This safety study investigates seizure rates in men at increased risk for seizure who are taking enzalutamide for metastatic castration-resistant prostate cancer.
Key Points
Question
Are seizure rates affected by treatment with enzalutamide in patients with metastatic castration-resistant prostate cancer who have seizure risk factors?
Finding
In this open-label safety study of 423 patients receiving enzalutamide for metastatic castration-resistant prostate cancer, 4 patients experienced a confirmed seizure within the 4-month study period and 3 patients experienced a confirmed seizure following the 4-month study period. This incidence is similar to that in patients with metastatic castration-resistant prostate cancer and similar seizure risk factors but no enzalutamide exposure.
Meaning
Enzalutamide did not increase seizure incidence in men with mCRPC and seizure risk factors.
Abstract
Importance
The androgen receptor inhibitor enzalutamide prolongs survival in men with metastatic castration-resistant prostate cancer (mCRPC). In controlled clinical studies, 0.5% (10 of 2051) of patients experienced seizure, but patients with a history of or risk factors for seizure were excluded. Men with mCRPC and seizure risk factors have an estimated seizure rate of 2.8 per 100 patient-years without enzalutamide exposure.
Objective
To assess seizure incidence in patients with seizure risk factors who were receiving enzalutamide for mCRPC.
Design, Setting, and Participants
The UPWARD study (A Study to Evaluate the Potential Increased Risk of Seizures Among Metastatic Castration-Resistant Prostate Cancer Patients Treated With Enzalutamide) is an international, multicenter (73 sites in 20 countries), single-arm, open-label safety study in institutional practice. Data were collected from September 25, 2013, to February 1, 2016. Patients had at least 1 risk factor for seizure at baseline, including medications that lower seizure threshold, history of stroke, or history of seizure. Exclusion criteria included seizure (assessed by neurologic examination and history) requiring antiseizure medication within the past 12 months.
Intervention
Treatment with oral enzalutamide, 160 mg/d.
Main Outcomes and Measures
The primary end point was the proportion of evaluable patients with 1 or more independently confirmed seizures during the 4-month study period; evaluable patients were defined as those who had 3 months or more of treatment or 1 or more confirmed seizures during this treatment period.
Results
Of 423 patients with mCRPC receiving enzalutamide, 366 were evaluated. At baseline, risk factors for seizure included medications that lowered seizure threshold (242 of 423 patients [57.2%]), history of brain injury (112 [26.5%]), and history of cerebrovascular accident or transient ischemic attack (94 [22.2%]). Four of the 366 evaluable patients (1.1%) had at least 1 confirmed seizure within 4 months of enzalutamide initiation, and 3 (0.8%) additional patients experienced a seizure within 4 months following the 4-month study period. The incidence of confirmed seizure was 2.6 per 100 patient-years (7 seizures). Of the 423 patients receiving enzalutamide, 357 (84.4%) experienced at least 1 treatment-emergent adverse event (an adverse event temporally related to the study treatment); 141 (33.3%) had at least 1 serious treatment-emergent adverse event, and 29 (6.9%) had at least 1 drug-related serious adverse event. Thirty-eight deaths (9.0%) were reported during treatment or within 30 days of drug discontinuation; 4 were considered possibly drug related.
Conclusions and Relevance
Incidence of seizure is similar in patients with mCRPC and similar seizure risk factors with or without enzalutamide exposure. The risk profile presented, along with the previously established efficacy of enzalutamide, suggests that enzalutamide can benefit patients with a history of seizures or other predisposing factors, but each patient should be closely monitored for the duration of treatment.
