This cohort study provides contemporary estimates of sudden and/or arrhythmic death vs other competing causes of death in patients with coronary heart disease without severe systolic dysfunction to search for high-risk subgroups that might be targeted in future trials of prevention of sudden and/or arrhythmic death.
Key Points
Question
What are the absolute and proportional risks for sudden and/or arrhythmic death (SAD) in patients with coronary heart disease who do not currently qualify for implantable cardioverter defibrillator therapy?
Findings
In this cohort study of 5761 participants, the 4-year cumulative incidence of SAD was lowest in those with a left ventricular ejection fraction more than 60% and highest among those with left ventricular ejection fraction of 30% to 40% and New York Heart Association Class III/IV heart failure. The proportion of deaths due to SAD was lowest in those with New York Heart Association class II heart failure and highest in those younger than 60 years.
Meaning
Absolute and proportional risk of SAD varied significantly across clinical subgroups, and both will need to be maximized in future risk stratification efforts.
Abstract
Importance
The majority of sudden and/or arrhythmic deaths (SAD) in patients with coronary heart disease occur in those without severe systolic dysfunction, for whom strategies for sudden death prevention are lacking.
Objective
To provide contemporary estimates of SAD vs other competing causes of death in patients with coronary heart disease without severe systolic dysfunction to search for high-risk subgroups that might be targeted in future trials of SAD prevention.
Design, Setting, and Participants
This prospective observational cohort study included 135 clinical sites in the United States and Canada. A total of 5761 participants with coronary heart disease who did not qualify for primary prevention implantable cardioverter defibrillator therapy based on left ventricular ejection fraction (LVEF) of more than 35% or New York Heart Association (NYHA) heart failure class (LVEF >30%, NYHA I).
Exposures
Clinical risk factors measured at baseline including age, LVEF, and NYHA heart failure class.
Main Outcomes and Measures
Primary outcome of SAD, which is a composite of SAD and resuscitated ventricular fibrillation arrest.
Results
The mean (SD) age of the cohort was 64 (11) years. During a median of 3.9 years, the cumulative incidence of SAD and non-SAD was 2.1% and 7.7%, respectively. Sudden and/or arrhythmic death was the most common mode of cardiovascular death accounting for 114 of 202 cardiac deaths (56%), although noncardiac death was the primary mode of death in this population. The 4-year cumulative incidence of SAD was lowest in those with an LVEF of more than 60% (1.0%) and highest among those with LVEF of 30% to 40% (4.9%) and class III/IV heart failure (5.1%); however, the cumulative incidence of non-SAD was similarly elevated in these latter high-risk subgroups. Patients with a moderately reduced LVEF (40%-49%) were more likely to die of SAD, whereas those with class II heart failure and advancing age were more likely to die of non-SAD. The proportion of deaths due to SAD varied widely, from 14% (18 of 131 deaths) in patients with NYHA II to 49% (37 of 76 deaths) in those younger than 60 years.
Conclusions and Relevance
In a contemporary population of patients with coronary heart disease without severe systolic dysfunction, SAD accounts for a significant proportion of overall mortality. Moderately reduced LVEF, age, and NYHA class distinguished SAD and non-SAD, whereas other markers were equally associated with both modes of death. Absolute and proportional risk of SAD varied significantly across clinical subgroups, and both will need to be maximized in future risk stratification efforts.
Introduction
Sudden death remains a significant public health problem and accounts for 15% to 20% of deaths worldwide.1 Coronary heart disease is the most common underlying substrate of sudden death, which is frequently due to ventricular arrhythmias. Implantable cardioverter defibrillators (ICDs) treat ventricular arrhythmias, and ICD implantation improves survival in patients with coronary heart disease with symptomatic heart failure and a left ventricular ejection fraction (LVEF) less than 30% to 35%. Unfortunately, more than 70% of sudden deaths in coronary heart disease occur in individuals with LVEF greater than 35% who do not qualify for ICDs.2,3 Improved risk stratification in this population is imperative to reduce the global burden of sudden death.
Presently, little is known about the contemporary epidemiology of sudden and/or arrhythmic death (SAD) and non-SAD in patients with stable coronary heart disease without severe systolic dysfunction. To provide a foundation for SAD risk stratification in this population, we designed a prospective, multicenter observational cohort study that enrolled 5761 participants from North America with coronary heart disease and LVEF more than 30% to 35%. We performed rigorous adjudication of mode of death, which included detailed standardized interviews regarding the circumstances surrounding the death, as well as a review of medical records. In the present analysis, we assess and compare absolute rates and relative risks of SAD and non-SAD across clinical subgroups of interest, accounting for competing modes of death. We then illustrate how information regarding competing modes of death might be used when designing future trials of SAD prevention in this population.
Methods
Study Cohort
The PRE-DETERMINE study (ClinicalTrials.gov identifier: NCT01114269) is an ongoing multicenter, prospective cohort study of patients with coronary heart disease with a history of myocardial infarction (MI) and/or mild to moderate left ventricular dysfunction who do not fulfill consensus guideline criteria for ICD implantation on the basis of LVEF or New York Heart Association (NYHA) heart failure class.4,5 Between July 2007 and November 2013, 5761 participants 18 years or older were enrolled at 135 sites in the United States and Canada (eFigure 1 in the Supplement). Inclusion criteria included confirmation of coronary heart disease or MI and the presence of left ventricular function (LVEF) more than 35% or an LVEF of 30% to 35% with NYHA class I. Exclusion criteria included a history of cardiac arrest not associated with acute MI, current or planned ICD, or life expectancy less than 6 months. All participants provided written informed consent, and the study was approved by the institutional review board at Brigham and Women’s Hospital.
Baseline LVEF, NYHA Class, and Covariate Ascertainment
Baseline data on demographics, clinical characteristics, medical history, lifestyle habits, cardiac test results, and medications were collected. The baseline LVEF was chosen to be the most recent assessment on which current medical treatment was based at the time of entry into the study (eMethods in the Supplement).
