Abstract
Importance
Studies investigating the association of cigarette smoking with prostate cancer incidence and outcomes have revealed controversial results.
Objective
To systematically review and analyze the association of smoking status with biochemical recurrence, metastasis, and cancer-specific mortality among patients with localized prostate cancer undergoing primary radical prostatectomy or radiotherapy.
Data Sources
A systematic search of original articles published between January 2000 and March 2017 was performed using PubMed, MEDLINE, Embase, and Cochrane Library databases in March 2017.
Study Selection
Observational studies reporting Cox proportional hazards regression or logistic regression analyses were independently screened.
Data Extraction and Synthesis
This systematic review and meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement and the Cochrane Handbook for Systematic Reviews of Interventions. Available multivariable hazard ratios (HRs) and corresponding 95% CIs were included in quantitative analysis. A risk-of-bias assessment was completed for nonrandomized studies.
Main Outcomes and Measures
Prespecified outcomes of interest were biochemical recurrence, metastasis, and cancer-specific mortality.
Results
A total of 5157 reports were identified, of which 16 articles were selected for qualitative analysis and 11 articles were selected for quantitative analysis. All included studies were observational and nonrandomized and comprised a total of 22 549 patients. Overall, 4202 patients (18.6%) were current smokers. The overall median follow-up was 72 months. Current smokers had a statistically significantly higher risk of biochemical recurrence (HR, 1.40; 95% CI, 1.18-1.66; P < .001 [10 studies]), as did former smokers (HR, 1.19; 95% CI, 1.09-1.30; P < .001 [7 studies]). Current smokers were also at a higher risk of metastasis (HR, 2.51; 95% CI, 1.80-3.51; P < .001 [3 studies]) and cancer-specific mortality (HR, 1.89; 95% CI, 1.37-2.60; P < .001 [5 studies]), whereas former smokers were not (metastasis: HR, 1.61; 95% CI, 0.65-3.97; P = .31 [2 studies]; cancer-specific mortality: HR, 1.05; 95% CI, 0.81-1.37; P = .70 [4 studies]).
Conclusions and Relevance
Current smokers at the time of primary curative treatment for localized prostate cancer are at higher risk of experiencing biochemical recurrence, metastasis, and cancer-specific mortality.
This systematic review and meta-analysis examines the association of smoking status with biochemical recurrence, metastasis, and cancer-specific mortality among patients with localized prostate cancer undergoing primary radical prostatectomy or radiotherapy.
Key Points
Question
What is the association of smoking with oncologic outcomes among patients undergoing radical prostatectomy or radiotherapy for localized prostate cancer?
Findings
In this systematic review and meta-analysis that included 11 studies with 22 549 patients with prostate cancer undergoing primary radical prostatectomy or radiotherapy, current smokers had a significantly higher risk of biochemical recurrence, metastasis, and cancer-specific mortality.
Meaning
These results should encourage radiation oncologists and urologists to counsel patients on smoking cessation, given the risk of poorer oncologic outcomes associated with smoking.
Introduction
Burning tobacco products and its additives produces thousands of chemicals, including more than 70 well-known carcinogens.1,2 Tobacco smoking is known as a preventable risk factor for the development and mortality of several genitourinary cancers such as bladder cancer,3,4 upper tract urothelial carcinoma,5 and renal cell carcinoma.3,6 In contrast, the effect of tobacco consumption on the incidence of prostate cancer is still a matter of debate.7,8 Nevertheless, the association between cigarette smoking and prostate cancer mortality seems to be robust. Two meta-analyses that evaluated the association of smoking with prostate cancer outcomes confirmed a higher risk of death among current smokers than among nonsmokers.7,8 These apparently discordant findings could be explained by the presence of higher-grade or higher-stage disease at the time of diagnosis, the adverse effects of tobacco use on oncologic outcomes after primary treatment, or both. Some studies revealed a correlation between smoking status and higher tumor volumes, more expansive high-grade tumor volumes, and extracapsular extension during radical prostatectomy (RP).9,10
The aim of this systematic review and meta-analysis was to investigate the association of smoking status, number of cumulative pack-years, and smoking cessation with biochemical recurrence (BCR), metastasis, and cancer-specific mortality (CSM) among patients with prostate cancer who are undergoing primary RP or radiotherapy (RT). Our hypothesis was that current smokers have a higher risk of BCR, metastasis, and CSM compared with former smokers or never smokers.
