Key Points
Question
Is the burden of atrial fibrillation associated with the risk of ischemic stroke and other thromboembolism in paroxysmal atrial fibrillation?
Findings
In a cohort study of 1965 adults with paroxysmal atrial fibrillation, a greater burden of atrial fibrillation (≥11%) on 14-day noninvasive, continuous electrocardiographic monitoring was associated with a significantly higher rate of thromboembolism while not taking anticoagulation vs a lower burden.
Meaning
Greater atrial fibrillation burden is associated with a higher risk of ischemic stroke independent of known risk factors in adults with paroxysmal atrial fibrillation; knowing the burden of atrial fibrillation may assist with shared decision making for stroke prevention strategies.
Abstract
Importance
Atrial fibrillation is a potent risk factor for stroke, but whether the burden of atrial fibrillation in patients with paroxysmal atrial fibrillation independently influences the risk of thromboembolism remains controversial.
Objective
To determine if the burden of atrial fibrillation characterized using noninvasive, continuous ambulatory monitoring is associated with the risk of ischemic stroke or arterial thromboembolism in adults with paroxysmal atrial fibrillation.
Design, Setting, and Participants
This retrospective cohort study conducted from October 2011 and October 2016 at 2 large integrated health care delivery systems used an extended continuous cardiac monitoring system to identify adults who were found to have paroxysmal atrial fibrillation on 14-day continuous ambulatory electrocardiographic monitoring.
Exposures
The burden of atrial fibrillation was defined as the percentage of analyzable wear time in atrial fibrillation or flutter during the up to 14-day monitoring period.
Main Outcomes and Measures
Ischemic stroke and other arterial thromboembolic events occurring while patients were not taking anticoagulation were identified through November 2016 using electronic medical records and were validated by manual review. We evaluated the association of the burden of atrial fibrillation with thromboembolism while not taking anticoagulation after adjusting for the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) or CHA2DS2-VASc stroke risk scores.
Results
Among 1965 adults with paroxysmal atrial fibrillation, the mean (SD) age was 69 (11.8) years, 880 (45%) were women, 496 (25%) were persons of color, the median ATRIA stroke risk score was 4 (interquartile range [IQR], 2-7), and the median CHA2DS2-VASc score was 3 (IQR, 1-4). The median burden of atrial fibrillation was 4.4% (IQR ,1.1%-17.23%). Patients with a higher burden of atrial fibrillation were less likely to be women or of Hispanic ethnicity, but had more prior cardioversion attempts compared with those who had a lower burden. After adjusting for either ATRIA or CHA2DS2-VASc stroke risk scores, the highest tertile of atrial fibrillation burden (≥11.4%) was associated with a more than 3-fold higher adjusted rate of thromboembolism while not taking anticoagulants (adjusted hazard ratios, 3.13 [95% CI, 1.50-6.56] and 3.16 [95% CI, 1.51-6.62], respectively) compared with the combined lower 2 tertiles of atrial fibrillation burden. Results were consistent across demographic and clinical subgroups.
Conclusions and Relevance
A greater burden of atrial fibrillation is associated with a higher risk of ischemic stroke independent of known stroke risk factors in adults with paroxysmal atrial fibrillation.
This cohort study analyzes the association between the burden of atrial fibrillation on 14-day noninvasive, continuous electrocardiographic monitoring and the rate of thromboembolism among adults with paroxysmal atrial fibrillation while not taking anticoagulation.
Introduction
Atrial fibrillation increases the risk for ischemic stroke and other arterial thromboembolism and affects millions of US adults.1,2,3 Atrial fibrillation can lead to thrombus development within the atria, particularly the left atrial appendage, which can cause thromboembolic events.4,5
Whether thromboembolic risk varies among those with paroxysmal vs nonparoxysmal atrial fibrillation has been an ongoing question. Compared with patients with nonparoxysmal atrial fibrillation, those with paroxysmal atrial fibrillation may be at higher thromboembolic risk given intermittent organized contraction of the atria following periods of atrial fibrillation. However, a recent meta-analysis suggested that there is a higher risk in patients with nonparoxysmal vs paroxysmal atrial fibrillation not taking anticoagulants,6 but current guidelines recommend anticoagulants for stroke prevention based on clinical risk stratification for either nonparoxysmal or paroxysmal atrial fibrillation.7 Yet, little is known about whether the burden of atrial fibrillation (ie, the amount of time spent in atrial fibrillation) independently increases the risk of stroke among patients with paroxysmal atrial fibrillation for whom decision making about stroke prevention strategies can be challenging. Previous studies have focused on selected populations with implanted devices, such as pacemakers,8,9,10,11,12,13,14 implantable cardioverter defibrillators,11,12,13,14 or cardiac resynchronization therapy devices,15 with varying thresholds for defining a meaningful amount of time in atrial fibrillation during long-term monitoring. Furthermore, studies have not examined the effect of the burden of atrial fibrillation measured using short-term, continuous, noninvasive strategies on the risk of stroke and other thromboembolic events while not taking anticoagulation. Within a large cohort with paroxysmal atrial fibrillation confirmed on extended continuous ambulatory electrocardiographic monitoring, we evaluated whether a greater burden of atrial fibrillation was independently associated with the risk of stroke and arterial thromboembolism while not taking anticoagulants to assist in improving stroke risk stratification.