Ascertainment and Classification of Incident Cardiovascular Events and Death
After enrollment, participants were followed up centrally by the Clinical Coordinating Center at Brigham and Women’s Hospital through questionnaires inquiring about intervening cardiac arrest, ICD implantation, and other pertinent cardiovascular end points administered by mail or telephone every 6 months (eMethods in the Supplement). Vital status was further assessed using contact with postal authorities, obituary searches, and serial searches of the National Death Index for names of nonrespondents.
Medical records pertaining to all deaths, cardiac arrests, and ICD implantations were sought to confirm study end points. For those participants who died outside of the hospital, standardized detailed interviews were conducted with family members and other potential witnesses regarding the circumstances surrounding the death. End points were confirmed using data from emergency medical service reports, emergency department and other medical records, autopsies, and witness reports of the circumstances surrounding the death. Owing to known unreliability for sudden death determination, information from the death certificate was not used in the determination of the primary end point
The primary end point was SAD. All deaths were classified according to timing (sudden vs nonsudden) and mechanism (arrhythmic vs nonarrhythmic) in accordance with criteria by Hinkle and Thaler.6,7 A definite sudden cardiac death was defined as a death or fatal cardiac arrest occurring within 1 hour of symptom onset without evidence for a noncardiac cause by history or autopsy. Unwitnessed deaths or deaths that occurred during sleep where the participant was observed to be symptom-free within the preceding 24 hours were considered probable sudden cardiac deaths.2,8,9 An arrhythmic death was defined as an abrupt spontaneous loss of pulse without evidence of preceding circulatory impairment or neurologic dysfunction. Deaths before which the pulse gradually disappeared and/or those preceded by circulatory or neurologic impairment were considered nonarrhythmic deaths and were excluded from the SAD end point even if the death occurred within 1 hour of symptom onset. Out-of-hospital cardiac arrests due to ventricular fibrillation requiring external electrical defibrillation for resuscitation were considered aborted arrhythmic deaths and included in the primary end point. Deaths were also classified as cardiac, noncardiac, or due to an unknown cause (eMethods in the Supplement).
Statistical Analysis
For the descriptive modes of death and clinical circumstances analyses, frequencies are reported using the same criteria used for death adjudication (ie, independent of the presence of an ICD). For all subsequent analyses, patients were censored at the time of ICD implantation, given the known impact of ICD therapy on SAD risk.10 For the cumulative incidence estimation and time-to-event analyses, participants contributed person-time from enrollment to date of death, out-of-hospital cardiac arrest, ICD implantation, last contact date, or April 11, 2016, whichever came first. Absolute rates of SAD and non-SAD were estimated and compared across clinical subgroups using cumulative incidence functions and the Gray test accounting for competing risk of the alternative end point (eMethods in the Supplement). Subdistribution hazard ratios from competing-risk Fine-Gray models and cause-specific hazards from Cox proportional hazards models were used to estimate relative risks. The possibility of nonlinear associations between covariates of interest (eg, LVEF, age) and modes of death were examined using restricted cubic spline Cox models with 3 knots. As there was no evidence of nonlinearity, linear associations are reported.
To examine whether clinical risk factors were differentially associated with SAD vs non-SAD, competing outcome Cox proportional hazard models with likelihood ratio comparisons were performed.11,12 Sensitivity analyses were performed including (1) excluding deaths with insufficient information on mode of death, (2) excluding probable sudden cardiac deaths, and (3) excluding out-of-hospital ventricular fibrillation arrests. Proportions of SAD were compared across subgroup strata using the χ2 test.
To illustrate the potential impact that competing modes of mortality might have on interventions that specifically target SAD, such as the ICD, we performed an exploratory analysis that modeled the theoretical efficacy of ICD therapy during the median follow-up of the study. Based on primary prevention trials in patients with severe systolic dysfunction,13 we assumed a 60% reduction in SAD and no reduction in non-SAD mortality, estimated from the cumulative incidence functions. The number needed to treat to save 1 life and the percentage reduction of total mortality were then estimated.14 Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc). A 2-tailed P value of less than .05 was considered statistically significant.
Results
Study Population
Baseline characteristics for participants enrolled in the PRE-DETERMINE Study (N = 5761) are shown in Table 1. The mean (SD) age of the cohort was 64 (11) years. Most patients were classified as NYHA class I at baseline (4597 [80%]) and the mean (SD) LVEF was 52% (10%). A total of 5328 patients (93%) in the cohort underwent either percutaneous or surgical revascularization prior to enrollment, and the majority was treated with β-blockers (4779 [83%]), aspirin (5074 [88%]), and lipid-lowering therapy (5370 [93%]). In 5525 participants (91%) with a history of MI, the median (interquartile range) time from MI to study enrollment was 2.1 (0.32-7.80) years. During study follow-up, 173 participants (3%) underwent ICD implantation at a median (interquartile range) time of 1.3 (0.64-2.71) years after study enrollment.
Table 1. Baseline Characteristics of Study Cohort (N = 5761).
| Baseline Characteristic | Study Cohort, No. (%) |
|---|---|
| Age, mean (SD), y | 64 (11) |
| Men | 4391 (76) |
| Race/ethnicity | |
| White | 5128 (89) |
| Black or African American | 315 (5) |
| Asian | 114 (2) |
| Othera | 115 (2) |
| Unknown | 89 (2) |
| History of smoking | 3814 (66) |
| Hypertension | 4371 (76) |
| History of myocardial infarction | 5225 (91) |
| History of revascularization | |
| Percutaneous coronary intervention | 4592 (80) |
| Coronary artery bypass graft surgery | 1886 (33) |
| Family history of sudden death | 1432 (25) |
| Diabetes mellitus | 1860 (32) |
| History of atrial fibrillation | 791 (14) |
| Continuous ejection fraction | 52 (10) |
| LVEF category, No. (%) | |
| ≥60% | 1591 (28) |
| 50% to 59% | 1997 (35) |
| 40% to 49% | 1756 (30) |
| 30% to 39% | 417 (7) |
| New York Heart Association class | |
| I | 4597 (80) |
| II | 925 (16) |
| III/IV | 223 (4) |
| Medication use | |
| Aspirin | 5074 (88) |
| β-Blocker | 4779 (83) |
| Lipid-lowering | 5370 (93) |
| Renin-angiotensin-aldosterone inhibitors | 4023 (70) |
Abbreviation: LVEF, left ventricular ejection fraction.