Methods
Literature Search
This systematic review and meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and the Cochrane Handbook for Systematic Reviews of Interventions.11,12 We systematically searched PubMed, MEDLINE, Embase, and the Cochrane Library to identify studies published between January 2000 and March 2017 that examined the association of smoking status with the prognosis of patients undergoing primary curative treatment (RP or RT) for localized prostate cancer. The bibliographic search was performed by B.F. and C.P. in March 2017. The following search terms were used: (“Cigarette” OR “Smoking” OR “Tobacco”) AND (“Prostate cancer”) AND (“Biochemical recurrence” OR “Recurrence” OR “Progression” OR “Survival” OR “Mortality” OR “Metastasis” OR “Prognosis”). No language restrictions were applied. All original articles found on the topic were observational cohort or case-control studies because the habit of smoking is not a factor that can be randomized. Biochemical recurrence, metastasis, and CSM were the primary end points of interest.
Eligibility Criteria
As proposed by the PRISMA guidelines, we used the population, intervention, comparator, outcome, and study design approach to specify the inclusion criteria. Reports were considered relevant when they included patients who received a diagnosis of prostate cancer (population), recorded smoking status (comparator), and the patients underwent primary curative treatment (intervention) to independently determine the association of smoking status with BCR, metastasis, and CSM (outcome) using Cox proportional hazards regression or logistic regression analyses (study design). We were primarily focused on comparing the risk of current, former, and never smoking.
Second, we aimed to investigate cumulative risk groups (pack-years) and different durations of smoking cessation. The main focus regarding primary curative treatment was on RP, RT, or both. Studies with mixed treatment populations had to consist of at least 80% of these 2 modalities and had to be adjusted for primary therapy. Only studies with smoking status examined in multivariable Cox proportional hazards regression analyses were considered for meta-analysis. If more than 1 report of the same study population existed, we selected the most recent regarding a specific survival outcome. Review articles, editorials, comments, and meeting abstracts were excluded. Search results were independently screened by B.F. and C.P. The references of the included articles were scanned for additional studies of interest. Disagreements were resolved by consulting the senior author (S.F.S.).
Data Extraction
After evaluation of full-text articles, data were independently extracted by B.F. and C.P. for further assessment of qualitative and quantitative analyses. All extracted variables were cross-checked to ensure their reliability. Discrepancies were generally resolved by consensus or finally decided by the senior author (S.F.S.).
We recorded the overall and risk-specific (smoking status) number of included participants with the corresponding frequency of BCR, the occurrence of metastasis and disease-specific mortality, and the median or mean duration of follow-up. Subsequently, the hazard ratio (HR) and 95% CI associated with the respective smoking status and outcome were retrieved. Furthermore, we searched for baseline characteristics, methods, and important confounders to establish comparability.
Statistical Analysis
Owing to the observational nature of the included studies, we extracted adjusted HRs and 95% CIs for the cumulative effect size calculation. Studies with univariable Cox proportional hazards regression or general logistic regression analyses were not considered for meta-analysis but were included in the systematic review. Effect summary estimation methods were not used in these cases because a high level of additional selection bias would have been introduced. If multivariable testing was performed but the results were not shown, we took univariable data into account. Statistical pooling of effect measures was based on the level of heterogeneity among studies, which was assessed with the Cochrane Q test and the I2 statistic. Significant heterogeneity was indicated by P < .05 in Cochrane Q tests and a ratio greater than 50% in I2 statistics, which led to the use of random-effects models according to the DerSimonian and Laird method.13,14,15 When these tests were negative for heterogeneity, fixed-effects models were chosen for calculation of pooled HRs through the inverse-variance method. Publication bias that included a small-study effect was evaluated by visual inspection of funnel plots for all assessed comparisons.16 Statistical analyses were performed using STATA/MP, version 14.2 (StataCorp). P < .05 was considered significant.