Methods
Source Population
The source population included members of Kaiser Permanente Northern California and Southern California, 2 integrated health care delivery systems providing care to more than 8 million persons. The membership is highly representative of the statewide population in regard to age, sex, race/ethnicity, and socioeconomic status.16,17,18 The project was approved by the Kaiser Permanente Northern and Southern California institutional review boards, and a waiver of informed consent was obtained due to the nature of the study.
Design and Analytic Sample
The Kaiser Permanente Real-World Heart Monitoring Strategy Evaluation, Treatment Patterns, and Health Metrics in Atrial Fibrillation (KP-RHYTHM) study first identified all adult members who underwent continuous ambulatory electrocardiographic monitoring using the ZIO service (iRhythm Technologies Inc) between October 2011 and October 2016. The ZIO Patch is a lightweight, lead wire–free, single-patient-use electrocardiographic monitor that is applied to the left upper chest and records and stores up to 14 days of continuous, beat-to-beat electrocardiographic data. After mailing the monitor back to iRhythm Technologies, Inc., the recording is analyzed using proprietary algorithms with verification of the algorithm output and report creation by conducted by certified cardiac technicians.13,14 The report is then transferred to the treating physician.
As the aim of this study was to examine the relationship of the burden of atrial fibrillation with thromboembolism while not receiving anticoagulation therapy in adults with paroxysmal atrial fibrillation, we identified patients with confirmed paroxysmal atrial fibrillation (>0% and <100% overall burden) on ZIO Patch electrocardiographic monitoring.13,14 For any episode to be labeled as atrial fibrillation (which included atrial flutter), the minimum episode duration was longer than 30 seconds. We excluded patients younger than age 18 years at the time of monitoring and those with fewer than 12 months of prior continuous membership or pharmacy benefit, disenrollment after device removal, death during the monitoring period, prior organ transplant, no analyzable wear time, or no periods of not taking anticoagulation during follow-up.
Predictor Variable
The primary predictor was the burden of atrial fibrillation in the setting of paroxysmal atrial fibrillation. To compare patients wearing the ZIO Patch for different lengths of time (up to 14 days), we examined 2 measures of atrial fibrillation burden. Our primary measure was the percentage of analyzable wear time spent in atrial fibrillation; the secondary measure was the longest continuous episode of atrial fibrillation during monitoring.
Follow-up
Follow-up occurred from the end of ZIO Patch monitoring through November 30, 2016. Patients were censored for death (n = 58 [3.0%]), health plan disenrollment (n = 82 [4.2%]), organ transplant (n = 4 [0.2%]) or end of follow-up (n = 1778 [90.5%]). Of note, patients who were censored because of health plan disenrollment were younger and had less comorbidity burden. Deaths were ascertained from electronic medical records, Social Security Administration files,19 and California state death certificate files.20
Outcomes
The outcome of interest included hospitalization for ischemic stroke or arterial thromboembolism while not taking anticoagulants. We searched hospital discharge and emergency department databases for diagnostic codes for potential events using International Classification of Diseases, Ninth and Tenth Edition codes (data not shown).21,22 Medical records were adjudicated by physicians using standardized criteria as previously described.21,22 A thromboembolic event included ischemic stroke defined as an acute neurological deficit lasting more than 24 hours that was not explained by other causes (eg, primary intracranial hemorrhage, trauma, infection, or vasculitis); transient ischemic attack, which included the same definition of ischemic stroke but with a 24-hour or less duration; and other symptomatic arterial thromboembolism confirmed by radiographic imaging, intraoperative examination, or pathological findings in the absence of underlying atherosclerotic disease in the affected artery. Only events that occurred while the patient was not taking anticoagulants that were confirmed by medical records were included. Warfarin exposure was based on a previously validated algorithm using pharmacy dispensings and intervening international normalized ratio measurements23; exposure to direct oral anticoagulants was based on the information on the number of days supplied per the dispensed prescription.
Covariates
We identified age, sex, and self-reported race/ethnicity from health plan databases. We identified stroke risk factors from electronic medical records using previously described methods.22 These included prior ischemic stroke, chronic heart failure, diabetes, hypertension, and reduced kidney function. Reduced kidney function included an estimated glomerular filtration rate less than 45 ml/min/1.73 m2 using the Chronic Kidney Disease Epidemiology Collaboration estimating equation,24 as well as proteinuria ascertained from urine dipstick results of 1+ or greater.25 We also calculated each patient’s predicted risk of thromboembolism using the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) and CHA2DS2-VASc scores. The ATRIA stroke risk score, which has a range of 0 to 15 (low risk = 0-5, moderate risk = 6, and high risk ≥ 7), has been shown to have greater discrimination than other risk schemes in multiple populations in the United States, United Kingdom, and Sweden.22,26,27 The CHA2DS2-VASc risk score,28 which has a range of 0 to 9 (low risk = 0), has been incorporated into various clinical guidelines.7
We assessed for missingness in the variables presented in the Table and found that 266 (12.5%) in the cohort were missing an estimated glomerular filtration rate and 10 (0.5%) in the cohort had no recordings of the longest episode of atrial fibrillation. In an earlier period, we showed that warfarin was obtained through non–health plan pharmacies for fewer than 6% of adults with atrial fibrillation.21 For medical conditions, including proteinuria, we classified patients with no documented evidence as not having the condition of interest.
