Key Points
Question
How do the risks of recurrence, metastasis, and death in cutaneous squamous cell carcinoma (cSCC) differ between tumors arising on the vermilion lip vs the cutaneous lip?
Findings
In this cohort study of 303 patients with 310 cSCCs of the lip (138 with cutaneous lip location, 172 vermilion), there was a 5-fold greater risk of nodal metastasis for vermilion lip tumors (13 of 172; 7.6%) compared with cutaneous lip tumors (2 of 138; 1.5%).
Meaning
Cutaneous SCCs on the cutaneous lip are low-risk tumors, whereas cSCCs on the vermilion lip have considerable risk of nodal metastasis and may therefore merit consideration of radiologic nodal staging.
Abstract
Importance
Although the lip is considered a high-risk location in cutaneous squamous cell carcinoma (cSCC), it has not been established whether this risk stems from vermilion or cutaneous locations or both.
Objective
To compare differences in risks of recurrence, metastasis, and death from cSCCs on the vermilion vs cutaneous lip.
Design, Setting, and Participants
Retrospective cohort study of 303 patients with 310 primary cSCCs of the lip (138 cutaneous, 172 vermilion) diagnosed between 2000 and 2015 at 2 academic tertiary care centers in Boston, Massachusetts.
Main Outcomes and Measures
Development of local recurrence, nodal metastasis, distant metastasis, disease-specific death, and all-cause death.
Results
Of the 303 study participants with 310 SCCs of the lip, 153 (50.5%) were men, and 150 (49.5%) were women; median age at diagnosis, 68 years (range, 27-93 years). Outcomes were as follows for vermilion vs cutaneous locations: local recurrence, 6.4% (11 of 172) vs 2.9% (4 of 138); nodal metastasis, 7.6% (13 of 172) vs 1.5% (2 of 138); distant metastasis, 0.6% (1 of 172) vs 0.7% (1 of 138); disease-specific death, 3.5% (6 of 172) vs 2.9% (4 of 138); and all-cause death, 26.7% (46 of 172) vs 29.0% (40 of 138). The difference was statistically significant for nodal metastasis (P = .01). In multivariable analysis, nodal metastasis was associated with vermilion lip location (subhazard ratio, 5.0; 95% CI, 1.1-23.8) and invasion beyond fat (fascia or beyond for vermilion lip) (subhazard ratio, 4.4; 95% CI, 1.3-14.9).
Conclusions and Relevance
The risk of nodal metastasis is 5-fold greater for cSCCs on the vermilion lip compared with those on the cutaneous lip. Squamous cell carcinomas of the cutaneous lip have a nodal metastasis risk similar to cSCCs in general (1.5%). Thus, vermilion involvement appears responsible for the increased risk associated with cSCC of lip. Vermilion involvement may merit radiologic nodal staging and inclusion in future tumor staging, since it was independently associated with higher-risk cSCC of the lip region.
This cohort study of patients with cutaneous squamous cell carcinomas of the lip evaluates the risk differences for recurrence, metastasis, and death in tumors of the vermilion vs cutaneous lip locations.
Introduction
Skin cancer is the most common cancer in the United States, with an incidence exceeding that of all other cancers combined.1,2 The 2 main types, cutaneous squamous cell carcinoma (cSCC) and basal cell carcinoma, are now approximately equal in incidence and together account for 5.4 million cases of nonmelanoma skin cancer in the United States per year.1 Previous studies have identified certain locations such as the lip, ear, and cheek as high-risk sites associated with increased risk of poor outcomes in cSCC.3,4,5 For cSCCs of the lip, local recurrence (LR) occurs in up to 22% of tumors compared with 3% for cSCCs of all locations.6,7,8 Similarly, cSCCs of the lip reportedly have as high as a 20% risk of metastasis, while the overall metastatic risk for cSCC is around 4%.6,7,8
To our knowledge, no study has determined whether the increased risk identified applies to the cutaneous lip, vermilion lip, or both. The risk differences between the individual zones of the lip (Figure 1) have thus far been poorly studied, with recent studies only analyzing the lip in its entirety or stratifying primarily by upper vs lower lip rather than by cutaneous vs vermilion lip.9,10 In addition, there has been a lack of consensus on the grouping of lip zones for cancer staging purposes. In the Cancer Staging Manual, 7th Edition of the American Joint Committee on Cancer (AJCC 7), cSCCs on the cutaneous lip were staged under the cSCC system, while cSCCs on the vermilion lip were staged under the lip and oral cavity system.11 Furthermore, within the cSCC system, the cutaneous lip was included as a high-risk location that could contribute toward upstaging a tumor, whereas the lip and oral cavity system did not include any location-based staging criterion.