Risk of Bias
The risk-of-bias evaluation of the included studies was assessed according to the Cochrane Handbook for Systematic Reviews of Interventions for including nonrandomized studies.17,18 Because the included studies were nonrandomized, risk of bias was determined using the pragmatic approach by examining the risk of preassigned confounders. The main confounding factors were identified as the most important prognostic factors at the time of primary prostate cancer treatment. We therefore reviewed the articles, adjusting for the effects of age, prostate-specific antigen level, clinical or pathologic stage, Gleason score, positive surgical margins, and the use of neoadjuvant androgen deprivation therapy within their Cox proportional hazards regression models. The presence of confounders was determined by consensus between two of us (B.F. and C.P.). The risk-of-bias summary and graph were built using the Cochrane Review Manager, version 5.3.19
Results
Study Population
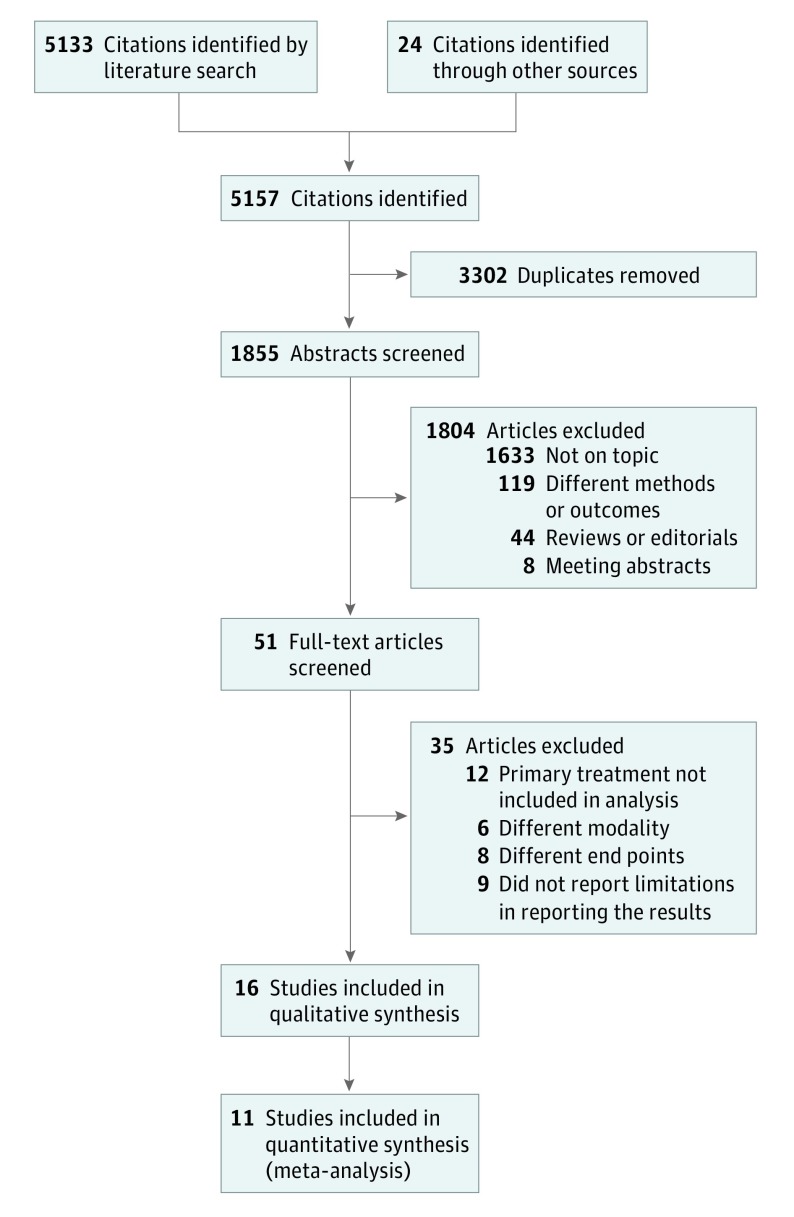
Overall, we identified 16 studies for qualitative analysis (eTable 1 in the Supplement) and 11 studies for quantitative analysis (Figure 1). The number of included participants from each selected study for meta-analysis ranged from 416 to 6538 participants (median, 1416 participants; mean, 2050 participants). Overall, the meta-analysis comprised 22 549 patients; 4202 (18.6%) were current smokers at the time of primary curative treatment, and 18 347 (81.4%) were nonsmokers (former and never smokers combined). Not all studies differentiated between former smoking and never smoking. The overall median follow-up period was 72 months for the entire cohort, which represented North America, Europe, and East Asia. All available data covered by the systematic review on BCR, metastasis, and CSM are summarized in eTables 1 to 6 in the Supplement. The risk-of-bias assessment revealed a moderate to high level of bias across studies (eFigure 1 in the Supplement) owing to the type of studies included (observational and nonrandomized) and confounding.