Other race includes Native American, Alaskan Native, Pacific Islander or Native Hawaiian, or participants reporting more than 1 race.
Modes of Death
Throughout a median (interquartile) follow-up of 3.9 (3.1-4.6) years, 559 deaths (10%) occurred. Of these, 202 (36.1%) were confirmed to be due to cardiac causes and 114 (20.4%) were classified as SADs. Deaths due to noncardiac causes were the primary competing mode of death (305 [54.6%]). Fifty-two deaths (9.3%) could not be definitively classified as due to cardiac or noncardiac causes, and the available information was insufficient to classify the death as SAD vs non-SAD in 36 cases (6.4%). Thirteen participants (2.3%) were resuscitated from out-of-hospital ventricular fibrillation arrest, of whom 2 subsequently died suddenly, yielding a total of 125 participants (22.4%) with the primary SAD end point.
Clinical Circumstances and Demographic Context of SAD
Of 125 end points, 89 primary end points (71%) occurred at home, and 66 (53%) were witnessed by a bystander (eTable 1 in the Supplement). Rhythm monitoring was present in 57 primary end points (46%), and ventricular tachycardia or fibrillation was documented in 34 (60%) of these monitored deaths. Interval echocardiography was reported in 93 primary end points (74%), and a newly documented LVEF less than 35% after enrollment was present in 21 patients (23%). Clinical history pertaining to angina was available in 94 primary end points (75%), and 18 patients (19%) reported symptoms that could have been consistent with unstable angina within 1 month of SAD. Most patients with SAD (72 [58%]) did not report symptoms immediately prior to SAD.
Incidence of SAD and Competing Modes of Death
As the implant of an ICD after study enrollment was assumed to influence the risk of SAD, subsequent analyses censored participants at the time of ICD implantation. An ICD was present in 6 participants (4.8%) prior to SAD and 15 participants (3.4%) prior to non-SAD, thus yielding 119 participants (21.8%) with the primary SAD end point and 426 (78.2%) competing non-SAD deaths for subsequent analyses. After accounting for competing causes of death, the 4-year cumulative incidence of SAD in the total cohort was 2.1% (95% CI, 1.8-2.6) compared with 7.7% (95% CI, 7.0-8.5) for non-SAD. Decreasing LVEF was associated with progressive elevations in the cumulative incidence of both SAD and non-SAD (Figure 1A and B). Each 10% decline in LVEF was associated with a 71% increase in the incidence of SAD (subdistribution hazard ratio per 10% decrease: 1.71; 95% CI, 1.40-2.11; P < .001) and a 44% increase in the incidence of non-SAD (subdistribution hazard ratios per 10% decrease: 1.44; 95% CI, 1.29-1.60; P < .001) (eFigure 2 in the Supplement). By comparison, increasing NYHA functional class was associated with a graded increased incidence of non-SAD, whereas SAD rates were only elevated among patients with NYHA class III/IV (Figure 1C and D). The 4-year cumulative incidences of SAD and non-SAD across all clinical subgroups are listed in Table 2. The highest absolute risks of SAD were found among those with the lowest LVEF (LVEF 30%-39%, 4-year incidence: 4.9%; 95% CI, 3.0-7.6) and most advanced heart failure (NYHA class III/IV, 4-year incidence: 5.1%; 95% CI, 2.6-8.9).