Introduction
Enzalutamide, an androgen receptor inhibitor, has demonstrated efficacy in patients with metastatic castration-resistant prostate cancer (mCRPC).1,2 A class effect of androgen receptor antagonists,3 seizure is a known adverse effect of enzalutamide.3,4,5,6 In a phase 1 and 2 study, seizures were reported in 3 of 140 (2%) enzalutamide-treated patients at doses greater than 360 mg/d, with none reported at 160 mg/d, the approved clinical dose.4,5 Subsequent trials excluded patients with a history of or predisposing factors for seizure at baseline1,2,7,8; to our knowledge, enzalutamide safety in patients with seizure risk factors has not been previously evaluated. The postchemotherapy AFFIRM (A Multinational Phase 3, Randomized, Double-blind, Placebo-Controlled Efficacy and Safety Study of Oral MDV3100 in Patients With Progressive Castration-Resistant Prostate Cancer Previously Treated With Docetaxel-Based Chemotherapy) study2 reported 5 seizures in 800 patients (0.6%) treated with 160 mg of oral enzalutamide daily. Using the same intervention, the PREVAIL (A Multinational Phase 3, Randomized, Double-blind, Placebo-Controlled Efficacy and Safety Study of Oral MDV3100 in Chemotherapy-Naive Patients With Progressive Metastatic Prostate Cancer Who Have Failed Androgen Deprivation Therapy),1 TERRAIN (A Randomized, Double-blind, Phase II, Efficacy and Safety Study of MDV3100 vs Bicalutamide in Castrate Men With Metastatic Prostate Cancer),8 and STRIVE (A Multicenter Phase 2, Randomized, Double-blind, Efficacy and Safety Study of Enzalutamide vs Bicalutamide in Men With Prostate Cancer Who Have Failed Primary Androgen Deprivation Therapy)7 trials combined reported seizures in 4 of 1253 chemotherapy-naive patients (0.3%).
The UPWARD study (A Study to Evaluate the Potential Increased Risk of Seizures Among Metastatic Castration-Resistant Prostate Cancer Patients Treated With Enzalutamide) was conducted to address requests from the US Food and Drug Administration and the European Medicines Agency for additional information on seizure in enzalutamide-treated men with seizure risk factors. This single-arm study evaluated the incidence of independently confirmed seizure events in enzalutamide-treated men with metastatic castration-resistant prostate cancer (mCRPC) and known risk factors for seizure.
Methods
Study Design
UPWARD was a prospective, global, multicenter, single-arm, open-label, postapproval safety study (ClinicalTrials.gov identifier: NCT01977651). There were 73 sites, in a total of 20 countries, including Argentina (6 sites), Australia (6 sites), Belgium (3 sites), Canada (4 sites), Chile (4 sites), Czech Republic (2 sites), Finland (3 sites), France (2 sites), Germany (3 sites), Hungary (1 site), Israel (8 sites), Italy (4 sites), New Zealand (1 site), Republic of Korea (5 sites), Singapore (1 site), Spain (6 sites), Sweden (2 sites), Taiwan (3 sites), the United Kingdom (1 site) and the United States (8 sites). Patients received oral enzalutamide, 160 mg/d, for an initial period of 4 months, with the option to continue treatment into a 1-year extension period. Inclusion and exclusion criteria are included in the eMethods in the Supplement. The data cutoff date for this study was February 1, 2016. The independent ethics committee or institutional review board reviewed the ethical, scientific, and medical appropriateness of the study before it was conducted. A list of the institutional review boards that approved the study is given in the eAppendix in the Supplement. Informed patient consent was obtained before study-related screening procedures were performed.
Primary and Secondary End Points
The primary end point was the percentage of evaluable patients with at least 1 independently confirmed seizure during the initial 4-month treatment period. Evaluable patients were defined as those who completed 3 months or more of treatment with enzalutamide or who had at least 1 confirmed seizure in the 4-month initial treatment period. Seizure evaluation methods and a list of medications that may lower seizure threshold are included in eTable 1 in the Supplement.
Secondary end points were the cumulative proportion of all patients with seizure events, including those occurring beyond the 4-month treatment period in the evaluable population, and the number of seizure events, including only the first seizure event, per patient-time exposure in the safety population (all patients who received ≥1 dose of enzalutamide). All subsequent seizure events were recorded. Adverse events were assessed in the safety population.
Statistical Analysis
Descriptive statistics were used for continuous variables (number of patients, mean, median, SD, minimum, and maximum). Frequencies (numbers) and percentages were displayed for categorical data. Percentages by category were based on the number of patients with complete data. The primary end point was estimated using the percentage point estimate and its 95% exact CI. Statistical significance was not calculated for the primary end point.
