Key Points
Question
Does adjuvant chemotherapy provide a survival benefit to patients with locally advanced rectal cancer who demonstrate a complete pathological response following neoadjuvant chemoradiation therapy and resection?
Findings
In this propensity score–matched cohort study including 667 matched pairs of patients with rectal cancer and a pathological complete response to neoadjuvant chemoradiation therapy and resection, patients who received adjuvant chemotherapy demonstrated better 5-year overall survival than those who did not receive adjuvant treatment (95.0% vs 88.2%).
Meaning
In this subgroup of patients with excellent prognosis, the administration of adjuvant chemotherapy may provide additional survival benefits; however, these benefits need to be weighed against the risks of chemotoxic effects.
Abstract
Importance
Although American guidelines recommend use of adjuvant chemotherapy in patients with locally advanced rectal cancer, individuals who achieve a pathological complete response (pCR) following neoadjuvant chemoradiotherapy are less likely to receive adjuvant treatment than incomplete responders. The association and resection of adjuvant chemotherapy with survival in patients with pCR is unclear.
Objective
To determine whether patients with locally advanced rectal cancer who achieve pCR after neoadjuvant chemoradiation therapy and resection benefit from the administration of adjuvant chemotherapy.
Design, Setting, and Participants
This retrospective propensity score–matched cohort study identified patients with locally advanced rectal cancer from the National Cancer Database from 2006 through 2012. We selected patients with nonmetastatic invasive rectal cancer who achieved pCR after neoadjuvant chemoradiation therapy and resection.
Exposures
We matched patients who received adjuvant chemotherapy to patients who did not receive adjuvant treatment in a 1:1 ratio. We separately matched subgroups of patients with node-positive disease before treatment and node-negative disease before treatment to investigate for effect modification by pretreatment nodal status.
Main Outcome and Measures
We compared overall survival between groups using Kaplan-Meier survival methods and Cox proportional hazards models.
Results
We identified 2455 patients (mean age, 59.5 years; 59.8% men) with rectal cancer with pCR after neoadjuvant chemoradiation therapy and resection. We matched 667 patients with pCR who received adjuvant chemotherapy and at least 8 weeks of follow-up after surgery to patients with pCR who did not receive adjuvant treatment. Over a median follow-up of 3.1 years (interquartile range, 1.94-4.40 years), patients treated with adjuvant chemotherapy demonstrated better overall survival than those who did not receive adjuvant treatment (hazard ratio, 0.44; 95% CI, 0.28-0.70). When stratified by pretreatment nodal status, only those patients with pretreatment node-positive disease exhibited improved overall survival with administration of adjuvant chemotherapy (hazard ratio, 0.24; 95% CI, 0.10-0.58).
Conclusions and Relevance
The administration of adjuvant chemotherapy in patients with rectal cancer with pCR is associated with improved overall survival, particularly in patients with pretreatment node-positive disease. Although this study suggests a beneficial effect of adjuvant treatment on survival in patients with pCR, these results are limited by the presence of potential unmeasured confounding in this nonrandomized study.
This propensity score–matched cohort study examines whether adjuvant chemotherapy is associated with improved overall survival in patients with rectal cancer and pathological complete response following neoadjuvant chemoradiation therapy and resection.
Introduction
The standard treatment for locally advanced rectal cancer includes neoadjuvant chemoradiation, surgery, and adjuvant chemotherapy (ACT). The rationale for the routine use of ACT is based on an extrapolation of the survival benefits among patients with colon cancer1,2,3,4,5; however, the efficacy of ACT in patients with rectal cancer is less clear.6 Early studies demonstrating benefits of the use of ACT for rectal cancer included patients who did not receive neoadjuvant chemoradiation7,8,9,10; trials evaluating ACT for patients with rectal cancer in the setting of neoadjuvant treatment followed by surgery have not clearly demonstrated a benefit to its use.11,12,13,14,15 Additionally, the utility of ACT for subgroups of patients known to have good prognoses is unclear.
Downstaging after neoadjuvant treatment is a known prognosticator of survival in rectal cancer.16,17,18,19 Between 10% and 25% of patients treated with neoadjuvant chemoradiotherapy achieve a pathological complete response (pCR; defined as ypT0N0),20,21 and these patients exhibit low rates of local and distant recurrence and better overall and disease-free survival compared with patients without pCR.21,22,23,24,25 Because of this, the use of ACT in patients who achieve pCR has been questioned.26,27,28,29 Previous trials evaluating the benefit of ACT for patients with rectal cancer have been underpowered for subgroup analyses of patients with pCR. While in the United States and Canada ACT remains the standard of care for locally advanced rectal cancer,30,31 in practice, patients who achieve a pCR are less likely to undergo ACT than those with residual disease after neoadjuvant chemoradiotherapy.22,24,32 Despite excellent survival among patients with pCR, these patients remain at risk for disease recurrence and cancer-related death. Additionally, downstaging exhibited by these patients is suggestive of tumor biology responsive to treatment. As such, patients with pCR may compose a chemosensitive subset of patients with rectal cancer likely to benefit from ACT. This study therefore seeks to determine whether ACT is associated with improved overall survival in patients with pCR.
Methods
Data Source
The patient cohort for this study was obtained from the National Cancer Database (NCDB) participant use data file for rectal cancers. The NCDB is a clinical oncology–specific database established in 1989 as a joint program of the American College of Surgeons Commission on Cancer and the American Cancer Society. A data use agreement between the American College of Surgeons and Commission on Cancer–accredited hospitals allows for the use of this data by independent investigators. More than 1500 accredited facilities contribute to these data. The NCDB estimates that approximately 70% of newly diagnosed malignant neoplasms in the United States are captured by this database annually.