Figure 1. Cumulative Incidence of Sudden and/or Arrhythmic Death (SAD) and Non-SAD Across Left Ventricular Ejection Fraction (LVEF) and New York Heart Association (NYHA) Functional Class.
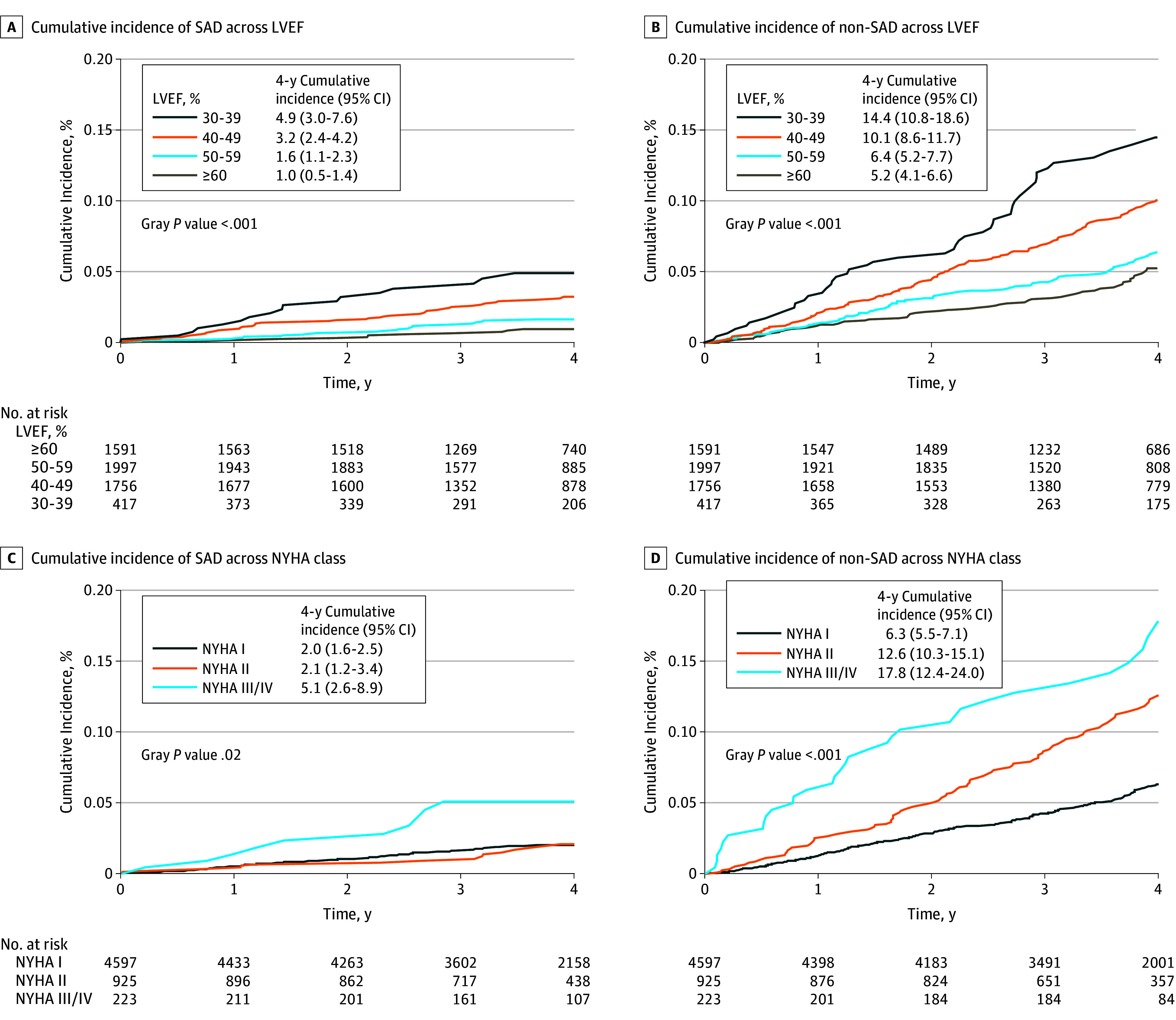
The 4-year cumulative incidence of each mode of death (ie, SAD and non-SAD), accounting for the competing risk of death, are shown in strata of LVEF (A and B) and NYHA functional class (C and D). Differences in cumulative incidence across strata are shown (Gray P value).
Table 2. Absolute Risk of Sudden and/or Arrhythmic Death (SAD) and Competing Modes of Death.
| Clinical Subgroup | Patients in Subgroup, No. (%) | 4-y Incidence SAD (95% CI) | P Valuea | 4-y Incidence Non-SAD (95% CI) | P Valuea |
|---|---|---|---|---|---|
| Age, y | |||||
| ≤59 | 1882 (33) | 2.0 (1.4-2.8) | .05 | 2.1 (1.4-2.9) | <.001 |
| 60-69 | 1990 (35) | 1.6 (1.1-2.2) | 5.6 (4.5-6.9) | ||
| >69 | 1889 (33) | 2.9 (2.1-3.8) | 15.2 (13.5-17.1) | ||
| Sex | |||||
| Male | 4391 (76) | 2.2 (1.8-2.7) | .91 | 7.4 (6.6-8.3) | .21 |
| Female | 1370 (24) | 2.0 (1.3-2.9) | 8.7 (7.1-10.6) | ||
| Race/ethnicity | |||||
| White | 5128 (89) | 2.2 (1.8-2.6) | .88 | 7.9 (7.1-8.8) | .31 |
| Other | 633 (11) | 2.2 (1.1-3.8) | 6.2 (4.3-8.5) | ||
| Smoking | |||||
| Never | 1945 (34) | 1.8 (1.2-2.5) | .27 | 6.3 (5.1-7.5) | .05 |
| Ever | 3814 (66) | 2.4 (1.9-2.9) | 8.5 (7.5-9.5) | ||
| Hypertension | |||||
| Yes | 4371 (76) | 2.4 (1.9-2.9) | .04 | 8.5 (7.6-9.5) | <.001 |
| No | 1390 (24) | 1.4 (0.8-2.2) | 5.3 (4.1-6.8) | ||
| History of revascularization | |||||
| Yes | 5328 (92) | 2.2 (1.8-2.6) | .93 | 7.5 (6.7-8.3) | .02 |
| No | 433 (8) | 2.0 (0.9-3.7) | 10.6 (7.7-14.1) | ||
| Family history sudden cardiac death | |||||
| Yes | 1432 (25) | 2.5 (1.7-3.5) | .25 | 8.0 (6.5-9.8) | .79 |
| No | 4329 (75) | 2.0 (1.6-2.5) | 7.6 (6.8-8.5) | ||
| Diabetes mellitus | |||||
| Yes | 1860 (32) | 3.3 (2.5-4.3) | <.001 | 11.4 (9.8-13.1) | <.001 |
| No | 3901 (68) | 1.6 (1.2-2.1) | 6.0 (5.2-6.9) | ||
| Atrial fibrillation | |||||
| Yes | 791 (14) | 3.8 (2.6-5.5) | .003 | 15.7 (13.0-18.7) | <.001 |
| No | 4969 (87) | 1.9 (1.5-2.3) | 6.4 (5.7-7.2) | ||
| Left ventricular ejection fraction, % | |||||
| ≥60 | 1591 (28) | 1.0 (0.5-1.6) | <.001 | 5.2 (4.1-6.6) | <.001 |
| 50-59 | 1997 (35) | 1.6 (1.1-2.3) | 6.4 (5.2-7.7) | ||
| 40-49 | 1756 (30) | 3.2 (2.4-4.2) | 10.1 (8.6-11.7) | ||
| 30-39 | 417 (7) | 4.9 (3.0-7.6) | 14.5 (10.8-18.6) | ||
| New York Heart Association class | |||||
| I | 4597 (80) | 2.0 (1.6-2.5) | .02 | 6.3 (5.5-7.1) | <.001 |
| II | 925 (16) | 2.1 (1.2-3.4) | 12.6 (10.3-15.1) | ||
| III/IV | 223 (4) | 5.1 (2.6-8.9) | 17.8 (12.4-24.0) |
The P value reflects the Gray test for equivalence of cumulative incidence functions across specified strata. Competing deaths (non-SAD) include nonsudden, nonarrhythmic cardiovascular deaths, noncardiovascular deaths, and unclassified deaths.