Table. Baseline Characteristics of Adults With Paroxysmal Atrial Fibrillation Found on Extended Continuous Ambulatory Electrocardiographic Monitoring, Overall and Stratified by Burden of Atrial Fibrillation.
| Characteristic | No. (%) | P Value | |||
|---|---|---|---|---|---|
| Overall (N = 1965) | Burden of Atrial Fibrillation | ||||
| Tertile 1 (n = 679 [0.01%-2.03%]) | Tertile 2 (n = 652 [2.05%-11.28%]) | Tertile 3(n = 634 [11.36%-99.99%]) | |||
| Age, mean (SD), y | 68.8 (11.8) | 68.5 (12.4) | 69.4 (11.2) | 68.5 (11.6) | .27 |
| Women | 880 (44.8) | 341 (50.2) | 290 (44.5) | 249 (39.3) | <.001 |
| Race/ethnicity | |||||
| White/European | 1469 (74.8) | 500 (73.6) | 485 (74.4) | 484 (76.3) | .10 |
| Black/African American | 100 (5.1) | 46 (6.8) | 35 (5.4) | 19 (3.0) | |
| Asian/Pacific Islander | 266 (13.5) | 91 (13.4) | 85 (13.0) | 90 (14.2) | |
| Other | 130 (6.6) | 42 (6.2) | 47 (7.2) | 41 (6.5) | |
| Hispanic ethnicity | 198 (10.1) | 72 (10.6) | 64 (9.8) | 62 (9.8) | <.001 |
| ATRIA stroke risk score | |||||
| Mean (SD) | 4.3 (2.8) | 4.3 (2.8) | 4.4 (2.8) | 4.3 (2.9) | .61 |
| Median (IQR) | 4.0 (2.0-7.0) | 4.0 (1.0-7.0) | 4.0 (2.0-7.0) | 4.0 (2.0-7.0) | .70 |
| CHA2DS2-VASc | |||||
| Mean (SD) | 2.6 (1.6) | 2.6 (1.6) | 2.6 (1.6) | 2.6 (1.7) | .97 |
| Median (IQR) | 3.0 (1.0-4.0) | 3.0 (1.0-4.0) | 3.0 (1.0-4.0) | 2.0 (1.0-4.0) | .95 |
| Diabetes | 387 (19.7) | 123 (18.1) | 126 (19.3) | 138 (21.8) | .24 |
| Heart failure | 179 (9.1) | 55 (8.1) | 60 (9.2) | 64 (10.1) | .45 |
| Hypertension | 1221 (62.1) | 430 (63.3) | 398 (61.0) | 393 (62.0) | .69 |
| Proteinuria | 201 (10.2) | 76 (11.2) | 71 (10.9) | 54 (8.5) | .22 |
| Prior ischemic stroke | 54 (2.7) | 13 (1.9) | 17 (2.6) | 24 (3.8) | .11 |
| Catheter ablation during previous 12 mo | 49 (2.5) | 20 (3.0) | 14 (2.2) | 15 (2.4) | .63 |
| Cardioversion attempt during previous 12 mo | 54 (2.8) | 13 (1.9) | 14 (2.2) | 27 (4.3) | .02 |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | |||||
| 90-150 | 289 (14.7) | 123 (18.1) | 80 (12.3) | 86 (13.6) | .24 |
| 60-89 | 954 (48.5) | 313 (46.1) | 335 (51.4) | 306 (48.3) | |
| 45-59 | 308 (15.7) | 97 (14.3) | 97 (14.9) | 114 (18.0) | |
| 30-44 | 120 (6.1) | 38 (5.6) | 40 (6.1) | 42 (6.6) | |
| 15-29 | 32 (1.6) | 12 (1.8) | 11 (1.7) | 9 (1.4) | |
| <15 | 3 (0.2) | 2 (0.3) | 1 (0.2) | 0 (0.0) | |
| Missing | 253 (12.9) | 92 (13.5) | 86 (13.2) | 75 (11.8) | |
| Dialysis | 6 (0.3) | 2 (0.3) | 2 (0.3) | 2 (0.3) | |
| Anticoagulant use during previous 90 d | 13 (0.7) | 2 (0.3) | 3 (0.5) | 8 (1.3) | .09 |
| Anticoagulant use 30 d postmonitoring | 759 (38.6) | 212 (31.2) | 238 (36.5) | 309 (48.7) | <.001 |
| Atrial fibrillation burden, median (IQR), % | 4.42 (1.11-17.16) | 0.56 (0.13-1.18) | 4.70 (3.21-6.95) | 27.06 (17.74-46.90) | <.001 |
| Total duration of atrial fibrillation, median (IQR), min | 710 (175-2540) | 89 (19-208) | 775 (515-1140) | 4456 (26655-7617) | <.001 |
| Longest episode of atrial fibrillation, median (IQR), min | 171 (49-590) | 31 (6-90) | 250 (95-538) | 700 (202-1824) | <.001 |
| Missing | 10 (0.5) | 10 (1.4) | 0 (0.0) | 0 (0.0) | |
Abbreviations: ATRIA, Anticoagulation and Risk Factors in Atrial Fibrillation; IQR, interquartile range.
