Abstract
This case series describes the occurrence of graft-vs-host-disease (GVHD) in patients with cutaneous T-cell lymphomas (CTCLs) treated with the CC chemokine receptor 4 (CCR4) antibody mogalizumab prior to allogeneic hematopoietic stem cell transplantation (HSCT).
Mogamulizumab is a monoclonal antibody against CC chemokine receptor 4 (CCR4) that has efficacy in patients with relapsed and refractory T-cell lymphomas and has an overall response rate of 36.8% in mycosis fungoides (MF) and Sézary syndrome (SS).1 Concerns exist regarding the use of mogamulizumab in patients prior to allogeneic hematopoietic stem cell transplantation (allo-HSCT) because CCR4 is highly expressed on both malignant and regulatory T cells. Depletion of regulatory T cells following mogamulizumab is thought to augment antitumor response but may also potentiate graft-vs-host disease (GVHD). Significant reduction of peripheral blood and tissue regulatory T cells was observed in patients with cutaneous T-cell lymphoma treated with mogamulizumab.2 Retrospective studies in patients with adult T-cell leukemia/lymphoma have reported that treatment with mogamulizumab prior to transplantation may be associated with an increased risk of severe, steroid-refractory acute GVHD, as well as poor clinical outcomes.3 We evaluated patients with advanced MF or SS who received mogamulizumab before allo-HSCT.
Methods
We included 8 patients with MF or SS who received mogamulizumab as part of a phase 1/2 clinical trial1 and subsequently received allo-HSCT. Mogamulizumab was administered weekly as an intravenous infusion for 4 weeks and every 2 weeks thereafter. All patients received a nonmyeloablative allo-HSCT using total-skin electron beam therapy, total lymphoid irradiation, and antithymocyte globulin preparation as a participant in a phase 2 trial at Stanford University (NCT00896493). Graft-vs-host disease prophylaxis consisted of cyclosporine or tacrolimus and mycophenolate mofetil. This study was approved by the Stanford University institutional review board, and patients provided written informed consent.
Results
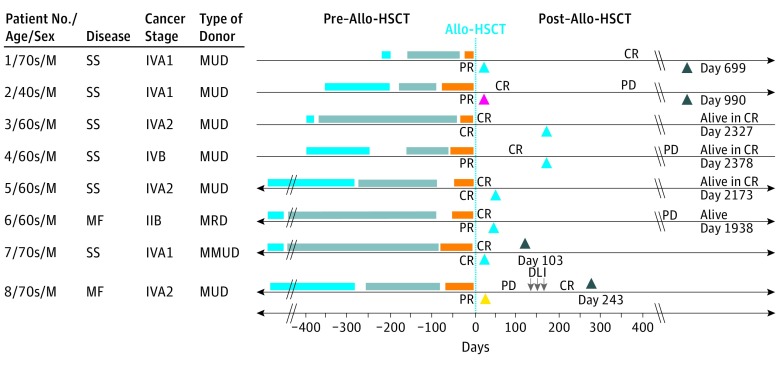
Patient characteristics are summarized in the Table. The clinical course of each patient is summarized in the Figure. Three-year progression-free survival and overall survival was 37.5% and 50.0%, respectively. One patient developed grade IV gastrointestinal acute GVHD. Notably, this was the only patient who received a peripheral blood stem cell graft from a human leukocyte antigen–mismatched unrelated donor. Another patient developed de novo chronic GVHD, and 1 patient developed donor lymphocyte infusion–related GVHD.
Table. Patient and Disease Characteristics for 8 Patients With MF or SS Who Received Mogamulizumab Prior to Allo-HSCT.
| Characteristics | No. (%) |
|---|---|
| Age at allo-HSCT, median (range), y | 66 (48-74) |
| Sex, No. (%) | |
| Male | 8 (100) |
| Race/ethnicity, No. (%) | |
| White | 7 (88) |
| Hispanic/Latino | 1 (13) |
| Diagnosis, No. (%) | |
| Mycosis fungoides | 2 (25) |
| Stage IIB | 1 (13) |
| Stage IVA | 1 (13) |
| Sézary syndrome | 6 (75) |
| Stage IVA | 5 (63) |
| Stage IVB | 1 (13) |
| Prior systemic therapies, median (range) | 9 (6-13) |
| Total mogamulizumab infusions, median (range) | 15 (3-38) |
| Time from last mogamulizumab to allo-HSCT, median (range), d | 281 (201-848) |
| Donor and compatibility, No. (%) | |
| Related | 1 (13) |
| Matched | 1 (13) |
| Mismatched | 0 |
| Unrelated | 7 (88) |
| Matched | 6 (75) |
| Mismatched | 1 (13) |
| Best chimerism, No. (%) | |
| Failure | 1 (13) |
| Mixed | 1 (13) |
| Engraftment | 6 (75) |
| Best response with allo-HSCT, No. (%) | |
| Complete response | 7 (88) |
| Partial response | 1 (13) |
| Stable disease | 0 |
| Progressive disease | 0 |
| Follow-up time after allo-HSCT, median (range), mo | 49 (3-79) |
Abbreviations: allo-HSCT, allogeneic hematopoietic stem cell transplantation; MF, mycosis fungoides; SS, Sézary syndrome.
Figure. Timelines of the Clinical Course of 8 Patients Who Received Mogamulizumab Before Allo-HSCT.