Distinguishing SAD From Competing Modes of Death
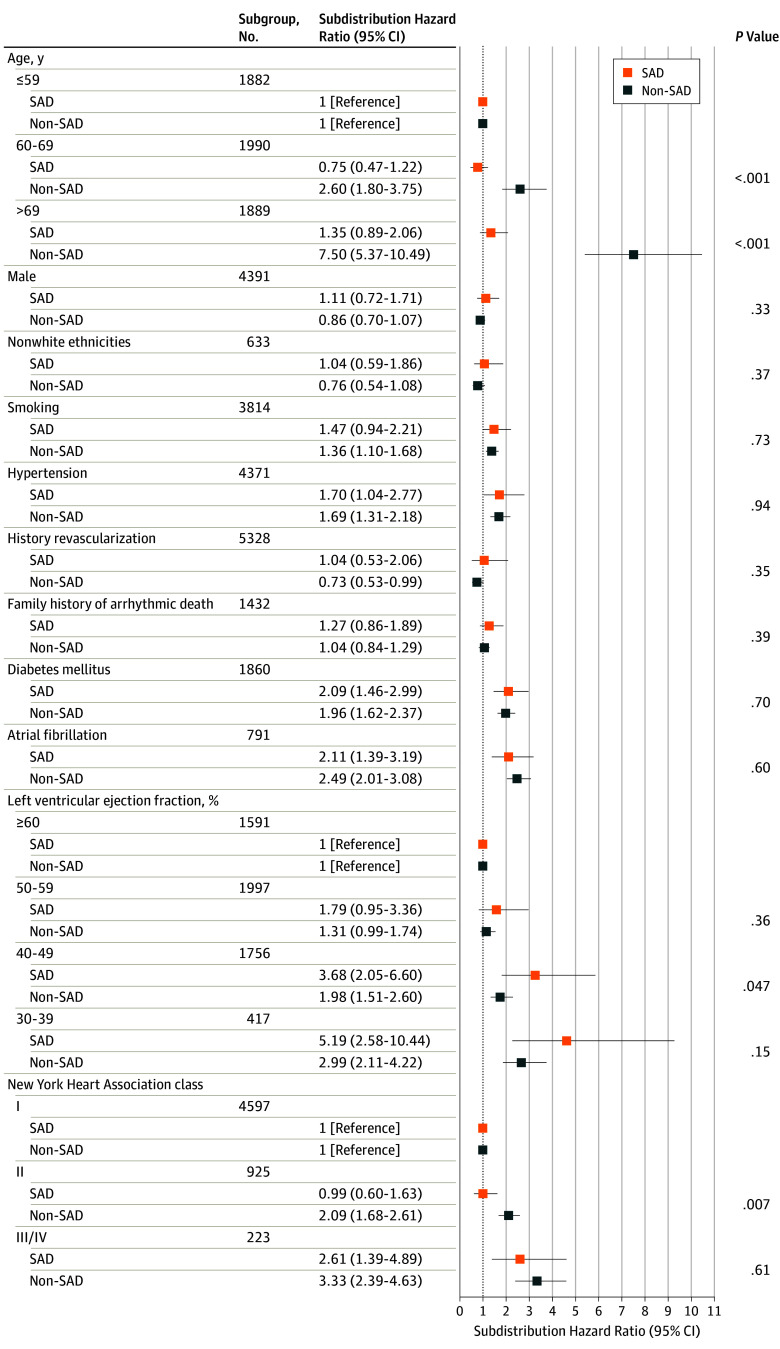
The relative hazard of SAD and non-SAD across clinical subgroups is shown in Figure 2 (cause-specific hazards shown in eTable 2 in the Supplement). In competing outcome models comparing subgroup associations with SAD and non-SAD, a moderately reduced LVEF (40%-49%) was more strongly associated with SAD than non-SAD (adjusted subdistribution hazard ratio [95% CI], 3.68 [2.05-6.60] vs. 1.98 [1.51-2.60]; P = .047). Other subgroups at increased risk of SAD (hypertension, diabetes mellitus, atrial fibrillation, and NYHA functional class III/IV) were at similarly increased risk of non-SAD (Figure 2). Therefore, the proportion of total mortality due to SAD was not significantly enriched in any of these subgroups (eTable 3 in the Supplement). By comparison, advancing age and NYHA functional class II were more strongly associated with an increased risk for non-SAD compared with SAD (Figure 2). With advancing age, the relative risk of non-SAD increased while the relative risk of SAD only marginally increased (eFigure 2 in the Supplement), and therefore the proportion of deaths that were SAD declined with increasing age (eTable 3 in the Supplement). Self-reported family history of sudden death, sex, and race/ethnicity were not associated with an increased risk of either SAD or non-SAD. These results did not differ significantly when unclassified deaths (36 [6.6%]; eTable 4 in the Supplement), probable sudden cardiac deaths (30 [5.5%]; eTable 5 in the Supplement), or out-of-hospital cardiac arrests (13 [2.4%]; eTable 6 in the Supplement) were excluded.
Figure 2. Differential Association of Clinical Risk Factors With Sudden and/or Arrhythmic Death (SAD) vs Competing Modes of Death.
The relative incidence of each mode of death (ie, SAD and non-SAD), accounting for the competing risk of other deaths, is shown for clinical subgroups of interest. P values for the differential association of each clinical subgroup with mode of death are shown.
