Key Points
Question
What is the role of aldosterone antagonist therapy in patients with ST-segment elevation myocardial infarction without heart failure or left ventricular ejection fraction greater than 40%?
Findings
In this systematic review and meta-analysis of 10 randomized clinical trials with 4147 unique patients who presented with ST-segment elevation myocardial infarction, treatment with aldosterone antagonists was associated with significantly reduced mortality and significantly increased left ventricular ejection fraction at follow-up.
Meaning
Patients who present with ST-segment elevation myocardial infarction could have survival benefit from initiation of treatment with aldosterone antagonists.
Abstract
Importance
Treatment with aldosterone antagonists is recommended and has been shown to have beneficial effects in patients with ST-segment elevation myocardial infarction (STEMI) and left ventricular ejection fraction (LVEF) less than 40%. However, the role of aldosterone antagonists in patients with ejection fraction greater than 40% or without congestive heart failure is not well known.
Objectives
To perform a systematic review and meta-analysis using standard techniques to determine the role of therapy with aldosterone antagonists in this patient population.
Data Sources
PubMed, Embase, CINAHL, and Cochrane Central databases were searched and a manual search for relevant references from the selected articles and published reviews was performed from database inception through June 2017.
Study Selection
Randomized clinical trials that evaluated treatment with aldosterone antagonists in patients with STEMI without clinical heart failure or LVEF greater than 40% were included.
Data Extraction and Synthesis
Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines were used to conduct and report the meta-analysis, which used a random-effects model. Two investigators independently performed the database search and agreed on the final study selection. A manual search was performed for relevant references from the selected articles and published reviews.
Main Outcomes and Measures
The outcomes analyzed were mortality, new congestive heart failure, recurrent myocardial infarction, ventricular arrhythmia, and changes in LVEF, serum potassium level, and creatinine level at follow-up.
Results
In all, 10 randomized clinical trials with a total of 4147 unique patients were included in the meta-analysis. In patients who presented with STEMI without heart failure, treatment with aldosterone antagonists compared with control was associated with lower risk of mortality (2.4% vs 3.9%; odds ratio [OR], 0.62; 95% CI, 0.42-0.91; P = .01) and similar risks of myocardial infarction (1.6% vs 1.5%; OR, 1.03; 95% CI, 0.57-1.86; P = .91), new congestive heart failure (4.3% vs 5.4%; OR, 0.82; 95% CI, 0.56-1.20; P = .31), and ventricular arrhythmia (4.1% vs 5.1%; OR, 0.76; 95% CI, 0.45-1.31; P = .33). Similarly, treatment with aldosterone antagonists compared with control was associated with a small yet significant increase in LVEF (mean difference, 1.58%; 95% CI, 0.18%-2.97%; P = .03), a small increase in serum potassium level (mean difference, 0.07 mEq/L; 95% CI, 0.01-0.13 mEq/L; P = .02), and no change in serum creatinine level (standardized mean difference, 1.4; 95% CI, −0.43 to 3.24; P = .13).
Conclusions and Relevance
Treatment with aldosterone antagonists is associated with a mortality benefit in patients with STEMI with LVEF greater than 40% or without heart failure.
This systematic review and meta-analysis of 10 randomized clinical trials explores the role of treatment with aldosterone antagonists in patients with ST-segment elevation myocardial infarction (STEMI) without heart failure or left ventricular ejection fraction greater than 40%.
Introduction
Primary percutaneous coronary intervention (PCI) or timely thrombolysis is recommended for patients presenting with ST-segment elevation myocardial infarction (STEMI).1,2 Despite significant improvement in guidelines-directed revascularization with PCI or thrombolysis, STEMI is associated with significant morbidity and mortality of up to 23% within 5 years after PCI, with a significant number of deaths occurring within the first 30 days.3 After STEMI, irreversible injury during the ischemia period may lead to necrosis, inflammation, and fibrosis in the reperfusion phase, leading to molecular and mechanical changes that eventually contribute to the ventricular remodeling process.4,5,6 Ventricular remodeling predisposes patients to higher morbidity and mortality and therefore has been the focus of therapy to improve long-term outcomes after STEMI.7
Aldosterone, which is a mineralocorticoid hormone, has been linked to the development of ventricular remodeling through the promotion of tissue fibrosis.5 Elevated aldosterone levels have been shown to correlate with worse adverse clinical outcomes, including mortality; therefore, reduction of aldosterone levels has been a target to improve long-term clinical outcomes in patients with coronary artery disease and patients who have experienced myocardial infarction (MI).8,9,10 On the basis of the Eplerenone Post–Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) trial, treatment with aldosterone antagonists (AAs) has been recommended for patients with acute MI with ejection fraction less than 40% and clinical heart failure or diabetes.2,11 Thus far, the usefulness of AAs in treating patients with STEMI with left ventricular ejection fraction (LVEF) greater than 40% has not been defined, although a number of randomized clinical trials (RCTs) have evaluated the role of AA therapy in patients with STEMI without clinical heart failure or LVEF greater than 40%; only 1 study12 showed a survival benefit associated with AA therapy in patients with STEMI, whereas other studies had inconclusive findings.13,14 Thus, to better define the role of AA therapy in patients with STEMI without clinical heart failure or LVEF greater than 40%, we performed this systematic review and meta-analysis of randomized clinical trials.