Patient Populations
The 2013 participant use data file release used for this study includes patients diagnosed with rectal neoplasms from January 1, 2004, through December 31, 2013. We limited the study cohort to patients diagnosed between January 1, 2006, and December 31, 2012, as information about neoadjuvant chemotherapy was not available prior to 2006 and vital status information was not available for those diagnosed in 2013. We used International Classification of Diseases for Oncology, third edition, morphologic codes to identify patients with rectal adenocarcinoma (eTable 1 in the Supplement). We limited cohort inclusion to those patients with invasive tumor behavior and confirmed that all patients included in our study were diagnosed based on histology. Our cohort was restricted to patients who received neoadjuvant chemoradiation followed by resectional surgery. Exclusion criteria included patients with multiple cancer diagnoses, patients with in situ tumors, patients diagnosed but not treated at the reporting facility, and those who underwent nonresectional ablative procedures or local excision. From the remaining cohort, we selected only those patients with documented ypT0N0 disease and excluded individuals with ypTXNX disease. Patients were then categorized based on receipt of ACT; these individuals are identified in the NCDB as those who received at least 2 cycles of ACT. Given the potential for immortal time bias, we excluded all patients with less than 8 weeks of follow-up after surgery based on the median time from surgery to adjuvant treatment in previous studies.33,34
Propensity Score Matching
We used propensity scores, reflecting the conditional probability of each patient having received ACT, to match patients who did and did not receive ACT. We constructed a multivariable logistic regression model to generate propensity scores. We selected covariates for inclusion in the propensity model a priori based on factors presumed to be associated with treatment received. Patient-level factors included in the model were age at diagnosis, sex, race, ethnicity, insurance status, area of residence (metropolitan vs not metropolitan based on 2013 Rural-Urban Continuum codes defined by population and worker commuting criteria), levels of income and education in the patient’s area of residence, and Charlson-Deyo comorbidity score. Treatment-related factors included facility type (community vs academic or integrated cancer program), distance between the patient’s residence and treatment facility based on zip code, total radiation dose (45 Gy, 50.4 Gy, 54 Gy, or other), time from end of radiation to surgery (<5, 5-7, 7-9, 9-12, or >12 weeks), hospital length of stay following surgery, and 30-day unplanned readmissions. Histologic grade (well, moderately, or poorly differentiated), tumor size (<2, 2-4, 4-6, or >6 cm), clinical T stage, clinical N stage, and number of nodes retrieved at surgery (<6, 6-11, or ≥12 nodes) were also included in the model. Based on previous NCDB studies, we expected certain covariates to have at least 15% missing data, limiting the use of multiple imputation methods. We therefore constructed dummy variables to indicate missingness and matched on these variables to ensure that the proportions of patients with missing data were balanced between propensity score–matched groups.
Patients who received ACT were matched to patients who did not receive ACT in a 1:1 ratio using a greedy, nearest-neighbor matching algorithm with no replacement. We used a caliper width equal to 0.2 of the standard deviation of the logit of the propensity score. We compared baseline characteristics between the propensity score–matched groups using standardized differences. A standardized difference less than 0.1 was used as indicative of negligible imbalance between groups.
Statistical Analysis
We generated Kaplan-Meier survival curves to compare overall survival between the propensity score–matched groups. Additionally, we constructed a marginal Cox proportional hazards model with treatment group as the sole predictor to determine the relative change in hazard associated with mortality—stratified by matched pairs, accounting for clustering at the facility level.35,36 The proportional hazards assumption was verified by adding a predictor-by-time interaction term into the model and by assessing Schoenfeld residuals over time. We used the hazard ratio (HR) and the survival probability in the non-adjuvant-treated group to determine the number needed to treat with adjuvant therapy to prevent 1 death at 3 years.37
To investigate potential effect modifiers, we generated and analyzed additional propensity score–matched groups for the subgroups of patients with pretreatment clinically node-positive and node-negative disease using similar methods.
Given the potential for unmeasured confounding, we performed a sensitivity analysis used by previous NCDB studies to determine the magnitude of effect that a hypothetical unmeasured confounder would need to have to render our results nonsignificant.38,39,40 The unmeasured confounder that we considered in this analysis was poor performance status (Eastern Cooperative Oncology Group [ECOG] grade ≥2). We assumed that the group of patients who did not receive ACT had a higher proportion of patients exhibiting poor performance status and that this measure was associated with mortality. We then varied the proportions of patients with poor performance status who did and did not receive ACT, as well as the strength of the association between poor performance status and mortality, based on estimates from previous studies,41,42,43,44,45,46 to recalculate our HR and 95% CI. Through this iterative process, we determined the level of association between our unmeasured confounder and mortality that would be necessary for our results to be nonsignificant.
In cases of fewer than 10 patients per cell, levels of categorical variables were combined, in accordance with NCDB use policies. All statistical analyses were performed using SAS Studio version 3.6 (SAS Institute Inc). All tests were 2-sided with a P value less than .05 considered statistically significant. As these data did not include personal identifiers, this study was exempt from review by the Cleveland Clinic Florida institutional review board and the collection of these data by the ACS is exempt from needing informed consent.
