Key Points
Question
What is the safety and antitumor activity of ipilimumab in women with human papillomavirus–related metastatic or recurrent cervical cancer?
Findings
In a phase 1/2 trial, 42 women received ipilimumab with manageable toxic effects (no dose-limiting toxic effects); 1 patient had a response, and 10 had stable disease with a median progression-free survival of 2.5 months. The baseline tumor immune infiltrate neither changed nor predicted benefit of treatment, yet a treatment-dependent increase of inducible T-cell costimulator, human leukocyte antigen–antigen D related, and programmed cell death 1 expression was observed on peripheral blood lymphocytes.
Meaning
Monotherapy with ipilimumab had minimal clinical activity but increased peripheral lymphocyte activation markers; failure to note these changes in tumors may explain the lack of clinical activity.
Abstract
Importance
Based on evidence of human papillomavirus (HPV)–induced immune evasion, immunotherapy may be an attractive strategy in cervical cancer. Ipilimumab is a fully humanized monoclonal antibody that blocks cytotoxic T-lymphocyte antigen-4 (CTLA-4), which acts to downregulate the T-cell immune response.
Objective
To assess the safety and antitumor activity of ipilimumab in recurrent cervical cancer.
Design, Setting, and Participants
A multicenter trial was designed for patients with metastatic cervical cancer (squamous cell carcinoma or adenocarcinoma) with measurable disease and progression after at least 1 line of platinum chemotherapy. A run-in safety cohort using ipilimumab, 3 mg/kg, every 21 days for 4 cycles in 6 patients was followed by a phase II cohort of ipilimumab, 10 mg/kg, every 21 days for 4 cycles and then 4 cycles of maintenance therapy every 12 weeks for patients demonstrating radiologic response or stabilization. Immune correlative studies were performed on peripheral blood before and after therapy on archival tissue and fresh tumor obtained prior to registration and 7 days after cycle 2. The study was conducted from December 3, 2012, to September 15, 2014. The data were analyzed from April 2016 to June 2016 and in July 2017.
Main Outcomes and Measures
The primary end points were safety and objective response rate. Immune analyses were performed on blood and tumor tissue.
Results
A total of 42 women (median age, 49 years; range, 23-78 years) were enrolled (29 [69%] squamous cell cervical cancer and 13 [31%] adenocarcinoma; 37 [93%] of 40 patients with tissue available for analysis had HPV-positive confirmation; there was no archival tissue for 2 women). Grade 3 toxic effects included diarrhea in 4 patients, 3 of whom had colitis. Of 34 patients evaluated for best response (Response Evaluation Criteria in Solid Tumors, version 1.1), 1 patient had partial response and 10 had stable disease. The median progression-free survival and overall survival were 2.5 months (95% CI, 2.1-3.2 months) and 8.5 months (95% CI, 3.6-not reached; 1 patient was still alive), respectively. Intratumoral pretreatment CD3, CD4, CD8, FoxP3, indoleamine 2,3-dioxygenase, and programmed cell death ligand 1 (PD-L1) expression was not predictive of benefit and did not significantly change with treatment. Multicolor flow cytometry on peripheral lymphocytes revealed a treatment-dependent increase of inducible T-cell costimulator, human leukocyte antigen–antigen D related, and PD-1 during initial treatment, which returned to baseline during maintenance.
Conclusions and Relevance
Ipilimumab was tolerable in this population but did not show significant single-agent activity. Immune changes were induced by anti–CTLA-4 therapy but did not correlate with clinical activity. Changes in these markers may guide further treatment strategies.
This phase1/2 trial evaluates the safety and antitumor activity of ipilimumab in women with recurrent cervical cancer.
Introduction
The molecular biology of human papillomavirus (HPV)–related tumors, such as cervical cancers,1 and associated immune escape mechanisms have driven the exploration of new treatment paradigms.2,3 Ipilimumab, the first checkpoint receptor inhibitor to be clinically tested, is a fully human monoclonal antibody against cytotoxic T-lymphocyte antigen-4. We designed a phase 1/2 trial to explore the tolerability and activity of ipilimumab in recurrent cervical cancer.