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-analyses Flowchart.
Biochemical Recurrence
Among studies that investigated BCR, 4656 of the included 21 797 participants (21.4%) experienced BCR during a median follow-up of 61 months.
Current Smoking
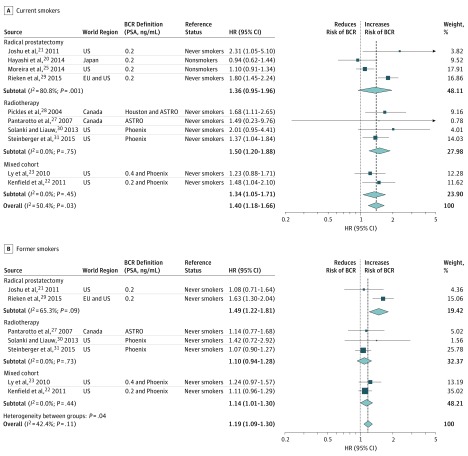
The association of current smoking status with BCR was investigated in 13 of the included articles.10,20,21,22,23,24,25,26,27,28,29,30,31 Ten of these articles provided multivariable HRs for inclusion into meta-analysis.20,21,22,23,25,27,28,29,30,31 The corresponding forest plot (Figure 2A32,33,34) revealed that current smokers had a significantly higher risk of experiencing BCR compared with nonsmokers whether they had undergone RP or RT (HR, 1.40; 95% CI, 1.18-1.66; P < .001). The Cochrane Q test and the I2 statistic showed heterogeneity; therefore, a random-effects analysis was performed. However, we included 2 studies20,25 that compared current smoking vs nonsmoking. Both showed no significant association with BCR. When comparing current smokers with never smokers only, the association was higher (HR, 1.59; 95% CI, 1.40-1.80; P < .001). Inspection of the funnel plot did not demonstrate publication bias (eFigure 2A in the Supplement).
Figure 2. Forest Plots of Studies Investigating the Association of Current and Former Smoking With Biochemical Recurrence (BCR).
A, Current smokers. Weights are from random-effects analysis. B, Former smokers. Weights are from fixed-effects analysis. The reference status “nonsmokers” contains never smokers and former smokers. BCR definitions include American Society for Therapeutic Radiology and Oncology (ASTRO) (3 consecutive increases greater than the nadir [1997])32; Houston (a PSA increase of ≥2 ng/mL above the nadir, defined as the last nonrising PSA [2002])33; and Phoenix (an increase of ≥2 ng/mL above the absolute nadir [2006]).34 EU indicates Europe; HR, hazard ratio; PSA, prostate-specific antigen. The dashed line indicates the overall pooled effect size (HR).