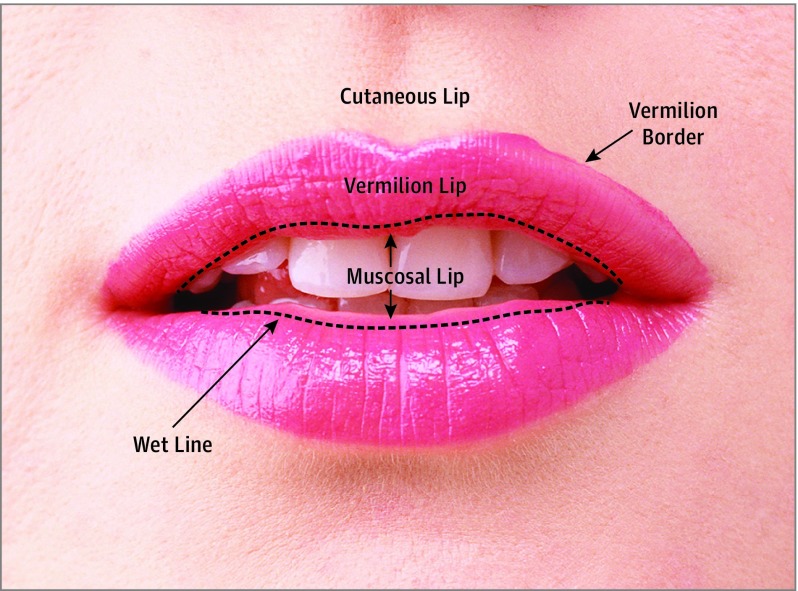
Figure 1. Zones of the Lip.
In AJCC 8, the cSCC staging system was updated to encompass only tumors arising on the head and neck.12 In addition, the vermilion lip was removed from the lip and oral cavity system and placed together with the cutaneous lip in the cSCC system.
In theory, the absence of subcutaneous fat in the vermilion lip makes this location more prone to deep invasion. Without a fat barrier, cSCCs arising on the vermilion lip can more quickly access the rich lymphovascular space of muscle and thus have greater metastatic potential than cSCCs on the cutaneous lip, where relatively avascular subcutaneous fat is present.13 Cutaneous SCCs on the vermilion lip that invade past the dermis (into fascia or muscle) are considered past the subcutaneous fat and are upstaged to T3 in AJCC 8.
The decision to drop location-based criterion for cSCC of the head and neck in AJCC 8 was made in part because studies in the existing literature have inconsistently analyzed, failed to analyze, or were underpowered to analyze risk differences between the lip zones in cSCC. Therefore, this study aims to (1) quantify and compare the risks of developing poor outcomes in cSCCs of the cutaneous lip vs vermilion lip, with the hypothesis that vermilion lip cases have worse outcomes; and (2) define risk factors independently associated with poor outcomes in cSCC of the lip via multivariable analysis adjusted for the presence of other concurrent risk factors.
Methods
The Partners Human Research Committee approved this study, waiving written informed consent for retrospective deidentified patient data. Patients diagnosed with cSCC of the lip between January 1, 2000, and December 31, 2015, at Brigham and Women’s Hospital (BWH) and Massachusetts General Hospital (MGH) in Boston, Massachusetts, were identified by (1) querying the Partners HealthCare System Research Patient Data Registry for patients with at least 2 International Classification of Diseases, Ninth Revision (ICD-9) codes for cSCC of the lip (eTable 1 in the Supplement); and (2) searching each hospital’s Department of Pathology electronic pathology report database using combinations of the key phrases “squamous cell carcinoma” and “lip” and the following synonyms: “squamous cell cancer,” “SCC,” “vermilion,” “oral commissure,” “nasolabial fold,” “nlf,” and “philtrum.” The medical records of all returned cases were manually reviewed by 2 of us (D.M.W. and P.S.K.) to validate that a histologic diagnosis of cSCC had been made.
