.53 g. Murphy et al. recently reported that free-living older men who ingested 5 g of leucine with each meal for 3 days while on either a low or high-protein diet had elevated basal and post-exercise muscle protein synthesis rates compared with the same individuals on the same diets (low or high-protein) without supplemental leucine24. Subjects on low and high-protein diets supplemented with 5 grams of leucine per meal did not differ from one another24. This finding suggests that under-representation of leucine content in the EAA group may have negatively influenced outcomes. Variations in EAA composition may help to explain why the present study did not demonstrate, as the previous study did, that patients receiving EAA had significantly greater improvements on functional mobility tests than those receiving placebo9.
Despite EAA supplement variability, EAA treatment significantly attenuated muscle atrophy, consistent with the findings of our previous report9. Increasing evidence supports defined protocols for EAA supplement dosing regimens. Supplement ingestion 1 hour after physical therapy was a design feature of both our previous study9 and the current investigation because, in an earlier study, we found that ingestion of similar amounts of EAA 1 hour after exercise enhanced muscle protein synthesis response and mTORC1 (mammalian target of rapamycin complex 1) signaling10. Enhanced muscle protein synthesis response to exercise with protein or EAA ingestion has been shown in several laboratories25-28. In addition, Jordan et al.29, in a study of older individuals on an isocaloric, isonitrogenous diet (with physical activity levels controlled for), showed that nitrogen balance increased 57% when protein was consumed immediately after daily exercise as opposed to at rest earlier in the day.
To our knowledge, there are no PROMs for functional status of the knee that can be used to detect minimum clinically important differences in the acute postoperative period (2 and 6 weeks after TKA); our study demonstrated no difference in KOOS or VR-12 scores between treatment groups at 6 weeks after surgery. Future work is needed to assess differences in patient-reported quality-of-life and knee-specific outcomes at 3 and 6 months after TKA for participants ingesting placebo versus EAA.
Energy intake in the present study was moderate, in line with other studies of older healthy adults23 and older patient populations10,24. While reduced energy intake across treatment conditions was found at 2 weeks after TKA in both our 2013 study9 and the current study, both the absolute amount of energy intake and the change from baseline differed. The mean kcal/day at 2 weeks after TKA were about 400 lower in our 2013 study than in the current study in both the placebo group (1,396 compared with 1,731) and the EAA group (1,546 compared with 1,949). Differences in energy intake in the immediate post-acute period may have contributed to differences in outcomes between the 2 studies.
The present study had several limitations. Analyses were limited to the acute postoperative period because previous research has shown that significant muscle loss occurs within the first 2 weeks after surgery9. Future research should explore muscle volume and functional outcomes across longer follow-up periods, ideally 2 years or longer. The retention rate of 63.9%—after accounting for the withdrawal of participants deemed ineligible after randomization—was lower than anticipated. Participants withdrew for personal reasons related to health, supplement compliance, and lack of time. Dropout rates did not differ between the groups, and baseline characteristics were not significantly different between dropouts and completers. Medications used to promote recovery after TKA, such as muscle relaxants, could have influenced results, but it is important to note that the proportion of muscle relaxant users did not differ between the groups, and muscle relaxants did not significantly interact with treatment group to predict outcomes. Finally, the inconsistent EAA composition across all vials is a limitation that could have affected results.
The present findings extend our understanding of the potential beneficial effects of EAA use in patients managed with TKA. Future studies designed to identify mechanisms of action, durability of effect, and longer dosing regimens are needed. Our results suggest that EAA supplementation is potentially effective for helping older adult patients to recover from TKA surgery.
Note
The authors are grateful to Michelle Bremer and Anthony Paluso for study coordination; to Chelsey Policar, Katie Kowalski, Amanda Morris, Katia Krane, Eli Edwards, and Callie Porter for data collection; to Dr. Fred Sabb, Dr. Jolinda Smith, and Scott Watrous for MRI technical assistance; to Dr. Timothy Burnett for assistance with MIPAV; to Dr. Thomas Metz and Nancy Isern of the Pacific Northwest National Laboratory, Richland, Washington, for EAA analyses; to Dr. Lewis E. Kazis, ScD, Director, Center for the Assessment of Pharmaceutical Practices, Boston University School of Public Health, for permission to use the VR-12 health survey in this and ongoing trials at Slocum.