Former Smoking
Former smoking as a risk factor for BCR after primary treatment was assessed in 8 studies,21,22,23,27,28,29,30,31 7 of which were integrated into the meta-analysis.21,22,23,27,29,30,31 Among the included patients, former smoking was independently associated (Figure 2B32,33,34) with BCR (HR, 1.19; 95% CI, 1.09-1.30; P < .001). The data were homogeneous according to the I2 statistic and the Cochrane Q test; therefore, a fixed-effects model was used for cumulative analysis. Inspection of the corresponding funnel plot did not show evidence of publication bias (eFigure 2B in the Supplement).
Cumulative Pack-years
Data about cumulative smoking exposure in pack-years were too heterogeneous to analyze. Moreover, the results were inconsistent. Joshu et al21 compared patients with a history of 10 pack-years or more vs never smokers during RP and found no significant association with BCR (HR, 0.87; 95% CI, 0.51-1.47). Rieken et al29 investigated patients with a history of more than 20 vs less than 20 pack-years and found no significant difference (HR, 0.92; 95% CI, 0.72-1.12; P = .50). In contrast, Ngo et al10 found a slightly higher risk (HR, 1.27; 95% CI, 1.03-1.54; P = .02) among patients with more than 20 pack-years of cumulative smoking exposure. Kenfield et al22 compared patients with a history of 39 pack-years or less and those with 40 pack-years or more vs never smokers in a mixed cohort (RP and RT). They reported contradictory results, with an elevated risk among patients with 39 pack-years or less (HR, 2.13; 95% CI, 1.24-3.64). Patients with a history of 40 pack-years or more did not have such an association (HR, 1.48; 95% CI, 0.88-2.48).
Smoking Cessation
Owing to heterogeneous data on smoking cessation, a meta-analysis could not be performed. Overall, 3 studies explored smoking cessation, whereas 2 studies investigated a time frame of smoking cessation greater than 10 years. In both reports, smoking cessation for more than 10 years was a protective factor for BCR (HR, 0.6; 95% CI, 0.4-0.9 [compared with current smokers]; HR, 0.96; 95% CI, 0.68-1.37 [compared with never smokers]), but smoking cessation for less than 10 years was not protective (eTable 3 in the Supplement).22,29 Furthermore, Joshu et al21 investigated a 5-year time frame of smoking cessation that did not show a benefit.
Metastasis
In 2 of 3 included reports that provided data on metastasis, 90 of 2086 patients (4.3%) developed metastases.27,31 The median follow-up was 75 months.
Current Smoking
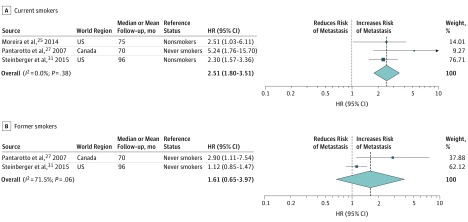
Current smoking as a risk factor for developing metastasis was reported in 3 studies.25,27,31 Meta-analysis of available HRs (Figure 3A) revealed that current smoking was associated with metastasis (HR, 2.51; 95% CI, 1.80-3.51; P < .001). No heterogeneity was observed, and the funnel plot did not show evidence of publication bias (eFigure 2C in the Supplement).
Figure 3. Forest Plots of Studies Investigating the Association of Current and Former Smoking With Metastasis.
A, Current smokers. Weights are from fixed-effects analysis. B, Former smokers. Weights are from random-effects analysis. HR indicates hazard ratio. The dashed line indicates the overall pooled effect size (HR).