Analytic Approach
All analyses were conducted using SAS software, version 9.3 (SAS Institute.). Given the non-normal distribution of the burden of atrial fibrillation, we compared baseline characteristics across tertiles of atrial fibrillation burden using analyses of variance or nonparametric tests for continuous variables and χ2 tests for categorical variables. We used a Poisson regression to estimate the annual incidence of confirmed thromboembolic events for periods during follow-up while patients were not taking anticoagulation as a rate per 100 person-years with 95% confidence limits, overall and by tertile of the burden of atrial fibrillation. We conducted separate Cox regression models for the primary and secondary measures of atrial fibrillation burden with thromboembolism using time not taking anticoagulation as our analytic timeline, unadjusted and after adjustment for either ATRIA or CHA2DS2-VASc risk score as a continuous variable (eFigure in the Supplement). In addition, we performed subgroup analyses by age, sex, and the presence or absence of chronic kidney disease, diabetes, or hypertension. Finally, we conducted sensitivity analyses in which we separately excluded patients with a previous ischemic stroke or those who had baseline exposure to anticoagulants, with censoring at the time of anticoagulant initiation during follow-up.
Results
Study Participants
Between October 2011 and October 2016, we identified 25 268 ZIO Patch monitoring episodes. We identified 1965 eligible adult patients who had paroxysmal atrial fibrillation during up to 14 days of undergoing continuous ambulatory electrocardiographic monitoring (median [interquartile range (IQR)] analyzable wear time, 14 [11-14] days) before the start of follow-up (Figure 1). Overall, the mean (SD) age was 69 (11.8) years, with 880 women (45%), 100 African American participants (5%), 266 Asian/Pacific Islander participants (14%), 198 Hispanic participants (10%), a median (IQR) ATRIA stroke risk score of 4 (2-7), and median (IQR) CHA2DS2-VASc score of 3 (1-4) (Table). The material differences in baseline characteristics included a lower prevalence of women, Hispanic race/ethnicity, a higher prevalence of prior cardioversion attempts, and anticoagulant use within 30 days after monitoring with a higher tertile of atrial fibrillation burden (Table).
Figure 1. Eligible Adults With Paroxysmal Atrial Fibrillation Found on Continuous Ambulatory Electrocardiographic (ECG) Monitoring.
The ZIO XT Patch is distributed through iRhythm Technologies, Inc.
Atrial Fibrillation Burden Distribution
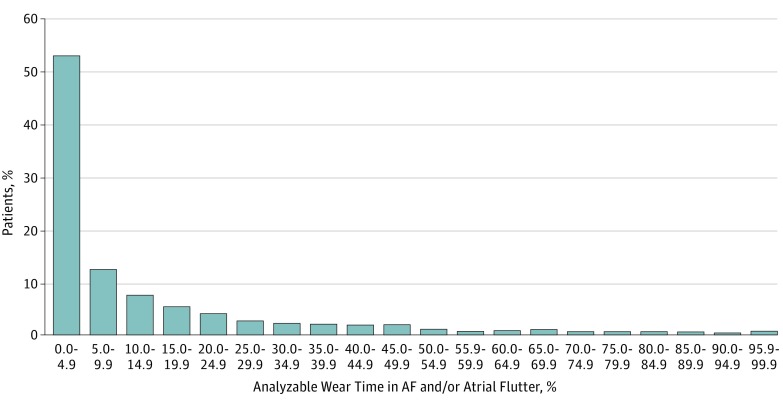
The burden of atrial fibrillation was non-normally distributed, with a median (IQR) of 4.4% (1.1%-17.2%) (Figure 2). The median (IQR) duration of the longest continuous episode of atrial fibrillation was 171 (49-590) minutes.
Figure 2. Distribution of Atrial Fibrillation (AF) Burden Found on Continuous Ambulatory Electrocardiographic Monitoring Among 1965 Adults With Confirmed Paroxysmal AF.
Atrial Fibrillation Burden and Thromboembolic Risk
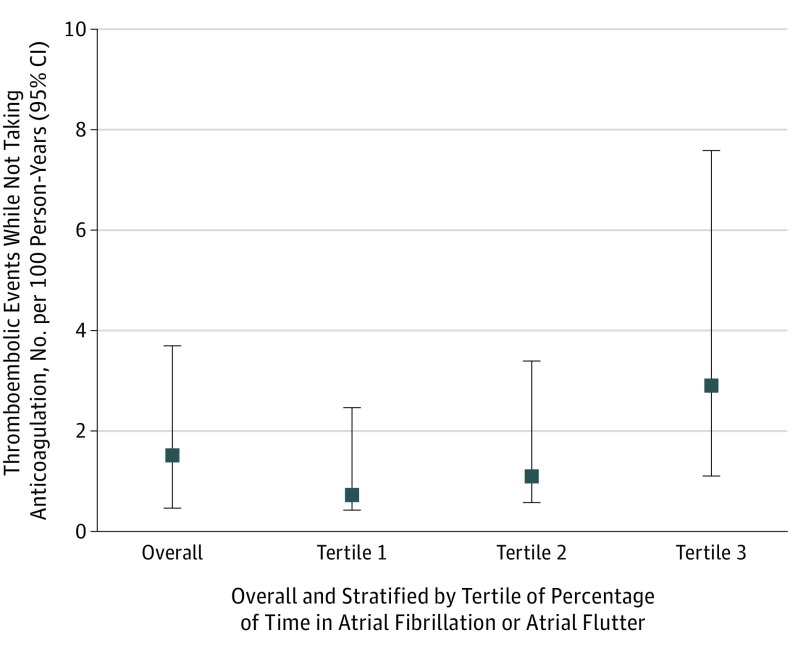
During follow-up, we identified 29 valid thromboembolic events (19 ischemic strokes, 8 transient ischemic attacks, 2 other arterial thromboembolic events) occurring while patients were not taking anticoagulation, with a median (IQR) time between the end of electrocardiographic monitoring to the thromboembolic event of 8 (2-15) months. During 1915 person years of follow-up while patients were not taking anticoagulation, the unadjusted thromboembolism incidence while not taking anticoagulation was 1.51 per 100 person-years (95% CI,1.05-2.18) (Figure 3). Across tertiles of burden of atrial fibrillation, the unadjusted thromboembolism incidence while not taking anticoagulation was higher in the highest tertile of burden of atrial fibrillation (Figure 3).