Results
Patient Disposition, Demographics, and Baseline Characteristics
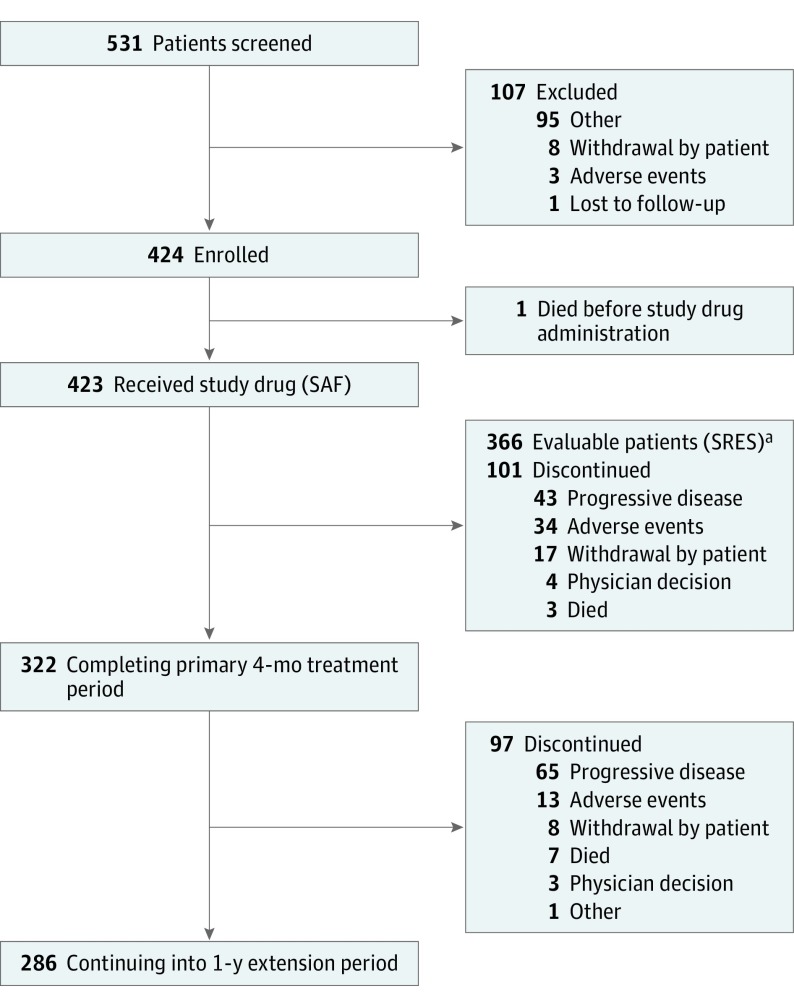
The study population consisted only of men owing to the nature of the disease. Overall, 531 patients were screened (mean age [SD], 73.2 [8.99] years); 423 received at least 1 dose of enzalutamide, and 366 (mean age [SD], 73.3 [8.94] years) were included in the evaluable population (Figure). A total of 101 (23.9%) patients discontinued treatment during the 4-month study treatment period. As of the data cutoff date of February 1, 2016, 220 (52.1%) patients were still receiving enzalutamide and 203 (47.9%) had discontinued treatment; of these, 111 (54.7%) discontinued because of progressive disease and 46 (23.2%) because of adverse events.
Figure. Disposition of Patients in the UPWARD Study.
SAF indicates safety analysis set; SRES, seizure-risk evaluation set.
aEvaluable patients were defined as those who completed at least 3 months of treatment with enzalutamide or who had at least 1 confirmed seizure in the 4-month initial treatment period.
Baseline demographic and disease characteristics are shown in eTable 2 in the Supplement. The most common seizure risk factors at baseline are reported in eTable 3 in the Supplement. Of the 423 receiving enzalutamide, concomitant medications were taken by 417 patients (98.6%) during the study.
Among evaluable patients, 4 (1.1%; 95% CI, 0.3%-2.8%) had at least 1 confirmed seizure within 4 months of enzalutamide therapy initiation. Potentially relevant medical history, seizure risk factors, and seizure type are described in eTable 4 in the Supplement. Seizure events were considered enzalutamide-related in 3 of the 4 patients (eTable 4 in the Supplement).
An additional 3 patients had a seizure after 4 months of enzalutamide treatment (eTable 4 in the Supplement). Of the 7 patients who experienced a seizure, 3 continued treatment and all 3 had a second seizure. One patient with a second seizure had a history of seizure before study participation. Of the 3 patients with a second seizure, 1 was not given antiseizure medication after the first seizure and 2 received antiseizure medication (levetiracetam), of whom 1 received levetiracetam starting 2 days before the second seizure.
Incidence of confirmed seizure among the evaluable patients was 2.6 per 100 patient-years, based on a total exposure time of 272.25 patient-years and 7 patients with a confirmed seizure. After data cutoff, 1 additional patient experienced an independently confirmed seizure (convulsion).
Adverse Events
Overall, 423 patients were included in the safety analysis. Median duration of enzalutamide treatment was 223.0 days, with 322 patients (76.1%) receiving treatment for at least 4 months, 263 (62.2%) for at least 6 months, and 75 (17.7%) for more than 1 year.