Footnotes
Investigation performed at the Department of Human Physiology, University of Oregon, Eugene; Oregon Research Institute, Eugene; Slocum Research and Education Foundation, Eugene; Oregon Health & Science University, Portland; and Slocum Center for Orthopedics and Sports Medicine, Eugene, Oregon.
Disclosure: The study was funded by the National Institute on Aging (AG046401), which did not play a role in the investigation. The Disclosure of Potential Conflicts of Interest forms are provided with the online version of the article (http://links.lww.com/JBJSOA/A46).
Disclaimer: The content of this manuscript is solely the responsibility of the authors and does not represent the official views of the funders.
References
- 1.Lorentzen JS, Petersen MM, Brot C, Madsen OR. Early changes in muscle strength after total knee arthroplasty. A 6-month follow-up of 30 knees. Acta Orthop Scand. 1999. April;70(2):176-9. [DOI] [PubMed] [Google Scholar]
- 2.Mizner RL, Snyder-Mackler L. Altered loading during walking and sit-to-stand is affected by quadriceps weakness after total knee arthroplasty. J Orthop Res. 2005. September;23(5):1083-90. Epub 2005 Mar 28. [DOI] [PubMed] [Google Scholar]
- 3.Stevens JE, Mizner RL, Snyder-Mackler L. Quadriceps strength and volitional activation before and after total knee arthroplasty for osteoarthritis. J Orthop Res. 2003. September;21(5):775-9. [DOI] [PubMed] [Google Scholar]
- 4.Moxley Scarborough D, Krebs DE, Harris BA. Quadriceps muscle strength and dynamic stability in elderly persons. Gait Posture. 1999. September;10(1):10-20. [DOI] [PubMed] [Google Scholar]
- 5.Brown M, Sinacore DR, Host HH. The relationship of strength to function in the older adult. J Gerontol A Biol Sci Med Sci. 1995. November;50(Spec No):55-9. [DOI] [PubMed] [Google Scholar]
- 6.Mizner RL, Petterson SC, Snyder-Mackler L. Quadriceps strength and the time course of functional recovery after total knee arthroplasty. J Orthop Sports Phys Ther. 2005. July;35(7):424-36. [DOI] [PubMed] [Google Scholar]
- 7.Moreland JD, Richardson JA, Goldsmith CH, Clase CM. Muscle weakness and falls in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2004. July;52(7):1121-9. [DOI] [PubMed] [Google Scholar]
- 8.Dillon EL, Sheffield-Moore M, Paddon-Jones D, Gilkison C, Sanford AP, Casperson SL, Jiang J, Chinkes DL, Urban RJ. Amino acid supplementation increases lean body mass, basal muscle protein synthesis, and insulin-like growth factor-I expression in older women. J Clin Endocrinol Metab. 2009. May;94(5):1630-7. Epub 2009 Feb 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dreyer HC, Strycker LA, Senesac HA, Hocker AD, Smolkowski K, Shah SN, Jewett BA. Essential amino acid supplementation in patients following total knee arthroplasty. J Clin Invest. 2013. November;123(11):4654-66. Epub 2013 Oct 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dreyer HC, Drummond MJ, Pennings B, Fujita S, Glynn EL, Chinkes DL, Dhanani S, Volpi E, Rasmussen BB. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am J Physiol Endocrinol Metab. 2008. February;294(2):E392-400. Epub 2007 Dec 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tipton KD, Ferrando AA, Phillips SM, Doyle D, Jr, Wolfe RR. Postexercise net protein synthesis in human muscle from orally administered amino acids. Am J Physiol. 1999. April;276(4 Pt 1):E628-34. [DOI] [PubMed] [Google Scholar]
- 12.McAuliffe M, Lalonde F, McGarry D, Gandler W, Csaky K, Trus B. Medical imaging processing, analysis and visualization in clinical research. In: Proceedings of the 14th IEEE Symposium on Computer-Based Medical Systems; 2001. July 26-27 Bethesda, MD: Institute of Electrical and Electronics Engineers; 2001. p 381-6. [Google Scholar]
- 13.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998. February;17(1):87-97. [DOI] [PubMed] [Google Scholar]
