Key Points
Question
What is the cost-effectiveness of electroconvulsive therapy compared with antidepressant medications and/or psychotherapy for treatment-resistant major depressive disorder in the United States?
Findings
In this mathematical modeling analysis integrating data from multiple published sources, offering electroconvulsive therapy as third-line treatment for depression would cost an estimated $54 000 per quality-adjusted life-year gained. Over 4 years, this would reduce time with uncontrolled depression from 50% to 34% of life-years.
Meaning
Electroconvulsive therapy may be an effective and cost-effective treatment for treatment-resistant depression and should be considered after failure of 2 or more lines of pharmacotherapy and/or psychotherapy.
Abstract
Importance
Electroconvulsive therapy (ECT) is a highly effective treatment for depression but is infrequently used owing to stigma, uncertainty about indications, adverse effects, and perceived high cost.
Objective
To assess the cost-effectiveness of ECT compared with pharmacotherapy/psychotherapy for treatment-resistant major depressive disorder in the United States.
Design, Setting, and Participants
A decision analytic model integrating data on clinical efficacy, costs, and quality-of-life effects of ECT compared with pharmacotherapy/psychotherapy was used to simulate depression treatment during a 4-year horizon from a US health care sector perspective. Model input data were drawn from multiple meta-analyses, randomized trials, and observational studies of patients with depression. Where possible, data sources were restricted to US-based studies of nonpsychotic major depression. Data were analyzed between June 2017 and January 2018.
Interventions
Six alternative strategies for incorporating ECT into depression treatment (after failure of 0-5 lines of pharmacotherapy/psychotherapy) compared with no ECT.
Main Outcomes and Measures
Remission, response, and nonresponse of depression; quality-adjusted life-years; costs in 2013 US dollars; and incremental cost-effectiveness ratios. Strategies with incremental cost-effectiveness ratios of $100 000 per quality-adjusted life-year or less were designated cost-effective.
Results
Based on the Sequenced Treatment Alternatives to Relieve Depression trial, we simulated a population with a mean (SD) age of 40.7 (13.2) years, and 62.2% women. Over 4 years, ECT was projected to reduce time with uncontrolled depression from 50% of life-years to 33% to 37% of life-years, with greater improvements when ECT is offered earlier. Mean health care costs were increased by $7300 to $12 000, with greater incremental costs when ECT was offered earlier. In the base case, third-line ECT was cost-effective, with an ICER of $54 000 per quality-adjusted life-year. Third-line ECT remained cost-effective in a range of univariate, scenario, and probabilistic sensitivity analyses. Incorporating all input data uncertainty, we estimate a 74% to 78% likelihood that at least 1 of the ECT strategies is cost-effective and a 56% to 58% likelihood that third-line ECT is the optimal strategy.
Conclusions and Relevance
For US patients with treatment-resistant depression, ECT may be an effective and cost-effective treatment option. Although many factors influence the decision to proceed with ECT, these data suggest that, from a health-economic standpoint, ECT should be considered after failure of 2 or more lines of pharmacotherapy/psychotherapy.
This study assesses the cost-effectiveness of electroconvulsive therapy compared with pharmacotherapy/psychotherapy for treatment-resistant major depressive disorder in the United States.
Introduction
Electroconvulsive therapy (ECT) is a highly effective treatment for depression.1 Research indicates that ECT can be significantly more effective than pharmacotherapy, with 50% to 60% of patients achieving rapid remission of depression after a course of ECT compared with 10% to 40% with pharmacotherapy/psychotherapy.2,3,4 Beyond its immediate clinical effects, ECT is also associated with decreased psychiatric hospitalization rates5,6 and reduced long-term risks of suicide and all-cause mortality.7
Despite these benefits, ECT use remains low. A 2017 analysis found that only 1.5% of psychiatric inpatients with severe affective disorders received ECT while hospitalized,5 and among individuals with depression in a large insurance claims database, fewer than 1% had ever received ECT.8 Numerous explanations have been proposed for the infrequent use of ECT including stigma, adverse effects, and lack of access.1,5,9
Although ECT can be a first-line treatment for depression with life-threatening psychotic or suicidal features, it is most often used in the United States for depression that has failed to respond to pharmacotherapy and/or psychotherapy.10,11,12 While treatment-resistant depression is a common indication for ECT, many clinical guidelines do not clearly specify how many pharmacotherapy/psychotherapy trials must fail before ECT is offered.9,12,13,14 In practice, patients often spend months to years with uncontrolled depression before considering ECT; in 1 US-based trial, patients averaged 38 weeks and 5 prior medication trials before initiating ECT.15
Another issue that may limit use of ECT is its cost, estimated at $300 to $1000 per treatment.16,17 With 5 to 15 treatments per initial course and 10 to 20 maintenance treatments per year, the annual cost of ECT can exceed $10 000 vs several hundred dollars for many antidepressant medications.18 Amidst ongoing efforts to improve the value of US health care, it is critical to assess whether ECT confers a clinical benefit commensurate with its higher cost. Although prior studies have suggested that ECT for depression may be cost-effective in some health care systems,19,20 we are aware of no study that has reported the cost-effectiveness of ECT for treatment-resistant depression in the United States; furthermore, to our knowledge, no prior study has evaluated when in the course of depression treatment ECT should be offered.
To address these issues, we developed a decision analytic model to simulate the clinical and economic effects of ECT. Our analysis has 2 main objectives: (1) to quantify the cost-effectiveness of ECT for treatment-resistant depression in the United States and (2) to define criteria for offering ECT that will maximize its health-economic value.
Methods
Overview of Analysis
We used a decision analytic model to assess the cost-effectiveness of varying strategies for offering ECT to patients with major depressive disorder. Strategies are characterized by the number of prior treatment lines (defined as a unique combination of antidepressant medications and/or psychotherapy)4,21 a patient must have tried before receiving ECT. We simulated 7 strategies: 1 strategy with no ECT available and 6 strategies with 0 to 5 lines prior to ECT. These strategies specifically target treatment-resistant depression because it is the most common indication for ECT in the United States.12 Because the decision to offer ECT to patients with life-threatening suicidality or psychosis relies on different costs, benefits, and clinical factors from those included in our model, we preferentially used data sources that do not target these indications.12 However, because there is substantial overlap between psychosis, suicidality, and treatment-resistant depression, many of our data sources included some patients with psychotic or suicidal features.22,23,24
We simulated the clinical and economic effects of these 7 strategies over a 4-year horizon. This horizon was chosen to allow adequate time for the costs and benefits of the various strategies to accrue while remaining within the range of prior modeling studies and long-term depression outcomes data.25,26 From these simulations, we calculated overall quality-adjusted life-years (QALYs, a measure combining longevity with health-related quality of life27) and costs from a health care sector perspective.28
The incremental cost-effectiveness ratio (ICER) of each strategy was calculated as the ratio of its incremental cost (in 2013 US dollars) to its incremental benefit (in QALYs) relative to the next most effective nondominated strategy. A strategy is considered dominated if it is less effective and more costly than another strategy (strong dominance) or if it is less effective and would have a higher ICER than another strategy (extended dominance).27 Commentators recommend using ICER thresholds ranging from $50 000 per QALY to $150 000 per QALY to define cost-effective health interventions in the United States29,30; for simplicity, we designated strategies with an ICER of $100 000 per QALY or less as cost-effective and also provided exact ICER values. In describing our model, analysis, and results, we adhered to the 2013 Consolidated Health Economic Evaluation Reporting Standards.31 Because this was secondary research without use of any identifiable data, this study was not regulated by the University of Michigan Health Sciences institutional review board. For the same reason, no consent was obtained.