Methods
Study Design
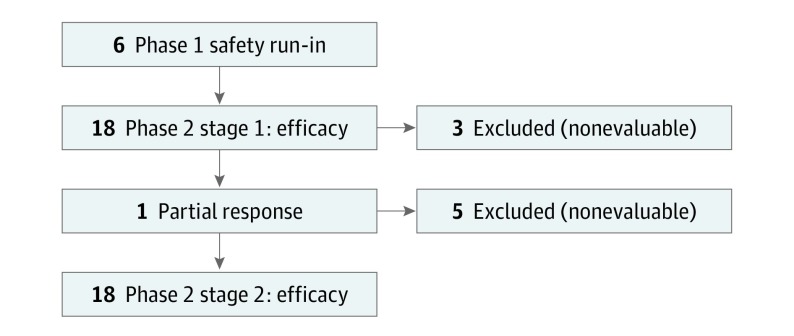
The run-in cohort (phase 1) assessed the safety of ipilimumab in this population given prior pelvic radiotherapy and platinum-based chemotherapy. The second cohort of the trial was a single-arm, multicenter, 2-stage, phase 2 study to evaluate the antitumor activity of ipilimumab (Figure 1). This study was conducted from December 3, 2012, to September 15, 2014, by the Princess Margaret Cancer Centre, Toronto, Ontario, Canada; University of Chicago Medical Center, Chicago, Illinois; and California Cancer Phase II Consortia, Duarte, California. The study was approved by the institutional review board of the City of Hope and the institutional review board at each site. All patients provided written informed consent; there was no financial compensation.
Figure 1. Study Flow Diagram.
Treatment Plan
The run-in cohort was to receive ipilimumab, 3 mg/kg, intravenously every 21 days for 4 cycles in 6 patients. The second cohort was to receive ipilimumab, 10 mg/kg, every 21 days for 4 cycles (week 1 to week 12) followed by 4 additional cycles as maintenance therapy (week 24 to 1 year) at the same dose every 12 weeks for patients with radiologic response or stabilization. All patients received 650 mg of acetaminophen before administration of ipilimumab.
Assessment of Toxic Effects and Efficacy
Toxicity was assessed per Common Terminology Criteria for Adverse Events, version 4.0.4 For the safety cohort, a dose-limiting toxic effect (DLT) was defined as unexpected nonautoimmune-related grade 3 adverse effects, grade 4 adverse effects, or autoimmune-related adverse effects that were not responsive to corticosteroid treatment and did not resolve to a grade 1 level within a 4-week period of initiating corticosteroids.
Radiologic assessment was performed by computed tomography within 28 days prior to registration, at weeks 12 (±1 week), 18, 24 weeks, and every 12 weeks during maintenance therapy. Assessment of response was performed using the Response Evaluation Criteria in Solid Tumors, version 1.1.5 Patients demonstrating clinical or radiologic progression or significant toxic effects were withdrawn from the study.
Correlative Analysis
Archival tissue, paired tumor biopsies at baseline and 7 days after cycle 2, and blood sample collection were requested in all patients at different times. In addition, HPV testing and immune assessment were performed on tumor tissue and blood samples at different time points.
Statistical Analysis
Coprimary end points were to assess safety and antitumor activity of ipilimumab by objective response rates (RRs) (null hypothesis RR<5%; alternative RR>20%; α error of 10%; and power of 90%) using a Simon 2-stage design.6 Secondary end points were to assess the antitumor activity of ipilimumab by progression-free survival and correlate changes in immunologic profiles with response.
For correlatives, change in the expression of the indicated molecule (percentage positive) before and after treatment (difference between C2D1 and baseline) was compared using a 2-sided t test (level of significance P < .05). Correlation of clinical activity by best objective RR (partial response or stable disease vs progressive disease) was assessed for changes (percentage expression) of these markers using the Mann-Whitney test with Prism, version 6 software (GraphPad Software Inc).