- 14.Collins NJ, Misra D, Felson DT, Crossley KM, Roos EM. Measures of knee function: International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, Knee Injury and Osteoarthritis Outcome Score (KOOS), Knee Injury and Osteoarthritis Outcome Score Physical Function Short Form (KOOS-PS), Knee Outcome Survey Activities of Daily Living Scale (KOS-ADL), Lysholm Knee Scoring Scale, Oxford Knee Score (OKS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Activity Rating Scale (ARS), and Tegner Activity Score (TAS). Arthritis Care Res (Hoboken). 2011. November;63(Suppl 11):S208-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins NJ, Roos EM. Patient-reported outcomes for total hip and knee arthroplasty: commonly used instruments and attributes of a “good” measure. Clin Geriatr Med. 2012. August;28(3):367-94. Epub 2012 Jun 22. [DOI] [PubMed] [Google Scholar]
- 16.Jones D, Kazis L, Lee A, Rogers W, Skinner K, Cassar L, Wilson N, Hendricks A. Health status assessments using the Veterans SF-12 and SF-36: methods for evaluating otucomes in the Veterans Health Administration. J Ambul Care Manage. 2001. July;24(3):68-86. [DOI] [PubMed] [Google Scholar]
- 17.Kazis LE, Miller DR, Skinner KM, Lee A, Ren XS, Clark JA, Rogers WH, Spiro A, 3rd, Selim A, Linzer M, Payne SM, Mansell D, Fincke RG. Patient-reported measures of health: the Veterans Health Study. J Ambul Care Manage. 2004. Jan-Mar;27(1):70-83. [DOI] [PubMed] [Google Scholar]
- 18.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001. September;16(9):606-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary care evaluation of mental disorders. Patient health questionnaire. JAMA. 1999. November 10;282(18):1737-44. [DOI] [PubMed] [Google Scholar]
- 20.Greenland S, Senn SJ, Rothman KJ, Carlin JB, Poole C, Goodman SN, Altman DG. Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. Eur J Epidemiol. 2016. April;31(4):337-50. Epub 2016 May 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lunn TH, Kristensen BB, Gaarn-Larsen L, Kehlet H. Possible effects of mobilisation on acute post-operative pain and nociceptive function after total knee arthroplasty. Acta Anaesthesiol Scand. 2012. November;56(10):1234-40. Epub 2012 Aug 10. [DOI] [PubMed] [Google Scholar]
- 22.Pua YH, Ong PH. Association of early ambulation with length of stay and costs in total knee arthroplasty: retrospective cohort study. Am J Phys Med Rehabil. 2014. November;93(11):962-70. [DOI] [PubMed] [Google Scholar]
- 23.Husted H, Jørgensen CC, Gromov K, Troelsen A; Collaborative Group of the Lundbeck Foundation Center for Fast-Track Hip and Knee Replacement. Low manipulation prevalence following fast-track total knee arthroplasty. Acta Orthop. 2015. February;86(1):86-91. Epub 2014 Sep 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy CH, Saddler NI, Devries MC, McGlory C, Baker SK, Phillips SM. Leucine supplementation enhances integrative myofibrillar protein synthesis in free-living older men consuming lower- and higher-protein diets: a parallel-group crossover study. Am J Clin Nutr. 2016. December;104(6):1594-606. Epub 2016 Nov 9. [DOI] [PubMed] [Google Scholar]
- 25.Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol. 1997. July;273(1 Pt 1):E122-9. [DOI] [PubMed] [Google Scholar]
- 26.Witard OC, Wardle SL, Macnaughton LS, Hodgson AB, Tipton KD. Protein considerations for optimising skeletal muscle mass in healthy young and older adults. Nutrients. 2016. March 23;8(4):181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore DR, Churchward-Venne TA, Witard O, Breen L, Burd NA, Tipton KD, Phillips SM. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci. 2015. January;70(1):57-62. Epub 2014 Jul 23. [DOI] [PubMed] [Google Scholar]
- 28.Dickinson JM, Gundermann DM, Walker DK, Reidy PT, Borack MS, Drummond MJ, Arora M, Volpi E, Rasmussen BB. Leucine-enriched amino acid ingestion after resistance exercise prolongs myofibrillar protein synthesis and amino acid transporter expression in older men. J Nutr. 2014. November;144(11):1694-702. Epub 2014 Sep 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jordan LY, Melanson EL, Melby CL, Hickey MS, Miller BF. Nitrogen balance in older individuals in energy balance depends on timing of protein intake. J Gerontol A Biol Sci Med Sci. 2010. October;65(10):1068-76. Epub 2010 Jul 9. [DOI] [PMC free article] [PubMed] [Google Scholar]