Model Description
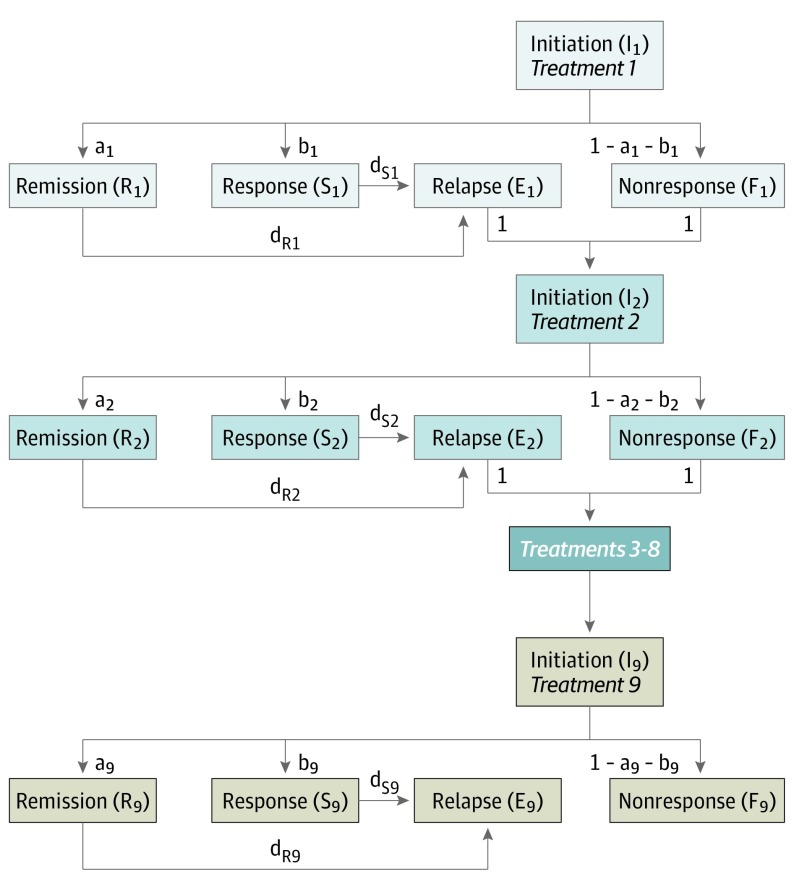
We developed a deterministic, state-transition model of depression treatment using Microsoft Excel 2016 (Figure 1); its mathematical details are fully described in the eAppendix in the Supplement and summarized here. The model includes 5 health states:
Figure 1. Model Structure.
Health states are represented by boxes and capital letters; transition probabilities between states are represented by arrowheads and lower-case letters. Patients in every model state are subject to a probability of mortality during each cycle; for clarity, these transitions are not shown in the diagram. Each group of 5 boxes of the same color represents 1 treatment line; for brevity, treatment lines 3 through 8 are omitted from the diagram and represented by a single dark blue box. Further information on the mathematical details of the model is provided in the eAppendix in the Supplement.
Initiation: the first month of a treatment line, before treatment benefits typically emerge3;
Remission: near-complete recovery of depressive symptoms (defined by score on a validated rating scale, eg, 24-item Hamilton Rating Scale of Depression <10)32;
Response: partial recovery of depressive symptoms without remission (defined by at least 50% reduction in 16-item Quick Inventory of Depressive Symptomatology-Self-Report)4;
Nonresponse: initial failure to achieve remission or response; and
Relapse: return of depressive symptoms after initial remission or response.
A simulated cohort of patients with major depressive disorder enters the model at initiation of first-line treatment and progresses through up to 9 treatment lines. Consistent with results of the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial,4 we do not explicitly distinguish between specific medications or between the presence/absence of psychotherapy. Instead, each change in treatment line represents switching between or augmenting the medications/psychotherapy used.
Model Inputs
Table 12,4,16,17,22,23,24,28,32,33,34,35,36,37,38,39,40,41,42,43,44,45 shows base case model inputs, along with ranges and distributions used in sensitivity analysis. Where available data permit, ranges reflect 95% confidence intervals; in other cases, the source of the range is described in subsequent sections.
Table 1. Model Input Data.