Results
From December 3, 2012, to September 15, 2014, 42 patients with a median age of 49 (range, 23-78) years were enrolled. Of these, 29 (69%) had squamous cell carcinoma and 13 (31%) had adenocarcinoma. Thirty-five (83%) patients had received prior radiotherapy, and 21 (50%) had received 2 or 3 prior chemotherapy regimens.
No DLTs were observed in the run-in cohort. Grade 3 toxic effects included diarrhea (3 of 4 patients with colitis) (Table and eTable 1 in the Supplement). Two patients died during the trial; 1 death occurred after 1 cycle and was not considered drug related, and the other death was a grade 5 colonic perforation following cycle 3, which occurred in a previously radiated area and was possibly related to ipilimumab.
Table. Immune-Mediated Toxic Effects Possibly Related to Ipilimumab for Run-in Phase 1 and Phase 2.
| Adverse Event | Patients, No. (%) | |
|---|---|---|
| All Grades | Grade ≥3 | |
| Diarrhea | 12 (29) | 4 (33) |
| Colitis | 2 (5) | 1 (50) |
| Enterocolitis | 1 (2) | 1 (100) |
| Pancolitis | 1 (2) | 1 (100) |
| Colonic perforation | 1 (2) | 1 (100) |
| Maculopapular rash | 12 (29) | 1 (8) |
| Pruritus | 9 (21) | 1 (11) |
| Increased aspartate aminotransferase | 9 (21) | 1 (11) |
| Increased alanine aminotransferase | 9 (21) | 0 |
| Arthralgia | 3 (7) | 1 (33) |
| Peripheral neuropathy | 2 (5) | 0 |
| Hypothyroidism | 2 (5) | 0 |
Best responses assessed for 34 evaluable patients included 1 partial response (squamous cell carcinoma); 10 stable disease (6 squamous-cell carcinoma and 4 adenocarcinoma), including 1 unconfirmed partial response; and 23 progressive disease. Eight patients were unevaluable because they were removed from the study before the first mandated computed tomographic imaging. Six patients continued treatment as maintenance. Median progression-free survival was 2.5 months (95% CI, 2.1-3.2 months), and the median overall survival was 8.5 months (95% CI, 3.6, upper limit not reached; 1 patient was still alive).
Thirty-seven (93%) of the 40 tumors were confirmed HPV-positive; 1 HPV evaluation was negative, 2 were inconclusive, and 2 had insufficient archival tissue). Twelve cases were genotyped; of these, 7 were HPV16, 3 were HPV18, and 1 was HPV39; 1 was not HPV.
Thirty-six of the 42 patients underwent biopsy: 25 (60%) received tumor biopsies before and during treatment, and 11 (26%) had only a pretreatment biopsy. In 1 patient, the biopsy did not contain tumor tissue. Five patients did not undergo a biopsy because it was considered unsafe.
Although intratumoral CD3, CD4, CD8, and FoxP3 expression before and during treatment was not significantly increased with ipilimumab, there was a significant increase in CD3 in tumor stroma (eFigure 1 in the Supplement). The presence of baseline immune infiltrate in the tumor or stroma was not associated with response or stable disease. Pretreatment programmed cell death ligand 1 (PD-L1) expression was negative in 20 patients, expressed in 1% to 10% of the cells in 4 patients, expressed in more than 10% in 4 patients, and was not available for 14 patients and did not correlate with response. In 5 patients, expression of PD-L1 changed from negative at baseline to positive after ipilimumab treatment. Indoleamine 2,3-dioxygenase (IDO) expression was negative at baseline in 23 patients and positive in 6 patients. Expression of IDO was not correlated with response, and its expression was different on paired biopsies for 2 patients (eTable 2 in the Supplement).