Former Smoking
The association of former smoking status with metastasis was reported in 2 studies27,31 and revealed contradictory results (Figure 3B). Both studies comprised patients undergoing RT only. Pooling of HRs showed no association with developing metastasis (HR, 1.61; 95% CI, 0.65-3.97; P = .31). Owing to heterogeneity between the studies, a random-effects model was used. The funnel plot was negative for publication bias (eFigure 2D in the Supplement).
Cancer-Specific Mortality
Among all included 7924 participants, 654 (8.3%) died of prostate cancer within a median follow-up of 95 months.
Current Smoking
Current smoking status as a risk factor for CSM was reported in 8 studies,22,25,27,28,31,35,36,37 5 of which provided multivariable HRs for inclusion in meta-analysis.22,25,27,31,35 Data were homogeneous according to the Cochrane Q test and the I2 statistic and could therefore be analyzed with a fixed-effects analysis. The cumulative pooling of available HRs across both treatment modalities (Figure 4A) demonstrated that being an active smoker during primary therapy was significantly associated with the risk of CSM by approximately a factor of 2 (HR, 1.89; 95% CI, 1.37-2.60; P < .001). Visual inspection of the appropriate funnel plot revealed no publication bias (eFigure 2E in the Supplement).
Figure 4. Forest Plots of Studies Investigating the Association of Current and Former Smoking With Cancer-Specific Mortality (CSM).
A, Current smokers. B, Former smokers. Weights are from fixed-effects analysis. HR indicates hazard ratio. The dashed line indicates the overall pooled effect size (HR).
Former Smoking
A total of 4 studies investigated the association of former smoking status with CSM.22,27,31,35 All 4 studies were selected for cumulative analysis. Pooling of available HRs within RT and mixed cohorts (Figure 4B) revealed that patients who had a history of smoking were not at higher risk of CSM (HR, 1.05; 95% CI, 0.81-1.37; P = .70). Data were homogeneous in the Cochrane Q test and the I2 statistic, and the corresponding funnel plot revealed no publication bias (eFigure 2F in the Supplement).
Cumulative Pack-years
Overall, 2 studies examined the association of cumulative exposure and smoking cessation with CSM.22,35 Data were too heterogeneous to analyze. Gong et al35 reported that a summarized dosage of 15 pack-years or more was significantly associated with CSM (HR, 5.82; 95% CI, 1.96-17.30; P < .001). In addition, a dosage between 1 and 9 pack-years was correlated with CSM (HR, 2.70; 95% CI, 1.10-6.64). Kenfield et al22 found no significant correlation between a cumulative dose of 40 pack-years or more and CSM (HR, 1.75; 95% CI, 0.73-4.19).
Smoking Cessation
In contrast, 2 studies showed that patients who had stopped smoking for 10 years or more were not at higher risk to die of prostate cancer (eTable 6 in the Supplement). Gong et al35 reported an HR of 0.45 (95% CI, 0.19-1.05) for patients who quit smoking more than 10 years ago compared with never smokers. Kenfield et al22 revealed a protective effect for patients who stopped smoking 10 years or more ago (HR, 0.6; 95% CI, 0.4-0.9) in comparison with current smokers.