| Parameter | Base Case Value (Sensitivity Analysis Range) | Distribution | Source |
|---|---|---|---|
| General inputs | |||
| Annual discount rate, % | 3 | NA | Sanders et al28 |
| Time horizon, y | 4 (3 to 5) | NA | NA |
| Annual mortality probability, % | 0.479 (0.446 to 0.515) | Normal | Rush et al,4 Arias et al,33 Cuijpers et al34 |
| Antidepressant efficacy | |||
| Initial remission probability, % | |||
| Line 1 | 42.4 (40.0 to 44.9) | β | Weinmann et al35 |
| Line 2 | 36.8 (35.2 to 38.4) | β | Rush et al4 |
| Line 3 | 30.6 (28.2 to 33.0) | β | Rush et al4 |
| Line 4 | 13.7 (10.5 to 17.3) | β | Rush et al4 |
| Lines 5-9 | 13.0 (7.7 to 19.5) | β | Rush et al4 |
| Initial response probability, %a | |||
| Line 1 | 60.3 (58.0 to 62.6) | β | Weinmann et al35 |
| Line 2 | 48.6 (47.0 to 50.2) | β | Rush et al4 |
| Line 3b | 30.6 (28.2 to 33.0) | β | Rush et al4 |
| Line 4 | 16.8 (13.3 to 20.7) | β | Rush et al4 |
| Lines 5-9 | 16.3 (10.3 to 23.3) | β | Rush et al4 |
| Annual relapse probability, % | |||
| Line 1 remission | 47.5 (44.6 to 50.5) | β | Rush et al4 |
| Line 1 reponse | 74.0 (69.5 to 78.3) | ||
| Line 2 remission | 47.5 (44.6 to 50.5) | β | Rush et al4 |
| Line 2 response | 74.0 (69.5 to 78.3) | ||
| Line 3 remission | 67.7 (62.9 to 72.3) | β | Rush et al4 |
| Line 3 response | 82.0 (76.9 to 86.6) | ||
| Line 4 remission | 60.2 (42.1 to 76.4) | β | Rush et al4 |
| Line 4 response | 86.2 (76.8 to 93.1) | ||
| Lines 5-9 remission | 78.1 (38.8 to 96.3) | β | Rush et al4 |
| Lines 5-9 response | 88.1 (75.2 to 96.1) | ||
| Electroconvulsive therapy efficacy, % | |||
| Initial remission probability | 50.9 (47.4 to 54.4) | β | Dierckx et al2 |
| Initial response probability | 66.6 (63.3 to 69.8) | β | Bailine et al,23 Seinaert et al,36 Medda et al,37 Sackeim and Prudic,36 Grunhaus et al,38 Daly et al24 |
| Annual relapse probability | 30.8 (19.4 to 43.3) | β | Kellner et al,22 Navarro et al,39 Nordenskjöld et al40 |
| Electroconvulsive therapy utility effect | |||
| Initiation | 0 (−0.12 to 0.12) | Uniform | NA |
| Remission response | 0 (−0.04 to 0.04) | Uniform | Perlis et al41 |
| Utility with depression | |||
| Remission | 0.85 (0.829 to 0.871) | Normal | Sapin et al42 |
| Response | 0.72 (0.653 to 0.787) | Normal | Sapin et al42 |
| Nonresponse, relapse, initiation | 0.58 (0.499 to 0.661) | Normal | Sapin et al42 |
| Costs, 2013 USD, $ | |||
| Annual health care cost with depression | |||
| Line 1 | 7833 (7451-8216) | Normal | Crown et al43 |
| Line 2 | 7833 (7451-8216) | Normal | Crown et al43 |
| Line 3 | 8242 (6958-9512) | Normal | Russell et al44 |
| Line 4 | 11 894 (11 403 to 12 370) | Normal | Russell et al44 |
| Line 5 | 11 793 (11 245 to 12 327) | Normal | Russell et al44 |
| Line 6 | 13 583 (12 789 to 14 362) | Normal | Russell et al44 |
| Line 7 | 14 781 (13 525 to 16 022) | Normal | Russell et al44 |
| Line 8 | 15 719 (13 785 to 17 639) | Normal | Russell et al44 |
| Line 9 | 16 816 (13 410 to 20 209) | Normal | Russell et al44 |
| Electroconvulsive therapy costs, USD | |||
| Initial treatment course | 4691 (2622-8000) | Uniform | Centers for Medicare and Medicaid Services,16 Rasmussen,17 Kolshus et al,32 Bailine45 |
| Maintenance, annual | 9383 (4163 to 14 602) | Uniform | Kellner et al,22 Navarro et al,39 Nordenskjöld et al,40 Bailine45 |
Abbreviation: NA, not applicable.
In the model, response and remission are independent states, ie, a patient cannot occupy both at once; in most clinical trial reports, patients in remission also meet response criteria. For comparability with clinical trial reporting, response probabilities in Table 1 are presented as the sum of the model’s independent remission and response probabilities.
In step 2 of the Sequenced Treatment Alternatives to Relieve Depression trial, slightly more patients met remission criteria than response criteria; hence, the independent response probability in the model is set to 0.
General
To reflect their current value, future costs and QALYs are discounted at an annual rate of 3%.28 The model has a 1-month cycle length; this ensures patients do not switch from a failed treatment until at least 8 weeks have elapsed, consistent with clinical guidelines and STAR*D outcomes.4,46
Population Characteristics and Mortality
Based on STAR*D,4 we simulated a population with a mean (SD) age of 40.7 (13.2) years, and 62.2% women. We apply 2013 CDC life table data33 to this age/sex distribution, along with a relative mortality rate of 1.58 (95% CI, 1.47-1.70) for individuals with depression compared with the general population, from a 2014 meta-analysis.34 Combining these data yields an overall annual mortality probability of 0.00479.
Although some observational studies suggest that depression treatment may reduce suicide risk,7 randomized studies have not found a significant effect.47 Hence, we make the conservative assumption that treatment does not affect mortality rates.
Efficacy of Antidepressant Treatment
We derived probabilities of initial remission (13.0%-36.8%), response (16.3%-48.6%), and nonresponse (51.4%-83.7%) across 4 steps of antidepressant therapy from the STAR*D trial.4 Of note, the first-step selective serotonin reuptake inhibitor remission and response rates observed in STAR*D (36.8% and 48.6%) are markedly lower than those reported by multiple meta-analyses of selective serotonin reuptake inhibitor efficacy,35,48 possibly reflecting preexisting treatment-resistance among STAR*D participants. For this reason, first-line remission (42.4%) and response (60.3%) rates in our base case are drawn from 1 such meta-analysis,35 and the 4 steps of STAR*D are used for treatment lines 2 to 5.
Annual probabilities of relapse after initially successful treatment (47.5%-88.1%) are also derived from STAR*D.4 Relapse probabilities increased for later treatment lines and after response compared with remission.4
Cost of Depression Treatment
Annual health care costs of people with depression (including outpatient, inpatient, medication, and laboratory costs) are derived from 2 studies that used the MarketScan insurance claims database to assess costs by number of prior medication trials.43,44 Consistent with other analyses,8,49 annual costs increased with increasing numbers of prior failed treatments. We used the Bureau of Economic Analysis medical care expenditure indices for mental health care (eAppendix in the Supplement) to inflate costs to 2013 US dollars; this is the most recent year for which these indices are available.50,51 This yielded annual costs ranging from $7833 to $16 816 per year for 0 to 8 prior trials.
Utility
Based on a prospective cohort of patients receiving antidepressant pharmacotherapy, we used utility values of 0.85, 0.72, and 0.58 for the remission, response, and nonresponse/relapse/initiation states.42 These values are consistent with clinical trial results52 and provide a more conservative estimate of the benefit of remission than reported in a meta-analysis that included lower-quality studies.49
Efficacy of ECT
Based on a meta-analysis of observational evaluations of ECT efficacy in unipolar depression, we used an initial remission probability of 50.9%2; meta-analyses of randomized trials comparing repetitive transcranial magnetic stimulation vs ECT (53.0%)53 and right-unilateral vs bitemporal ECT (52.5%)32 report similar values. Our primary source did not assess response rates, so we calculated a weighted mean response rate (66.6%) from the included studies.23,24,36,37,38,54
Relapse rate after ECT was derived from 3 studies that are, to our knowledge, the only randomized trials of maintenance ECT plus pharmacotherapy vs pharmacotherapy alone; 6-month relapse rates in the ECT groups were 8%,39 29%,40 and 13%.22 A weighted mean of these results yields a 6-month relapse probability of 16.8%, corresponding with an annual probability of 30.8%.
