Abstract
Background:
Reported information on the characteristics of benign bone tumors is disjointed, and the long-term trends in the occurrence of malignant bone tumors by histological type have not been reported in Japan. Our aim was to describe the characteristics of both benign and malignant bone tumors as described in cases registered in the Hiroshima Tumor Tissue Registry from 1973 to 2012.
Methods:
Cases were identified with the International Classification of Diseases for Oncology (ICD-O-3) topography code C40-C41 (bones, joints, and articular cartilage), and histological types were classified according to the World Health Organization 2013 system. We described the distribution of the cases by behavior, sex, skeletal site of tumor occurrence, histological type, period at diagnosis (in 10-year groups), and age at diagnosis (in 10-year groups).
Results:
We observed 2,542 benign bone tumors, 272 intermediate bone tumors, and 506 malignant bone tumors. We confirmed that 81.6% of benign bone tumors were chondrogenic, consisting primarily of osteochondromas and enchondromas. Giant cell tumor of bone was the most dominant type of intermediate tumor, whereas osteogenic tumors and chondrogenic tumors were the most dominant types of malignant tumors. Among malignant bone tumors, 41.7% of tumors occurred in the long bones of the lower limb, and there were different peaks of age at the time of diagnosis for osteogenic tumors and chondrogenic tumors. A similar distribution of histological types was seen throughout the 40-year observation period.
Conclusions:
Osteochondroma and enchondroma differed in terms of the age of the patient at the time of diagnosis and the skeletal sites where the tumors most frequently occurred. Giant cell tumor had a large impact on occurrence as a common type of intermediate bone tumor.
Clinical Relevance:
The results of the present study, based on pathological tissue registry data, provide knowledge about the epidemiological and pathological features of bone tumors in Japan.
Benign and malignant bone tumors are relatively rare. Benign bone tumors comprise a wide variety of different histological types1, occur most frequently between the ages 5 and 25 years, and can occur in any part of the skeletal system2. They usually do not affect life expectancy, but several histological types of intermediate bone tumors such as osteochondromatosis and giant cell tumor of bone have a risk of malignancy. The information on the characteristics of benign bone tumors that has been reported to date is disjointed, and a long-term trend of benign bone tumors by histological type has not been reported in Japan. The Swedish Cancer Registry reported a longitudinal incidence rate of giant cell tumor3. Conversely, the characteristics of malignant bone tumors are well known4-6. Such tumors occur frequently in teenagers and constitute 3% to 4% of childhood cancer in Hiroshima and all of Japan7,8. Recently, an increased proportion of elderly patients with osteosarcoma has been reported in Japan6.
The goal of the present study was to describe the characteristics of bone tumors according to behavior and histological type on the basis of surgical resection and biopsy procedures that were registered in the Hiroshima Tumor Tissue Registry from 1973 to 2012.
Materials and Methods
The Hiroshima Tumor Tissue Registry is a population-based pathological tissue registry that was initiated in 1973 by the Hiroshima Prefecture Medical Association with the cooperation of the Radiation Effects Research Foundation. It encompasses the entire Hiroshima Prefecture (population in 2012, 2.86 million, representing 2.6% of the Japanese population) in Japan. Pathologists voluntarily report the pathological diagnosis of all tumors and provide sample slides of resected malignant tumors, including biopsy specimens. When there are multiple tumor records for the same patient, such as biopsy and surgery records, the pathologist panels decide whether the records are reports of the same primary tumor or of multiple primary tumors by examining pathological reports or by performing a histological examination, if necessary. All tumors are coded with use of the first, second, or third revision of the International Classification of Diseases for Oncology (ICD-O). The Hiroshima Tumor Tissue Registry has provided all data on malignant tumors to the Hiroshima Prefecture Cancer Registry, which is a population-based cancer registry, and the Hiroshima Tumor Tissue Registry has contributed to improving the Hiroshima Prefecture Cancer Registry data quality since its inception in 2002. Details have been described elsewhere9.
The subjects in the present study were patients who were diagnosed with primary bone tumors of all types that were registered in the Hiroshima Tumor Tissue Registry from 1973 to 2012. Cases were identified with the ICD-O-3 topography code C40-C41 (bones, joints, and articular cartilage) and were assigned as bone tumors according to the World Health Organization (WHO) 2013 classification system10. Hematopoietic tumors, including malignant lymphoma, were excluded. Histological types were classified according to the WHO 2013 system10 (Table I). When there was uncertainty about whether the behavior was benign or malignant (ICD-O-3 behavior code, 1), as in cases of osteochondromatosis and giant cell tumor, the tumor was classified as intermediate according to the WHO 2013 system. Malignant fibrous histiocytoma has been classified as a miscellaneous tumor since 2013; however, we classified it as a fibrohistiocytic tumor in the present study. We described the number, registration rate (per million population, standardized by age-class of the Segi’s World Standard Population), and proportion of bone tumor cases by behavior, sex, skeletal site of tumor occurrence, histological type, period at diagnosis (in 10-year groups), and age of diagnosis (in 10-year groups). Bone tumor skeletal sites were classified according to the ICD-O-3 topography code. The present study was approved by the Data Usage Committee of the Hiroshima Tumor Tissue Registry.
TABLE I.