Each qualifying tumor was assigned to the cutaneous lip or the vermilion lip. For tumors with inconclusive locations from clinical notes and pathology reports, histopathology slides of these tumors were obtained from their respective hospitals and reviewed by 3 of us (S.K., P.R., and C.D.S.). Classification of lip location was made based on the architectural appearance of the section (with superficial muscle and absence of fat indicating vermilion) and presence or absence of hair follicles and adnexal structures, which are found in the cutaneous lip but not in the vermilion lip. If a tumor had involvement of both cutaneous and vermilion lip, it was classified as a vermilion case, since tumor exposure to the richer lymphatics of the vermilion lip would increase the risk of metastasis according to the study hypothesis.
Features such as tumor diameter, histologic differentiation, anatomic level of invasion, and the presence of perineural invasion (PNI) were obtained from pathology reports and clinical notes. Tumor diameter was obtained from clinical notes if available; otherwise, it was recorded as the greatest diameter of the gross lesion reported on the excisional specimen pathology report (which should slightly underestimate the tumor size, given formalin shrinkage). Slides of tumors with PNI (which is reported, when present, on cSCC pathology reports at BWH and MGH as standard practice) were reviewed by the same 3 of us who reviewed the histopathology slides (S.K., P.R., and C.D.S.) to record the diameter of the largest involved nerve. Unless otherwise stated in the pathology report (biopsy report, excision report, or Mohs operative note), tumors were considered to be well differentiated, confined to the dermis, and free of PNI. The T stage based on the BWH Tumor Staging system14 and AJCC 8 was determined for all tumors and stratified by BWH low (T1/T2a) vs high (T2b/T3) stage and AJCC 8 low (T1/T2) vs high (T3/T4a,b) stage. Treatment of the primary tumor was recorded from surgical operative notes and clinical notes. Additional information, including demographic information, immune status, and history of skin cancer, was obtained from the medical record.
Outcomes of interest, including LR, nodal metastasis (NM), distant metastasis (DM), disease-specific death (DSD), and all-cause death (ACD), were determined to have occurred if indicated in clinical notes by the treatment team or primary care physicians. Local recurrence was considered to have occurred if a pathology report documented invasive cSCC in the same anatomic location as a prior cSCC and a clinical note from a treating physician confirmed that the second lesion was considered to be a recurrence of the index primary tumor. Nodal metastasis was defined as pathologically confirmed cSCC in a draining nodal basin of the primary cSCC with no other potential source. Distant metastasis was established by histological analysis and/or radiological studies and documentation by the treatment team. Disease-specific death was considered to have occurred if the primary tumor or related complications were listed as the cause of death by a treating physician. All-cause death was determined by a review of medical records and online obituary search. Patients who did not develop an outcome of interest were censored on the date of death or the date of the medical record review if alive.
Baseline demographic characteristics, medical history, tumor characteristics, and treatment and outcome data were stratified by location on the cutaneous lip vs vermilion lip and compared using the Pearson χ2 test or Fisher exact test for categorical variables and Wilcoxon rank-sum test for continuous variables. The competing risk analysis of Fine and Gray15 was used for the NM and DSD end points because cSCC is primarily a disease of the elderly population, and death from non-cSCC causes frequently occurs prior to the development of disease-related outcomes and must be taken into account. Multivariable models were built using a combination of backward elimination and the change-in-estimate method. Variables with P < .20 and variables that, when included, resulted in a change of 10% or more in the effect estimate of location on the vermilion lip (vs cutaneous lip) were included in the multivariable model.16,17 All statistical analyses were conducted using Stata software (version 15.0; StataCorp LP).
Results
The Partners HealthCare System Research Patient Data Registry query and searches of the pathology report databases combined found 2259 patients who met the initial search criteria. After medical record review, most of these cases were determined to be cSCCs of nonlip locations, recurrent tumors, or incorrectly coded lesions and were excluded. A total of 394 cases of primary invasive (not in situ) cSCC of the lip were identified, of which 56 required histologic review to determine the location on cutaneous lip vs vermilion lip, and 84 were excluded due to insufficient primary tumor information, leaving 310 cSCCs of the lip in 303 patients for inclusion in the study cohort. Table 1 summarizes the characteristics of the study population. The patients were evenly split between men (50.5%; n = 153) and women (49.5%; n = 150). The median age was 68 years (range, 27-93 years). Immunosuppression (due to organ transplantation, leukemia/lymphoma, or medications for autoimmune diseases) was present in 79 (26.1%) patients. There was a history of skin cancer in 173 (57.1%) patients.