We assumed all patients offered ECT would accept it (ie, we simulated exclusively ECT-willing patients). Although real-world uptake is substantially lower, this will not affect the cost-effectiveness of ECT among those who do accept it; to confirm this, we varied uptake between 10% and 100% in sensitivity analysis.
Cost and Utility Effects of ECT
Because recent, nationally representative cost data were lacking, we used a per-treatment ECT cost derived from a 1998 report by Bailine45 updated to 2013 US dollars, yielding a value of $586.50 We excluded inpatient care from the per-treatment ECT cost estimate because ECT is increasingly performed outpatient or initiated after a patient has already been hospitalized and hence would not cause increased inpatient length of stay; we also excluded the cost of psychiatric evaluations because these were already incorporated into our overall cost estimates.5,6 In sensitivity analysis, we varied this cost between $328 (reflecting per-treatment Centers for Medicare and Medicaid Services reimbursement to inpatient psychiatric facilities5,16) and $1000 (as estimated by an ECT specialist17). We assumed that ECT use had no direct effect on background health care costs, but that it could affect these costs as mediated by enhanced remission/response of depression.
Based on multiple ECT trials, we simulated 8 ECT sessions during 1 month during the initial treatment course.32 For maintenance treatment, we used a base-case value of 16 treatments per year, as reported in multiple maintenance ECT trials.39,55 In sensitivity analysis, we varied this between 7.1 treatments per year,22 from a trial of adaptive maintenance ECT, to 24.9 treatments per year, for symmetry. We made the conservative assumption that the number of maintenance treatments in the first year applied to every subsequent year.
In sensitivity analysis, we assessed incorporating a direct utility decrement for patients receiving ECT (reflecting the possibility of a worse adverse event profile than background pharmacotherapy/psychotherapy56) or a utility benefit (reflecting the faster clinical response and substantial utility increases observed in ECT trials3,57). For maintenance ECT, we varied this utility effect between −0.04 and 0.04 per month, from an estimate of the disutility associated with antidepressant medications.41 Because adverse cognitive effects of ECT are predominantly observed in the first 15 days of treatment,56 we tripled this range (−0.12 to 0.12) during the initial course.
Sensitivity and Uncertainty Analyses
To assess the robustness of our results to model input uncertainty, we performed univariate, probabilistic, and scenario sensitivity analyses. In univariate sensitivity analysis, we varied each individual model parameter over the ranges shown in Table 1, and calculated cost-effectiveness outcomes at its upper and lower bounds.
In probabilistic sensitivity analysis, the model was run using parameter values drawn at random from distributions reflecting the uncertainty in the estimation of each parameter, ie, second-order uncertainty (Table 1)58; to estimate overall uncertainty, we repeated this process 10 000 times. For each run, the optimal strategy, defined as the strategy producing the most QALYs with ICER of $100 000 per QALY or less, is determined. Prior research suggests that modeling probabilistic inputs independently may underestimate overall uncertainty.59 To address this, we performed 1 probabilistic sensitivity analysis with minimal parameter covariance (independent parameter variance), and 1 with maximal covariance (linked parameter variance); further details are provided in the eAppendix in the Supplement.
Finally, in scenario sensitivity analysis, we assessed alternative approaches to modeling pharmacotherapy efficacy, depression cost, and maintenance ECT use. First, we assessed using first-line efficacy data from the previously described meta-analysis,35 with subsequent response and remission probabilities calculated based on a 19% reduction in the odds of remission/response with each successive line.60 Alternatively, we assessed using only STAR*D efficacy data, without incorporating first-line efficacy data from the aforementioned meta-analysis.4,35 Next, we used costs of depression derived from a study by Gibson et al.8 This study is from 2010, rather than 2002 for our base-case data source, but uses a metric of prior treatment experience that is less analogous to lines in our model; annual costs range from $6916 to $15 576 for treatment lines 1 to 9. We next assessed incorporating an absolute mortality reduction of 0.07% for patients with remission/response of depression, reflecting 1 estimate of the reduction in suicide mortality with effective treatment.61 Finally, we simulated using maintenance ECT only after 1 relapse with maintenance pharmacotherapy alone; annual relapse probability with maintenance pharmacotherapy alone is set to 51.1%.62
Results
Model Validation
To provide external validation of our model, we compared model-generated outcomes with independent published results. During the 24 months after initiation of first-line antidepressant treatment, the model estimated an annual per-patient cost of $9413, compared with $9287 from a 2017 study of primarily privately insured patients63 and $11 263 among Medicaid patients.64 During a 4-year horizon without ECT, the model estimated patients spend 50.2% of their life-years in nonresponse/relapse/initiation states compared with a mean fraction of time ill of 46% (95% CI, 34%-58%) from a meta-analysis of long-term unipolar depression outcomes.26 Finally, the model estimated that mean utility increased by 0.159 after initial ECT course and 0.201 six months later, compared with 0.177 and 0.204 from a large ECT trial.65,66
Base Case
Base case results are presented in Table 2. Over 4 years, ECT was projected to reduce the fraction of time with nonresponse of depression from 50.2% of life-years to 37.1% to 32.9% of life-years, with greater reductions when ECT is offered earlier. These reductions translate to mean quality-adjusted survival gains of 0.12 to 0.15 QALYs, at incremental costs of $7300 to $12 000. Cost increases were attributable primarily to the cost of ECT; mean ECT-related cost increased from $9100 to $17 100 with earlier ECT offer, while the mean cost of other health care declined from $42 500 (without ECT) to $37 400 (with first-line ECT).