Methods
Data Sources and Search Strategy
We complied with Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines to conduct and report this meta-analysis after writing a study protocol.15 We searched the PubMed, Embase, CINAHL, and Cochrane Central Register of Clinical Trials electronic databases for English-language references from inception through June 2017 using the search terms aldosterone antagonists OR AA OR spironolactone OR eplerenone OR canrenoate AND ST elevation myocardial infarction OR acute myocardial infarction OR STEMI. Two of us (K.D. and S.S.) independently performed the database search and agreed on the final study selection. A manual search was performed for relevant references from the selected articles and published reviews.
Study Selection
Those RCTs evaluating the effects of treatment with AAs in patients with STEMI without heart failure or with LVEF greater than 40% in adult patients (≥18 years) were selected for the systematic review and meta-analysis. Those studies that did not report on clinical or biochemical outcomes were excluded. Also excluded were studies evaluating patients after STEMI with LVEF less than 40%, meeting abstracts, and nonrandomized studies.
Data Extraction
Four investigators worked in 2 groups (K.D. and A.H.; S.P.S. and S.S.) to extract data from the selected studies in duplicate using a standardized data extraction form. Data were obtained on study characteristics that included study design, patient selection, inclusion and exclusion criteria, follow-up duration, number of patients, type and dosing of AAs and outcomes, patient demographic and baseline characteristics, and outcomes of interest reported at follow-up.
Outcomes
Mortality, MI, new congestive heart failure (CHF), ventricular arrhythmia, and change in LVEF, serum creatinine level, and potassium level were the major outcomes of interest. Analysis of an outcome was only performed if at least 2 studies reported that outcome.
Statistical Analysis
We performed the statistical analyses with use of Review Manager software (RevMan, version 5.3; Cochrane Collaboration, Nordic Cochrane Center) and used a random-effects model to compute overall effects. The continuous variables were analyzed as mean difference (MD) or standardized MD with 95% CI depending on the units of reporting. For the categorical variables, we calculated the odds ratio (OR) with 95% CI using the total number of events and patients as reported in the individual studies. Study heterogeneity was evaluated with the Cochran Q and I2 index, and significant heterogeneity (I2>60%) was explored with sensitivity analyses. We planned prespecified subgroup analyses based on the duration of follow-up (≥6 months vs <6 months), type of AA used (spironolactone, eplerenone, or canrenoate potassium), and size of study (small defined as a total number of participants of <100 in each arm).
We performed additional sensitivity analyses with use of a Bayesian Markov Chain Monte Carlo model16 and reported categorical variables as OR with 95% credible interval. A random-effects model with informative priors was adopted for final interpretation of the results.2,3 To achieve convergence, a burn-in phase of 20 000 simulations was performed followed by 50 000 simulations for the final analyses.17,18 Conversion was confirmed by inspection of Gelman Rubin graphs.19 The Bayesian analysis was performed with use of WinBUGS software, version 1.4 (MRC Biostatistics Unit) and the Microsoft Excel–based tool NetMetaXL.20
Results
Description of Included Studies
eFigure 1 in the Supplement shows the PRISMA flow diagram of study selection. A total of 319 citations were retrieved after the initial search of electronic databases and the manual search. After 29 duplicates were removed, 291 eligible citations were screened, which resulted in 25 publications for full-text review. Finally, we excluded 15 studies that did not meet the specified criteria and were left with 10 publications for qualitative and quantitative analysis (meta-analysis).
Table 1 and Table 2 show the individual study and patient characteristics of the included studies. Inclusion and exclusion criteria are presented in the eTable in the Supplement. Ten studies had enrolled 4147 patients, with 2093 patients in the AA arms and 2054 patients in the control arms. Three studies each originated from Italy13,25,26 and Turkey21,22,23; 1 study each originated from Japan,24 China,6 and France12; and 1 study was an international study.14 The AAs used were spironolactone, eplerenone, canrenoate, and canrenoate followed by spironolactone. Follow-up duration was variable: 6 to 12 months for 8 studies,6,12,13,14,21,22,23,25 1 month for 1 study,24 and 10 days for 1 study.26 Revascularization was performed with primary PCI, thrombolysis, or both in all studies except the 2001 study by Di Pasquale et al,26 which included patients for whom thrombolysis was contraindicated or who did not achieve reperfusion after thrombolysis.