Number of Bone Tumor Cases by Behavior and Histological Type as Recorded in Hiroshima Tumor Tissue Registry, 1973-2012*
| Histological Group† | Benign | Intermediate | Malignant | ||||||
| ICD-O-3-M | Morphology | No. of Cases | ICD-O-3-M | Morphology | No. of Cases | ICD-O-3-M | Morphology | No. of Cases | |
| 1. Chondrogenic tumors | |||||||||
| 9210/0 | Osteochondroma | 1,057 | 9210/1 | Osteochondromatosis, NOS | 40 | 9220/3 | Chondrosarcoma, NOS; Conventional chondrosarcoma (grade 2, grade 3); Secondary chondrosarcoma | 124 | |
| 9220/0 | Chondroma, NOS; enchondroma; synovial chondromatosis | 952 | 9220/1 | Multiple chondromatosis | 3 | 9221/3 | Periosteal chondrosarcoma (juxtacortical chondrosarcoma) | 1 | |
| 9221/0 | Periosteal chondroma | 19 | 9231/3 | Myxoid chondrosarcoma | 3 | ||||
| 9230/0 | Chondroblastoma, NOS‡ | 23 | 9243/3 | Dedifferentiated chondrosarcoma | 2 | ||||
| 9241/0 | Chondromyxoid fibroma | 23 | 9240/3 | Mesenchymal chondrosarcoma | 4 | ||||
| 2. Osteogenic tumors | 9242/3 | Clear cell chondrosarcoma | 1 | ||||||
| 9180/0 | Osteoma, NOS | 254 | 9200/1 | Aggressive osteoblastoma | 1 | 9180/3 | Osteosarcoma | 176 | |
| 9191/0 | Osteoid osteoma | 83 | Osteoblastic osteosarcoma | ||||||
| 9200/0 | Osteoblastoma | 18 | 9181/3 | Chondroblastic osteosarcoma | 7 | ||||
| 9182/3 | Fibroblastic osteosarcoma | 4 | |||||||
| 9183/3 | Telangiectatic osteosarcoma | 6 | |||||||
| 9185/3 | Small cell osteosarcoma (round-cell osteosarcoma) | 1 | |||||||
| 9192/3 | Parosteal osteosarcoma | 7 | |||||||
| 3. Fibrogenic tumors | |||||||||
| 8810/0 | Fibroma, NOS | 13 | 8821/1 | Aggressive fibromatosis | 1 | 8810/3 | Fibrosarcoma | 4 | |
| 8823/0 | Desmoplastic fibroma | 2 | |||||||
| 4. Fibrohistiocytic tumors | |||||||||
| 8830/0 | Benign fibrous histiocytoma/non-ossifying fibroma (metaphyseal fibrous defect) | 10 | 8823/1 | Desmoplastic fibroma of bone | 7 | 8830/3 | Malignant fibrous histiocytoma | 33 | |
| 8831/0 | Histiocytoma, NOS | 2 | 8830/1 | Atypical fibrous histiocytoma | 3 | ||||
| 5. Hematopoietic tumors | |||||||||
| 6. Osteoclastic giant cell rich tumors | 9250/1 | Giant cell tumor of bone | 212 | 9250/3 | Malignancy in giant cell tumor of bone | 14 | |||
| 7. Notochordal tumors | |||||||||
| 9370/3 | Chordoma | 43 | |||||||
| 9371/3 | Chondroid chordoma | 4 | |||||||
| 8. Vascular tumors | |||||||||
| 9120/0 | Hemangioma, NOS | 16 | 9130/1 | Hemangioendothelioma, NOS | 2 | 9120/3 | Angiosarcoma | 7 | |
| 9121/0 | Cavernous hemangioma | 14 | 9133/1 | Epithelioid hemangioendothelioma, NOS | 1 | 9130/3 | Hemangioendothelioma, malignant | 1 | |
| 9122/0 | Venous hemangioma | 2 | 9136/1 | Spindle cell hemangioendothelioma | 1 | ||||
| 9131/0 | Capillary hemangioma | 6 | |||||||
| 9. Myogenic tumors | 8890/0 | Leiomyoma of bone | 1 | 8890/3 | Leiomyosarcoma of bone | 4 | |||
| 10. Lipogenic tumors | |||||||||
| 8850/0 | Lipoma of bone | 11 | |||||||
| 8861/0 | Angiolipoma, NOS | 1 | |||||||
| 11. Tumors of undefined neoplastic nature | |||||||||
| 8811/0 | Fibromyxoma | 2 | 9040/3 | Synovial sarcoma, NOS | 7 | ||||
| 8840/0 | Myxoma, NOS | 1 | 9041/3 | Synovial sarcoma, spindle cell | 1 | ||||
| 9262/0 | Ossifying fibroma | 28 | 9043/3 | Synovial sarcoma, biphasic | 1 | ||||
| 12. Miscellaneous tumors | |||||||||
| 8711/0 | Glomus tumor, NOS | 1 | 9150/1 | Hemangiopericytoma, NOS | 1 | 8800/3 | Sarcoma, NOS | 21 | |
| 8800/0 | Soft tissue tumor, benign | 1 | 8801/3 | Spindle cell sarcoma | 1 | ||||
| 8824/0 | Myofibroma | 1 | 9260/3 | Ewing sarcoma | 25 | ||||
| 9530/0 | Meningioma, NOS | 1 | 9261/3 | Adamantinoma of long bones | 4 | ||||
| 13. Other rare tumors | |||||||||
| 14. Unclassified tumors | |||||||||
| Total | 2,542 | 272 | 506 | ||||||
ICD-O-3-M = International Classification of Diseases for Oncology morphology code, NOS = not otherwise specified.
The histological group was based on the WHO 2013 classification.
Chondroblastoma is regarded as demonstrating intermediate behavior according to the WHO 2013 classification; however, it is included as a benign tumor here because its ICD-O-3 behavior code is 0.
Results
Benign Bone Tumors
We observed 2,542 benign bone tumors over a period of 40 years. Most benign tumors occurred in 3 sites, such as short bones of the upper limb (33.8%), long bones of the lower limb (26.8%), and short bones of the lower limb (14.3%) (Table II). The registration rates for benign bone tumors at the first period were 18.4 for males and 14.8 for females; thereafter, the rates increased and became stable around 28 for males and around 26 for females (Table III).
TABLE II.
Number of Bone Tumor Cases by Behavior, Sex, and Skeletal Site as Recorded in Hiroshima Tumor Tissue Registry, 1973-2012*
| Skeletal Sites (ICD-O-3-T) | Benign | Intermediate | Malignant | ||||||
| Male | Female | Total | Male | Female | Total | Male | Female | Total | |
| C40.0: Long bones of upper limb, scapula | 98 (7.8%) | 66 (5.1%) | 164 (6.5%) | 13 (8.9%) | 10 (7.9%) | 23 (8.5%) | 22 (7.7%) | 16 (7.3%) | 38 (7.5%) |
| C40.1: Short bones of upper limb | 387 (30.7%) | 473 (36.9%) | 860 (33.8%) | 3 (2.1%) | 10 (7.9%) | 13 (4.8%) | 2 (0.7%) | 3 (1.4%) | 5 (1.0%) |
| C40.2: Long bones of lower limb | 369 (29.3%) | 312 (24.3%) | 681 (26.8%) | 95 (65.1%) | 69 (54.8%) | 164 (60.3%) | 126 (43.9%) | 85 (38.8%) | 211 (41.7%) |
| C40.3: Short bones of lower limb | 192 (15.3%) | 171 (13.3%) | 363 (14.3%) | 8 (5.5%) | 7 (5.6%) | 15 (5.5%) | 5 (1.7%) | 4 (1.8%) | 9 (1.8%) |
| C40.9: Bone of limb, NOS* | 6 (0.5%) | 6 (0.5%) | 12 (0.5%) | 1 (0.7%) | 2 (1.6%) | 3 (1.1%) | 1 (0.3%) | 1 (0.5%) | 2 (0.4%) |
| C41.0: Bones of skull and face | 84 (6.7%) | 137 (10.7%) | 221 (8.7%) | 2 (1.4%) | 3 (2.4%) | 5 (1.8%) | 29 (10.1%) | 25 (11.4%) | 54 (10.7%) |
| C41.1: Mandible | 19 (1.5%) | 54 (4.2%) | 73 (2.9%) | 0 (0%) | 2 (1.6%) | 2 (0.7%) | 12 (4.2%) | 15 (6.8%) | 27 (5.3%) |
| C41.2: Vertebral column | 29 (2.3%) | 21 (1.6%) | 50 (2.0%) | 4 (2.7%) | 5 (4.0%) | 9 (3.3%) | 18 (6.3%) | 14 (6.4%) | 32 (6.3%) |
| C41.3: Rib, sternum, and clavicle | 39 (3.1%) | 29 (2.3%) | 68 (2.7%) | 3 (2.1%) | 6 (4.8%) | 9 (3.3%) | 17 (5.9%) | 22 (10.0%) | 39 (7.7%) |
| C41.4: Pelvic bones, sacrum, and coccyx | 29 (2.3%) | 11 (0.9%) | 40 (1.6%) | 7 (4.8%) | 10 (7.9%) | 17 (6.3%) | 48 (16.7%) | 34 (15.5%) | 82 (16.2%) |
| C41.9: Bone, NOS | 7 (0.6%) | 3 (0.2%) | 10 (0.4%) | 10 (6.8%) | 2 (1.6%) | 12 (4.4%) | 7 (2.4%) | 0 (0%) | 7 (1.4%) |
| Total | 1,259 (100%) | 1,283 (100%) | 2,542 (100%) | 146 (100%) | 126 (100%) | 272 (100%) | 287 (100%) | 219 (100%) | 506 (100%) |
All skeletal sites include associated joints except for “mandible” and “vertebral column.” The values are given as the number of tumors, with the percentage in parentheses. ICD-O-3-T = International Classification of Diseases for Oncology topography code, and NOS = not otherwise specified.