Table 1. Patient Characteristics.
| Patient Characteristics | Patients, No. (%) (N = 303) |
Tumors, No. (%) (N = 310) | P Value | |
|---|---|---|---|---|
| Cutaneous Lip cSCCs (n = 138) |
Vermilion Lip cSCCs (n = 172) |
|||
| Sex | .02 | |||
| Female | 150 (49.5) | 78 (56.5) | 75 (43.6) | |
| Male | 153 (50.5) | 60 (43.5) | 97 (56.4) | |
| Race | .93 | |||
| White non-Hispanic | 292 (96.4) | 134 (97.1) | 164 (95.4) | |
| Hispanic or Latino | 2 (0.7) | 1 (0.7) | 1 (0.6) | |
| American Indian/Alaskan Native | 1 (0.3) | 0 (0) | 1 (0.6) | |
| Unknown | 8 (2.6) | 3 (2.2) | 6 (3.5) | |
| Age, median (range), y | 68 (27-93) | 67.5 (36-93) | 68 (27-92) | .92 |
| Immunosuppression | .43 | |||
| Yes | 79 (26.1) | 40 (29.0) | 43 (25.0) | |
| No | 224 (73.9) | 98 (71.0) | 129 (75.0) | |
| Previous skin cancer | .19 | |||
| Yes | 173 (57.1) | 84 (60.9) | 92 (53.5) | |
| No | 130 (42.9) | 54 (39.1) | 80 (46.5) | |
Abbreviation: cSCC, cutaneous squamous cell carcinoma.
Table 2 summarizes tumor characteristics stratified by location on the cutaneous lip vs vermilion lip. Of the 310 cSCCs of the lip, 138 (44.5%) were located on the cutaneous lip and 172 (55.5%) were located on the vermilion lip (including 23 that involved cutaneous lip as well). The median follow-up time was 49.6 months (range, 12.2-196.3 months). Most tumors were less than 2 cm in diameter (267 [86.1%]), well-differentiated (239 [77.1%]), confined to the dermis (284 [91.6%]), and free of perineural invasion (289 [93.2%]). The majority of tumors were low (T1/T2a) BWH T stage (295 [95.2%]) and low (T1/T2) AJCC 8 T stage (282 [91.0%]). For primary treatment, Mohs surgery (199 [64.2%]) was most commonly used, followed by standard excision (69 [22.3%]) and other treatments (42 [13.6%]), which included combination therapy (Mohs surgery or standard excision with radiotherapy and/or chemotherapy), radiation monotherapy, destruction, topical medications, unknown treatments, and no treatment.
Table 2. Tumor Characteristics of the Study Cohort.
| Tumor Characteristic | cSCCs, No. (%) | P Value | ||
|---|---|---|---|---|
| Total (N = 310) |
Cutaneous Lip (n = 138) |
Vermilion Lip (n = 172) |
||
| Follow-up time, median (range), mo | 49.6 (12.2-196.3) | 54.8 (12.6-196.3) | 46.2 (12.2-188.0) | .04 |
| Tumor diameter, cm | .01 | |||
| <2 | 267 (86.1) | 128 (92.8) | 139 (80.8) | |
| ≥2 | 36 (11.6) | 8 (5.8) | 28 (16.3) | |
| Unknown | 7 (2.3) | 2 (1.5) | 5 (2.9) | |
| Tumor differentiation | .02 | |||
| Well | 239 (77.1) | 116 (84.1) | 123 (71.5) | |
| Moderate to poor | 71 (22.9) | 22 (15.9) | 49 (28.5) | |
| Level of invasion | .41 | |||
| Dermis | 284 (91.6) | 129 (93.5) | 155 (90.1) | |
| Fat | 1 (0.3) | 1 (0.7) | 0 (0) | |
| Fascia | 2 (0.7) | 0 (0) | 2 (1.2) | |
| Muscle | 22 (7.1) | 8 (5.8) | 14 (8.1) | |
| Bone | 1 (0.3) | 0 (0) | 1 (0.6) | |
| Perineural invasion, mm | .62 | |||