Table 1. Characteristics of the Included Studies.
| Source (Country of Study) | Study Design | AA Used | AA Therapy, No. of Patients | Control Group, No. of Patients | Initial Treatment | Dosing Protocol | Duration of Follow-up |
|---|---|---|---|---|---|---|---|
| Beygui et al,12 2016 (France) | Multicenter, randomized, open-label, blinded end point | Canrenoate, 200 mg IV, followed by spironolactone, 25 mg oral | 612 | 617 | Primary PCI or thrombolysis | Randomized within 72 h of STEMI | 6 mo |
| Montalescot et al,14 2014 (multinational) | Randomized, placebo-controlled, double-blind, multicenter | Eplerenone, 25-50 mg/d | 506 | 506 | PCI or thrombolysis | Within 24 h of symptom onset | Mean of 10.5 mo (up to 18 mo) |
| Vatankulu et al,21 2013 (Turkey) | Randomized, controlled | Spironolactone, 12.5-25 mg | 104 | 56 | PCI or thrombolysis | After admission to CCU | 6 mo |
| Wu et al,6 2013 (China) | Randomized | Spironolactone, 20 mg | 308 | 308 | PCI or thrombolysis | Within 17.1 ± 3.8 h | 1 y |
| Kayrak et al,22 2010 (Turkey) | Randomized | Spironolactone, 25 mg | 55 | 55 | PCI | 18.2 ± 3.2 h | 6 mo |
| Uzunhasan et al,23 2009 (Turkey) | Randomized | Spironolactone, 50 mg | 41 | 41 | PTCA or thrombolysis | Unclear | 6 mo |
| Di Pasquale et al,13 2005 (Italy) | Double-blind, randomized | IV canrenoate, 1 mg/h, followed by 25 m/d oral | 341 | 346 | Thrombolysis, PTCA, or CABG | Within 4-6 h of admission | 180 d |
| Hayashi et al,24 2003 (Japan) | Randomized | IV canrenoate, 200 mg, then spironolactone, 25 mg/d | 75 | 75 | PCI | IV given immediately after admission to CCU, then oral given within 24 h | 1 mo |
| Modena et al,25 2001 (Italy) | Randomized | Potassium canrenoate, 50 mg/d | 24 | 22 | PCI or thrombolysis | 9 ± 1 d After hospitalization before discharge | 12 mo |
| Di Pasquale et al,26 2001 (Italy) | Double-blind, randomized | Canreonate, 25 mg/d IV, followed by oral | 27 | 28 | Patients were not suitable for or did not undergo perfusion after thrombolysis | Within 8-10 h of admission | 10 d |
Abbreviations: AA, aldosterone antagonist; CABG, coronary artery bypass grafting; CCU, coronary care unit; IV, intravenous; PCI, percutaneous coronary intervention; PTCA, percutaneous transluminal coronary angioplasty; STEMI, ST-segment elevation myocardial infarction.
Table 2. Patient Characteristicsa.
| Source | Age, Mean (SD), y | Male, % | DM, % | HTN, % | Smoker, % | LVEF, Mean (SD), % | Revascularization, % | Reperfusion, % | BB Use, % | ACEI/ARB Use, % |
|---|---|---|---|---|---|---|---|---|---|---|
| Beygui et al,12 2016b | ||||||||||
| AA group (n = 612) | 58c | 83.9 | 16 | 41.8 | 47 | 50c | 93 | 11.7 | 93.5 | 87.5 |
| Control group (n = 617) | 58c | 82.1 | 15.7 | 43.9 | 51.7 | 50c | 93.7 | 10.5 | 92.9 | 89.6 |
| Montalescot et al,14 2014 | ||||||||||
| AA group (n = 506) | 58.5 (10.8) | 83 | 12.8 | 47.6 | NR | All patients ≥40 | 85 | 6.3 | 87.9 | 82.6 |
| Control group (n = 506) | 57.8 (11) | 79.6 | 15.4 | 51.3 | NR | All patients ≥40 | 86.9 | 4.2 | 88.3 | 83.2 |
| Vatankulu et al,21 2013 | ||||||||||
| AA group (n = 104) | 12.5 mg: 54 (11); 25 mg: 58 (9) |
12.5 mg: 86; 25 mg: 85 |
12.5 mg: 16; 25 mg: 16.6 |
12.5 mg: 20; 25 mg: 27.7 |
12.5 mg: 68; 25 mg: 64.8 |
12.5 mg: 51.1; 25 mg: 49.1 |
12.5 mg: 92; 25 mg: 87 |
12.5 mg: 8; 25 mg: 13 |