Results
Study Population and Propensity Score Matching
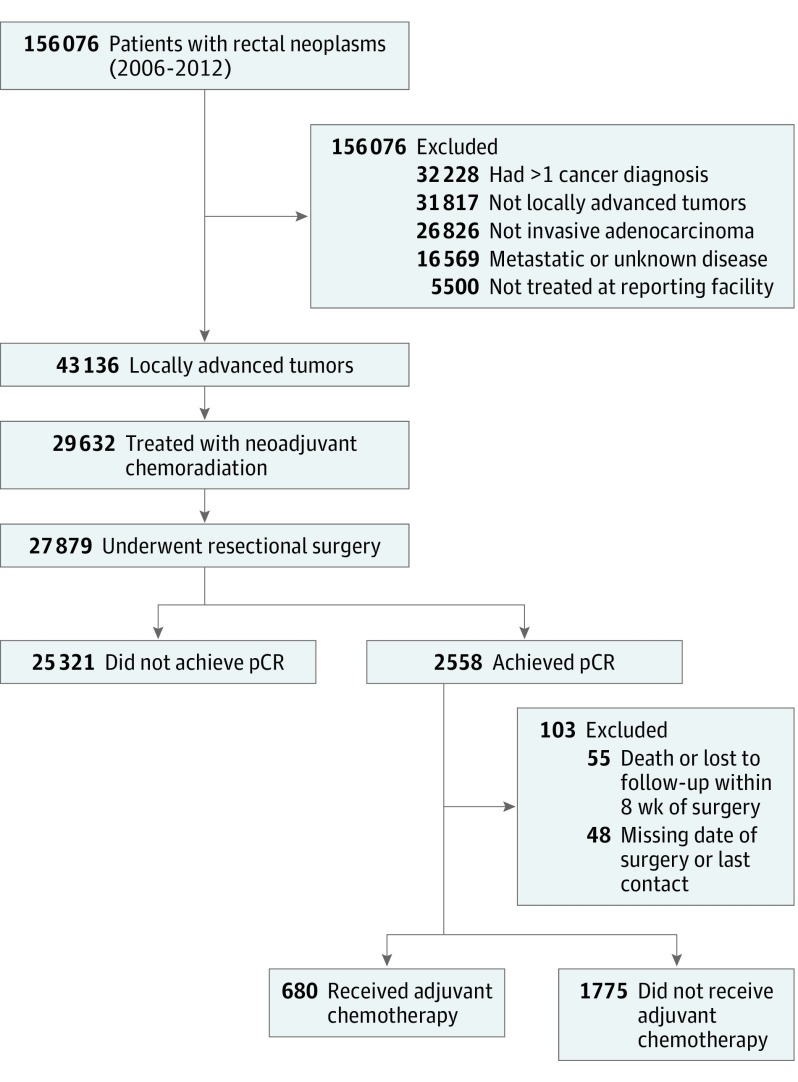
A total of 156 076 cases of rectal cancer were captured by the NCDB from 2006 through 2012. Of these, 29 632 patients with locally advanced T3/T4 or node-positive disease treated by neoadjuvant chemoradiation met all inclusion criteria (Figure 1). Of the 27 879 patients who underwent resectional surgery, only 2558 (9.2%) had a documented pCR. A total of 2455 (8.8%) had at least 8 weeks of follow-up (mean age, 59.5 years; 59.8% male); 680 (27.7%) received ACT. A summary of the baseline characteristics of the adjuvant- and non-adjuvant-treated groups prior to propensity score matching is presented in the Table (complete list of baseline characteristics provided in eTable 2 in the Supplement).
Figure 1. Patient Flowchart.
pCR indicates pathological complete response.
Table. Selected Baseline Characteristics Before and After Propensity Score Matching.
| Characteristic | No. (%) | |||||
|---|---|---|---|---|---|---|
| Before Matching | After Matching | |||||
| No Adjuvant Treatment (n = 1775) |
Adjuvant Treatment (n = 680) |
Standardized Differencea | No Adjuvant Treatment (n = 667) |
Adjuvant Treatment (n = 667) |
Standardized Differencea | |
| Patient Characteristics | ||||||
| Age, median (IQR), y | 61 (52-70) | 57 (49-65) | −0.34 | 56 (49-65) | 57 (49-65) | 0.006 |
| Male | 1088 (61.3) | 381 (56.0) | 0.11 | 370 (55.5) | 379 (56.8) | −0.03 |
| White | 1546 (87.1) | 597 (87.8) | 0.06 | 591 (88.6) | 585 (87.7) | 0.04 |
| Insurance status | 0.27 | 0.06 | ||||
| Uninsured | 59 (3.3) | 21 (3.1) | 23 (3.5) | 21 (3.2) | ||
| Private | 911 (51.3) | 433 (63.7) | 421 (63.1) | 422 (63.3) | ||
| Medicaid | 97 (5.5) | 35 (5.2) | 38 (5.7) | 35 (5.3) | ||
| Medicare | 666 (37.5) | 176 (25.9) | 173 (25.9) | 174 (26.1) | ||
| Charlson-Deyo comorbidity score | 0.09 | 0.02 | ||||
| 0 | 1401 (78.9) | 560 (82.4) | 544 (81.6) | 547 (82.0) | ||
| 1 | 303 (17.1) | 100 (14.7) | 101 (15.1) | 100 (15.0) | ||
| ≥2 | 71 (4.0) | 20 (2.9) | 22 (3.3) | 20 (3.0) | ||
| Tumor Characteristics | ||||||
| Grade | 0.12 | 0.05 | ||||
| Well differentiated | 119 (6.7) | 32 (4.7) | 30 (4.5) | 32 (4.8) | ||
| Moderately differentiated | 1064 (60.0) | 439 (64.6) | 419 (62.8) | 430 (64.5) | ||
| Poorly differentiated | 107 (6.0) | 45 (6.6) | 41 (6.2) | 43 (6.4) | ||
| Clinical T stage | 0.17 | 0.02 | ||||
| 1 or 2 | 199 (11.2) | 62 (9.1) | 59 (8.9) | 60 (9.0) | ||
| 3 or 4 | 1462 (82.4) | 596 (87.7) | 584 (87.6) | 585 (87.7) | ||
| Clinical N stage | 0.25 | 0.02 | ||||
| 0 | 985 (55.5) | 321 (47.2) | 319 (47.8) | 319 (47.8) | ||
| 1 | 647 (36.5) | 319 (46.9) | 311 (46.6) | 309 (46.3) | ||
| 2 | 59 (3.3) | 26 (3.8) | 25 (3.8) | 25 (3.8) | ||
| Treatment Characteristics | ||||||
| Time from end of radiation to surgery, wk | 0.25 | 0.09 | ||||
| <5 | 103 (5.8) | 45 (6.6) | 36 (5.4) | 45 (6.8) | ||
| 5-7 | 460 (25.9) | 200 (29.4) | 207 (31.0) | 197 (29.5) | ||
| 7-9 | 511 (28.8) | 233 (34.3) | 214 (32.1) | 228 (34.2) | ||
| 9-12 | 386 (21.8) | 131 (19.3) | 143 (21.4) | 129 (19.3) | ||
| >12 | 200 (11.3) | 37 (5.4) | 36 (5.4) | 36 (5.4) | ||
| No. of nodes examined | 0.16 | 0.03 | ||||
| <6 | 386 (21.8) | 109 (16.0) | 105 (15.7) | 108 (16.2) | ||
| 6-11 | 469 (26.4) | 176 (25.9) | 164 (24.6) | 172 (25.8) | ||
| ≥12 | 900 (50.7) | 387 (56.9) | 391 (58.6) | 380 (57.0) | ||
| Length of stay, median (IQR), d | 6 (5-8) | 6 (4-7) | 0.04 | 6 (4-8) | 6 (4-7) | −0.08 |
| 30-d unplanned readmission | 108 (6.1) | 57 (8.4) | 0.09 | 52 (7.8) | 57 (8.5) | 0.06 |
Abbreviation: IQR, interquartile range.