TABLE III.
Number and Registration Rates of Bone Tumors by Behavior, Histological Type, Sex, and Period at Diagnosis as Recorded in the Hiroshima Tumor Tissue Registry, 1973-2012*
| Male | Female | Total | |||||||||||||||||||
| 1973-1982 | 1983-1992 | 1993-2002 | 2003-2012 | 1973-2012 | 1973-1982 | 1983-1992 | 1993-2002 | 2003-2012 | 1973-2012 | 1973-2012 | |||||||||||
| Behavior and Histological Type | No. of Tumors | Rate | No. of Tumors | Rate | No. of Tumors | Rate | No. of Tumors | Rate | No. of Tumors | Rate | No. of Tumors | Rate | No. of Tumors | Rate | No. of Tumors | Rate | No. of Tumors | Rate | No. of Tumors | Rate | No. of Tumors |
| Benign | |||||||||||||||||||||
| 1. Chondrogenic tumors | 198 (85.0%) | 15.7 | 323 (86.6%) | 24.4 | 283 (82.7%) | 24.1 | 252 (81.0%) | 24.0 | 1,056 (83.9%) | 21.7 | 159 (81.1%) | 12.2 | 295 (81.9%) | 20.8 | 298 (78.4%) | 22.3 | 266 (76.7%) | 21.4 | 1,018 (79.3%) | 19.1 | 2,074 (81.6%) |
| 2. Osteogenic tumors | 24 (10.3%) | 1.9 | 38 (10.2%) | 2.7 | 46 (13.5%) | 3.4 | 44 (14.1%) | 3.9 | 152 (12.1%) | 3.0 | 24 (12.2%) | 1.7 | 45 (12.5%) | 2.9 | 64 (16.8%) | 3.5 | 70 (20.2%) | 4.2 | 203 (15.8%) | 3.1 | 355 (14.0%) |
| 3. Fibrogenic tumors | 1 (0.4%) | 0.1 | 3 (0.8%) | 0.2 | 4 (1.2%) | 0.4 | 1 (0.3%) | 0.1 | 9 (0.7%) | 0.2 | 1 (0.5%) | 0.1 | 1 (0.3%) | 0.1 | 2 (0.5%) | 0.1 | 2 (0.6%) | 0.1 | 6 (0.5%) | 0.1 | 15 (0.6%) |
| 4. Fibrohistiocytic tumors | 1 (0.4%) | 0.1 | 1 (0.3%) | 0.1 | 2 (0.2%) | 0.0 | 2 (1.0%) | 0.2 | 4 (1.1%) | 0.3 | 3 (0.8%) | 0.2 | 1 (0.3%) | 0.0 | 10 (0.8%) | 0.2 | 12 (0.5%) | ||||
| 8. Vascular tumors | 5 (2.1%) | 0.3 | 3 (0.8%) | 0.2 | 4 (1.2%) | 0.2 | 7 (2.3%) | 0.4 | 19 (1.5%) | 0.3 | 3 (1.5%) | 0.2 | 7 (1.9%) | 0.4 | 4 (1.1%) | 0.2 | 5 (1.4%) | 0.2 | 19 (1.5%) | 0.3 | 38 (1.5%) |
| 9. Myogenic tumors | 1 (0.3%) | 0.2 | 1 (0.1%) | 0.0 | 1 (0.0%) | ||||||||||||||||
| 10. Lipogenic tumors | 1 (0.4%) | 0.1 | 1 (0.3%) | 0.1 | 2 (0.6%) | 0.1 | 1 (0.3%) | 0.1 | 5 (0.4%) | 0.1 | 1 (0.5%) | 0.1 | 1 (0.3%) | 0.1 | 4 (1.1%) | 0.2 | 1 (0.3%) | 0.0 | 7 (0.5%) | 0.1 | 12 (0.5%) |
| 11. Tumors of undefined neoplastic nature | 3 (1.3%) | 0.2 | 4 (1.1%) | 0.3 | 1 (0.3%) | 0.1 | 3 (1.0%) | 0.2 | 11 (0.9%) | 0.2 | 6 (3.1%) | 0.4 | 7 (1.9%) | 0.6 | 5 (1.3%) | 0.3 | 2 (0.6%) | 0.1 | 20 (1.6%) | 0.4 | 31 (1.2%) |
| 12. Miscellaneous tumors | 2 (0.6%) | 0.1 | 2 (0.6%) | 0.2 | 4 (0.3%) | 0.1 | 4 (0.2%) | ||||||||||||||
| Subtotal | 233 (100%) | 18.4 | 373 (100%) | 27.9 | 342 (100%) | 28.5 | 311 (100%) | 29.1 | 1,259 (100%) | 25.6 | 196 (100%) | 14.8 | 360 (100%) | 25.1 | 380 (100%) | 26.9 | 347 (100%) | 26.2 | 1,283 (100%) | 23.3 | 2,542 (100%) |
| Intermediate | |||||||||||||||||||||
| 1. Chondrogenic tumors | 1 (2.6%) | 0.1 | 5 (20.0%) | 0.5 | 13 (33.3%) | 1.4 | 5 (11.4%) | 0.6 | 24 (16.4%) | 0.6 | 4 (16.0%) | 0.3 | 10 (27.8%) | 0.9 | 5 (14.7%) | 0.5 | 19 (15.1%) | 0.4 | 43 (15.8%) | ||
| 2. Osteogenic tumors | 1 (2.3%) | 0.0 | 1 (0.7%) | 0.0 | 1 (0.4%) | ||||||||||||||||
| 3. Fibrogenic tumors | 1 (2.6%) | 0.1 | 1 (0.7%) | 0.0 | 1 (0.4%) | ||||||||||||||||