Table 2. Base Case Results.
| Strategy | Fraction of Life-Years With Depression Outcome, % | QALYs per Patient | Healthcare Costs per Patient, 2013 US $ | Incremental Cost-effectiveness Ratio (US $/QALY) | ||||
|---|---|---|---|---|---|---|---|---|
| Remission | Response | Nonresponsea | Totalb | ECT | Other | |||
| ECT | ||||||||
| None | 42.5 | 7.3 | 50.2 | 2.63 | 42 490 | NA | 42 490 | NA |
| Sixth-line | 52.2 | 10.7 | 37.1 | 2.75 | 50 080 | 9090 | 40 990 | Dominated |
| Fifth-line | 53.0 | 11.0 | 36.1 | 2.76 | 49 850 | 9910 | 39 930 | Dominated |
| Fourth-line | 53.6 | 11.4 | 35.1 | 2.76 | 50 900 | 10 900 | 40 000 | Dominated |
| Third-line | 53.8 | 12.1 | 34.1 | 2.77 | 49 830 | 12 220 | 37 610 | 54 000 |
| Second-line | 53.8 | 12.7 | 33.5 | 2.77 | 52 000 | 14 610 | 37 390 | 564 000 |
| First-line | 53.8 | 13.3 | 32.9 | 2.78 | 54 520 | 17 130 | 37 390 | 815 000 |
Abbreviations: ECT, electroconvulsive therapy; QALYs, quality-adjusted life-years.
Includes the model states nonresponse, relapse, and initiation.
Due to rounding, the total cost presented does not equal the sum of the ECT and other subcategories for certain strategies.
Using a willingness-to-pay threshold of $100 000 per QALY, third-line ECT was projected to be cost-effective, with an ICER of $54 000 per QALY. Second-line and first-line ECT were not cost-effective, with ICERs of $564 000 per QALY and $815 000 per QALY. Analyzed incrementally, fourth-, fifth-, and sixth-line ECT were all dominated (ie, they offered fewer QALYs at a worse ICER than other strategies). However, when compared with not offering ECT, each of these strategies would be cost-effective, with cost-effectiveness ratios between $60 000 to $70 000 per QALY.
Sensitivity and Uncertainty Analyses
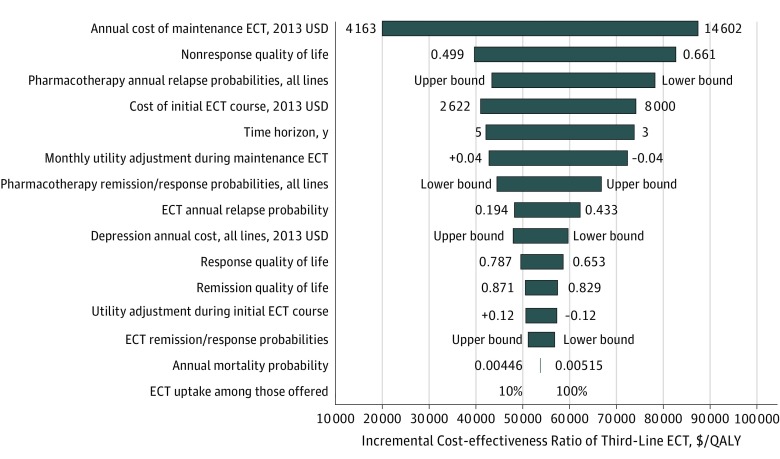
Univariate sensitivity analysis results are shown in Figure 2. Third-line ECT remained cost-effective (ICER ≤$100 000 per QALY) across all individual parameter variations. The cost of ECT was an important source of uncertainty, with annual maintenance cost and initial course cost both among the 4 parameters with the most influence over the ICER of third-line ECT (ICER ranges were $20 000 per QALY to $87 000 per QALY for maintenance cost and $41 000 per QALY to $74 000 per QALY for initial course cost). Extending beyond the preestablished uncertainty ranges, third-line ECT would reach an ICER of $100 000 per QALY at an annual maintenance cost of $16 590 or an initial course cost of $12 180.
Figure 2. Univariate Sensitivity Analysis Results.
Horizontal bars indicate the range of incremental cost-effectiveness ratios (ICERs) for the third-line electroconvulsive therapy (ECT) strategy produced by varying the values of the model parameters shown on the vertical axis. The parameter values producing the lower value and upper value of the ICER are shown to the left and right of each horizontal bar. Values specified as lower bound or upper bound indicate that multiple model parameters are being varied in concert and cannot all be displayed on the figure; Table 1 provides parameter values. Parameters are arranged from those producing the greatest variation in the ICER (top) to those producing the least (bottom). QALY indicates quality-adjusted life-year.
In probabilistic sensitivity analysis, we found a 74% to 78% likelihood that at least 1 ECT strategy was cost-effective (ICER ≤$100 000/QALY) depending on the degree of parameter covariance (Table 3). Third-line ECT was the single optimal strategy in 56% to 58% of PSA simulations.
Table 3. Probabilistic Sensitivity Analysis Results.
| Strategy | Fraction of 10 000 Simulations in Which Strategy Is Optimal, Given Willingness to Pay of $100 000/QALY, % | |
|---|---|---|
| Independent Parameter Variance | Linked Parameter Variance | |
| ECT | ||
| None | 21.7 | 26.5 |
| Sixth-line | 0.3 | 0 |
| Fifth-line | 17.2 | 12.9 |
| Fourth-line | 2.8 | 0.1 |
| Third-line | 55.8 | 57.9 |
| Second-line | 2.0 | 2.2 |
| First-line | 0.2 | 0.5 |
Abbreviations: ECT, electroconvulsive therapy; QALY, quality-adjusted life-year.
Finally, the 5 scenario sensitivity analyses (with alternative approaches to pharmacotherapy efficacy, depression cost, maintenance ECT use, and suicide) did not markedly change our results, with third-line ECT remaining cost-effective (ICER ≤$100 000/QALY) in all scenarios (eTable in the Supplement).
Discussion
We used a decision analytic model to evaluate the clinical and economic effects of varying strategies for using ECT to treat treatment-resistant depression in the United States. We found that ECT would substantially improve clinical outcomes, reducing time with uncontrolled depression from 50.2% to 32.9% to 37.1% of life-years over a 4-year horizon. We projected a 74% to 78% likelihood that ECT would be cost-effective in the United States given commonly accepted cost-effectiveness thresholds (ICER ≤$100 000 per QALY).29 Across a range of realistic variations in model input data, we found that offering ECT after failure of 2 lines of pharmacotherapy/psychotherapy would most reliably maximize its health-economic value. For patients with 3 or more prior treatment trials, offering ECT later in the course of treatment would still be cost-effective. When compared with other depression interventions that have been evaluated, the cost-effectiveness of ECT for treatment-resistant depression is superior to pharmacogenetic testing41 and similar to a rural collaborative care intervention.67
Based on these findings, we recommend that clinicians consider offering ECT to patients with major depressive disorder who have failed to respond to 2 trials of pharmacotherapy and/or psychotherapy. Notably, our findings align well with 2017 recommendations from Conway et al, who propose defining treatment-resistant depression by “failure of 2 adequate dose-duration antidepressants or psychotherapy from different classes”21 and recommend considering ECT at this point. Similarly, the 2017 Florida best practice guidelines for treatment of major depressive disorder include ECT as a level 3 treatment, to be considered after failure of levels 1 and 2.68
Despite these recommendations, we recognize that many clinicians will regard offering ECT as third-line treatment to be overly aggressive, especially given the common view of ECT as a last-resort treatment for depression.15,69 To understand how third-line ECT can be reasonable and cost-effective, it is crucial to recognize how challenging treatment-resistant depression is to manage. Observational and trial data indicate that fewer than one-third of patients with this condition respond to pharmacotherapy and/or psychotherapy, and those who do respond are at high risk of relapse.4,70 Given the limited effectiveness of commonly used treatments, it may be less surprising that earlier ECT use would greatly improve clinical outcomes for patients with treatment-resistant depression.