Figure 3. Thromboembolic Event Rates While Not Taking Anticoagulation, Overall and Stratified by Atrial Fibrillation (AF) Burden Tertile in 1965 Adults With Confirmed Paroxysmal AF.
Overall, there were 29 thromboembolic events for 1915 person-years. In tertile 1, there were 5 events over 690 person-years in which patients spent 0.01%-2.03% of time in AF or atrial flutter. In tertile 2, there were 7 events over 639 person-years in which patients spent 2.05%-11.28% of time in AF or atrial flutter. In tertile 3, there were 17 events over 586 person-years in which patients spent 11.36%-99.99% of time in AF or atrial flutter.
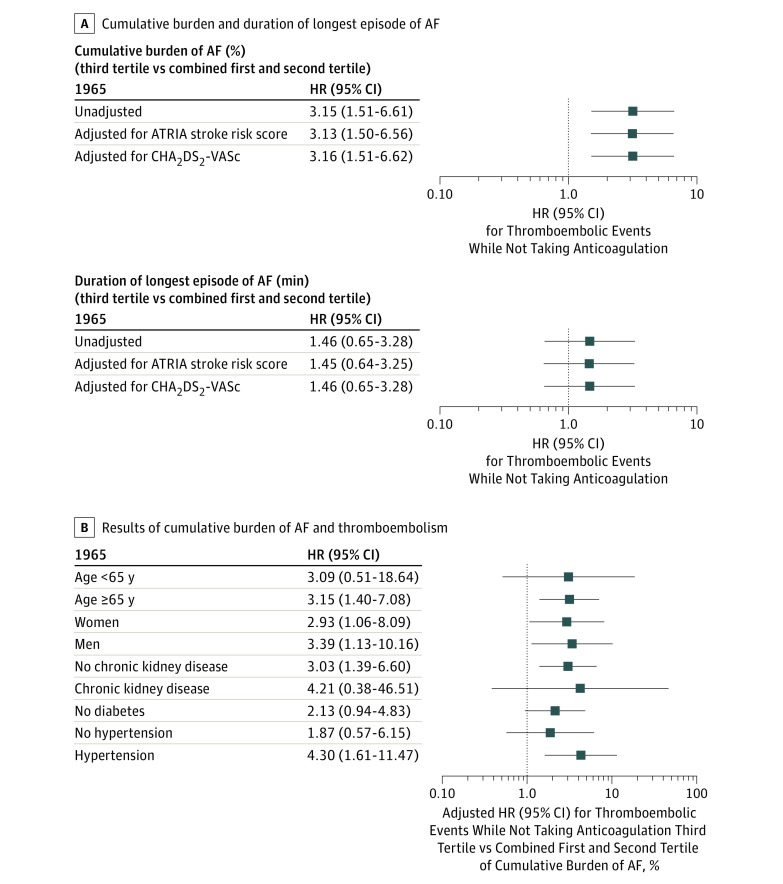
We compared patients within the combined first and second tertiles with those in the third tertile of burden of atrial fibrillation and found a 215% (95% CI, 51%-561%) increase in the unadjusted risk of thromboembolic events while not taking anticoagulation (Figure 4A). Adjusting for ATRIA or CHA2DS2-VASc risk scores did not materially affect the results (Figure 4A). Analyses in the subgroups of age, sex, and the presence or absence of chronic kidney disease or hypertension, as well as in those without diabetes, demonstrated similar associations and there were no significant interactions (Figure 4B). Results were similar in patients without previous ischemic stroke (eTable 1 in the Supplement), and the association was stronger among patients with no baseline or follow-up exposure to anticoagulants (eTable 2 in the Supplement). In contrast, a secondary measure of atrial fibrillation burden—the duration of the longest observed episode—was not significantly associated with thromboembolism while not taking anticoagulation, overall (Figure 4A), and in those without previous ischemic stroke (eTable 1 in the Supplement) or no baseline or follow-up exposure to anticoagulants (eTable 2 in the Supplement).
Figure 4. Association Between Atrial Fibrillation (AF) Burden and Thromboembolic Events While Not Taking Anticoagulation in Adults With Confirmed Paroxysmal AF.