For categorical variables, the standardized difference was calculated as difference = (P1 − P2)/√[P1(1 − P1) + P2(1 − P2)]/2 where P1 and P2 refer to the proportion of the variable in the treatment and control groups.
Six hundred sixty-seven patients who underwent ACT were successfully matched to control patients who did not undergo ACT using propensity scores. Standardized differences between patients treated with and without ACT for all covariates included in the propensity score match were less than 0.1, indicating balanced groups after matching (Table). The distribution of propensity scores before and after matching are presented in the eFigure in the Supplement.
Effect of ACT on Overall Survival of Patients With pCR
The median follow-up for all patients was 3.08 years (interquartile range [IQR], 1.87-4.63), 3.05 years (IQR, 1.80-4.78 years) for patients who did not receive ACT, and 3.10 years (IQR, 1.94-4.40 years) for patients who did receive ACT.
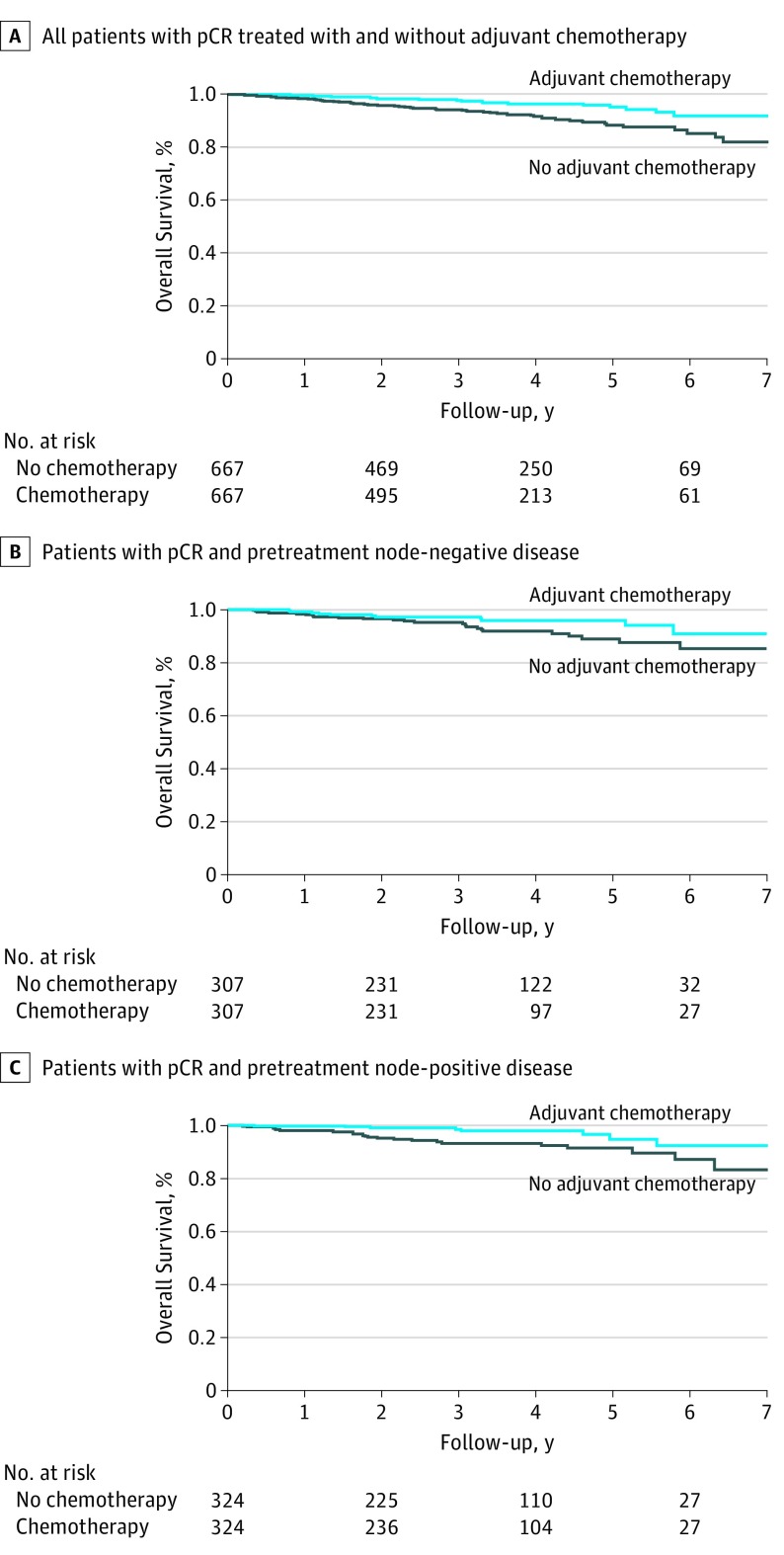
Three-year overall survival was 97.6% for patients treated with ACT and 94.0% for patients who did not receive ACT; 5-year overall survival was 95.0% and 88.2%, respectively. Use of ACT was associated with a statistically significant improvement in overall survival (HR, 0.44; 95% CI, 0.28-0.70) (Figure 2A). The number needed to treat with adjuvant chemotherapy to prevent 1 death at 3 years was 30 patients (95% CI, 24-78).
Figure 2. Kaplan-Meier Survival Curves.
pCR indicates pathological complete response.
To investigate potential effects of temporal trends, we conducted a sensitivity analysis that included year of diagnosis in the propensity score match. The results of our analysis were similar with the addition of this variable (HR, 0.48; 95% CI, 0.29-0.78).