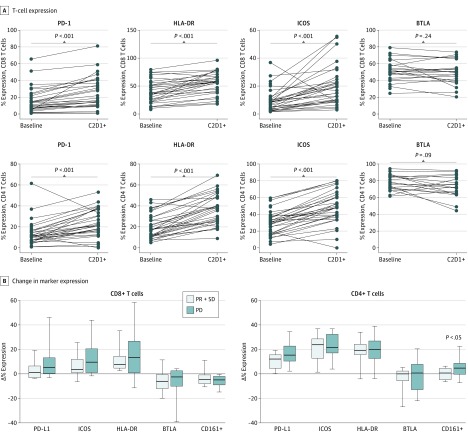
Multicolor flow cytometry on peripheral lymphocytes revealed a significant treatment-dependent increase of inducible T-cell costimulator, human leukocyte antigen–antigen D related, and PD-1 during treatment, which returned to baseline levels during maintenance (Figure 2) but did not correlate with response. Increased expression of CD161+ on CD4+ T cells was seen in tumors from patients with progressive disease (P = .04) but not in patients with partial response or stable disease (eFigure 2 in the Supplement).
Figure 2. Effect of Ipilimumab Administration on Peripheral T-Cell Phenotype.
Best response was assessed by Response Evaluation Criteria in Solid Tumors, 1.1 for the 34 evaluable patients; 8 patients were not evaluable because they were removed from study therapy before the first protocol-mandated computed tomography scan at week 12. All patients were assessed for toxic effects. Statistical analysis of the changes in the expression of immune markers was assessed in correlation with clinical response. Both CD4+ and CD8+ T cells demonstrated significantly increased expression of programmed cell death protein 1 (PD-1), human leukocyte antigen–antigen D related (HLA-DR), and inducible T-cell costimulator (ICOS) following treatment compared with pretreatment baseline. A, With ipilimumab treatment, CD4+ and CD8+ T cells showed an increase in expression of PD-1, HLA-DR, and ICOS but not B- and T lymphocyte–associated markers after the therapy. B, Changes in the expression of CD8 and CD4 markers were not associated with clinical response. Change in the CD161+ expression in CD4+ T cells, however, was significantly higher in patients whose disease progressed compared with those who had stable disease (SD) or partial response (PR). The horizontal line in each box indicates the median, and the top border of the box indicates the 75th percentile, the bottom the 25th. The whiskers above and below the box mark represent minimum and maximum values for that particular graph. BTLA indicates B- and T-lymphocyte attenuator; PD, progressive disease.
Discussion
To our knowledge, this is the first trial investigating ipilimumab in cervical cancer with correlative studies in paired biopsies and blood sample collection for immune profiling. Safety of treatment was demonstrated, and the toxic effects were similar to those observed using similar doses of ipilimumab in other cancers.7 Although the study did not meet the prespecified RR of 20%, some patients may have derived benefit from treatment, with durable, stable disease for 6 patients. Pretreatment tumor expression of PD-L1 was negative for 20 of 28 patients tested, although a recent study reported that more than 90% of tumor samples expressed PD-L1 in archival tissue.8
Ipilimumab significantly induced immune activation in peripheral blood but did not elicit a significant response in patients’ tumors. The lack of induction of significant activated immune infiltrate or the inability of activated cells to gain access to the tumor may account for the lack of tumor response. Factors such as tumor hypoxia9 and prior pelvic radiotherapy may reduce immune infiltration into the tumor following administration of ipilimumab.
In this study, the induction of PD-1 expression on peripheral lymphocytes following administration of ipilimumab has been observed and may be a mechanism contributing to resistance or immune exhaustion. Preliminary activity of anti-PD1 treatment has been reported in advanced cervical cancer.10,11 Increases in circulating CD4+CD161+ T-cell populations appear to be associated with disease progression. These cells may represent a specific subpopulation of TH17,12 which are T-helper lymphocytes characterized by functional plasticity.13,14 The observed immune changes reflect immunologic activation without significant antitumor effects, which warrants further investigation into the mechanisms of immune escape.