A, Unadjusted and multivariable-adjusted results for the cumulative burden of AF and the duration of the longest episode of AF in the overall cohort. B, Results of the cumulative burden of AF and thromboembolism in prespecified patient subgroups with adjustment for Anticoagulation and Risk Factors in Atrial Fibrillation risk score. Given the distribution of AF burden by diabetes status, a model estimate was not possible in the subgroup with diabetes.
Discussion
Among a contemporary population of adults with confirmed paroxysmal atrial fibrillation, a greater cumulative burden of atrial fibrillation that was identified using up to 14-day continuous, noninvasive electrocardiographic monitoring was independently associated with a higher risk of subsequent ischemic stroke or arterial thromboembolism while patients were not taking anticoagulation, even after adjusting for known stroke risk factors. Furthermore, results were consistent across key subgroups. In contrast, the duration of the longest continuous episode of atrial fibrillation during the monitoring period did not predict subsequent thromboembolism.
We uniquely examined the prospective association between the burden of atrial fibrillation using a noninvasive, short-term continuous monitoring approach and the subsequent risk of thromboembolism in paroxysmal atrial fibrillation. In contrast, the limitations of previous studies include examining only selected samples of patients who were receiving invasive, expensive implanted devices; using a range of thresholds for defining a meaningful burden of atrial fibrillation and the variable duration of follow-up; not restricting to periods during which patients were not taking anticoagulation; or not always validating ischemic stroke and other thromboembolic events. In a pooled analysis29 of 10 016 patients with cardiac implantable electronic devices (ie, dual-chamber pacemaker, implantable cardioverter-defibrillator, or a cardiac resynchronization therapy device) from TRENDS,30 PANORAMA, and the Italian Clinical Service Project,31 Boriani et al29 evaluated device-detected atrial tachyarrhythmias and thromboembolic risk in patients with paroxysmal or persistent atrial fibrillation. In this analysis, the rate of ischemic stroke or transient ischemic attack was low, ranging from 0.32 per 100 person-years for having 0 to fewer than 5 total minutes to 0.49 per 100 person-years for having 23 or more total hours of atrial fibrillation.29 After adjusting for the CHADS2 score and the receipt of anticoagulation at entry, increasing time in atrial fibrillation was associated with a higher risk of stroke as a continuous measure (hazard ratio [HR], 1.03 per hour; 95% CI, 1.00-1.05) and using prespecified cutoffs of 1 hour or longer (HR, 2.11; 95% CI, 1.22-3.64) and 5 minutes or longer (HR, 1.76; 95% CI, 1.02-3.02).29 In the Atrial Fibrillation Reduction Atrial Pacing Trial (ASSERT) of 2580 patients with a pacemaker or cardioverter defibrillator and no prior atrial fibrillation or atrial flutter, asymptomatic atrial tachyarrhythmias lasting longer than 6 minutes during a 3-month monitoring period were associated with a higher adjusted rate of ischemic stroke or systemic embolism (HR, 2.49; 95% CI, 1.28-4.85).13 However, this study was not focused on patients with paroxysmal atrial fibrillation and did not characterize the burden of atrial fibrillation. A case-crossover analysis examined 187 veterans who had a cardiac implantable electronic device and experienced an ischemic stroke.11 A positive burden of atrial fibrillation was defined as having 5.5 or more hours of atrial fibrillation on any day in the 30 days before the stroke. Among 16 patients who had discordance in having a positive burden in the 30 days vs no atrial fibrillation in the 91 to 120 days before their stroke, a positive burden of recent atrial fibrillation was associated with an elevated risk of stroke after adjusting for warfarin use (adjusted odds ratio, 5.22; 95% CI, 1.22-47.4). However, precision was limited and the study was not focused on patients with known paroxysmal atrial fibrillation.11 In contrast, among 178 patients with heart failure and reduced ejection fraction, prior atrial fibrillation, and an implanted cardiac resynchronization therapy device, experiencing atrial high-rate events of 1% or more during a 24-hour period was not significantly associated with a higher rate of thromboembolism.15 Our study materially extends previous efforts by focusing on a widely available, noninvasive extended monitoring strategy in patients with confirmed paroxysmal atrial fibrillation using a common metric of burden of atrial fibrillation that was standardized across patients and also demonstrates the independent prognostic information of atrial fibrillation burden beyond known stroke risk factors.