Of the 423 patients, 357 (84.4%) reported at least 1 treatment-emergent adverse event, with 205 (48.5%) experiencing a drug-related (as assessed by the investigator) adverse event (Table). The most frequently reported treatment-emergent adverse events are shown in the Table.
Table. Overview of Adverse Events in Men Taking Enzalutamide for Metastatic Castration-Resistant Prostate Cancer.
| Eventa | Event, No. (%)b | Total No. (%)c (N = 423) | |
|---|---|---|---|
| Grade <3 | Grade ≥3 | ||
| Adverse event | 357 (84.4) | ||
| Drug-related adverse eventd | 205 (48.5) | ||
| Serious adverse evente | 141 (33.3) | ||
| Drug-related serious adverse eventd | 29 (6.9) | ||
| Adverse event leading to permanent discontinuation of study drug | 66 (15.6) | ||
| Drug-related adverse event leading to permanent discontinuation of study drugd | 17 (4.0) | ||
| Death | 38 (9.0) | ||
| Frequently reported (≥5% of patients) treatment-emergent adverse events | |||
| Fatigue | 79 (18.7) | 8 (1.9) | 87 (20.6) |
| Asthenia | 64 (15.1) | 16 (3.8) | 80 (18.9) |
| Decreased appetite | 65 (15.4) | 5 (1.2) | 70 (16.5) |
| Anemia | 36 (8.5) | 17 (4.0) | 53 (12.5) |
| Back pain | 49 (11.6) | 4 (0.9) | 53 (12.5) |
| Nausea | 45 (10.6) | 3 (0.7) | 48 (11.3) |
| Constipation | 39 (9.2) | 2 (0.5) | 41 (9.7) |
| Diarrhea | 39 (9.2) | 1 (0.2) | 40 (9.5) |
| Arthralgia | 31 (7.3) | 0 | 31 (7.3) |
| Dyspnea | 22 (5.2) | 8 (1.9) | 30 (7.1) |
| Edema peripheral | 26 (6.1) | 2 (0.5) | 28 (6.6) |
| Vomiting | 25 (5.9) | 1 (0.2) | 26 (6.1) |
| Insomnia | 24 (5.7) | 0 | 24 (5.7) |
| Hot flash | 23 (5.4) | 0 | 23 (5.4) |
| Abdominal pain | 18 (4.3) | 5 (1.2) | 23 (5.4) |
| Pain | 16 (3.8) | 6 (1.4) | 22 (5.2) |
| Pain in extremity | 19 (4.5) | 3 (0.7) | 22 (5.2) |
| Hypertension | 13 (3.1) | 9 (2.1) | 22 (5.2) |
Preferred terms per Medical Dictionary for Regulatory Activities, Version 16.0 (http://www.meddra.org).
Grade refers to the severity of the adverse event with unique clinical descriptions of severity for each adverse event based on this general guideline: grade 1, mild adverse event; grade 2, moderate adverse event; grade 3, severe adverse event; grade 4, life-threatening or disabling adverse event; and grade 5, death related to adverse event.
All enrolled patients who took at least 1 dose of study drug and for whom any data were reported after the first dose of study drug (safety analysis set).
Possible or probable, as assessed by the investigator, or records where relationship was missing.
An adverse event was considered serious if, in the view of either the investigator or sponsor, it resulted in any of the following outcomes: resulted in death, was life-threatening, resulted in persistent or significant disability/incapacity or substantial disruption of the ability to conduct normal life functions, resulted in congenital anomaly or birth defect, required inpatient hospitalization or led to prolongation of hospitalization, or a medically important event.
Thirty-eight deaths (9.0%) occurred during treatment or within 30 days after treatment was discontinued; 4 deaths were considered drug related by the investigator (1 each of cerebral hemorrhage, mCRPC progression, sudden cardiac death, and general deterioration). No seizure-related deaths were reported.
Sixty-six patients (15.6%) permanently discontinued enzalutamide treatment because of a treatment-emergent adverse event; 3 of these discontinued because of a seizure event (convulsion, grand mal convulsion, and loss of consciousness).
Discussion
UPWARD, a postapproval safety study, investigated the incidence of independently confirmed seizure events in enzalutamide-treated men with mCRPC and at least 1 known risk factor for seizure at baseline. The incidence of seizures (2.6 per 100 patient-years) was comparable to that in a large retrospective analysis of patients in the United States with mCRPC and similar seizure risk factors but no exposure to enzalutamide (2.8 per 100 patient-years).9 UPWARD results demonstrate that enzalutamide did not increase seizure incidence in men with mCRPC and predisposing factors.