12.5 mg: 96; 25 mg: 96 |
12.5 mg: 92; 25 mg: 93 |
| Control group (n = 56) | 57 (11) | 80.3 | 19.6 | 26.7 | 60.7 | 50.1 | 89 | 11 | 91 | 82 |
| Wu et al,6 2013 | ||||||||||
| AA group (n = 308) | 59.8 (11.7) | 73.66 | 19.08 | 46.9 | 53.8 | 52.05 (12.1) | 44.2 | 30 | 94.2 | 93.8 |
| Control group (n = 308) | 59.9 (10.3) | 72.1 | 19.9 | 43.9 | 51.1 | 50.12 (11.3) | 37.2 | 28.5 | 92.8 | 93.9 |
| Kayrak et al,22 2010 | ||||||||||
| AA group (n = 55) | 55.3 (10) | 18.18 | 20 | 25.4 | 54.5 | 50.5 (8.3) | 100 | NR | 98.1 | 90.9 |
| Control group (n = 55) | 57.2 (11.1) | 25.4 | 18.18 | 29 | 45.4 | 49.5 (8) | 100 | NR | 94.5 | 92.7 |
| Uzunhasan et al,23 2009 | ||||||||||
| AA group (n = 41) | 52 (10) | 79 | 18 | 40 | 72 | 47 | 100 | 0 | NR | NR |
| Control group (n = 41) | 52 (10) | 71 | 16 | 35 | 66 | 44 | 100 | 0 | NR | NR |
| Di Pasquale et al,13 2005 | ||||||||||
| AA group (n = 341) | 62.6 (6) | 71.26 | 38.7 | 35.7 | 24.9 | 44.5 (6) | 76.5 | 55.3 | 36.9 | 100 |
| Control group (n = 346) | 62.8 (5) | 70.5 | 40.7 | 34.9 | 25.4 | 44.7 (9) | 76.8 | 54.9 | 36.1 | 100 |
| Hayashi et al,24 2003 | ||||||||||
| AA group (n = 75) | 64.4 | 75.38 | 41.5 | 27.6 | 52.3 | 46 | 100 | 0 | 29.2 | 100 |
| Control group (n = 75) | 62.9 | 73.9 | 42 | 30.4 | 52.1 | 46.5 | 100 | 0 | 31.8 | 100 |
| Modena et al,25 2001 | ||||||||||
| AA group (n = 24) | 59 (10) | 70.8 | 16.6 | 50 | 41.6 | 47 (6) | 100 | 0 | 41.6 | 100 |
| Control group (n = 22) | 62 (13) | 77.2 | 13.6 | 54.5 | 45.4 | 46 (5) | 100 | 0 | 50 | 100 |
| Di Pasquale et al,26 2001 | ||||||||||
| AA group (n = 27) | 61.11 (11.1) | 74 | 33.3 | 70.3 | 18.18 | 40.11 (12) | 51 | 37 | 48.14 | 100 |
| Control group (n = 28) | 62.18 (8.41) | 75 | 39.2 | 71.4 | 7.14 | 40.32 (13) | 42 | 32.1 | 42.8 | 100 |
Abbreviations: AA, aldosterone antagonist; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BB, β-blocker; DM, diabetes; HTN, hypertension; LVEF, left ventricular ejection fraction; NR, not reported; STEMI, ST-segment elevation myocardial infarction.
Group sizes are included for each study. Percentage data are given as provided in each study’s report; some variation in denominators may have occurred for individual characteristics.
Data reported for both patients with STEMI and patients without STEMI.
Median.
Clinical Outcomes
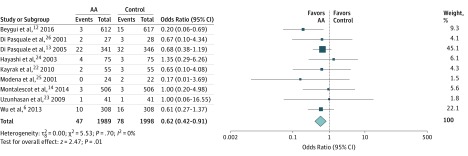
Therapy with AAs compared with a control group (from 9 studies) reduced the risk of mortality (2.4% vs 3.9%; OR, 0.62 [95% CI, 0.42-0.91]; P = .01; I2 = 0) (Figure 1). No difference was noted in the risk of MI (1.6% vs 1.5%; OR, 1.03 [95% CI, 0.57-1.86]; P = .91; I2 = 0) (eFigure 2 in the Supplement), CHF (4.3% vs 5.4%; OR, 0.82 [95% CI, 0.56-1.20]; P = .31; I2 = 0) (eFigure 3 in the Supplement), and ventricular arrhythmia (4.1% vs 5.1%; OR, 0.76 [95% CI, 0.45-1.31]; P = .33; I2 = 13) (eFigure 4 in the Supplement) between the AA group and the control group.
Figure 1. Mortality Comparing Aldosterone Antagonist (AA) Therapy vs Control.
Square data markers represent odds ratios; horizontal lines, the 95% CIs, with marker size reflecting the statistical weight of the study using random-effects meta-analysis. The diamond data marker represents the overall odds ratio and 95% CI for the outcome of interest. Evaluated using the random-effects Mantel-Haenszel test.