Limitations
Our analysis has limitations related to the model’s structure, the input data used, and the scope of the analysis. By necessity, a model must include some simplifying assumptions. Where possible, we have chosen these assumptions to be conservative with respect to ECT’s cost-effectiveness, such as assuming indefinite maintenance ECT use, excluding ECT’s effects on mortality and speed of remission,3,7 and excluding the possibility of greater efficacy with earlier ECT use.10 In addition, our model assumes that transition probabilities between states (eg, relapse probabilities71) do not vary over time. Despite these simplifications, the model’s outcomes are well validated by independent data on costs, long-term clinical outcomes, and quality of life.
Next, there are several shortcomings in our model’s input data. Much of our cost data are more than a decade old and reflect primarily privately insured patients,43,44,45 and there is uncertainty regarding the cost of ECT.16,17,45 In addition, many of our estimates of relapse rates with pharmacotherapy or maintenance ECT reflect sample sizes of 100 patients or fewer.4,22,39,40 Finally, our reliance on clinical trial data may limit the generalizability and external validity of our results.4 However, our main findings are robust to sensitivity analysis using alternative data sources or appropriately broad confidence intervals.
Finally, the scope of our analysis introduces several limitations. Our choice of a health care sector perspective is intended to be relevant to patients, clinicians, and payers; however, this perspective excludes indirect costs and benefits such as patient transportation and productivity gains after depression treatment.17,72 Additionally, we did not evaluate other novel interventions for treatment-resistant depression, such as repetitive transcranial magnetic stimulation or ketamine.13,46
Conclusions
In this mathematical modeling analysis, we found that ECT is a cost-effective treatment option for treatment-resistant depression in the United States. Offering ECT after 2 failed lines of pharmacotherapy/psychotherapy is most likely to maximize its health-economic value and is concordant with recommendations from some national guidelines and ECT specialists. Increasing use of ECT by offering it earlier in the course of treatment-resistant depression could greatly improve outcomes for this difficult-to-treat patient population.
eAppendix. Model States and Transitions
eTable. Scenario Sensitivity Analysis Results
References
- 1.Sackeim HA. Modern electroconvulsive therapy: vastly improved yet greatly underused. JAMA Psychiatry. 2017;74(8):779-780. [DOI] [PubMed] [Google Scholar]
- 2.Dierckx B, Heijnen WT, van den Broek WW, Birkenhäger TK. Efficacy of electroconvulsive therapy in bipolar versus unipolar major depression: a meta-analysis. Bipolar Disord. 2012;14(2):146-150. [DOI] [PubMed] [Google Scholar]
- 3.Husain MM, Rush AJ, Fink M, et al. Speed of response and remission in major depressive disorder with acute electroconvulsive therapy (ECT): a Consortium for Research in ECT (CORE) report. J Clin Psychiatry. 2004;65(4):485-491. [DOI] [PubMed] [Google Scholar]
- 4.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917. [DOI] [PubMed] [Google Scholar]
- 5.Slade EP, Jahn DR, Regenold WT, Case BG. Association of electroconvulsive therapy with psychiatric readmissions in US hospitals. JAMA Psychiatry. 2017;74(8):798-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olfson M, Marcus S, Sackeim HA, Thompson J, Pincus HA. Use of ECT for the inpatient treatment of recurrent major depression. Am J Psychiatry. 1998;155(1):22-29. [DOI] [PubMed] [Google Scholar]
- 7.Fink M, Kellner CH, McCall WV. The role of ECT in suicide prevention. J ECT. 2014;30(1):5-9. [DOI] [PubMed] [Google Scholar]
- 8.Gibson TB, Jing Y, Smith Carls G, et al. Cost burden of treatment resistance in patients with depression. Am J Manag Care. 2010;16(5):370-377. [PubMed] [Google Scholar]
- 9.Kellner CH, Greenberg RM, Murrough JW, Bryson EO, Briggs MC, Pasculli RM. ECT in treatment-resistant depression. Am J Psychiatry. 2012;169(12):1238-1244. [DOI] [PubMed] [Google Scholar]
- 10.Heijnen WT, Birkenhäger TK, Wierdsma AI, van den Broek WW. Antidepressant pharmacotherapy failure and response to subsequent electroconvulsive therapy: a meta-analysis. J Clin Psychopharmacol. 2010;30(5):616-619. [DOI] [PubMed] [Google Scholar]
- 11.Leiknes KA, Jarosh-von Schweder L, Høie B. Contemporary use and practice of electroconvulsive therapy worldwide. Brain Behav. 2012;2(3):283-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Psychiatric Association Committe on Electroconvulsive Therapy In: Weinter RD, Coffey CE, Fochtmann LJ, et al. , eds. The Practice of Electroconvulsive Therapy: Recommendations for Treatment, Training, and Privileging. 2nd ed Washington, DC: American Psychiatric Association; 2001. [Google Scholar]
- 13.Milev RV, Giacobbe P, Kennedy SH, et al. ; CANMAT Depression Work Group . Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder, section 4: neurostimulation treatments. Can J Psychiatry. 2016;61(9):561-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Psychiatric Association Practice guideline for the treatment of patients with major depressive disorder, third edition. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Accessed August 27, 2017.
- 15.Sackeim HA, Dillingham EM, Prudic J, et al. Effect of concomitant pharmacotherapy on electroconvulsive therapy outcomes: short-term efficacy and adverse effects. Arch Gen Psychiatry. 2009;66(7):729-737. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Medicare and Medicaid Services Inpatient psychiatric facility prospective payment system. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/Downloads/Inpatient-Psychiatric-Facility-Prospective-Payment-System-Text-Only.pdf. Accessed August 11, 2017.