Blue bars represent mogamulizumab therapy (mogamulizumab was initiated for patients 1 through 4 within 400 days); gray, other systemic therapies; and orange, total-skin electron beam therapy, total lymphoid irradiation, and antithymocyte globulin preparation (patient 1 received only total-skin electron beam therapy and total lymphoid irradiation). Black arrowheads represent death. Post–allo-HSCT, patient 1 experienced oral chronic GVHD between days 95 and 125, cutaneous chronic GVHD between days 130 and 699, and gastrointestinal chronic GVHD between days 269 and 699 and died at day 699 from GVHD. Post–allo-HSCT, patient 2 died at day 990 from cutaneous T-cell lymphoma. Post–allo-HSCT, patient 7 experienced gastrointestinal acute GVHD between days 28 and 103 and died at day 103 from GVHD. Post–allo-HSCT, patient 8 experienced gastrointestinal GVHD between days 237 and 243 and died at day 243 from donor leukocyte infusion–related graft-vs-host disease. Cyan arrowheads represent graft success with full donor chimerism; yellow arrowheads, partial graft success with mixed donor chimerism; and magenta arrowheads, graft failure. Allo-HSCT indicates allogeneic hematopoietic stem cell transplantation; CR, complete response; DLI, donor lymphocyte infusion; GVHD, graft-vs-host disease; MF, mycosis fungoides; MMUD, mismatched unrelated donor; MRD, matched related donor; MUD, matched unrelated donor; PD, progressive disease; PR, partial response; SS, Sézary syndrome.
Discussion
In this small, retrospective study of 8 patients with MF or SS who received mogamulizumab prior to allo-HSCT, we observed 1 patient (13%) who developed grade IV acute GVHD. The incidence of acute GVHD in patients with MF or SS patients who underwent allo-HSCT was previously reported as 28% to 50%.4,5
Conventional risk factors, including degree of human leukocyte antigen disparity and peripheral blood stem cell graft source, were likely the dominant factors that contributed to acute GVHD in this patient. Another patient developed GVHD after donor lymphocyte infusion, the purpose of which is to enhance graft-vs-lymphoma effects but is also associated with a 30% incidence of GVHD.6
Prior studies in adult T-cell leukemia/lymphoma suggest that a short interval between the last administration of mogamulizumab and allo-HSCT is associated with a greater risk of severe acute GVHD, possibly related to residual mogamulizumab and its effects on regulatory T-cell depletion. Though the half-life of mogamulizumab is approximately 16 to 18 days, in vivo studies demonstrate that regulatory T cells may be depleted for half a year or longer.3 Time from last mogamulizumab administration to allo-HSCT in our cohort was more than 200 days, and patients likely had sufficient time to clear mogamulizumab and replete regulatory T cells. No patients were in the high-risk time frame (<50 days) reported in adult T-cell leukemia/lymphoma studies.2
In 8 patients with remote exposure to mogamulizumab prior to allo-HSCT, we did not observe a notable increased incidence of grade II to IV acute GVHD. As phase 1/21 and recently completed phase 3 (NCT01728805) studies demonstrate that mogamulizumab may be an effective agent for disease reduction in patients with CTCL, mogamulizumab will likely be used as a bridge to allo-HSCT when it is made more available. Future prospective studies should monitor concentrations of mogamulizumab and regulatory T cells following treatment to determine whether shorter time intervals between mogamulizumab and allo-HSCT increases risk of acute GVHD.
References
- 1.Duvic M, Pinter-Brown LC, Foss FM, et al. Phase 1/2 study of mogamulizumab, a defucosylated anti-CCR4 antibody, in previously treated patients with cutaneous T-cell lymphoma. Blood. 2015;125(12):1883-1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ni X, Jorgensen JL, Goswami M, et al. Reduction of regulatory T cells by mogamulizumab, a defucosylated anti-CC chemokine receptor 4 antibody, in patients with aggressive/refractory mycosis fungoides and Sézary syndrome. Clin Cancer Res. 2015;21(2):274-285. [DOI] [PubMed] [Google Scholar]
- 3.Fuji S, Inoue Y, Utsunomiya A, et al. Pretransplantation anti-ccr4 antibody mogamulizumab against adult t-cell leukemia/lymphoma is associated with significantly increased risks of severe and corticosteroid-refractory graft-versus-host disease, nonrelapse mortality, and overall mortality. J Clin Oncol. 2016;34(28):3426-3433. [DOI] [PubMed] [Google Scholar]
- 4.Hosing C, Bassett R, Dabaja B, et al. Allogeneic stem-cell transplantation in patients with cutaneous lymphoma: updated results from a single institution. Ann Oncol. 2015;26(12):2490-2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Masson A, Beylot-Barry M, Bouaziz JD, et al. ; French Study Group on Cutaneous Lymphomas and Société Française de Greffe de Moëlle et Thérapie Cellulaire . Allogeneic stem cell transplantation for advanced cutaneous T-cell lymphomas: a study from the French Society of Bone Marrow Transplantation and French Study Group on Cutaneous Lymphomas. Haematologica. 2014;99(3):527-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scarisbrick JJ, Dignan FL, Tulpule S, et al. A multicentre UK study of GVHD following DLI: rates of GVHD are high but mortality from GVHD is infrequent. Bone Marrow Transplant. 2015;50(1):62-67. [DOI] [PubMed] [Google Scholar]