| 4. Fibrohistiocytic tumors | 1 (2.6%) | 0.1 | 3 (12.0%) | 0.2 | 1 (2.6%) | 0.0 | 5 (3.4%) | 0.1 | 4 (16.0%) | 0.3 | 1 (2.8%) | 0.1 | 5 (4.0%) | 0.1 | 10 (3.7%) | ||||||
| 6. Osteoclastic giant cell rich tumors | 35 (92.1%) | 2.7 | 17 (68.0%) | 1.3 | 22 (56.4%) | 1.5 | 38 (86.4%) | 2.8 | 112 (76.7%) | 2.1 | 31 (100%) | 2.2 | 17 (68.0%) | 1.1 | 24 (66.7%) | 1.7 | 28 (82.4%) | 2.1 | 100 (79.4%) | 1.8 | 212 (77.9%) |
| 8. Vascular tumors | 2 (5.1%) | 0.2 | 2 (1.4%) | 0.0 | 1 (2.8%) | 0.1 | 1 (2.9%) | 0.1 | 2 (1.6%) | 0.0 | 4 (1.5%) | ||||||||||
| 12. Miscellaneous tumors | 1 (2.6%) | 0.1 | 1 (0.7%) | 0.0 | 1 (0.4%) | ||||||||||||||||
| Subtotal | 38 (100%) | 2.9 | 25 (100%) | 1.9 | 39 (100%) | 3.3 | 44 (100%) | 3.5 | 146 (100%) | 2.9 | 31 (100%) | 2.2 | 25 (100%) | 1.7 | 36 (100%) | 2.7 | 34 (100%) | 2.7 | 126 (100%) | 2.3 | 272 (100%) |
| Malignant | |||||||||||||||||||||
| 1. Chondrogenic tumors | 6 (13.3%) | 0.4 | 27 (31.8%) | 1.6 | 17 (23.3%) | 0.9 | 22 (26.2%) | 1.0 | 72 (25.1%) | 1.1 | 16 (36.4%) | 1.1 | 16 (34.0%) | 0.9 | 13 (25.0%) | 0.6 | 18 (23.7%) | 0.9 | 63 (28.8%) | 0.9 | 135 (26.7%) |
| 2. Osteogenic tumors | 23 (51.1%) | 1.9 | 33 (38.8%) | 2.5 | 31 (42.5%) | 2.3 | 27 (32.1%) | 2.5 | 114 (39.7%) | 2.3 | 13 (29.5%) | 0.9 | 20 (42.6%) | 1.4 | 26 (50.0%) | 1.8 | 28 (36.8%) | 2.6 | 87 (39.7%) | 1.6 | 201 (39.7%) |
| 3. Fibrogenic tumors | 1 (1.2%) | 0.1 | 1 (1.4%) | 0.0 | 2 (0.7%) | 0.0 | 1 (2.3%) | 0.1 | 1 (1.3%) | 0.0 | 2 (0.9%) | 0.0 | 4 (0.8%) | ||||||||
| 4. Fibrohistiocytic tumors | 2 (4.4%) | 0.2 | 2 (2.4%) | 0.1 | 12 (16.4%) | 0.6 | 7 (8.3%) | 0.2 | 23 (8.0%) | 0.3 | 2 (4.5%) | 0.1 | 2 (3.8%) | 0.1 | 6 (7.9%) | 0.3 | 10 (4.6%) | 0.1 | 33 (6.5%) | ||
| 6. Osteoclastic giant cell rich tumors | 2 (4.4%) | 0.1 | 6 (7.1%) | 0.4 | 8 (2.8%) | 0.1 | 2 (4.5%) | 0.1 | 4 (8.5%) | 0.2 | 6 (2.7%) | 0.1 | 14 (2.8%) | ||||||||
| 7. Notochordal tumors | 4 (8.9%) | 0.3 | 7 (8.2%) | 0.4 | 5 (6.8%) | 0.2 | 14 (16.7%) | 0.5 | 30 (10.5%) | 0.3 | 3 (6.8%) | 0.2 | 1 (1.9%) | 0.0 | 13 (17.1%) | 0.7 | 17 (7.8%) | 0.2 | 47 (9.3%) | ||
| 8. Vascular tumors | 3 (3.5%) | 0.1 | 1 (1.2%) | 0.0 | 4 (1.4%) | 0.0 | 2 (3.8%) | 0.1 | 2 (2.6%) | 0.1 | 4 (1.8%) | 0.0 | 8 (1.6%) | ||||||||
| 9. Myogenic tumors | 1 (1.4%) | 0.1 | 1 (0.3%) | 0.0 | 3 (3.9%) | 0.1 | 3 (1.4%) | 0.0 | 4 (0.8%) | ||||||||||||
| 11. Tumors of undefined neoplastic nature | 3 (6.7%) | 0.2 | 2 (2.4%) | 0.1 | 5 (1.7%) | 0.1 | 2 (4.5%) | 0.1 | 2 (3.8%) | 0.1 | 4 (1.8%) | 0.1 | 9 (1.8%) | ||||||||
| 12. Miscellaneous tumors | 5 (11.1%) | 0.4 | 4 (4.7%) | 0.3 | 6 (8.2%) | 0.4 | 13 (15.5%) | 1.2 | 28 (9.8%) | 0.5 | 5 (11.4%) | 0.4 | 7 (14.9%) | 0.6 | 6 (11.5%) | 0.5 | 5 (6.6%) | 0.2 | 23 (10.5%) | 0.4 | 51 (10.1%) |
| Subtotal | 45 (100%) | 3.5 | 85 (100%) | 5.6 | 73 (100%) | 4.6 | 84 (100%) | 5.5 | 287 (100%) | 4.9 | 44 (100%) | 3.0 | 47 (100%) | 3.1 | 52 (100%) | 3.2 | 76 (100%) | 4.8 | 219 (100%) | 3.5 | 506 (100%) |
| Total | 316 | 483 | 454 | 439 | 1,692 | 271 | 432 | 468 | 457 | 1,628 | 3,320 | ||||||||||
The values in the “Rate” columns indicate the age-standardized registration rate per million population according to the Segi’s World Standard Population. Patients whose age was unknown were excluded.