In sensitivity analysis, our findings were robust to unmeasured confounding. Assuming a prevalence of poor performance status of 10% in the group that did not receive ACT and 5% in the group treated with ACT, the HR for the independent association between poor performance status and mortality would need to be 12 or greater to render our results nonsignificant; this was considered an extreme value (eTable 3 in the Supplement). If, instead, 10.0% of patients in the untreated group and only 2.5% of patients in the treated group exhibited poor performance status, the HR for the independent association between poor performance status and mortality would need to be greater than 6.5 for our results to be sensitive to this confounder.
Subgroup Analyses by Clinical Node Status
Of the 2455 patients (8.8%) who met inclusion criteria, 1306 (53.2%) presented with clinically node-negative disease prior to treatment; 321 (24.6%) of these patients received ACT. Three hundred seven patients with node-negative disease who received ACT were propensity score matched to patients with node-negative disease who did not receive ACT. Three-year overall survival for clinically node negative patients was 97.1% for patients treated with ACT and 95.2% for patients who did not receive ACT; 5-year overall survival was 95.8% and 88.8%, respectively. Node-negative patients treated with ACT did not demonstrate a statistically significant improvement in overall survival compared with those who did not receive ACT (HR, 0.64; 95% CI, 0.34-1.21) (Figure 2B).
We identified 1051 patients with clinically node-positive disease prior to treatment; 345 (32.8%) of these patients received ACT. We propensity score matched 324 pairs. Three-year overall survival for clinically node-positive patients was 98.3% for patients treated with ACT and 93.1% for patients who did not receive ACT; 5-year overall survival was 94.7% and 91.2%, respectively. Receipt of ACT was associated with a significant improvement in overall survival in this subgroup (HR, 0.24; 95% CI, 0.10-0.58) (Figure 2C). The number of pretreatment node-positive patients needed to treat with adjuvant chemotherapy to prevent 1 death at 3 years was 20 patients (95% CI, 17-36).
Discussion
Our study demonstrates that in patients with rectal cancer who achieved pCR following neoadjuvant chemoradiation therapy and resection, the administration of ACT was associated with improved overall survival. The strength of this association was strongest for patients with pretreatment node-positive disease.
Several randomized clinical trials (RCTs) attempting to establish the role of ACT for rectal cancer have failed to demonstrate a benefit to its use, which may in part be explained by insufficient power and poor adherence to treatment. The EORTC 22921 trial,47 which used a 2×2 factorial design to evaluate the benefits of both preoperative chemoradiotherapy and ACT in patients with resectable T3-T4 tumors, concluded that there was no benefit to the addition of ACT in patients treated with neoadjuvant chemoradiation. However, 36.9% of patients randomized to ACT did not undergo any cycles of treatment. Importantly, although this trial was unable to demonstrate the 10% difference in survival for which it was powered, patients treated with ACT did demonstrate a 4% improvement in 5-year overall survival and 6% improvement in 5-year disease-free survival.48 Similarly, although no benefit to ACT was demonstrated in the Italian I-CNR-RT trial,15 the authors point out that 28% of patients randomized to ACT did not undergo treatment, most commonly because of patient refusal.
The choice of chemotherapy agents used in previous RCTs may also explain discrepancies in their results. In EORTC 22921, I-CNR-RT, and the PROCTOR arm of the Dutch PROCTOR-SCRIPT trial,12 all of which failed to demonstrate benefits to ACT, patients were randomized to adjuvant treatment with fluorouracil plus leucovorin. However, ADORE, an RCT comparing this with a regimen of oxaliplatin and fluorouracil plus leucovorin in patients with pathologic stage II/III rectal cancer, demonstrated a 9.3% absolute improvement in 3-year overall survival among patients treated with a regimen of oxaliplatin and fluorouracil plus leucovorin.49 Similarly, the German CAO/ARO/AIO-04 trial demonstrated a benefit to the addition of oxaliplatin.50 Although CHRONICLE,14 a trial of capecitabine plus oxaliplatin vs no adjuvant treatment found a 7% improvement in disease-free survival but no improvement in overall survival, this study was closed prematurely after randomization of only 113 of the 780 patients required owing to slow accrual attributed to a bias among treating physicians to not offer ACT.
Our study demonstrated that the greatest survival benefit to ACT is in patients with the greatest burden of pretreatment disease (pretreatment node-positive patients). One theory for this finding is that tumor downstaging after neoadjuvant treatment may indicate favorable tumor biology and can be correlated with further responsivity to additional chemotherapy. Adjuvant chemotherapy for patients with proven responsivity to treatment may be beneficial by potentially eradicating residual micrometastatic disease.13,51 However, it should be noted that our subgroup analyses were exploratory and require further investigation for confirmation.
Whether ACT is beneficial for patients with tumor downstaging has been difficult to evaluate in previous RCTs, which have been underpowered for subgroup analyses of downstaged patients. However, a recent meta-analysis of 7 RCTs and 10 retrospective studies, including 5457 patients, demonstrated improved 5-year overall survival with ACT among patients who experienced tumor downstaging (odds ratio, 0.57; 95% CI, 0.38-0.85).51
Studies specifically evaluating the effect of ACT in the population with pCR have also been limited by a lack of statistical power. Several small studies demonstrate absolute improvements in overall survival ranging from 4.5% to 15%; however, these differences were not statistically significant.24,27,52,53 To avoid issues related to small sample sizes, others have attempted to use large registries. A previous propensity score–adjusted NCDB study by Xu et al54 evaluated adherence with ACT among patients with rectal cancer and similarly compared survival among patients with pCR. In congruence with the results from our study, they also demonstrated improved overall survival in patients with pCR who received ACT. However, this was a subgroup analysis and propensity scores were generated from the larger cohort of patients with locally advanced rectal cancer rather than from the patients with pCR alone; therefore, propensity score adjustment may not have appropriately adjusted for confounding by indication among patients with pCR in this previous study.