| None | 289 (93.2) | 130 (94.2) | 159 (92.4) | |
| <0.1 | 1 (0.3) | 1 (0.7) | 0 (0) | |
| ≥0.1 | 4 (1.3) | 1 (0.7) | 3 (1.7) | |
| Present, nerve diameter unknown | 16 (5.2) | 6 (4.4) | 10 (5.8) | |
| BWH T stage | .15 | |||
| T1/T2a (low) | 295 (95.2) | 134 (97.1) | 161 (93.6) | |
| T2b/T3 (high) | 15 (4.8) | 4 (2.9) | 11 (6.4) | |
| AJCC 8 T stage | .08 | |||
| T1/T2 (low) | 282 (91.0) | 130 (94.2) | 152 (88.4) | |
| T3/T4a,b (high) | 28 (9.0) | 8 (5.8) | 20 (11.6) | |
| Treatment modality | <.001 | |||
| Standard excision | 69 (22.3) | 15 (10.9) | 54 (31.4) | |
| Mohs surgery | 199 (64.2) | 110 (79.7) | 89 (51.7) | |
| Other treatment | 42 (13.6) | 13 (9.4) | 29 (16.9) | |
| Outcomes of interest | ||||
| Local recurrence | 15 (4.8) | 4 (2.9) | 11 (6.4) | .15 |
| Nodal metastasis | 15 (4.8) | 2 (1.5) | 13 (7.6) | .01 |
| Disease-specific death | 10 (3.2) | 4 (2.9) | 6 (3.5) | >.99 |
| Any poor outcome | 25 (8.1) | 5 (3.6) | 20 (11.6) | .01 |
| All-cause death | 86 (27.7) | 40 (29.0) | 46 (26.7) | .66 |
Abbreviations: AJCC 8, American Joint Committee on Cancer Cancer Staging Manual, 8th Edition; BWH, Brigham and Women’s Hospital staging system; cSCC, cutaneous squamous cell carcinoma.
The following outcomes of interest occurred during the study period: LR occurred in 15 (4.8%) tumors, NM occurred in 15 (4.8%) tumors, DM occurred in 2 (0.6%) tumors, DSD occurred in 10 (3.2%) tumors, any poor outcome (LR, NM, DM, and/or DSD) occurred in 25 (8.1%) tumors, and ACD occurred in 86 (27.7%) tumors.
When tumor characteristics were compared between cSCCs on the 2 lip zones, tumors on the vermilion lip were more likely to have a larger diameter (≥2 cm) (16.8% [28 of 167] vs 5.9% [8 of 136]; P = .004) and more aggressive histologic characteristics (moderate or poor differentiation) (28.5% (49 of 172) vs 15.9% (22 of 138); P = .01) than those on the cutaneous lip. Cutaneous SCCs on the vermilion lip were more frequently treated with standard excision than Mohs surgery compared with those on the cutaneous lip (37.8% (54 of 143) vs 12.0% (15 of 125); P < .001). All outcomes of interest except ACD occurred more frequently in cSCCs on the vermilion lip than on the cutaneous lip: LR, 6.4% (11 of 172) vs 2.9% (4 of 138); NM, 7.6% (13 of 172) vs 1.5% (2 of 138); distant metastasis, 0.6% (1 of 172) vs 0.7% (1 of 138); DSD, 3.5% (6 of 172) vs 2.9% (4 of 138); any poor outcome, 11.6% (20 of 172) vs 3.6% (5 of 138); and ACD, 26.7% (46 of 172) vs 29.0% (40 of 138)). However, this difference was statistically significant for only the NM and any-poor-outcome end points (P = .01 for each). While vermilion lip cSCCs were more often located on the lower lip (81%; 139 of 172), there was no significant risk difference in the NM end point between lower vermilion lip cSCCs and upper vermilion lip cSCCs (7.9%; 11 of 139 vs 6.3%; 2 of 32, respectively; P > .99).