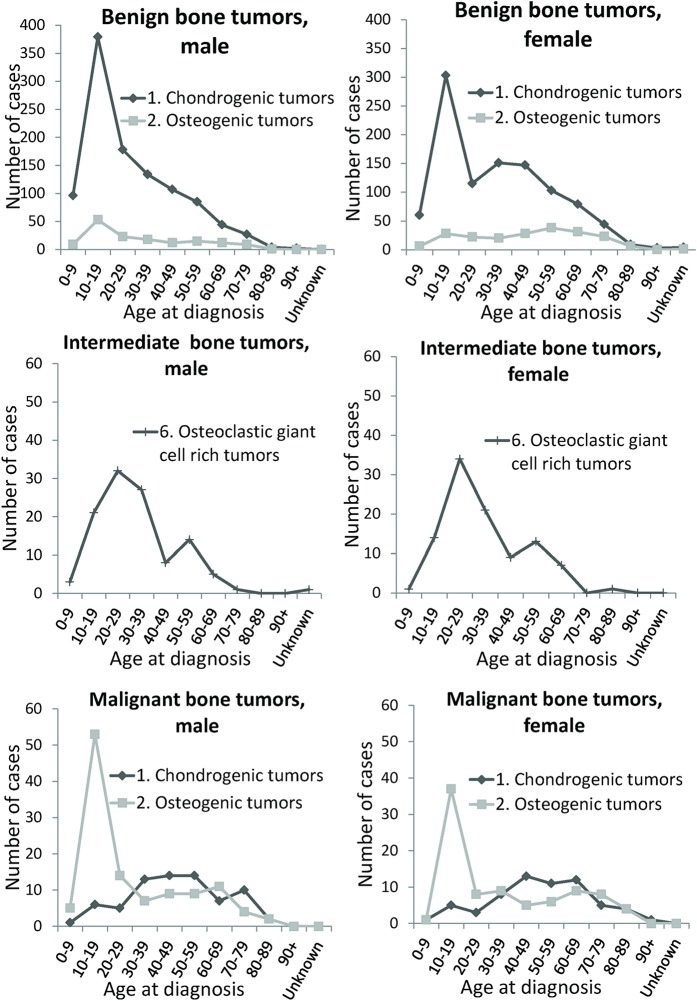
Among the benign tumors, the most common histological type was chondrogenic tumors (2,074; 81.6%) and the distribution of histological types was uniform and similar for both sexes for the entire observation period (Table III). A primary peak for benign chondrogenic tumors occurred in patients 10 to 19 years of age and then rapidly decreased. Thereafter, the number of patients >20 years of age gradually decreased among men, whereas a secondary peak in patients 30 to 49 years of age was observed in women (Fig. 1).
Fig. 1.
Line graphs illustrating the age distribution for major histological groups of bone tumors by sex and behavior as recorded in the Hiroshima Tumor Tissue Registry between 1973 and 2012.
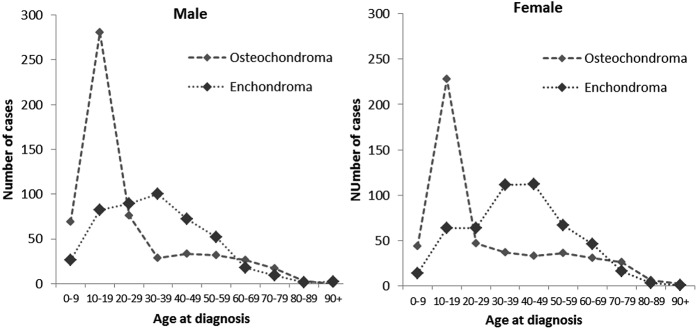
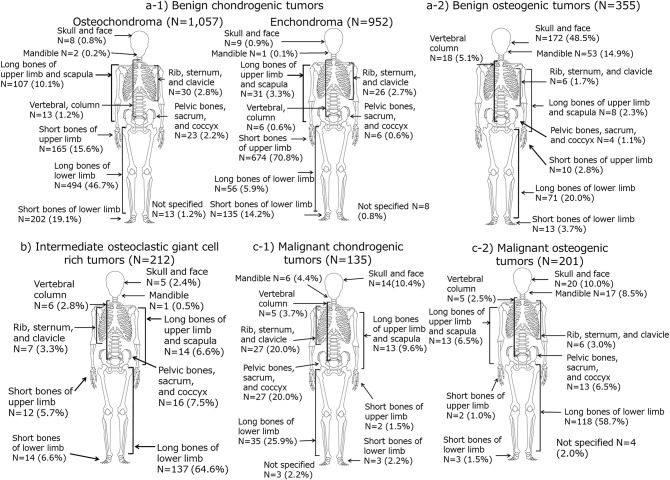
Among benign chondrogenic tumors, there were 1,057 osteochondromas (51.0%, ICD-O-3-M (morphology code)/behavior: 9210/0) and 952 enchondromas (45.9%, ICD-O-3-M/behavior: 9220/0). The number of osteochondromas peaked in teenagers for both sexes, whereas the number of enchondromas peaked in patients 30 to 49 years of age for both sexes (Fig. 2). Therefore, a secondary peak of benign chondrogenic tumors in patients 30 to 49 years of age among women was due to the peak of enchondroma. The peak of enchondroma in patients 30 to 49 years of age among women was enhanced compared with men, because the peak of osteochondroma in teenagers among females was relatively lower than that among males. Regarding the skeletal sites, osteochondromas occurred frequently in the lower limbs (long bones, 46.7%; short bones, 19.1%), whereas enchondromas occurred frequently in the short bones of the upper limbs (70.8%) (Fig. 3). Tumor distributions by skeletal site for osteochondroma and enchondroma were similar for both sexes.
Fig. 2.
Line graphs illustrating the age distribution for osteochondroma and enchondroma in patients with benign chondrogenic tumors as recorded in the Hiroshima Tumor Tissue Registry between 1973 and 2012.
Fig. 3.
Diagrams illustrating the skeletal site distribution for bone tumors by behavior and histological group as recorded in the Hiroshima Tumor Tissue Registry between 1973 and 2012.
Benign osteogenic tumors were the second most common benign tumor (355; 14.0%) (Table III) and occurred in the skull and face (48.5%) as well as mandible (14.9%) (Fig. 3). The number of patients with osteogenic tumors peaked at 10 to 19 years of age for both males and females, with an apparent secondary peak at 50 to 59 years of age among women (Fig. 1).