To our knowledge, this study is the largest to date evaluating the effect of ACT among a contemporary cohort of patients with pCR. With the use of propensity score matching, our groups were balanced on measured confounders that could have influenced receipt of ACT and our results proved to be robust on sensitivity analysis, helping our study avoid many of the methodological limitations of previous studies. Given the low mortality among patients with pCR, the use of propensity score matching rather than adjustment with a multivariable Cox model also allowed us to avoid model overspecification. We explored the role of pretreatment nodal status by separately analyzing subgroups based on clinical nodal status, ensuring balance of covariates in these subgroup analyses. Finally, although we could not determine whether patients underwent a full course of ACT, all patients in our ACT group received at least 2 cycles of treatment.
Limitations
Our study has limitations. The use of a retrospective database limited our ability to investigate other sources of potential bias. For example, we were unable to determine the chemotherapy agents administered, their dosages, or patient adherence. Because we could only identify patients who had received at least 2 cycles of ACT, patients who received a single cycle may have been misclassified. Although this may have been the case for only a few patients, this misclassification could have led to an overestimation of the effect of ACT. Additionally, we were unable to explore potential benefits of ACT on cancer recurrence or cancer-specific mortality as the NCDB does not provide data on local or distant recurrence or cause of death. Our follow-up was relatively short and few events were observed over this time. Despite this, we were able to detect a significant difference in overall survival in our short follow-up period. Interestingly, patients with pretreatment node-positive and node-negative disease exhibited similar survival times. Although this may reflect the strength of pCR attainment as a prognosticator, this could also be explained by possible misclassification of nodal status given the poor ability of radiologic methods to accurately determine lymph node positivity.55,56 Given the observational nature of this study, significant potential for bias exists; patients who received ACT may have been systematically different from those who did not receive ACT. We attempted to mitigate these effects by propensity score matching; however, significant residual confounding from unmeasured covariates may have resulted in an overestimation of the benefit of ACT in our study. However, our sensitivity analysis demonstrated that the association between ACT and survival would remain significant in the presence of substantial unmeasured confounding. Finally, the generalizability of our study findings needs to be considered. Although the use of propensity scores increased the internal validity of our study by ensuring balance between groups in important baseline covariates, the matching process systematically excluded certain patients (eg, elderly patients, male patients, and those receiving Medicare), which limits the inferences that can be made.
Conclusions
While previous studies have suggested withholding ACT in patients who achieve pCR, our findings suggest that the use of ACT may confer a survival benefit in this population. As techniques to increase the rates of pCR improve, it is becoming increasingly important to determine the optimal treatment paradigm for these patients. Ideally, a prospective RCT would be used to evaluate the role of ACT among patients with pCR; however, given the difficulty in accruing the number of patients that such a study would require, observational studies remain the best evidence to guide management. Our study demonstrates that the use of adjuvant treatment should be considered in patients treated with neoadjuvant chemoradiotherapy who achieve pCR. Recognizing the trade-offs between improving survival and the toxic effects of chemotherapy, the decision to administer ACT in this subset of patients with excellent survival should be individualized.
eTable 1. Codes Used for Cohort Development
eTable 2. Baseline Characteristics Before and After Propensity Score Matching
eTable 3. Sensitivity Analysis Evaluating the Sensitivity of Study Results to the Presence of Unmeasured Confounding
eFigure. Histograms Demonstrating the Distribution of Propensity Scores Among Nonadjuvant (upper panes) and adjuvant (lower panes) Treated Patients
References
- 1.André T, Boni C, Navarro M, et al. . Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27(19):3109-3116. [DOI] [PubMed] [Google Scholar]
- 2.Moertel CG, Fleming TR, Macdonald JS, et al. . Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med. 1990;322(6):352-358. [DOI] [PubMed] [Google Scholar]
- 3.O’Connell MJ, Mailliard JA, Kahn MJ, et al. . Controlled trial of fluorouracil and low-dose leucovorin given for 6 months as postoperative adjuvant therapy for colon cancer. J Clin Oncol. 1997;15(1):246-250. [DOI] [PubMed] [Google Scholar]
- 4.Twelves C, Wong A, Nowacki MP, et al. . Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med. 2005;352(26):2696-2704. [DOI] [PubMed] [Google Scholar]
- 5.Marsoni S; International Multicenter Pooled Analysis of Colon Cancer Trials Investigators . Efficacy of adjuvant fluorouracil and leucovorin in stage B2 and C colon cancer. Semin Oncol. 2001;28(1)(suppl):14-19. [DOI] [PubMed] [Google Scholar]
- 6.Bujko K, Glynne-Jones R, Bujko M. Does adjuvant fluoropyrimidine-based chemotherapy provide a benefit for patients with resected rectal cancer who have already received neoadjuvant radiochemotherapy? a systematic review of randomised trials. Ann Oncol. 2010;21(9):1743-1750. [DOI] [PubMed] [Google Scholar]
- 7.Akasu T, Moriya Y, Ohashi Y, Yoshida S, Shirao K, Kodaira S; National Surgical Adjuvant Study of Colorectal Cancer . Adjuvant chemotherapy with uracil-tegafur for pathological stage III rectal cancer after mesorectal excision with selective lateral pelvic lymphadenectomy: a multicenter randomized controlled trial. Jpn J Clin Oncol. 2006;36(4):237-244. [DOI] [PubMed] [Google Scholar]