Limitations
The primary limitation of the trial was the single-arm design. This was a phase 1/2 trial assessing ipilimumab monotherapy with no control arm.
Conclusions
This phase 1/2 study shows the need for new treatment strategies and the feasibility of immunotherapy studies with paired biopsies in recurrent cervical cancers. Ipilimumab as monotherapy did not demonstrate significant benefit in this setting.
eTable 1. Overall Toxicities Possibly Related to Ipilimumab for Run-In Phase 1 and Phase 2
eTable 2. DAB-Stained for Immune Markers as Part of the Trial
eFigure 1. Immunohistochemistry on Paired Tumor Biopsies
eFigure 2. Flow Cytometry Plots for PD-1 and ICOS on CD4+ and CD8+ CD3+ T Cells
References
- 1.Menderes G, Black J, Schwab CL, Santin AD. Immunotherapy and targeted therapy for cervical cancer: an update. Expert Rev Anticancer Ther. 2016;16(1):-. [DOI] [PubMed] [Google Scholar]
- 2.Pfaendler KS, Tewari KS. Changing paradigms in the systemic treatment of advanced cervical cancer. Am J Obstet Gynecol. 2016;214(1):22-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE), Version 4. Washington, DC: National Institutes of Health, National Cancer Institute; May 28, 2009.
- 5.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. [DOI] [PubMed] [Google Scholar]
- 6.Simon R. Designs for efficient clinical trials. Oncology (Williston Park). 1989;3(7):43-49. [PubMed] [Google Scholar]
- 7.Fecher LA, Agarwala SS, Hodi FS, Weber JS. Ipilimumab and its toxicities: a multidisciplinary approach. Oncologist. 2013;18(6):733-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enwere EK, Kornaga EN, Dean M, et al. Expression of PD-L1 and presence of CD8-positive T cells in pre-treatment specimens of locally advanced cervical cancer. Mod Pathol. 2017;30(4):577-586. [DOI] [PubMed] [Google Scholar]
- 9.Fyles A, Milosevic M, Hedley D, et al. Tumor hypoxia has independent predictor impact only in patients with node-negative cervix cancer. J Clin Oncol. 2002;20(3):680-687. [DOI] [PubMed] [Google Scholar]
- 10.Frenel JS, Le Tourneau C, O'Neil BH, et al. Pembrolizumab in patients with advanced cervical squamous cell cancer: preliminary results from the phase 1b KEYNOTE-028 study. J Clin Oncol. 2016;34(suppl 15):5515. [Google Scholar]
- 11.Hollebecque A, Meyer T, Moore KN, et al. An open-label, multicohort, phase I/II study of nivolumab in patients with virus-associated tumors (CheckMate 358): efficacy and safety in recurrent or metastatic (R/M) cervical, vaginal, and vulvar cancers (abstract). J Clin Oncol. 2017;35(suppl):5504. [Google Scholar]
- 12.Maggi L, Santarlasci V, Capone M, et al. CD161 is a marker of all human IL-17–producing T-cell subsets and is induced by RORC. Eur J Immunol. 2010;40(8):2174-2181. [DOI] [PubMed] [Google Scholar]
- 13.Guéry L, Hugues S. Th17 cell plasticity and functions in cancer immunity. Biomed Res Int. 2015;2015:314620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bailey SR, Nelson MH, Himes RA, Li Z, Mehrotra S, Paulos CM. Th17 cells in cancer: the ultimate identity crisis. Front Immunol. 2014;5:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Overall Toxicities Possibly Related to Ipilimumab for Run-In Phase 1 and Phase 2
eTable 2. DAB-Stained for Immune Markers as Part of the Trial
eFigure 1. Immunohistochemistry on Paired Tumor Biopsies
eFigure 2. Flow Cytometry Plots for PD-1 and ICOS on CD4+ and CD8+ CD3+ T Cells