Our analysis was strengthened by including a diverse, community-based sample of contemporary adults with paroxysmal atrial fibrillation. We systematically captured thromboembolic events that were validated by physician review using standardized clinical criteria and confirmed that the thromboembolic event occurred while the patients were not taking anticoagulation, with an incidence of thromboembolism similar to that seen in recent clinical trials and observational studies. We linked data from a US Food and Drug Administration–approved noninvasive, extended continuous electrocardiographic monitoring device (including classification of beat-to-beat data up to 14-days duration using validated algorithms) with longitudinal data on anticoagulation and clinical outcomes. We adjusted for predicted stroke risk using either the CHA2DS2-VASc or ATRIA risk score—the latter being derived and validated in Kaiser Permanente populations22 and having greater discrimination than the CHA2DS2-VASc risk score in several US and European populations.22,26,27 While our study sample included a range of predicted stroke risk, most patients were considered at low to moderate risk using the validated ATRIA risk score,22,26,27 and risk scores were similar across tertiles of burden of atrial fibrillation, which highlights the potential use of information from atrial fibrillation burden in patients with paroxysmal atrial fibrillation, in which the anticoagulation decision is most challenging. This is further highlighted given that patients with paroxysmal atrial fibrillation may be less likely to receive antithrombotic therapy compared with patients who have nonparoxysmal atrial fibrillation, regardless of underlying predicted stroke risk.32
Limitations
Our analysis had several limitations. Information on the exact reasons for undergoing arrhythmia monitoring and for not receiving anticoagulation was unavailable. Given the number of events, we had limited power to determine thromboembolic risk at more granular cutoffs of atrial fibrillation burden, but our data suggested a threshold of approximately 11% as meaningful in the overall cohort and across age, sex, and subgroups without diabetes, chronic kidney disease, and hypertension. We were underpowered to evaluate the association of atrial fibrillation burden with thromboembolism in this subset of patients with a CHA2DS2-VASc score of 0 or 1. Patients in the highest tertile of atrial fibrillation burden were more likely to receive anticoagulation, which led to modestly fewer person-years while not taking anticoagulation in this subgroup (586) than those in the first (690) or second (639) tertiles, but this would bias the association between atrial fibrillation burden and thromboembolism toward the null. While a greater burden of atrial fibrillation could theoretically lead to longer periods of blood stasis, the promotion of thrombus generation, and the development of worsening of certain stroke risk factors (eg, heart failure or impaired kidney function), we were unable to determine if the burden of atrial fibrillation is a marker of risk or causative. Although we studied primarily insured adults in California, our results are likely to be generalizable to other populations and practice settings. Our analysis that examined any intermittent periods of time not taking anticoagulation during follow-up as a continuous period for an individual patient was based on the assumption of a constant hazard of the outcome, which may not be true; however, we did not find any evidence of violation of the proportionality assumption. Our continuous electrocardiographic monitoring strategy was only up to 14 days, so we were unable to evaluate the potential value of longer (or shorter) periods to characterize the burden of atrial fibrillation. Finally, as stroke risk factors can accumulate over time in patients, risk stratification is a dynamic process and further study is needed to evaluate the incremental utility of repeated episodes of characterizing atrial fibrillation burden.
Conclusions
In conclusion, a greater burden of atrial fibrillation identified using a noninvasive, 14-day continuous monitoring strategy is associated with a higher risk of ischemic stroke and arterial thromboembolism in adults with paroxysmal atrial fibrillation that is independent of known stroke risk factors. Characterizing the burden of atrial fibrillation in patients with paroxysmal atrial fibrillation could assist patients and physicians in having a more informed, shared decision-making discussion about stroke prevention strategies.
eTable 1. Association of cumulative burden of atrial fibrillation with thromboembolic events off anticoagulation in adults with paroxysmal atrial fibrillation and no previous ischemic stroke
eTable 2. Association of cumulative burden of atrial fibrillation with thromboembolic events off anticoagulation in adults with paroxysmal atrial fibrillation who had no baseline or follow-up exposure to anticoagulants
eFigure. Depiction of analytical timeline of adults with paroxysmal atrial fibrillation while off or on anticoagulation therapy
References
- 1.Go AS, Hylek EM, Phillips KA, et al. . Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. JAMA. 2001;285(18):2370-2375. [DOI] [PubMed] [Google Scholar]
- 2.Miyasaka Y, Barnes ME, Gersh BJ, et al. . Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114(2):119-125. [DOI] [PubMed] [Google Scholar]
- 3.Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the US adult population. Am J Cardiol. 2013;112(8):1142-1147. [DOI] [PubMed] [Google Scholar]
- 4.Stoddard MF, Dawkins PR, Prince CR, Ammash NM. Left atrial appendage thrombus is not uncommon in patients with acute atrial fibrillation and a recent embolic event: a transesophageal echocardiographic study. J Am Coll Cardiol. 1995;25(2):452-459. [DOI] [PubMed] [Google Scholar]
- 5.Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996;61(2):755-759. [DOI] [PubMed] [Google Scholar]
- 6.Ganesan AN, Chew DP, Hartshorne T, et al. . The impact of atrial fibrillation type on the risk of thromboembolism, mortality, and bleeding: a systematic review and meta-analysis. Eur Heart J. 2016;37(20):1591-1602. [DOI] [PubMed] [Google Scholar]