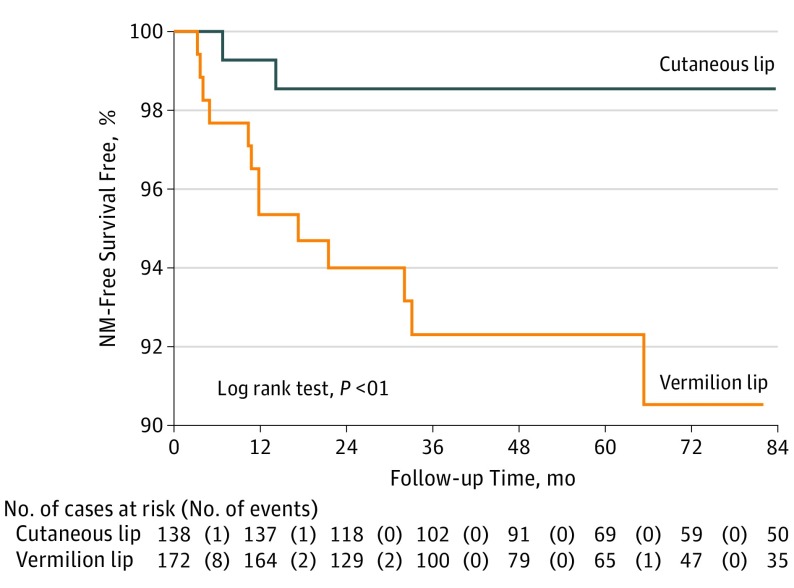
eTable 2 in the Supplement summarizes univariable and multivariable analyses for selected outcomes of interest (NM and DSD). The BWH and AJCC 8 T stages were excluded from multivariable analyses owing to multicollinearity with individual risk factors. On multivariable analysis, elevated risk of NM in cSCC of the lip was associated with location on the vermilion lip (vs cutaneous lip; subhazard ratio [SHR], 5.0; 95% CI, 1.1-23.8) and depth of invasion beyond subcutaneous fat (eg, invasion of fascia or muscle or beyond) (SHR, 4.4; 95% CI, 1.3-14.9). Elevated risk of DSD was associated with PNI (SHR, 4.6; 95% CI, 1.1-18.9) and non-Mohs treatment (vs standard excision; SHR, 0.1; 95% CI, 0.0-0.7). Figure 2 shows Kaplan-Meier curves and the life table for NM stratified by lip location. Table 3 provides details of the 15 cases of NM and shows that more than half of these cases were AJCC 8 and BWH T1 without any risk factors. A sensitivity analysis showed no difference in outcomes between those cases requiring vs not requiring histologic review to classify vermilion vs cutaneous lip location.
Figure 2. Kaplan-Meier Curves With Accompanying Life Table for Nodal Metastasis (NM).
Cases represent tumors rather than patients; several patients in the study had more than 1 tumor.
Table 3. Risk Factors, BWH and AJCC 8 Tumor Stage, and Outcomes in Nodal Metastasis Cases.
| Location | Age, y/Sex | Immunosuppression | Previous Skin Cancer | Tumor Diameter, cm | Tumor Differentiation | Level of Invasion | Perineural Invasion | BWH T Stage | AJCC 8 T Stage | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Vermilion | 50/M | No | No | Unknown | Well | Dermis | No | T1 | T1 | NM |
| Vermilion | 43/M | No | No | 0.4 | Well | Dermis | No | T1 | T1 | LR, NM, DSD, ACD |
| Vermilion | 66/F | No | Yes | 0.8 | Well | Dermis | No | T1 | T1 | LR, NM, DSD, ACD |
| Vermilion | 71/M | No | No | 1.3 | Well | Dermis | No | T1 | T1 | NM, DSD, ACD |
| Vermilion | 76/M | No | No | 1.5 | Well | Dermis | No | T1 | T1 | NM |
| Vermilion | 75/M | Yes | Yes | 1.8 | Well | Dermis | No | T1 | T1 | NM, DSD, ACD |
| Vermilion | 84/M | Yes | Yes | 2.4 | Well | Dermis | No | T2a | T2 | LR, NM, ACD |
| Vermilion | 72/M | No | Yes | 0.5 | Moderate | Dermis | No | T1 | T1 | LR, NM, ACD |
| Vermilion | 74/F | No | Yes | 0.8 | Moderate | Dermis | No | T1 | T1 | NM |
| Vermilion | 59/M | No | No | 1.8 | Moderate | Muscle | No | T2a | T3 | NM |
| Vermilion | 74/F | No | Yes | 1.8 | Moderate | Muscle | No | T2a | T3 | NM |
| Vermilion | 71/F | No | No | 1.1 | Poor | Dermis | No | T2a | T1 | NM |
| Vermilion | 61/F | No | No | 2.5 | Poor | Dermis | Yes | T2b | T2 | NM |
| Cutaneous | 62/M | No | No | 1.1 | Well | Muscle | No | T2a | T3 | NM, DSD, ACD |
| Cutaneous | 81/F | No | Yes | 1.4 | Moderate | Muscle | No | T2a | T3 | LR, NM, DSD, ACD |
Abbreviations: ACD, all-cause death; AJCC 8, American Joint Committee on Cancer Cancer Staging Manual, 8th Edition; BWH, Brigham and Women’s Hospital staging system; DSD, disease-specific death; LR, local recurrence; NM, nodal metastasis.