Intermediate Bone Tumors
We observed 272 intermediate bone tumors, including 212 giant cell tumors (77.9%) and 43 chondrogenic tumors (osteochondromatosis) (15.8%). The registration rates for intermediate bone tumors were 2.9 for males and 2.3 for females and remained stable over the observation periods (Table III). A primary peak of giant cell tumor was seen in patients 20 to 29 years of age, whereas a secondary peak was observed in patients 50 to 59 years of age (Fig. 1). Among the giant cell tumors, 64.6% of cases occurred in the long bones of the lower limb (Fig. 3).
Malignant Bone Tumors
We observed 506 malignant bone tumors over a period of 40 years. The registration rates from the first to the fourth decade were 3.5, 5.6, 4.6, and 5.5 for males and 3.0, 3.1, 3.2, and 4.8 for females, respectively (Table III). Malignant bone tumors occurred in the long bones of the lower limb (41.7%); in the pelvis, sacrum, and coccyx (16.2%); in the skull and face (10.7%); and in the rib, sternum, and clavicle (7.7%) (Table II). The numbers of malignant bone tumors were 287 for males and 219 for females, and the male:female ratio was 1.31:1. A primary peak in malignant bone tumors occurred in patients 10 to 19 years of age and then rapidly decreased. A plateau was observed in patients 20 to 50 years of age, with a secondary peak observed for patients 60 to 69 years of age and a decline observed in elderly individuals.
Tumor distributions by age were similar for both sexes. Among malignant bone tumors, the most frequent tumor types were osteogenic tumors (201; 39.7%) and chondrogenic tumors (135; 26.7%) (Table III). These 2 major histological groups covered approximately two-thirds of all malignant bone tumors. There was only a peak in teens for malignant osteogenic tumors, which occurred in the long bones of the lower limb (58.7%) (Figs. 1 and 3). There was a primary peak in teenagers and a secondary peak in middle-aged patients for malignant chondrogenic tumors, which were distributed in the long bones of lower limb (25.9%); in the bones of the trunk of the body such as the rib, sternum, and clavicle (20.0%); and around the pelvis (20.0%) (Figs. 1 and 3).
Time trends of the number of cases and registration rates were stable for entire period, particularly for female patients (Table III). However, in the last decade, the number of malignant cases increased because of an increase in the number of notochordal tumors in subjects aged 50 to 79 years.
Among the 51 miscellaneous tumors, 25 Ewing sarcomas were observed (14 in males and 11 in females) (Table I). Most cases of Ewing sarcoma occurred in children and adolescents (including 2 in patients 0 to 9 years of age and 13 in patients 10 to 19 years of age), and the other 10 cases occurred in patients 20 to 39 years of age.
Discussion
We presented the statistics—by sex, skeletal site, and histological type—on bone tumors of all behavior types that were diagnosed between 1973 and 2012. We confirmed that 81.6% of benign bone tumors were chondrogenic tumors, primarily consisting of osteochondromas and enchondromas. The most dominant types of malignant tumors were osteogenic tumors and chondrogenic tumors. Approximately 40% of the malignant bone tumors occurred in the long bones of the lower limb, with different age peaks at the time of diagnosis for each major histological type. A similar distribution of histological types was seen during the 40-year observation period. We observed only 25 cases of Ewing sarcoma over 40 years.
Few cancer registries routinely collect data on both benign and malignant tumors. The Bone and Soft Tissue Tumor (BSTT) Registry in Japan, which is a nationwide and organ-specific registry, collects data on both benign and malignant bone tumors6. A recent report from that registry11 demonstrated that 8,979 benign bone tumors were registered between 2006 and 2013. With the exclusion of lesions that were not recognized as benign bone tumors in the present study (i.e., giant cell tumors [1,187], neurilemmomas [51], neurofibromas [2], and tumors of an unknown histological type [150]), the number of benign bone tumors decreased to 7,589. Of those, the most dominant type was chondrogenic tumors (80.4%; 6,100 of 7,589) and the second most common type was osteogenic tumors (10.1%; 768 of 7,589). The distribution of histological types among benign tumor cases reported by the BSTT Registry was consistent with the benign tumors in the present study. The BSTT Registry also showed that a primary peak of osteochondroma occurred in teenagers and that a primary peak of enchondroma occurred among men 30 to 34 years of age and among women 35 to 44 years of age11. The age distributions of osteochondroma and enchondroma were in accordance with our results.
The Swedish Cancer Registry, the Bone Tumor Registry of Western Australia, and the BSTT Registry collect information on giant cell tumor, which is recognized as an intermediate tumor. Liede et al. reported that the giant cell tumor-to-osteosarcoma ratio, based on the data from the BSTT registry in Japan as well as the registries of Sweden and Mexico, ranged from 0.49 to 0.7912. The giant cell tumor-to-osteosarcoma ratio from the Hiroshima Tumor Tissue Registry was 1.05, which was slightly higher than that reported in other registries.
Osteochondromatosis has a risk of malignant transformation into secondary chondrosarcoma, particularly in cases with multiple exostoses13. We observed 43 cases of osteochondromatosis, which has been reported to have a probability of malignant transformation of 1% to 5% among all primary osteochondromas14,15. As the patients with osteochondromatosis in the Hiroshima Tumor Tissue Registry had had tissue resected during biopsy or surgery, we carefully reviewed their data individually to determine whether there were any cases in which osteochondromatosis had transformed into malignant chondrosarcoma, but we did not find any cases of malignant transformation. We will continue to consider the possibility of malignant transformation among patients with osteochondromatosis who are registered in the Hiroshima Tumor Tissue Registry.
A peak in the incidence of osteosarcoma has been observed in elderly Caucasian individuals as a result of the relatively high prevalence of osteosarcoma in patients with Paget disease4. In addition, Ogura et al., in a report from the BSTT Registry, reported an increasing proportion of osteosarcoma cases among the elderly in Japan6. However, we found neither a peak of osteosarcoma in the elderly nor any case of osteosarcoma with Paget disease. These findings are attributed to the lower prevalence of osteosarcoma with Paget disease. Hashimoto et al.16, on the basis of a mail survey, reported that the prevalence of Paget disease was 2.8 per million in Japan during the period between 1990 and 2002. This prevalence was extremely low when compared with the prevalence of 0.1% to 5% among individuals in high-prevalence countries17. Furthermore, Hashimoto et al. reported that half of the cases in their report were not confirmed by histological diagnosis16. Therefore, osteosarcoma in patients with Paget disease may not have been registered in the Hiroshima Tumor Tissue Registry.