- 8.Fisher B, Wolmark N, Rockette H, et al. . Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer: results from NSABP protocol R-01. J Natl Cancer Inst. 1988;80(1):21-29. [DOI] [PubMed] [Google Scholar]
- 9.Gray R, Barnwell J, McConkey C, Hills RK, Williams NS, Kerr DJ; Quasar Collaborative Group . Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007;370(9604):2020-2029. [DOI] [PubMed] [Google Scholar]
- 10.Thomas PR, Lindblad AS. Adjuvant postoperative radiotherapy and chemotherapy in rectal carcinoma: a review of the Gastrointestinal Tumor Study Group experience. Radiother Oncol. 1988;13(4):245-252. [DOI] [PubMed] [Google Scholar]
- 11.Bosset J-F, Calais G, Mineur L, et al. ; EORTC Radiation Oncology Group . Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol. 2014;15(2):184-190. [DOI] [PubMed] [Google Scholar]
- 12.Breugom A, Van Gijn W, Muller E, et al. . Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo) radiotherapy and total mesorectal excision: a Dutch Colorectal Cancer Group (DCCG) randomised phase III trial. Ann Oncol. 2014;26(4):696-701. [DOI] [PubMed] [Google Scholar]
- 13.Collette L, Bosset J-F, den Dulk M, et al. ; European Organization for Research and Treatment of Cancer Radiation Oncology Group . Patients with curative resection of cT3-4 rectal cancer after preoperative radiotherapy or radiochemotherapy: does anybody benefit from adjuvant fluorouracil-based chemotherapy? a trial of the European Organization for Research and Treatment of Cancer Radiation Oncology Group. J Clin Oncol. 2007;25(28):4379-4386. [DOI] [PubMed] [Google Scholar]
- 14.Glynne-Jones R, Counsell N, Quirke P, et al. . Chronicle: results of a randomized phase III trial in locally advanced rectal cancer after neoadjuvant chemoradiation randomising postoperative adjuvant capecitabine plus oxaliplatin (XELOX) versus control. Ann Oncol. 2014;25(7):1356-1362. [DOI] [PubMed] [Google Scholar]
- 15.Sainato A, Cernusco Luna Nunzia V, Valentini V, et al. . No benefit of adjuvant fluorouracil leucovorin chemotherapy after neoadjuvant chemoradiotherapy in locally advanced cancer of the rectum (LARC): long term results of a randomized trial (I-CNR-RT). Radiother Oncol. 2014;113(2):223-229. [DOI] [PubMed] [Google Scholar]
- 16.Quah HM, Chou JF, Gonen M, et al. . Pathologic stage is most prognostic of disease-free survival in locally advanced rectal cancer patients after preoperative chemoradiation. Cancer. 2008;113(1):57-64. [DOI] [PubMed] [Google Scholar]
- 17.Rödel C, Martus P, Papadoupolos T, et al. . Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23(34):8688-8696. [DOI] [PubMed] [Google Scholar]
- 18.Valentini V, Coco C, Picciocchi A, et al. . Does downstaging predict improved outcome after preoperative chemoradiation for extraperitoneal locally advanced rectal cancer? a long-term analysis of 165 patients. Int J Radiat Oncol Biol Phys. 2002;53(3):664-674. [DOI] [PubMed] [Google Scholar]
- 19.Vecchio FM, Valentini V, Minsky BD, et al. . The relationship of pathologic tumor regression grade (TRG) and outcomes after preoperative therapy in rectal cancer. Int J Radiat Oncol Biol Phys. 2005;62(3):752-760. [DOI] [PubMed] [Google Scholar]
- 20.Hartley A, Ho K, McConkey C, Geh J. Pathological complete response following pre-operative chemoradiotherapy in rectal cancer: analysis of phase II/III trials. Br J Radiol. 2014;78(934):934-938.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16177017&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 21.Martin ST, Heneghan HM, Winter DC. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br J Surg. 2012;99(7):918-928. [DOI] [PubMed] [Google Scholar]
- 22.Maas M, Nelemans PJ, Valentini V, et al. . Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11(9):835-844. [DOI] [PubMed] [Google Scholar]
- 23.Capirci C, Valentini V, Cionini L, et al. . Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: long-term analysis of 566 ypCR patients. Int J Radiat Oncol Biol Phys. 2008;72(1):99-107. [DOI] [PubMed] [Google Scholar]
- 24.de Campos-Lobato LF, Stocchi L, da Luz Moreira A, et al. . Pathologic complete response after neoadjuvant treatment for rectal cancer decreases distant recurrence and could eradicate local recurrence. Ann Surg Oncol. 2011;18(6):1590-1598. [DOI] [PubMed] [Google Scholar]
- 25.García-Aguilar J, Hernandez de Anda E, Sirivongs P, Lee S-H, Madoff RD, Rothenberger DA. A pathologic complete response to preoperative chemoradiation is associated with lower local recurrence and improved survival in rectal cancer patients treated by mesorectal excision. Dis Colon Rectum. 2003;46(3):298-304. [DOI] [PubMed] [Google Scholar]
- 26.Díaz Beveridge R, Akhoundova D, Bruixola G, Aparicio J. Controversies in the multimodality management of locally advanced rectal cancer. Med Oncol. 2017;34(6):102-102. [DOI] [PubMed] [Google Scholar]
- 27.Gamaleldin M, Church JM, Stocchi L, Kalady M, Liska D, Gorgun E. Is routine use of adjuvant chemotherapy for rectal cancer with complete pathological response justified? Am J Surg. 2017;213(3):478-483. [DOI] [PubMed] [Google Scholar]
- 28.Maas M, Nelemans PJ, Valentini V, et al. . Adjuvant chemotherapy in rectal cancer: defining subgroups who may benefit after neoadjuvant chemoradiation and resection: a pooled analysis of 3,313 patients. Int J Cancer. 2015;137(1):212-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fietkau R, Barten M, Klautke G, et al. . Postoperative chemotherapy may not be necessary for patients with ypN0-category after neoadjuvant chemoradiotherapy of rectal cancer. Dis Colon Rectum. 2006;49(9):1284-1292. [DOI] [PubMed] [Google Scholar]
- 30.Benson AB, Venook AP, Bekaii-Saab T, et al. . Rectal cancer, version 2.2015. J Natl Compr Canc Netw. 2015;13(6):719-728. [DOI] [PubMed] [Google Scholar]
- 31.Monson JR, Weiser MR, Buie WD, et al. ; Standards Practice Task Force of the American Society of Colon and Rectal Surgeons . Practice parameters for the management of rectal cancer (revised). Dis Colon Rectum. 2013;56(5):535-550. [DOI] [PubMed] [Google Scholar]