The safety profile of enzalutamide in UPWARD was generally consistent with that previously reported in enzalutamide-treated patients, including participants in large placebo-controlled phase 3 trials in mCRPC. As of data cutoff, 203 patients had withdrawn from the study; however, the number of withdrawals owing to disease progression and adverse events (37.4%) was lower than that observed for patients receiving enzalutamide in the AFFIRM (62.6%), PREVAIL (46.3%), and TERRAIN (48.6%) trials.1,2,8
Limitations
There are limitations associated with this study; however, a randomized controlled trial was not deemed feasible. Although enrollment of patients with particular risk factors was monitored, enrollment within each category was not capped. Some preexisting seizure risk factors, including history of seizure and history of brain arteriovenous malformations, were underrepresented here but are higher than in the Truven report.9
Conclusions
Treatment with enzalutamide did not increase seizure incidence, which was within that expected for the population studied (ie, comparable to the incidence in patients with mCRPC and similar seizure risk factors without enzalutamide exposure).9 Enzalutamide was generally well tolerated; adverse-event data were consistent with its known safety profile.1,2,7,8 These data suggest that enzalutamide did not increase seizure incidence in men with mCRPC and seizure risk factors and is an option for patients with seizure risk factors. However, it should be used with caution and input from neurology specialists. The risk profile presented, along with the previously established efficacy profile, suggests that enzalutamide can benefit patients with seizure risk factors, who should be closely monitored throughout the duration of treatment to ensure continued benefit and safety.
eMethods. Additional Methods Descriptions
eAppendix. Institutional Review Boards That Approved the Study
eTable 1. List of Medications That May Lower Seizure Threshold
eTable 2. Demographic and Baseline Characteristics
eTable 3. Seizure Risk Categories
eTable 4. Patients Experiencing a Seizure
References
- 1.Beer TM, Armstrong AJ, Rathkopf DE, et al. ; PREVAIL Investigators . Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scher HI, Fizazi K, Saad F, et al. ; AFFIRM Investigators . Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187-1197. [DOI] [PubMed] [Google Scholar]
- 3.Foster WR, Car BD, Shi H, et al. Drug safety is a barrier to the discovery and development of new androgen receptor antagonists. Prostate. 2011;71(5):480-488. [DOI] [PubMed] [Google Scholar]
- 4.Higano CS, Beer TM, Taplin ME, et al. Long-term safety and antitumor activity in the phase 1-2 study of enzalutamide in pre- and post-docetaxel castration-resistant prostate cancer. Eur Urol. 2015;68(5):795-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scher HI, Beer TM, Higano CS, et al. ; Prostate Cancer Foundation/Department of Defense Prostate Cancer Clinical Trials Consortium . Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1-2 study. Lancet. 2010;375(9724):1437-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tombal B, Borre M, Rathenborg P, et al. Enzalutamide monotherapy in hormone-naive prostate cancer: primary analysis of an open-label, single-arm, phase 2 study. Lancet Oncol. 2014;15(6):592-600. [DOI] [PubMed] [Google Scholar]
- 7.Penson DF, Armstrong AJ, Concepcion R, et al. Enzalutamide versus bicalutamide in castration-resistant prostate cancer: the STRIVE trial. J Clin Oncol. 2016;34(18):2098-2106. [DOI] [PubMed] [Google Scholar]
- 8.Shore ND, Chowdhury S, Villers A, et al. Efficacy and safety of enzalutamide versus bicalutamide for patients with metastatic prostate cancer (TERRAIN): a randomised, double-blind, phase 2 study. Lancet Oncol. 2016;17(2):153-163. [DOI] [PubMed] [Google Scholar]
- 9.Dharmani C, Bonafede M, Krivoshik A. Risk factors for and incidence of seizures in metastatic castration-resistant prostate cancer: a real-world retrospective cohort study [published online October 13, 2017]. Clin Drug Investig. doi: 10.1007/s40261-017-0578-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Additional Methods Descriptions
eAppendix. Institutional Review Boards That Approved the Study
eTable 1. List of Medications That May Lower Seizure Threshold
eTable 2. Demographic and Baseline Characteristics
eTable 3. Seizure Risk Categories
eTable 4. Patients Experiencing a Seizure