- 17.Rasmussen KG. Some considerations in choosing electroconvulsive therapy versus transcranial magnetic stimulation for depression. J ECT. 2011;27(1):51-54. [DOI] [PubMed] [Google Scholar]
- 18.Vlahiotis A, Devine ST, Eichholz J, Kautzner A. Discontinuation rates and health care costs in adult patients starting generic versus brand SSRI or SNRI antidepressants in commercial health plans. J Manag Care Pharm. 2011;17(2):123-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenhalgh J, Knight C, Hind D, Beverley C, Walters S. Clinical and cost-effectiveness of electroconvulsive therapy for depressive illness, schizophrenia, catatonia and mania: systematic reviews and economic modelling studies. Health Technol Assess. 2005;9(9):1-156, iii-iv. [DOI] [PubMed] [Google Scholar]
- 20.Vallejo-Torres L, Castilla I, González N, Hunter R, Serrano-Pérez P, Perestelo-Pérez L. Cost-effectiveness of electroconvulsive therapy compared to repetitive transcranial magnetic stimulation for treatment-resistant severe depression: a decision model. Psychol Med. 2015;45(7):1459-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conway CR, George MS, Sackeim HA. Toward an evidence-based, operational definition of treatment-resistant depression: when enough is enough. JAMA Psychiatry. 2017;74(1):9-10. [DOI] [PubMed] [Google Scholar]
- 22.Kellner CH, Husain MM, Knapp RG, et al. ; CORE/PRIDE Work Group . A novel strategy for continuation ECT in geriatric depression: phase 2 of the PRIDE study. Am J Psychiatry. 2016;173(11):1110-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bailine S, Fink M, Knapp R, et al. Electroconvulsive therapy is equally effective in unipolar and bipolar depression. Acta Psychiatr Scand. 2010;121(6):431-436. [DOI] [PubMed] [Google Scholar]
- 24.Daly JJ, Prudic J, Devanand DP, et al. ECT in bipolar and unipolar depression: differences in speed of response. Bipolar Disord. 2001;3(2):95-104. [DOI] [PubMed] [Google Scholar]
- 25.Zimovetz EA, Wolowacz SE, Classi PM, Birt J. Methodologies used in cost-effectiveness models for evaluating treatments in major depressive disorder: a systematic review. Cost Eff Resour Alloc. 2012;10(1):1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forte A, Baldessarini RJ, Tondo L, Vázquez GH, Pompili M, Girardi P. Long-term morbidity in bipolar-I, bipolar-II, and unipolar major depressive disorders. J Affect Disord. 2015;178:71-78. [DOI] [PubMed] [Google Scholar]
- 27.Briggs AH, Claxton C, Sculpher MJ. Decision Modelling for Health Economic Evaluation. New York, NY: Oxford University Press; 2006. [Google Scholar]
- 28.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093-1103. [DOI] [PubMed] [Google Scholar]
- 29.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness: the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796-797. [DOI] [PubMed] [Google Scholar]
- 30.Anderson JL, Heidenreich PA, Barnett PG, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(21):2304-2322. [DOI] [PubMed] [Google Scholar]
- 31.Husereau D, Drummond M, Petrou S, et al. ; ISPOR Health Economic Evaluation Publication Guidelines-CHEERS Good Reporting Practices Task Force . Consolidated health economic evaluation reporting standards (CHEERS): explanation and elaboration: a report of the ISPOR Health Economic Evaluations Publication Good Reporting Practices Task Force. Value Health. 2013;16(2):231-250. [DOI] [PubMed] [Google Scholar]
- 32.Kolshus E, Jelovac A, McLoughlin DM. Bitemporal v. high-dose right unilateral electroconvulsive therapy for depression: a systematic review and meta-analysis of randomized controlled trials. Psychol Med. 2017;47(3):518-530. [DOI] [PubMed] [Google Scholar]
- 33.Arias E, Heron M, Xu J. United States life tables, 2013. Natl Vital Stat Rep. 2017;66(3):1-64. [PubMed] [Google Scholar]
- 34.Cuijpers P, Vogelzangs N, Twisk J, Kleiboer A, Li J, Penninx BW. Comprehensive meta-analysis of excess mortality in depression in the general community versus patients with specific illnesses. Am J Psychiatry. 2014;171(4):453-462. [DOI] [PubMed] [Google Scholar]
- 35.Weinmann S, Becker T, Koesters M. Re-evaluation of the efficacy and tolerability of venlafaxine vs SSRI: meta-analysis. Psychopharmacology (Berl). 2008;196(4):511-520. [DOI] [PubMed] [Google Scholar]
- 36.Sienaert P, Vansteelandt K, Demyttenaere K, Peuskens J. Ultra-brief pulse ECT in bipolar and unipolar depressive disorder: differences in speed of response. Bipolar Disord. 2009;11(4):418-424. [DOI] [PubMed] [Google Scholar]
- 37.Medda P, Perugi G, Zanello S, Ciuffa M, Cassano GB. Response to ECT in bipolar I, bipolar II and unipolar depression. J Affect Disord. 2009;118(1-3):55-59. [DOI] [PubMed] [Google Scholar]
- 38.Grunhaus L, Schreiber S, Dolberg OT, Hirshman S, Dannon PN. Response to ECT in major depression: are there differences between unipolar and bipolar depression? Bipolar Disord. 2002;4(suppl 1):91-93. [DOI] [PubMed] [Google Scholar]
- 39.Navarro V, Gastó C, Torres X, et al. Continuation/maintenance treatment with nortriptyline versus combined nortriptyline and ECT in late-life psychotic depression: a two-year randomized study. Am J Geriatr Psychiatry. 2008;16(6):498-505. [DOI] [PubMed] [Google Scholar]
- 40.Nordenskjöld A, von Knorring L, Ljung T, Carlborg A, Brus O, Engström I. Continuation electroconvulsive therapy with pharmacotherapy versus pharmacotherapy alone for prevention of relapse of depression: a randomized controlled trial. J ECT. 2013;29(2):86-92. [DOI] [PubMed] [Google Scholar]
- 41.Perlis RH, Patrick A, Smoller JW, Wang PS. When is pharmacogenetic testing for antidepressant response ready for the clinic? A cost-effectiveness analysis based on data from the STAR*D study. Neuropsychopharmacology. 2009;34(10):2227-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sapin C, Fantino B, Nowicki M-L, Kind P. Usefulness of EQ-5D in assessing health status in primary care patients with major depressive disorder. Health Qual Life Outcomes. 2004;2:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crown WH, Finkelstein S, Berndt ER, et al. The impact of treatment-resistant depression on health care utilization and costs. J Clin Psychiatry. 2002;63(11):963-971. [DOI] [PubMed] [Google Scholar]
- 44.Russell JM, Hawkins K, Ozminkowski RJ, et al. The cost consequences of treatment-resistant depression. J Clin Psychiatry. 2004;65(3):341-347. [DOI] [PubMed] [Google Scholar]
- 45.Bailine S. Reimbursement and documentation issues in an ambulatory ECT program. J ECT. 1998;14(4):255-258. [PubMed] [Google Scholar]
- 46.Kennedy SH, Lam RW, McIntyre RS, et al. ; CANMAT Depression Work Group . Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder, section 3: pharmacological treatments. Can J Psychiatry. 2016;61(9):540-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Braun C, Bschor T, Franklin J, Baethge C. Suicides and suicide attempts during long-term treatment with antidepressants: a meta-analysis of 29 placebo-controlled studies including 6,934 patients with major depressive disorder. Psychother Psychosom. 2016;85(3):171-179. [DOI] [PubMed] [Google Scholar]
- 48.Papakostas GI, Homberger CH, Fava M. A meta-analysis of clinical trials comparing mirtazapine with selective serotonin reuptake inhibitors for the treatment of major depressive disorder. J Psychopharmacol. 2008;22(8):843-848. [DOI] [PubMed] [Google Scholar]
- 49.Mrazek DA, Hornberger JC, Altar CA, Degtiar I. A review of the clinical, economic, and societal burden of treatment-resistant depression: 1996-2013. Psychiatr Serv. 2014;65(8):977-987. [DOI] [PubMed] [Google Scholar]
- 50.Bureau of Economic Analysis Medical care expenditure indices from MEPS. https://www.bea.gov/national/health_care_satellite_account.htm. Accessed August 11, 2017.