- 32.Khrizman P, Niland JC, ter Veer A, et al. . Postoperative adjuvant chemotherapy use in patients with stage II/III rectal cancer treated with neoadjuvant therapy: a national comprehensive cancer network analysis. J Clin Oncol. 2013;31(1):30-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Day AR, Middleton G, Smith RV, Jourdan IC, Rockall TA. Time to adjuvant chemotherapy following colorectal cancer resection is associated with an improved survival. Colorectal Dis. 2014;16(5):368-372. [DOI] [PubMed] [Google Scholar]
- 34.Aspinall SL, Good CB, Zhao X, et al. . Adjuvant chemotherapy for stage III colon cancer: relative dose intensity and survival among veterans. BMC Cancer. 2015;15(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Austin PC. A tutorial and case study in propensity score analysis: an application to estimating the effect of in-hospital smoking cessation counseling on mortality. Multivariate Behav Res. 2011;46(1):119-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cummings P, McKnight B, Greenland S. Matched cohort methods for injury research. Epidemiol Rev. 2003;25(1):43-50. [DOI] [PubMed] [Google Scholar]
- 37.Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ. 1999;319(7223):1492-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mokdad AA, Yopp AC, Polanco PM, et al. . Adjuvant chemotherapy vs postoperative observation following preoperative chemoradiotherapy and resection in gastroesophageal cancer: a propensity score-matched analysis. JAMA Oncol. 2018;4(1):31-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin DY, Psaty BM, Kronmal RA. Assessing the sensitivity of regression results to unmeasured confounders in observational studies. Biometrics. 1998;54(3):948-963. [PubMed] [Google Scholar]
- 40.Mitra N, Heitjan DF. Sensitivity of the hazard ratio to nonignorable treatment assignment in an observational study. Stat Med. 2007;26(6):1398-1414. [DOI] [PubMed] [Google Scholar]
- 41.Dobbins TA, Badgery-Parker T, Currow DC, Young JM. Assessing measures of comorbidity and functional status for risk adjustment to compare hospital performance for colorectal cancer surgery: a retrospective data-linkage study. BMC Med Inform Decis Mak. 2015;15(1):55-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giantonio BJ, Catalano PJ, Meropol NJ, et al. ; Eastern Cooperative Oncology Group Study E3200 . Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25(12):1539-1544. [DOI] [PubMed] [Google Scholar]
- 43.Grothey A, Sugrue MM, Purdie DM, et al. . Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE). J Clin Oncol. 2008;26(33):5326-5334. [DOI] [PubMed] [Google Scholar]
- 44.Kabbinavar FF, Hambleton J, Mass RD, Hurwitz HI, Bergsland E, Sarkar S. Combined analysis of efficacy: the addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancer. J Clin Oncol. 2005;23(16):3706-3712. [DOI] [PubMed] [Google Scholar]
- 45.Mathoulin-Pélissier S, Bécouarn Y, Belleannée G, et al. ; Regional Aquitaine Group for Colorectal cancer GRACCOR . Quality indicators for colorectal cancer surgery and care according to patient-, tumor-, and hospital-related factors. BMC Cancer. 2012;12(1):297-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ugolini G, Pasini F, Ghignone F, et al. . How to select elderly colorectal cancer patients for surgery: a pilot study in an Italian academic medical center. Cancer Biol Med. 2015;12(4):302-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bosset J-F, Collette L, Calais G, et al. ; EORTC Radiotherapy Group Trial 22921 . Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355(11):1114-1123. [DOI] [PubMed] [Google Scholar]
- 48.Bosset J-F, Calais G, Mineur L, et al. . Enhanced tumorocidal effect of chemotherapy with preoperative radiotherapy for rectal cancer: preliminary results: EORTC 22921. J Clin Oncol. 2005;23(24):5620-5627. [DOI] [PubMed] [Google Scholar]
- 49.Hong YS, Nam B-H, Kim KP, et al. . Oxaliplatin, fluorouracil, and leucovorin versus fluorouracil and leucovorin as adjuvant chemotherapy for locally advanced rectal cancer after preoperative chemoradiotherapy (ADORE): an open-label, multicentre, phase 2, randomized controlled trial. Lancet Oncol. 2014;15(11):1245-1253. [DOI] [PubMed] [Google Scholar]
- 50.Rödel C, Liersch T, Becker H, et al. ; German Rectal Cancer Study Group . Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol. 2012;13(7):679-687. [DOI] [PubMed] [Google Scholar]
- 51.Petrelli F, Coinu A, Lonati V, Barni S. A systematic review and meta-analysis of adjuvant chemotherapy after neoadjuvant treatment and surgery for rectal cancer. Int J Colorectal Dis. 2015;30(4):447-457. [DOI] [PubMed] [Google Scholar]
- 52.Kuan FC, Lai CH, Ku HY, et al. . The survival impact of delayed surgery and adjuvant chemotherapy on stage II/III rectal cancer with pathological complete response after neoadjuvant chemoradiation. Int J Cancer. 2017;140(7):1662-1669. [DOI] [PubMed] [Google Scholar]
- 53.Zhou J, Qiu H, Lin G, et al. . Is adjuvant chemotherapy necessary for patients with pathological complete response after neoadjuvant chemoradiotherapy and radical surgery in locally advanced rectal cancer? long-term analysis of 40 ypCR patients at a single center. Int J Colorectal Dis. 2016;31(6):1163-1168. [DOI] [PubMed] [Google Scholar]
- 54.Xu Z, Mohile SG, Tejani MA, et al. . Poor compliance with adjuvant chemotherapy use associated with poorer survival in patients with rectal cancer: an NCDB analysis. Cancer. 2017;123(1):52-61. [DOI] [PubMed] [Google Scholar]
- 55.Al-Sukhni E, Milot L, Fruitman M, et al. . Diagnostic accuracy of MRI for assessment of T category, lymph node metastases, and circumferential resection margin involvement in patients with rectal cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2012;19(7):2212-2223. [DOI] [PubMed] [Google Scholar]
- 56.Li XT, Sun YS, Tang L, Cao K, Zhang XY. Evaluating local lymph node metastasis with magnetic resonance imaging, endoluminal ultrasound and computed tomography in rectal cancer: a meta-analysis. Colorectal Dis. 2015;17(6):129-135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Codes Used for Cohort Development
eTable 2. Baseline Characteristics Before and After Propensity Score Matching
eTable 3. Sensitivity Analysis Evaluating the Sensitivity of Study Results to the Presence of Unmeasured Confounding
eFigure. Histograms Demonstrating the Distribution of Propensity Scores Among Nonadjuvant (upper panes) and adjuvant (lower panes) Treated Patients