We found that giant cell tumor represented a considerable proportion of bone tumors. Giant cell tumors primarily appeared in the long bones of the lower limb in patients 10 to 40 years of age. Giant cell tumor previously was considered to be benign, but recurrence after excision has been reported in 20% to 50% of patients, with 10% of cases becoming malignant upon recurrence18,19. Histologically benign giant cell tumor rarely occurs in cases of lung metastases20. Therefore, giant cell tumor has been classified as intermediate since the latest WHO 2013 classification10. Although the first choice of treatment is curettage followed by filling with bone cement21,22 and additional treatment with adjuvants, the rate of recurrence is still high1,18. Chemotherapy with denosumab, a monoclonal antibody that inhibits the osteoclastic activity of giant cell tumor, recently has emerged as another choice of treatment21. Active treatments are necessary in cases of giant cell tumor because of the risk of recurrence and malignant metastasis; however, the safety of long-term denosumab use has not yet been reported23, although it was approved for use in giant cell tumor treatment in Japan in May 201424. We will continue to carefully observe the cases of giant cell tumor and the associated characteristics.
The present study had several limitations. First, our data were based on patients with tumors that had been resected during surgery or biopsy and registered in the Hiroshima Tumor Tissue Registry. Patients who did not undergo therapeutic or diagnostic resection were not registered in the database. According to Cancer Incidence in Five Continents, the incidence of malignant bone tumors in Hiroshima City from 1978 to 1999 and the rate in Hiroshima Prefecture per 100,000 population in the period 2003 to 2007 were reported to be 0.5 to 0.8 for males and 0.4 to 0.6 for females9,25-29; in comparison, the registration rates per 100,000 population for malignant bone tumor in the Hiroshima Tumor Tissue Registry were 0.35 to 0.56 for males and 0.30 to 0.48 for females. Second, we observed 2,542 benign bone tumors over a period of 40 years; however, this number is most likely an underestimation because the majority of benign bone tumors are asymptomatic30 and are not usually detected or resected for diagnosis. Conventional radiology such as radiographs, computed tomography, and magnetic resonance imaging are used to diagnose chondroid tumors1. Among the benign bone tumors registered in the Hiroshima Tumor Tissue Registry and diagnosed after 1993, 95.6% were in patients who underwent surgery.
The Hiroshima Tumor Tissue Registry is a population-based tumor tissue registry that collects information on all tumors that are diagnosed pathologically in hospitals and clinical laboratories in Hiroshima Prefecture. In 2012, 88 institutions, including all designated cancer care hospitals, hospitals, clinics, and clinical laboratories, reported their tumor diagnoses to the Hiroshima Tumor Tissue Registry. Of the cancer cases registered in the Hiroshima Prefecture Cancer Registry, which is a regional cancer registry, 75% of the cases were also covered by the Hiroshima Tumor Tissue Registry31. Moreover, at the time of registration, all patients reported by local pathologists to the Hiroshima Tumor Tissue Registry are confirmed to not already be registered in the database with use of personal identifying information such as name, date of birth, address, etc. Therefore, there is no duplication of patients, even if the tumor information is reported by different hospitals (e.g., following biopsy and surgical diagnosis). The diagnoses associated with all tumors registered in the Hiroshima Tumor Tissue Registry are confirmed histologically. If a patient with a benign bone tumor needed medical treatment, the diagnosis should be confirmed pathologically and the tumor information is registered in the Hiroshima Tumor Tissue Registry. Despite these limitations, we believe that the information on all benign, intermediate, and malignant bone tumors in the present study is valuable, and we have been able to show the variation of histological type and age distribution of bone tumors by behavior in Hiroshima Prefecture.
This report described characteristics of benign, intermediate, and malignant bone tumors with regard to frequency of cases, registration rate, age distribution, and skeletal site by pathological type. There were differences between osteochondroma and enchondroma in terms of the number of patients and skeletal sites where the tumor most frequently occurred, although both tumor types were originated from chondrogenic tumors. Giant cell tumor has a high frequency and was the most dominant type of intermediate bone tumor. These findings shed light on the epidemiological and pathological features of bone tumors.
Acknowledgments
Note: All authors are members of the Hiroshima Tumor Tissue Registry Working Committee. This study was administrated by the Committee. The tabulated numbers in this paper were based on the annual report of Hiroshima Tumor Tissue Registry No. 41, published in March 2017. The authors thank all pathologists who cooperated and submitted the pathological information and specimens to the Hiroshima Tumor Tissue Registry. They also thank the staff of the Hiroshima Prefecture Medical Association, the staff of the Tumor and Tissue Registry Office, the Radiation Effects Research Foundation, and Ms. Mikiko Hayashi for their help with data collection and analysis. The Radiation Effects Research Foundation, Hiroshima and Nagasaki, Japan, is a public interest incorporated foundation funded by the Japanese Ministry of Health, Labour and Welfare (MHLW) and the U.S. Department of Energy (DOE). This publication was supported by Radiation Effects Research Foundation Research Protocol 18-61. The views of the authors do not necessarily reflect those of the 2 governments.
Investigation performed at the Hiroshima Prefecture Medical Association and the Radiation Effects Research Foundation, Hiroshima, Japan
Disclosure: This study was conducted by the Hiroshima Tumor Tissue Registry Working Committee, which was operated by the Hiroshima Prefecture Medical Association with cooperation and financial support of Hiroshima Prefecture and the Radiation Effects Research Foundation. The Disclosure of Potential Conflicts of Interest forms are provided with the online version of the article (http://links.lww.com/JBJSOA/A47).
The Hiroshima Tumor Tissue Registry Working Committee includes all authors of the present study as well as Koji Arihiro, Kouichi Ichimura, Mai Utada, Atsuko Sadakane, Yukie Kan, Ikuko Ogawa, Mayumi Kaneko, Hideo Tanaka, Hideshi Kawakami, Kazuya Kuraoka, Naomi Sasaki, Kazuhiro Sentani, Yutaka Daimaru, Yoshiro Tachiyama, Tomohiro Toji, Hirofumi Nakayama, Takashi Nishisaka, Megumu Fujihara, Koichi Mandai, Yuki Hirai, Jun Noma, and Hiroyasu Yamada.
References
- 1.Hakim DN, Pelly T, Kulendran M, Caris JA. Benign tumours of the bone: A review. J Bone Oncol. 2015. March 02;4(2):37-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nogueira Drumond JM. Benign bone tumors and tumor-like bone lesions: treatment update and new trends. Rev Bras Ortop. 2015. December 08;44(5):386-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rockberg J, Bach BA, Amelio J, Hernandez RK, Sobocki P, Engellau J, Bauer HC, Liede A. Incidence trends in the diagnosis of giant cell tumor of bone in Sweden since 1958. J Bone Joint Surg Am. 2015. November 04;97(21):1756-66. [DOI] [PubMed] [Google Scholar]
- 4.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009. April 01;115(7):1531-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mirabello L, Troisi RJ, Savage SA. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int J Cancer. 2009. July 01;125(1):229-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogura K, Higashi T, Kawai A. Statistics of bone sarcoma in Japan: report from the Bone and Soft Tissue Tumor Registry in Japan. J Orthop Sci. 2017. January;22(1):133-43. Epub 2016 Nov 12. [DOI] [PubMed] [Google Scholar]
- 7.Sugiyama H, Nishi N, Kuwabara M, Ninomiya M, Arita K, Yasui W, Kasagi F, Kodama K. Incidence and survival of childhood cancer cases diagnosed between 1998 and 2000 in Hiroshima City, Japan. Asian Pac J Cancer Prev. 2009. Oct-Dec;10(4):675-80. [PubMed] [Google Scholar]
- 8.Steliarova-Foucher E, Colombet M, Ries LAG, Hesseling P, Moreno F, Shin HY, Stiller CA. International incidence of childhood cancer, volume III. 2017. http://iicc.iarc.fr/results/. Accessed 2017 Mar 3.