- 51.Dunn A, Grosse SD, Zuvekas SH. Adjusting health expenditures for inflation: a review of measures for health services research in the United States [published online November 21, 2016]. Health Serv Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benedict A, Arellano J, De Cock E, Baird J. Economic evaluation of duloxetine versus serotonin selective reuptake inhibitors and venlafaxine XR in treating major depressive disorder in Scotland. J Affect Disord. 2010;120(1-3):94-104. [DOI] [PubMed] [Google Scholar]
- 53.Ren J, Li H, Palaniyappan L, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2014;51:181-189. [DOI] [PubMed] [Google Scholar]
- 54.Sackeim HA, Prudic J. Length of the ECT course in bipolar and unipolar depression. J ECT. 2005;21(3):195-197. [DOI] [PubMed] [Google Scholar]
- 55.Kellner CH, Knapp RG, Petrides G, et al. Continuation electroconvulsive therapy vs pharmacotherapy for relapse prevention in major depression: a multisite study from the Consortium for Research in Electroconvulsive Therapy (CORE). Arch Gen Psychiatry. 2006;63(12):1337-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry. 2010;68(6):568-577. [DOI] [PubMed] [Google Scholar]
- 57.McCall WV, Reboussin D, Prudic J, et al. Poor health-related quality of life prior to ECT in depressed patients normalizes with sustained remission after ECT. J Affect Disord. 2013;147(1-3):107-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD; ISPOR-SMDM Modeling Good Research Practices Task Force . Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force Working Group-6. Med Decis Making. 2012;32(5):722-732. [DOI] [PubMed] [Google Scholar]
- 59.Taylor M. The implications of parameter independence in probabilistic sensitivity analysis. Paper presented at: International Society for Pharmacoeconomics and Outcomes Research 19th Annual European Congress; October 31, 2016; Vienna, Austria. [Google Scholar]
- 60.Amsterdam JD, Williams D, Michelson D, et al. Tachyphylaxis after repeated antidepressant drug exposure in patients with recurrent major depressive disorder. Neuropsychobiology. 2009;59(4):227-233. [DOI] [PubMed] [Google Scholar]
- 61.Hornberger J, Li Q, Quinn B. Cost-effectiveness of combinatorial pharmacogenomic testing for treatment-resistant major depressive disorder patients. Am J Manag Care. 2015;21(6):e357-e365. [PubMed] [Google Scholar]
- 62.Jelovac A, Kolshus E, McLoughlin DM. Relapse following successful electroconvulsive therapy for major depression: a meta-analysis. Neuropsychopharmacology. 2013;38(12):2467-2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gauthier G, Guérin A, Zhdanava M, et al. Treatment patterns, healthcare resource utilization, and costs following first-line antidepressant treatment in major depressive disorder: a retrospective US claims database analysis. BMC Psychiatry. 2017;17(1):222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Olfson M, Marcus S, Benson C, Mcrae J, Amos T Health care costs of treatment-resistant depression in a Medicaid population. Paper presented at: International Society for Pharmacoeconomics and Outcomes Research 22nd Annual Meeting; May 23, 2017; Boston, MA. [Google Scholar]
- 65.McCall WV, Rosenquist PB, Kimball J, et al. Health-related quality of life in a clinical trial of ECT followed by continuation pharmacotherapy: effects immediately after ECT and at 24 weeks. J ECT. 2011;27(2):97-102. [DOI] [PubMed] [Google Scholar]
- 66.Ara R, Brazier J. Deriving an algorithm to convert the eight mean SF-36 dimension scores into a mean EQ-5D preference-based score from published studies (where patient level data are not available). Value Health. 2008;11(7):1131-1143. [DOI] [PubMed] [Google Scholar]
- 67.Pyne JM, Fortney JC, Tripathi SP, Maciejewski ML, Edlund MJ, Williams DK. Cost-effectiveness analysis of a rural telemedicine collaborative care intervention for depression. Arch Gen Psychiatry. 2010;67(8):812-821. [DOI] [PubMed] [Google Scholar]
- 68.McIntyre RS, Suppes T, Tandon R, Ostacher M. Florida best practice psychotherapeutic medication guidelines for adults with major depressive disorder. J Clin Psychiatry. 2017;78(6):703-713. [DOI] [PubMed] [Google Scholar]
- 69.Dauenhauer LE, Chauhan P, Cohen BJ. Factors that influence electroconvulsive therapy referrals: a statewide survey of psychiatrists. J ECT. 2011;27(3):232-235. [DOI] [PubMed] [Google Scholar]
- 70.Dunner DL, Rush AJ, Russell JM, et al. Prospective, long-term, multicenter study of the naturalistic outcomes of patients with treatment-resistant depression. J Clin Psychiatry. 2006;67(5):688-695. [DOI] [PubMed] [Google Scholar]
- 71.Sackeim HA, Prudic J, Devanand DP, et al. A prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities. Arch Gen Psychiatry. 2000;57(5):425-434. [DOI] [PubMed] [Google Scholar]
- 72.Stewart WF, Ricci JA, Chee E, Hahn SR, Morganstein D. Cost of lost productive work time among US workers with depression. JAMA. 2003;289(23):3135-3144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Model States and Transitions
eTable. Scenario Sensitivity Analysis Results