Change in LVEF and Biochemical Profile
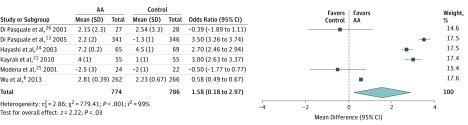
Treatment with AAs compared with a control group resulted in a small yet significant increase in LVEF (MD, 1.58% [95% CI, 0.18%-2.97%]; P = .03; I2 = 99) (Figure 2), a small increase in serum potassium level (MD, 0.07 mEq/L [95% CI, 0.01-0.13 mEq/L]; P = .02; I2 = 99; to convert to millimoles per liter, multiply by 1.0) (eFigure 5 in the Supplement), and no change in serum creatinine level (standardized MD, 1.4 [95% CI, −0.43 to 3.24]; P = 13; I2 = 100; to convert to micromoles per liter, multiply by 76.25) (eFigure 6 in the Supplement).
Figure 2. Left Ventricular Ejection Fraction Comparing Aldosterone Antagonist (AA) Therapy vs Control.
Square data markers represent mean difference; horizontal lines, the 95% CIs, with marker size reflecting the statistical weight of the study using random-effects meta-analysis. The diamond data marker represents the overall mean difference and 95% CI for the outcome of interest. Evaluated using the random-effects inverse variance test.
Sensitivity and Subgroup Analyses
We performed subgroup analyses on the clinical outcomes based on the individual AA agents, duration of follow-up (≥6 months vs <6 months), study size (small vs large), and combined mode of administration (intravenous vs oral) with follow-up duration. When analyses were restricted to individual agents vs control, mortality benefit was not observed for individual agents. Analysis based on the duration of follow-up (6-12 months) showed reduced mortality risk associated with therapy with AAs compared with control, consistent with the primary analysis. Similarly, we performed an analysis that included the larger studies (≥100 patients in each arm)6,12,13,14 that showed reduced mortality (OR, 0.57 [95% CI, 0.35-0.94]; P = .03; I2 = 18) and similar risk of MI (OR, 0.96 [95% CI, 0.52-1.77]; P = .89; I2 = 0), CHF (OR, 0.90 [95% CI, 0.60-1.37]; P = .63; I2 = 0), and ventricular arrhythmia (OR, 0.65 [95% CI, 0.16-2.68]; P = .55; I2 = 33) associated with AA therapy, consistent with the primary analyses. The comparison of intravenous administration of AAs followed by oral administration of AAs with up to 6 months of follow-up vs oral administration of AAs with up to 12 months of follow-up did not show a difference in the clinical outcomes. Because canrenoate is approximately 30% less potent than spironolactone,27 to address the effect of dosing in various outcomes, we performed additional analyses after excluding the studies that used low-dose canrenoate (Di Pasquale et al13,26), which had results that were consistent with the primary outcomes. The analysis excluding the 2001 study by Di Pasquale et al,26 which included patients who did not undergo or achieve reperfusion, showed a mortality benefit associated with receipt of AAs. Furthermore, we performed sensitivity analyses with use of a Bayesian model for mortality, MI, CHF, and ventricular arrhythmia, and the results were not significantly different from those of the primary analysis (eFigure 7 in the Supplement).
Study Quality and Publication Bias
The studies were of moderate to high quality according to the Cochrane Collaboration’s bias assessment tools.28 One study showed bias for blinding of participants or personnel.12 One study showed bias for blinding of participants and personnel and outcomes.23 Five studies showed bias for allocation concealment and blinding of participants, personnel, or outcome.6,21,22,24,25 Publication bias was tested by visual examination of the funnel plots, which were symmetrical and showed no evidence of publication bias for mortality outcome (eFigure 8 in the Supplement). Publication bias was not tested for other outcomes because of the small overall number of studies available for meaningful assessment of publication bias (<10 studies).