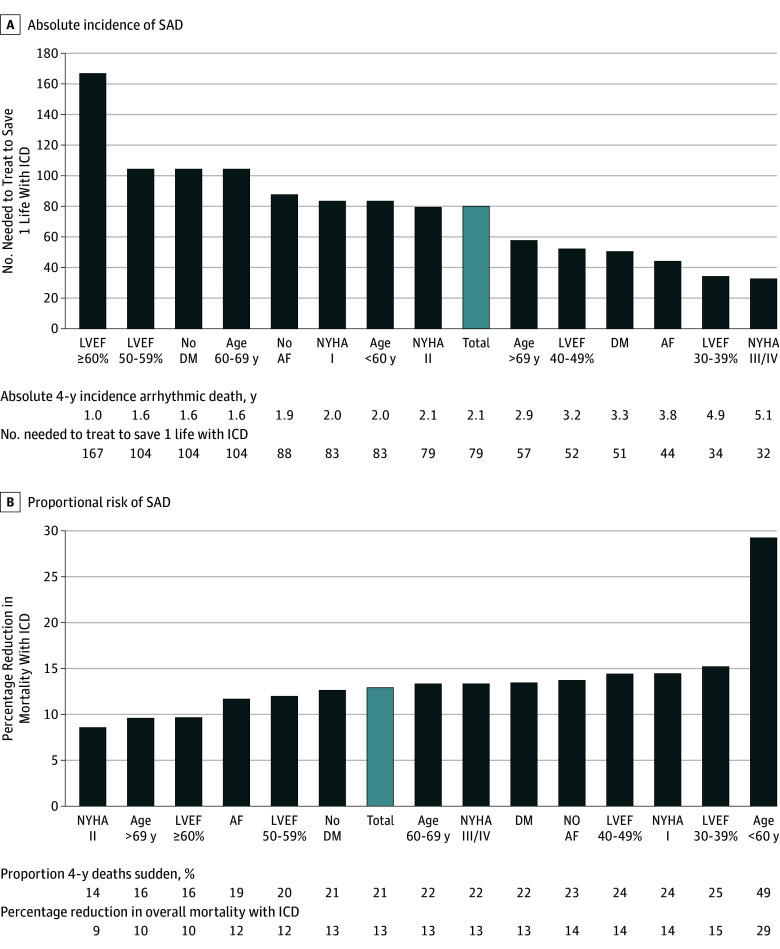
Impact of Absolute vs Proportional Risk of SAD: Modeling ICD Benefit Across Clinical Subgroups
To explore the implications of our findings on sudden death prevention strategies, we modeled the theoretical impact of ICD implantation on overall survival within clinical subgroups across the spectrum of both absolute and proportional risk of SAD (Figure 3). In the total cohort, ICD implantation was projected to reduce total mortality by 13%, and the number needed to treat to save 1 life was 79. As the absolute risk of SAD increased (Figure 3A), the estimated number needed to treat to save 1 life with an ICD decreased to nadir of 32 for patients with advanced heart failure; however, the estimated reduction in overall mortality remained modest (13%) due to similar elevations in competing modes of death. Conversely, as SAD accounted for a greater proportion of total mortality (Figure 3B), ICD implantation was projected to yield a greater relative decrease in overall mortality. The proportion of deaths due to SAD was highest in the younger participants (<60 years), in which 37 of 76 deaths (49%) were sudden and/or arrhythmic. In this subgroup, ICD therapy was projected to confer the greatest relative reduction in mortality (29%); however, the number needed to treat to save 1 life without further risk stratification was 83.
Figure 3. Absolute and Proportional Risk of Sudden and/or Arrhythmic Death (SAD).
AF indicates atrial fibrillation; DM, diabetes mellitus; LVEF, left ventricular ejection fracture; NYHA, New York Heart Association.
Implantable cardioverter defibrillator (ICD) benefit throughout the median follow-up of the study (4 years) was estimated using 2 metrics: the number needed to treat to save 1 life (A) and percentage reduction in total mortality (B). Implantable cardioverter defibrillator benefit was modeled in patients without an ICD. Subgroups are ordered by increasing absolute incidence of SAD (A) and increasing proportional risk of SAD (B).
Discussion
In this large, contemporary cohort of patients with coronary heart disease and LVEF greater than 30% to 35%, SAD accounted for approximately one-fifth of total mortality and was the most common mode of cardiovascular death. Sudden and/or arrhythmic death was unheralded in the majority, occurred most commonly at home, and when monitored, was associated with ventricular tachyarrhythmia in most patients. Moderately reduced left ventricular function (LVEF 40%-49%) was more strongly associated with SAD vs non-SAD, whereas age and NYHA class II heart failure were more strongly associated with non-SAD. The proportion of deaths due to SAD varied widely from 14% in patients with NYHA class II heart failure to 49% in those younger than 60 years. In an exploratory modeling analysis, the projected impact of ICD therapy on overall survival was sensitive to both absolute and proportional SAD risk.
The findings of our study have several implications relating to risk stratification and prevention of SAD in patients with coronary heart disease. First, despite 70% of arrests occurring at home, the proportion of monitored patients found in ventricular tachycardia or fibrillation was substantial (60%) and higher than recent reports in cardiac arrest registries (22%).15 These data underscore efforts to more rapidly deliver defibrillator therapy to the site of cardiac arrests16 and further support the search for strategies to prevent and treat ventricular arrhythmias as a means to reduce mortality in this population.
Second, the annualized incidence of SAD in this population was 0.53%. Although this is 10-fold higher than rates in the general population,8 effective risk stratification would need to elevate this risk approximately 6-fold to achieve SAD rates observed in primary prevention ICD trials in which mortality benefits were observed.13 In addition to enriching for absolute SAD risk, our data demonstrate the potential importance of considering competing causes of death in sudden death prevention efforts. In our exploratory modeling analysis, we demonstrate how competing causes of deaths diminished relative risk reductions conferred by the ICD in those at highest absolute risk. Conversely, subgroups in which the greatest proportion of deaths were due to SAD, such as those younger than 60 years in our study, would be expected to obtain the greatest relative benefit, even though these subgroups are not at the highest absolute SAD risk. These latter findings are concordant with the results of a 2016 randomized clinical trial of ICD therapy in nonischemic cardiomyopathy17 in which survival benefit was limited to younger participants. Looking ahead, identification of markers that uniquely discriminate SAD from non-SAD will be required to maximize absolute and proportional risk in subpopulations targeted for sudden death prevention.