- 7.January CT, Wann LS, Alpert JS, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1-e76. [DOI] [PubMed] [Google Scholar]
- 8.Glotzer TV, Hellkamp AS, Zimmerman J, et al. ; MOST Investigators . Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation. 2003;107(12):1614-1619. [DOI] [PubMed] [Google Scholar]
- 9.Capucci A, Santini M, Padeletti L, et al. ; Italian AT500 Registry Investigators . Monitored atrial fibrillation duration predicts arterial embolic events in patients suffering from bradycardia and atrial fibrillation implanted with antitachycardia pacemakers. J Am Coll Cardiol. 2005;46(10):1913-1920. [DOI] [PubMed] [Google Scholar]
- 10.Botto GL, Padeletti L, Santini M, et al. . Presence and duration of atrial fibrillation detected by continuous monitoring: crucial implications for the risk of thromboembolic events. J Cardiovasc Electrophysiol. 2009;20(3):241-248. [DOI] [PubMed] [Google Scholar]
- 11.Turakhia MP, Ziegler PD, Schmitt SK, et al. . Atrial fibrillation burden and short-term risk of stroke: case-crossover analysis of continuously recorded heart rhythm from cardiac electronic implanted devices. Circ Arrhythm Electrophysiol. 2015;8(5):1040-1047. [DOI] [PubMed] [Google Scholar]
- 12.Glotzer TV, Daoud EG, Wyse DG, et al. . The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol. 2009;2(5):474-480. [DOI] [PubMed] [Google Scholar]
- 13.Healey JS, Connolly SJ, Gold MR, et al. ; ASSERT Investigators . Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366(2):120-129. [DOI] [PubMed] [Google Scholar]
- 14.Van Gelder IC, Healey JS, Crijns HJGM, et al. . Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J. 2017;38(17):1339-1344. [DOI] [PubMed] [Google Scholar]
- 15.Shanmugam N, Boerdlein A, Proff J, et al. . Detection of atrial high-rate events by continuous home monitoring: clinical significance in the heart failure-cardiac resynchronization therapy population. Europace. 2012;14(2):230-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82(5):703-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon NP. Characteristics of adult health plan members in the Northern California region membership, as estimated from the 2011 Member Health Survey. https://divisionofresearch.kaiserpermanente.org/projects/memberhealthsurvey/SiteCollectionDocuments/mhs11reg.pdf. Accessed February 15, 2018.
- 18.Koebnick C, Langer-Gould AM, Gould MK, et al. . Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16(3):37-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curb JD, Ford CE, Pressel S, Palmer M, Babcock C, Hawkins CM. Ascertainment of vital status through the National Death Index and the Social Security Administration. Am J Epidemiol. 1985;121(5):754-766. [DOI] [PubMed] [Google Scholar]
- 20.Arellano MG, Petersen GR, Petitti DB, Smith RE. The California Automated Mortality Linkage System (CAMLIS). Am J Public Health. 1984;74(12):1324-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Go AS, Hylek EM, Chang Y, et al. . Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA. 2003;290(20):2685-2692. [DOI] [PubMed] [Google Scholar]
- 22.Singer DE, Chang Y, Borowsky LH, et al. . A new risk scheme to predict ischemic stroke and other thromboembolism in atrial fibrillation: the ATRIA study stroke risk score. J Am Heart Assoc. 2013;2(3):e000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Go AS, Hylek EM, Borowsky LH, Phillips KA, Selby JV, Singer DE. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Ann Intern Med. 1999;131(12):927-934. [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296-1305. [DOI] [PubMed] [Google Scholar]
- 26.van den Ham HA, Klungel OH, Singer DE, Leufkens HG, van Staa TP. Comparative performance of ATRIA, CHADS2, and CHA2DS2-VASc risk scores predicting stroke in patients with atrial fibrillation: results from a national primary care database. J Am Coll Cardiol. 2015;66(17):1851-1859. [DOI] [PubMed] [Google Scholar]
- 27.Aspberg S, Chang Y, Atterman A, Bottai M, Go AS, Singer DE. Comparison of the ATRIA, CHADS2, and CHA2DS2-VASc stroke risk scores in predicting ischaemic stroke in a large Swedish cohort of patients with atrial fibrillation. Eur Heart J. 2016;37(42):3203-3210. [DOI] [PubMed] [Google Scholar]
- 28.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263-272. [DOI] [PubMed] [Google Scholar]
- 29.Boriani G, Glotzer TV, Santini M, et al. . Device-detected atrial fibrillation and risk for stroke: an analysis of >10,000 patients from the SOS AF project (Stroke preventiOn Strategies based on Atrial Fibrillation information from implanted devices). Eur Heart J. 2014;35(8):508-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glotzer TV, Daoud EG, Wyse DG, et al. . Rationale and design of a prospective study of the clinical significance of atrial arrhythmias detected by implanted device diagnostics: the TRENDS study. J Interv Card Electrophysiol. 2006;15(1):9-14. [DOI] [PubMed] [Google Scholar]
- 31.Santini M, Gasparini M, Landolina M, et al. ; cardiological centers participating in Clinical Service Project . Device-detected atrial tachyarrhythmias predict adverse outcome in real-world patients with implantable biventricular defibrillators. J Am Coll Cardiol. 2011;57(2):167-172. [DOI] [PubMed] [Google Scholar]
- 32.Hsu JC, Chan PS, Tang F, Maddox TM, Marcus GM. Differences in anticoagulant therapy prescription in patients with paroxysmal versus persistent atrial fibrillation. Am J Med. 2015;128(6):654.e1-654.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Association of cumulative burden of atrial fibrillation with thromboembolic events off anticoagulation in adults with paroxysmal atrial fibrillation and no previous ischemic stroke
eTable 2. Association of cumulative burden of atrial fibrillation with thromboembolic events off anticoagulation in adults with paroxysmal atrial fibrillation who had no baseline or follow-up exposure to anticoagulants
eFigure. Depiction of analytical timeline of adults with paroxysmal atrial fibrillation while off or on anticoagulation therapy