Discussion
The major finding of our meta-analysis was the reduced risk of mortality associated with receipt of AAs compared with a control group in patients with STEMI without heart failure or reduced LVEF less than 40%. The risks of CHF, MI, and ventricular arrhythmia were, however, similar for both the AA group and the control group. Therapy with AAs was associated with small increases in LVEF and serum potassium level, and no difference was observed in serum creatinine level. Overall mortality rate after STEMI in this study was 3.14% at follow-up, which is lower than the mortality rate of 4% at 1 year of follow-up reported in a recent study.29
To our knowledge, this is the first meta-analysis to systematically review the role of therapy with AAs in patients who presented with STEMI and did not have significant LV dysfunction or CHF. The results of pooled analyses of 2 trials that were included in this meta-analysis were presented at the European Society of Cardiology meeting in 2017 and showed mortality benefit in the AA-treated group vs the standard therapy group (0.4% vs 1.6%; stratified OR, 0.22 [95% CI, 0.07-0.65]; P = .006),30 which is consistent with our results. One important observation in our meta-analysis was the reduction in mortality without a reduction in the outcomes of MI, CHF, or ventricular arrhythmia, similar to the results of the EPHESUS trial.11 The EPHESUS trial reported a significant reduction in all-cause and cardiovascular-associated mortality without a difference in the incidence of MI or CHF or a reduction in hospitalizations related to ventricular arrhythmia. A possible mechanism for mineralocorticoid receptor antagonists to reduce mortality is prevention of electrical remodeling that precedes cardiomyocyte hypertrophy following MI.31 In addition, animal studies have shown that mineralocorticoid receptor expression in the heart results in cardiac arrhythmia–related death that could be prevented by treatment with spironolactone.32 Similarly, immediate treatment with mineralocorticoid receptor blocker results in MI healing by modulation of the inflammatory response.33
Patients without clinical heart failure at presentation to the hospital constitute a large proportion of patients with STEMI. In 4 years of data (from 1994 to 1998) from the National Registry of Myocardial Infarction-2 study, 80.9% of 190 518 patients admitted to the hospital with STEMI presented without heart failure.34 Another recent report, based on a large database from the Nationwide Inpatient Sample, revealed a decreasing number of cases of heart failure at presentation, from 25.4% of cases in 2003 to 20.7% in 2010, among 1 990 002 admissions of patients with STEMI.35 This epidemiologic trend was most likely the result of improved pharmacotherapy and revascularization, which minimized the extent of injury caused by acute coronary occlusion in patients with STEMI. In addition, these data show that the incidence of patients with STEMI without heart failure may increase in the future, making such patients an important population group in which to establish interventions to improve outcomes.
The Randomized, Placebo-Controlled Trial Evaluating the Safety and Efficacy of Early Treatment with Eplerenone in Patients with Acute Myocardial Infarction (REMINDER)14 and the Aldosterone Lethal Effects Blocked in Acute Myocardial Infarction Treated with or Without Reperfusion to Improve Outcome and Survival at 6 Months’ Follow-up (ALBATROSS)12 trial were 2 of the most recent studies that investigated the role of AAs in patients with STEMI without heart failure or significant LV dysfunction. Although neither trial demonstrated a meaningful benefit of treatment with AAs in the study population, the REMINDER trial showed lower brain-type natriuretic peptide levels without other clinical benefits in participants treated with AAs, and the ALBATROSS trial, which seemingly did not establish the benefit of AAs in the overall study population, showed significant benefit of treatment with AAs in a post hoc analysis restricted to patients with STEMI. At this point, there are 2 ongoing or just completed trials in the ClinicalTrials.gov database. The Early Mineralocorticoid Receptor Antagonist Treatment to Reduce Myocardial Infarct Size (MINIMISE-STEMI) trial, which is now completed, aims to compare the effect of spironolactone vs placebo on infarct size in patients with STEMI.36 The Colchicine and Spironolactone in Patients with STEMI/SYNERGY Stent Registry (CLEAR-SYNERGY) trial plans to compare the effects of colchicine and spironolactone vs placebo in a 2 × 2 factorial design on clinical outcomes after PCI in patients with STEMI (ClinicalTrials.gov Identifier: NCT03048825). These trials may shed further light on the role of spironolactone in patients with STEMI.
There are several implications of this meta-analysis. STEMI and its adverse effects on morbidity and mortality remain major cardiovascular problems, and there is a constant search for therapies to improve survival and other clinical outcomes. The mortality benefit observed in the meta-analysis was substantial (38% reduction). One concern among physicians is the possibility of adverse effects associated with AA use, namely, hyperkalemia, acute kidney injury, and gynecomastia. Although our meta-analysis indicated that AA use was associated with elevated serum potassium levels, the clinical implications of such a small increase in serum potassium level are unclear. However, the serum potassium levels of patients who initiate treatment with AAs should be closely monitored during follow-up. No increase in serum creatinine levels was observed in the meta-analysis, which is reassuring. Our meta-analysis clearly showed a small but significant improvement in LVEF, the clinical significance of which is unclear.
Limitations
A potential limitation of our meta-analysis is the lack of patient-level data, which our study was not designed to provide. There was variation in the type of AA agents, the mode of administration (oral vs intravenous), the duration of follow-up, and the use of concomitant therapy, including β-blocker use and reperfusion and revascularization strategies; we tried to explore most of these factors with subgroup analyses. Not all studies reported on each outcome of interest, and some of the studies were not designed to measure clinical outcomes, which may be a limitation. However, our meta-analysis has been strengthened by the inclusion of all randomized trials published on this subject. In addition, the results were mainly driven by the ALBATROSS trial,12 although there was a strong trend toward improved survival when analysis was performed after exclusion of this study (OR, 0.70 [95% CI, 0.47-1.04]; P = .08; I2 = 0).