Discussion
Prior studies showing increased risk of poor outcomes in cSCCs of the lip have thus far been unable to analyze risk differences between the zones of the lip. In the present study, cSCCs on the vermilion lip had a greater risk of developing LR, NM, and DSD than did cSCCs of the cutaneous lip. In particular, the risk of NM for cSCCs on the vermilion lip (7.6%) was 5-fold higher than the risk for cSCCs on the cutaneous lip (1.5%) on multivariable analysis, independent of depth of invasion (P = .04). Since the 1.5% risk for cutaneous lip is slightly less than that reported for cSCC overall, prior studies that found the lip to be a high-risk location were likely driven by poor outcomes of vermilion lip (and not cutaneous lip) cases. Other studies that did not identify the lip as a high-risk location may have included mostly cutaneous lip cSCCs.18
The present study found on multivariable analysis that cSCC location on the vermilion lip was associated with development of NM, independent of depth of invasion (which was the only other risk factor associated with NM in this cohort). This finding suggests that the recently updated AJCC 8 criteria and BWH cSCC staging may not adequately account for the increased risk inherent to vermilion lip location. Although the etiology of cSCCs arising on the vermilion lip is primarily related to UV light exposure similar to other nonmelanoma skin cancers on other sun-exposed areas of the body,19 the absence of subcutaneous fat in the vermilion lip may permit quicker tumor access to the rich lymphovascular space of muscle and thus greater metastatic potential. However, it is interesting to note that most of the metastatic cases examined in the present study did not report invasion beyond the dermis. Microinvasion of lymphatics in the connective tissue and muscle layer immediately below the dermis of vermilion lip may be difficult to detect and might therefore have been underestimated in this study. However, these data indicate that obvious invasion of lip muscle, poor differentiation, large tumor diameter, and PNI need not be present for metastasis to occur from vermilion lip cSCC. Vermilion location alone was associated with an elevated risk of NM in the absence of other risk factors.
While the presence of PNI was associated with a 4.6-fold higher risk of DSD, and treatment with Mohs surgery was associated with a 10-fold lower risk of DSD (compared with standard excision) on multivariable analysis, DSD was not associated with vermilion vs cutaneous lip location. A larger study may show a higher risk of mortality, but a rather large study may be needed, since NMs are highly curable if not too advanced. A 5-year cure rate of 73% has been reported for head and neck cSCC with NM treated with surgery and adjuvant radiotherapy.20
Limitations
A limitation of this study is its retrospective nature, with 21% of reviewed cases being excluded for lack of adequate information on risk factors and outcomes from clinic records and histologic review. Similarly, 18% of cases required histologic review to determine anatomic location on cutaneous lip vs vermilion lip owing to unclear location in the medical record; however, sensitivity analysis indicated that this did not affect results. Some factors that may potentially contribute to the worse outcomes seen on vermilion lip (eg, tobacco use and herpes simplex virus) could not be assessed in this retrospective design. Although this is one of the largest studies of lip cSCC, the small numbers likely precluded incorporation of some factors with prognostic significance. However, that such a strong association was demonstrated with vermilion lip location in a relatively small cohort indicates that this is likely a robust prognostic factor.
Conclusions
These findings have important implications for clinical practice and future research. Vermilion involvement appears responsible for the increased risk associated with cSCC of the lip and is independently associated with NM. The 7.6% risk of NM for vermilion cSCC, if borne out in other studies, may warrant radiologic nodal staging and closer clinical follow-up than cSCC in general. Future studies should distinguish between cSCCs arising on the cutaneous and vermilion lip given the difference in outcomes they portend. If these findings are replicated, and the risk of NM with vermilion lip location as a sole risk factor can be better quantified, vermilion lip location may merit inclusion in future AJCC cSCC staging.