Third, our study distinguishes approaches to SAD risk stratification in patients with coronary heart disease from previously studied populations. Family history of sudden cardiac death, which has been associated with sudden death during first acute MI18 and in the general population,19 was not associated with SAD in this population. Therefore, the genetic predisposition for SAD may differ in patients who have survived their first coronary event. Likewise, advancing age and male sex did not significantly elevate SAD risk in our cohort, suggesting that undiagnosed or untreated coronary heart disease may partly underlie these reported associations with sudden cardiac death in general populations.20,21 In contrast to patients with severe left ventricular dysfunction, most competing causes of death were noncardiac rather than cardiac.22 Risk markers distinguishing cardiac from noncardiac death may therefore enrich for SAD risk in this population compared with a low LVEF population.
Finally, our data challenge the contemporary paradigm of SAD risk stratification, which dichotomizes risk at an LVEF of 35%.4 In this study, SAD risk was continuously and inversely associated with LVEF, and further, the relative risk of SAD was greater than non-SAD in patients with an LVEF of 40% to 49%. Given that more than 70% of individuals experiencing sudden death have an LVEF greater than 35%,3,23 integration of a more continuous assessment of LVEF into future risk stratification efforts may improve SAD risk prediction.
Limitations
Our study has limitations. First, although we used rigorous and widely accepted methods of death adjudication,6,7 the possibility of death misclassification cannot be excluded. Autopsy information was only available in a few participants, likely reflecting known secular declines in autopsy rates in North America and the presumption of coronary heart disease as the cause of death in patients with established coronary heart disease.24 To address the potential for misclassification, we performed several sensitivity analyses accounting for the certainty of death adjudication, and the results did not differ from the primary analysis. To the extent that death misclassification was nondifferential, the identified associations are biased toward the null. Future studies may consider the role of implantable rhythm monitoring that would provide more definitive assessment of cardiac rhythm at the time of death. Second, while the PRE-DETERMINE Study is not a population-based cohort, the baseline characteristics and rates of medical therapy in this study were similar to contemporary population-based registries of coronary heart disease.25,26 Importantly, the racial and ethnic diversity in our cohort limited our ability to examine the impact of these factors on modes of death. Third, throughout the course of usual clinical care, 173 participants (3%) underwent ICD implantation. We elected to censor at the time of ICD implantation to most conservatively assess rates of SAD, as detailed information regarding ICD therapies was not available. Fourth, standardized reassessment of LVEF was not prespecified in the study design, and we cannot comment specifically on the impact of changes in LVEF and interval risk of SAD. Fifth, patients with nonischemic cardiomyopathy are at risk for SAD, and our findings may not generalize to this population. Finally, the projected impact of ICD implantation on survival incorporated SAD risk reduction estimates (ie, 60% relative risk reduction) derived from prior randomized clinical trials of ICD therapy in patients with low LVEF.13,22 Whether ICD therapy would have the same cause-specific risk reduction in patients without severe systolic dysfunction is unknown.
Conclusions
In conclusion, in patients with coronary heart disease and LVEF greater than 35%, SAD accounted for a substantial proportion of total mortality. Sudden and/or arrhythmic death occurred most often at home without antecedent clinical worsening or symptoms. Moderately reduced LVEF, heart failure severity, and age distinguished SAD and non-SAD. Future risk stratification integrating both absolute and proportional risk of SAD will be important to maximize sudden death prevention efforts.
eMethods. Supplementary Methods
eTable 1. Circumstances and Clinical History Prior to Sudden and/or Arrhythmic Death
eTable 2. Cause-specific Hazard for the Association of Clinical Risk Factors with Sudden and/or Arrhythmic versus Competing Modes of Death
eTable 3. Sudden and/or Arrhythmic Proportion of Total Mortality in Clinical Subgroups
eTable 4. Differential Association of Clinical Risk Factors with SAD and Non-SAD: Sensitivity Analysis Excluding 36 Participants with Unclassified Mode of Death
eTable 5. Differential Association of Clinical Risk Factors with SAD versus non-SAD: Sensitivity Analysis Excluding 30 Participants with Probable Sudden Cardiac Death
eTable 6. Differential Association of Clinical Risk Factors with SAD versus Non-SAD: Sensitivity Analysis Excluding 13 Participants with Out of Hospital Cardiac Arrest
eFigure 1. Geographic Distribution of Enrollment Sites in PRE-DETERMINE
eFigure 2. Continuous Association between LV Ejection Fraction and Age with Cause-Specific Death
References
- 1.Hayashi M, Shimizu W, Albert CM. The spectrum of epidemiology underlying sudden cardiac death. Circ Res. 2015;116(12):1887-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Vreede-Swagemakers JJ, Gorgels AP, Dubois-Arbouw WI, et al. Out-of-hospital cardiac arrest in the 1990's: a population-based study in the Maastricht area on incidence, characteristics and survival. J Am Coll Cardiol. 1997;30(6):1500-1505. [DOI] [PubMed] [Google Scholar]
- 3.Stecker EC, Vickers C, Waltz J, et al. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol. 2006;47(6):1161-1166. [DOI] [PubMed] [Google Scholar]
- 4.European Heart Rhythm Association; Heart Rhythm Society; Zipes DP, Camm AJ, Borggrefe M, et al. ; American College of Cardiology; American Heart Association Task Force; European Society of Cardiology Committee for Practice Guidelines . ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death). J Am Coll Cardiol. 2006;48(5):e247-e346. [DOI] [PubMed] [Google Scholar]