Conclusions
On the basis of this meta-analysis, we conclude that AAs are associated with a mortality benefit in patients with STEMI with LVEF greater than 40% or without heart failure. Future adequately powered randomized studies should confirm these findings.
eFigure 1. PRISMA Flow Diagram of the Study Search
eFigure 2. Forest Plot of Congestive Heart Failure
eFigure 3. Forest Plot of Myocardial Infarction
eFigure 4. Forest Plot of Ventricular Arrhythmia
eFigure 5. Forest Plot of Potassium Level
eFigure 6. Forest Plot of Creatinine Level
eFigure 7. Sensitivity Analysis of Major Clinical Outcomes (Bayesian Model)
eFigure 8. Funnel Plot (Mortality)
eTable. Inclusion and Exclusion Criteria
References
- 1.Ibanez B, James S, Agewall S, et al. ; ESC Scientific Document Group . 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119-177. [DOI] [PubMed] [Google Scholar]
- 2.O’Gara PT, Kushner FG, Ascheim DD, et al. ; American College of Emergency Physicians; Society for Cardiovascular Angiography and Interventions . 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61(4):e78-e140. [DOI] [PubMed] [Google Scholar]
- 3.Pedersen F, Butrymovich V, Kelbæk H, et al. Short- and long-term cause of death in patients treated with primary PCI for STEMI. J Am Coll Cardiol. 2014;64(20):2101-2108. [DOI] [PubMed] [Google Scholar]
- 4.Burchfield JS, Xie M, Hill JA. Pathological ventricular remodeling: mechanisms: part 1 of 2. Circulation. 2013;128(4):388-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silvestre JS, Heymes C, Oubénaïssa A, et al. Activation of cardiac aldosterone production in rat myocardial infarction: effect of angiotensin II receptor blockade and role in cardiac fibrosis. Circulation. 1999;99(20):2694-2701. [DOI] [PubMed] [Google Scholar]
- 6.Wu CT, Wang ZH, Li ZQ, Wang LF. Effect of spironolactone on cardiac remodeling after acute myocardial infarction. World J Emerg Med. 2013;4(1):48-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie M, Burchfield JS, Hill JA. Pathological ventricular remodeling: therapies: part 2 of 2. Circulation. 2013;128(9):1021-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beygui F, Collet JP, Benoliel JJ, et al. High plasma aldosterone levels on admission are associated with death in patients presenting with acute ST-elevation myocardial infarction. Circulation. 2006;114(24):2604-2610. [DOI] [PubMed] [Google Scholar]
- 9.Beygui F, Montalescot G, Vicaut E, et al. ; OPERA Investigators . Aldosterone and long-term outcome after myocardial infarction: a substudy of the French nationwide observatoire sur la prise en charge hospitalière, l’evolution à un an et les caractéristiques de patients présentant un infarctus du myocarde avec ou sans onde Q (OPERA) study. Am Heart J. 2009;157(4):680-687. [DOI] [PubMed] [Google Scholar]
- 10.Mignano A, Pitruzzella V, Arnone G, et al. Prognostic role of aldosterone in patients with acute coronary syndrome: short and medium term follow-up. J Cardiovasc Med (Hagerstown). 2014;15(1):27-32. [DOI] [PubMed] [Google Scholar]
- 11.Pitt B, Remme W, Zannad F, et al. ; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators . Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348(14):1309-1321. [DOI] [PubMed] [Google Scholar]
- 12.Beygui F, Cayla G, Roule V, et al. ; ALBATROSS Investigators . Early aldosterone blockade in acute myocardial infarction: the ALBATROSS randomized clinical trial. J Am Coll Cardiol. 2016;67(16):1917-1927. [DOI] [PubMed] [Google Scholar]
- 13.Di Pasquale P, Cannizzaro S, Scalzo S, et al. Effects of canrenoate plus angiotensin-converting enzyme inhibitors versus angiotensin-converting enzyme inhibitors alone on systolic and diastolic function in patients with acute anterior myocardial infarction. Am Heart J. 2005;150(5):919. [DOI] [PubMed] [Google Scholar]
- 14.Montalescot G, Pitt B, Lopez de Sa E, et al. ; REMINDER Investigators . Early eplerenone treatment in patients with acute ST-elevation myocardial infarction without heart failure: the Randomized Double-blind REMINDER Study. Eur Heart J. 2014;35(34):2295-2302. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ. 2005;331(7521):897-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner RM, Jackson D, Wei Y, Thompson SG, Higgins JP. Predictive distributions for between-study heterogeneity and simple methods for their application in Bayesian meta-analysis. Stat Med. 2015;34(6):984-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane Database of Systematic Reviews. Int J Epidemiol. 2012;41(3):818-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat. 1998;7(4):434-455. doi: 10.2307/1390675 [DOI] [Google Scholar]