eTable 1. ICD-9 Codes for Cutaneous Squamous Cell Carcinoma of the Lip
eTable 2. Univariable and Multivariable Analyses for Selected Outcomes of Interest
References
- 1.Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151(10):1081-1086. doi: 10.1001/jamadermatol.2015.1187 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30. doi: 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 3.Rowe DE, Carroll RJ, Day CL Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. Implications for treatment modality selection. J Am Acad Dermatol. 1992;26(6):976-990. doi: 10.1016/0190-9622(92)70144-5 [DOI] [PubMed] [Google Scholar]
- 4.Brougham NDLS, Dennett ER, Cameron R, Tan ST. The incidence of metastasis from cutaneous squamous cell carcinoma and the impact of its risk factors. J Surg Oncol. 2012;106(7):811-815. doi: 10.1002/jso.23155 [DOI] [PubMed] [Google Scholar]
- 5.Thompson AK, Kelley BF, Prokop LJ, Murad MH, Baum CL. Risk factors for cutaneous squamous cell carcinoma recurrence, metastasis, and disease-specific death: a systematic review and meta-analysis. JAMA Dermatol. 2016;152(4):419-428. doi: 10.1001/jamadermatol.2015.4994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmults CD, Karia PS, Carter JB, Han J, Qureshi AA. Factors predictive of recurrence and death from cutaneous squamous cell carcinoma: a 10-year, single-institution cohort study. JAMA Dermatol. 2013;149(5):541-547. doi: 10.1001/jamadermatol.2013.2139 [DOI] [PubMed] [Google Scholar]
- 7.Zitsch RP III, Park CW, Renner GJ, Rea JL. Outcome analysis for lip carcinoma. Otolaryngol Head Neck Surg. 1995;113(5):589-596. doi: 10.1016/S0194-5998(95)70050-1 [DOI] [PubMed] [Google Scholar]
- 8.Brantsch KD, Meisner C, Schönfisch B, et al. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: a prospective study. Lancet Oncol. 2008;9(8):713-720. doi: 10.1016/S1470-2045(08)70178-5 [DOI] [PubMed] [Google Scholar]
- 9.Han AY, Kuan EC, Mallen-St Clair J, Alonso JE, Arshi A, St John MA. Epidemiology of squamous cell carcinoma of the lip in the United States: a population-based cohort analysis. JAMA Otolaryngol Head Neck Surg. 2016;142(12):1216-1223. doi: 10.1001/jamaoto.2016.3455 [DOI] [PubMed] [Google Scholar]
- 10.Unsal AA, Unsal AB, Henn TE, Baredes S, Eloy JA. Cutaneous squamous cell carcinoma of the lip: a population-based analysis. Laryngoscope. 2018;128(1):84-90. doi: 10.1002/lary.26704 [DOI] [PubMed] [Google Scholar]
- 11.Edge SB, ed. AJCC Cancer Staging Manual. 7th ed New York: Springer; 2010. [Google Scholar]
- 12.Amin MB, ed. AJCC Cancer Staging Manual. 8th ed New York: Springer; 2017. [Google Scholar]
- 13.Schmults CD, ed. High-Risk Cutaneous Squamous Cell Carcinoma: A Practical Guide for Patient Management. Berlin, Germany: Springer; 2016. [PubMed] [Google Scholar]
- 14.Jambusaria-Pahlajani A, Kanetsky PA, Karia PS, et al. Evaluation of AJCC tumor staging for cutaneous squamous cell carcinoma and a proposed alternative tumor staging system. JAMA Dermatol. 2013;149(4):402-410. doi: 10.1001/jamadermatol.2013.2456 [DOI] [PubMed] [Google Scholar]
- 15.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 16.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138(11):923-936. doi: 10.1093/oxfordjournals.aje.a116813 [DOI] [PubMed] [Google Scholar]
- 17.Budtz-Jørgensen E, Keiding N, Grandjean P, Weihe P. Confounder selection in environmental epidemiology: assessment of health effects of prenatal mercury exposure. Ann Epidemiol. 2007;17(1):27-35. doi: 10.1016/j.annepidem.2006.05.007 [DOI] [PubMed] [Google Scholar]
- 18.Haisma MS, Plaat BEC, Bijl HP, et al. Multivariate analysis of potential risk factors for lymph node metastasis in patients with cutaneous squamous cell carcinoma of the head and neck. J Am Acad Dermatol. 2016;75(4):722-730. doi: 10.1016/j.jaad.2016.06.010 [DOI] [PubMed] [Google Scholar]
- 19.Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63(1-3):8-18. doi: 10.1016/S1011-1344(01)00198-1 [DOI] [PubMed] [Google Scholar]
- 20.Veness MJ, Morgan GJ, Palme CE, Gebski V. Surgery and adjuvant radiotherapy in patients with cutaneous head and neck squamous cell carcinoma metastatic to lymph nodes: combined treatment should be considered best practice. Laryngoscope. 2005;115(5):870-875. doi: 10.1097/01.MLG.0000158349.64337.ED [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. ICD-9 Codes for Cutaneous Squamous Cell Carcinoma of the Lip
eTable 2. Univariable and Multivariable Analyses for Selected Outcomes of Interest