- 5.Kadish AH, Bello D, Finn JP, et al. Rationale and design for the Defibrillators to Reduce Risk by Magnetic Resonance Imaging Evaluation (DETERMINE) trial. J Cardiovasc Electrophysiol. 2009;20(9):982-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hinkle LE Jr, Thaler HT. Clinical classification of cardiac deaths. Circulation. 1982;65(3):457-464. [DOI] [PubMed] [Google Scholar]
- 7.Fishman GI, Chugh SS, Dimarco JP, et al. Sudden cardiac death prediction and prevention: report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop. Circulation. 2010;122(22):2335-2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chugh SS, Jui J, Gunson K, et al. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate-based review in a large U.S. community. J Am Coll Cardiol. 2004;44(6):1268-1275. [DOI] [PubMed] [Google Scholar]
- 9.Straus SM, Kors JA, De Bruin ML, et al. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006;47(2):362-367. [DOI] [PubMed] [Google Scholar]
- 10.Moss AJ, Zareba W, Hall WJ, et al. ; Multicenter Automatic Defibrillator Implantation Trial II Investigators . Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346(12):877-883. [DOI] [PubMed] [Google Scholar]
- 11.Sandhu RK, Conen D, Tedrow UB, et al. Predisposing factors associated with development of persistent compared with paroxysmal atrial fibrillation. J Am Heart Assoc. 2014;3(3):e000916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;51(2):524-532. [PubMed] [Google Scholar]
- 13.Packer DL, Prutkin JM, Hellkamp AS, et al. Impact of implantable cardioverter-defibrillator, amiodarone, and placebo on the mode of death in stable patients with heart failure: analysis from the sudden cardiac death in heart failure trial. Circulation. 2009;120(22):2170-2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Betts TR, Sadarmin PP, Tomlinson DR, et al. Absolute risk reduction in total mortality with implantable cardioverter defibrillators: analysis of primary and secondary prevention trial data to aid risk/benefit analysis. Europace. 2013;15(6):813-819. [DOI] [PubMed] [Google Scholar]
- 15.Daya MR, Schmicker RH, Zive DM, et al. ; Resuscitation Outcomes Consortium Investigators . Out-of-hospital cardiac arrest survival improving over time: results from the Resuscitation Outcomes Consortium (ROC). Resuscitation. 2015;91:108-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boutilier JJ, Brooks SC, Janmohamed A, et al. ; Rescu Epistry Investigators . Optimizing a drone network to deliver automated external defibrillators. Circulation. 2017;135(25):2454-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Køber L, Thune JJ, Nielsen JC, et al. ; DANISH Investigators . Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med. 2016;375(13):1221-1230. [DOI] [PubMed] [Google Scholar]
- 18.Dekker LR, Bezzina CR, Henriques JP, et al. Familial sudden death is an important risk factor for primary ventricular fibrillation: a case-control study in acute myocardial infarction patients. Circulation. 2006;114(11):1140-1145. [DOI] [PubMed] [Google Scholar]
- 19.Jouven X, Desnos M, Guerot C, Ducimetière P. Predicting sudden death in the population: the Paris Prospective Study I. Circulation. 1999;99(15):1978-1983. [DOI] [PubMed] [Google Scholar]
- 20.Deo R, Norby FL, Katz R, et al. Development and validation of a sudden cardiac death prediction model for the general population. Circulation. 2016;134(11):806-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deo R, Vittinghoff E, Lin F, Tseng ZH, Hulley SB, Shlipak MG. Risk factor and prediction modeling for sudden cardiac death in women with coronary artery disease. Arch Intern Med. 2011;171(19):1703-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenberg H, Case RB, Moss AJ, Brown MW, Carroll ER, Andrews ML; MADIT-II Investigators . Analysis of mortality events in the Multicenter Automatic Defibrillator Implantation Trial (MADIT-II). J Am Coll Cardiol. 2004;43(8):1459-1465. [DOI] [PubMed] [Google Scholar]
- 23.Gorgels AP, Gijsbers C, de Vreede-Swagemakers J, Lousberg A, Wellens HJ. Out-of-hospital cardiac arrest: the relevance of heart failure: the Maastricht Circulatory Arrest Registry. Eur Heart J. 2003;24(13):1204-1209. [DOI] [PubMed] [Google Scholar]
- 24.Burton JL, Underwood J. Clinical, educational, and epidemiological value of autopsy. Lancet. 2007;369(9571):1471-1480. [DOI] [PubMed] [Google Scholar]
- 25.Smilowitz NR, Mahajan AM, Roe MT, et al. Mortality of myocardial infarction by sex, age, and obstructive coronary artery disease status in the ACTION Registry-GWTG (Acute Coronary Treatment and Intervention Outcomes Network Registry-Get With the Guidelines). Circ Cardiovasc Qual Outcomes. 2017;10(12):e003443. [DOI] [PubMed] [Google Scholar]
- 26.Peterson ED, Roe MT, Chen AY, et al. The NCDR ACTION Registry-GWTG: transforming contemporary acute myocardial infarction clinical care. Heart. 2010;96(22):1798-1802. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Supplementary Methods
eTable 1. Circumstances and Clinical History Prior to Sudden and/or Arrhythmic Death
eTable 2. Cause-specific Hazard for the Association of Clinical Risk Factors with Sudden and/or Arrhythmic versus Competing Modes of Death
eTable 3. Sudden and/or Arrhythmic Proportion of Total Mortality in Clinical Subgroups
eTable 4. Differential Association of Clinical Risk Factors with SAD and Non-SAD: Sensitivity Analysis Excluding 36 Participants with Unclassified Mode of Death
eTable 5. Differential Association of Clinical Risk Factors with SAD versus non-SAD: Sensitivity Analysis Excluding 30 Participants with Probable Sudden Cardiac Death
eTable 6. Differential Association of Clinical Risk Factors with SAD versus Non-SAD: Sensitivity Analysis Excluding 13 Participants with Out of Hospital Cardiac Arrest
eFigure 1. Geographic Distribution of Enrollment Sites in PRE-DETERMINE
eFigure 2. Continuous Association between LV Ejection Fraction and Age with Cause-Specific Death