- 20.Brown S, Hutton B, Clifford T, et al. A Microsoft-Excel-based tool for running and critically appraising network meta-analyses: an overview and application of NetMetaXL. Syst Rev. 2014;3:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vatankulu MA, Bacaksiz A, Sonmez O, et al. Does spironolactone have a dose-dependent effect on left ventricular remodeling in patients with preserved left ventricular function after an acute myocardial infarction? Cardiovasc Ther. 2013;31(4):224-229. [DOI] [PubMed] [Google Scholar]
- 22.Kayrak M, Bacaksiz A, Vatankulu MA, et al. The effects of spironolactone on atrial remodeling in patients with preserved left ventricular function after an acute myocardial infarction: a randomized follow-up study. Coron Artery Dis. 2010;21(8):477-485. [DOI] [PubMed] [Google Scholar]
- 23.Uzunhasan I, Yildiz A, Coskun U, et al. Effects of aldosterone blockade on left ventricular function and clinical status during acute myocardial infarction. Scand J Clin Lab Invest. 2009;69(5):545-549. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi M, Tsutamoto T, Wada A, et al. Immediate administration of mineralocorticoid receptor antagonist spironolactone prevents post-infarct left ventricular remodeling associated with suppression of a marker of myocardial collagen synthesis in patients with first anterior acute myocardial infarction. Circulation. 2003;107(20):2559-2565. [DOI] [PubMed] [Google Scholar]
- 25.Modena MG, Aveta P, Menozzi A, Rossi R. Aldosterone inhibition limits collagen synthesis and progressive left ventricular enlargement after anterior myocardial infarction. Am Heart J. 2001;141(1):41-46. [DOI] [PubMed] [Google Scholar]
- 26.Di Pasquale P, Alessi V, Barberi O, et al. The combination ace-inhibitors plus canreonate in patients with anterior myocardial infarction: safety and tolerability study. Int J Cardiol. 2001;77(2-3):119-127. [DOI] [PubMed] [Google Scholar]
- 27.Ramsay L, Asbury M, Shelton J, Harrison I. Spironolactone and canrenoate-K: relative potency at steady state. Clin Pharmacol Ther. 1977;21(5):602-609. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. London, UK: Cochrane Collaboration; 2011. http://training.cochrane.org/handbook. Accessed April 11, 2018.
- 29.Perelshtein Brezinov O, Klempfner R, Zekry SB, Goldenberg I, Kuperstein R. Prognostic value of ejection fraction in patients admitted with acute coronary syndrome: a real world study. Medicine (Baltimore). 2017;96(9):e6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beygui F, Van belle E, Ecollan P, et al. Pooled patient-level analysis from the ALBATROSS and REMINDER randomized trials. Paper presented at: European Society of Cardiology Meeting; August 28, 2017; Barcelona, Spain. [Google Scholar]
- 31.Perrier E, Kerfant BG, Lalevee N, et al. Mineralocorticoid receptor antagonism prevents the electrical remodeling that precedes cellular hypertrophy after myocardial infarction. Circulation. 2004;110(7):776-783. [DOI] [PubMed] [Google Scholar]
- 32.Ouvrard-Pascaud A, Sainte-Marie Y, Bénitah JP, et al. Conditional mineralocorticoid receptor expression in the heart leads to life-threatening arrhythmias. Circulation. 2005;111(23):3025-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fraccarollo D, Galuppo P, Schraut S, et al. Immediate mineralocorticoid receptor blockade improves myocardial infarct healing by modulation of the inflammatory response. Hypertension. 2008;51(4):905-914. [DOI] [PubMed] [Google Scholar]
- 34.Wu AH, Parsons L, Every NR, Bates ER; Second National Registry of Myocardial Infarction . Hospital outcomes in patients presenting with congestive heart failure complicating acute myocardial infarction: a report from the Second National Registry of Myocardial Infarction (NRMI-2). J Am Coll Cardiol. 2002;40(8):1389-1394. [DOI] [PubMed] [Google Scholar]
- 35.Agarwal M, Agrawal S, Garg L, et al. National trends in the incidence, management, and outcomes of heart failure complications in patients hospitalized for ST-segment elevation myocardial infarction. Mayo Clin Proc Innovations Quality & Outcomes. 2017;1(1):26-36. doi: 10.1016/j.mayocpiqo.2017.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bulluck H, Fröhlich GM, Mohdnazri S, et al. Mineralocorticoid receptor antagonist pretreatment to MINIMISE reperfusion injury after ST-elevation myocardial infarction (the MINIMISE STEMI Trial): rationale and study design. Clin Cardiol. 2015;38(5):259-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. PRISMA Flow Diagram of the Study Search
eFigure 2. Forest Plot of Congestive Heart Failure
eFigure 3. Forest Plot of Myocardial Infarction
eFigure 4. Forest Plot of Ventricular Arrhythmia
eFigure 5. Forest Plot of Potassium Level
eFigure 6. Forest Plot of Creatinine Level
eFigure 7. Sensitivity Analysis of Major Clinical Outcomes (Bayesian Model)
eFigure 8. Funnel Plot (Mortality)
eTable. Inclusion and Exclusion Criteria