- 9.Forman D, Bray F, Brewster DH, Gombe Mbalawa C, Kohler B, Piñeros M, Steliarova-Foucher E, Swaminathan R, Ferlay J. Cancer incidence in five continents, vol. X Lyon: IARC Scientific Publications; 2014. [DOI] [PubMed] [Google Scholar]
- 10.Fletcher CDM, Bridge JA, Hogendoorn P, Mertens F. WHO classification of tumors of soft tissue and bone. 4th ed. Lyon: International Agency on Research on Cancer; 2013. [Google Scholar]
- 11.Japanese Orthopaedic Association Musculoskeletal Tumor Committee. Bone tumor registry in Japan. Tokyo: National Cancer Center; 2013. [Google Scholar]
- 12.Liede A, Bach BA, Stryker S, Hernandez RK, Sobocki P, Bennett B, Wong SS. Regional variation and challenges in estimating the incidence of giant cell tumor of bone. J Bone Joint Surg Am. 2014. December 03;96(23):1999-2007. [DOI] [PubMed] [Google Scholar]
- 13.de Souza AM, Bispo Júnior RZ. Osteochondroma: ignore or investigate? Rev Bras Ortop. 2014. October 27;49(6):555-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altay M, Bayrakci K, Yildiz Y, Erekul S, Saglik Y. Secondary chondrosarcoma in cartilage bone tumors: report of 32 patients. J Orthop Sci. 2007. September;12(5):415-23. Epub 2007 Sep 28. [DOI] [PubMed] [Google Scholar]
- 15.Murphey MD, Choi JJ, Kransdorf MJ, Flemming DJ, Gannon FH. Imaging of osteochondroma: variants and complications with radiologic-pathologic correlation. RadioGraphics. 2000;20(5):1407-34. [DOI] [PubMed] [Google Scholar]
- 16.Hashimoto J, Ohno I, Nakatsuka K, Yoshimura N, Takata S, Zamma M, Yabe H, Abe S, Terada M, Yoh K, Fukunaga M, Cooper C, Morii H, Yoshikawa H; Japanese Committee on Clinical Guidelines of Diagnosis and Treatment of Paget’s Disease of Bone of the Japan Osteoporosis Society. Prevalence and clinical features of Paget’s disease of bone in Japan. J Bone Miner Metab. 2006;24(3):186-90. [DOI] [PubMed] [Google Scholar]
- 17.Corral-Gudino L, Borao-Cengotita-Bengoa M, Del Pino-Montes J, Ralston S. Epidemiology of Paget’s disease of bone: a systematic review and meta-analysis of secular changes. Bone. 2013. August;55(2):347-52. Epub 2013 May 1. [DOI] [PubMed] [Google Scholar]
- 18.Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg Am. 1987. January;69(1):106-14. [PubMed] [Google Scholar]
- 19.Strøm TMA, Skeie AT, Lobmaier IK, Zaikova O. Giant cell tumor: a rare condition in the immature skeleton-a retrospective study of symptoms, treatment, and outcome in 16 children. Sarcoma. 2016;2016:3079835. Epub 2016 Nov 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashimoto M, Suzumura Y, Kitamura S, Teramoto K, Hanaoka J, Tezuka N. A rare case of simultaneous bilateral pneumothorax caused by multiple pulmonary metastases of a femoral giant cell tumor. J Japan Assoc Chest Surg. 2012;26(1):104-9. Japanese. [Google Scholar]
- 21.Chakarun CJ, Forrester DM, Gottsegen CJ, Patel DB, White EA, Matcuk GR., Jr Giant cell tumor of bone: review, mimics, and new developments in treatment. Radiographics. 2013. Jan-Feb;33(1):197-211. [DOI] [PubMed] [Google Scholar]
- 22.Zuo D, Zheng L, Sun W, Fu D, Hua Y, Cai Z. Contemporary adjuvant polymethyl methacrylate cementation optimally limits recurrence in primary giant cell tumor of bone patients compared to bone grafting: a systematic review and meta-analysis. World J Surg Oncol. 2013. July 16;11:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaston CL, Grimer RJ, Parry M, Stacchiotti S, Dei Tos AP, Gelderblom H, Ferrari S, Baldi GG, Jones RL, Chawla S, Casali P, LeCesne A, Blay JY, Dijkstra SP, Thomas DM, Rutkowski P. Current status and unanswered questions on the use of Denosumab in giant cell tumor of bone. Clin Sarcoma Res. 2016. September 14;6(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daiichi-Sankyo https://www.daiichisankyo.co.jp/news/detail/006130.html Accessed 2018 Apr 11.
- 25.Cancer incidence in five continents. Volume V. IARC Sci Publ. 1987;(88):1-970. [PubMed] [Google Scholar]
- 26.Parkin DM, Muir CS, Whelan SL, Gao YT, Ferlay J, Powell J. Cancer incidence in five continents, vol. VI Lyon: IARC Scientific Publications; 1992. [PubMed] [Google Scholar]
- 27.Parkin DM, Whelan SL, Ferlay J, Raymond L, Young J. Cancer incidence in five continents, vol. VII Lyon: IARC Scientific Publications; 1997. [Google Scholar]
- 28.Parkin DM, Whelan SL., Ferlay J, Teppo L, Thomas DB. Cancer incidence in five continents, vol. VIII Lyon: IARC Scientific Publications; 2002. [Google Scholar]
- 29.Curado MP, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M, Boyle P. Cancer incidence in five continents, vol. IX Lyon: IARC Scientific Publications; 2007. [Google Scholar]
- 30.Reijnders C, Hameetman L, Bove JVMG. Bone: osteochondroma. Atlas Genet Cytogenet Oncol Haematol. 2009;13(9):678-80. [Google Scholar]
- 31.Hiroshima Prefecture Cancer Registry (annual report in 2012): Hiroshima Prefecture, Hiroshima Prefecture Medical Association, Radiation Effects Research Foundation Committee, Hiroshima Prefecture Cancer Registry Steering Committee. Letter Press; 2016. [Google Scholar]