Discussion
In this systematic review and meta-analysis, we investigated the oncologic outcomes of patients with a different smoking status at the time of primary RP or definitive RT for localized prostate cancer. We found that current smokers are at higher risk for BCR, metastasis, and CSM compared with nonsmokers. In addition, former smokers undergoing primary curative treatment were at a significantly higher risk of BCR, but not metastasis or CSM, compared with never smokers. Quantitative analyses on cumulative exposure and smoking cessation were not possible owing to the heterogeneity of data. However, after systematically reviewing all available studies, only 2 studies for each outcome reported on the association of smoking cessation using a cutoff point of 10 years or more; both studies found a clinically significant benefit of smoking cessation of 10 years or more regarding BCR.22,29 However, the association with CSM was not significant in 1 of the studies, probably owing to a low event rate.35
These findings encourage physicians to use the diagnosis and treatment of localized prostate cancer as a teachable moment to counsel patients to stop smoking.38 Here, we identified a modifiable risk factor that may improve the outcome of patients with prostate cancer. In fact, smoking appears to affect all disease phases: recurrence, metastasis, and CSM. Regarding the association of smoking with the outcomes of different treatment modalities, the effect summaries for RP and RT do not look different. However, there are statistical limitations to draw this conclusion. The biological underpinning of the association between smoking and poorer oncologic outcomes is further bolstered by the finding of more adverse pathologic features during RP (tumor volume, high-grade volume, and ≥pT3) in smokers.9,10 For example, Silva et al39 found that Hispanic patients with a history of smoking had nearly twice the risk of their Gleason score increasing during active surveillance. Finally, Oefelein and Resnick40 found an independent association of cigarette smoking with time to castration resistance in a cohort with advanced prostate cancer undergoing androgen deprivation therapy. These reports are consistently building robust evidence. Nevertheless, further research in this field is needed to allow more decisive evidence for guideline recommendations and policymakers.
At the molecular level, several mechanisms can explain the association between smoking and progression of prostate cancer. Inflammatory processes within the prostate have been identified as a disease driver.41 Furthermore, nicotine is another candidate mechanism that may explain a higher risk of metastasis after diagnosis by increasing interleukin 8 levels. The association between smokeless tobacco and prostate cancer points toward a crucial role of nicotine in prostate cancer.42 However, another possible way in which smoking may affect prostate cancer outcomes is through CpG hypermethylation. Methylation analysis showed significant correlation between smoking and multigene hypermethylation.43,44
Limitations
The methods of the studies included in the meta-analysis are suboptimal. Most of these reports had information about smoking status at the time of primary therapy only but not during follow-up. Some patients may have quit smoking after treatment, and the differential effect on oncologic outcomes of quitting at that time remains unknown. Westmaas et al45 reported that a cancer diagnosis, even in tumors that are not strongly related to smoking, is associated with a higher subsequent rate of smoking cessation. This outcome could also explain, in part, why former smoking was not associated with CSM as it was with BCR. Another explanation for this lack of association is the limited follow-up and the long natural history of prostate cancer. Furthermore, studies did not provide data on whether former smokers remained nonsmokers during follow-up or not.
Other limitations include the observational and nonrandomized design of the included studies introducing different biases, such as selection and recall biases. Studies’ populations and methods varied widely. Some articles did not differentiate between former smokers and never smokers, grouping them as nonsmokers. Overall, the extracted data were heterogeneous and could not always be compared. Nevertheless, we combined different treatment modalities for comparison owing to the paucity of reports and thereby possibly creating additional bias. Our conclusions are limited by the small numbers of eligible studies in each treatment group and outcome. Furthermore, some studies did not report all calculated results and numerical data, which were required to compare nonrandomized studies. The overall risk of bias was moderate to high. However, through inclusion of multivariable results only, adjusted for the effects of major confounders, we could lower the bias and establish a satisfactory level of comparability.
Conclusions
To our knowledge, this is the first systematic review and meta-analysis that investigated the association of smoking with oncologic outcomes after primary treatment for localized prostate cancer. We found that current smokers at the time of RP or RT were at a higher risk of experiencing BCR, metastasis, and CSM after local therapy. Results regarding former smoking and time to cessation were inconclusive because available data were sparse and heterogenous. Our findings encourage radiation oncologists and urologists to counsel patients to stop smoking, using primary prostate cancer treatment as a teachable moment. Further studies with clear definitions of the study population and a precise assessment of the smoking exposure are needed to clarify the association of smoking cessation with long-term oncologic outcomes.
eTable 1. All Included Articles
eTable 2. Impact of Smoking Status on BCR
eTable 3. Impact of Cumulative Smoking and Smoking Cessation on BCR
eTable 4. Impact of Smoking Status on Metastasis
eTable 5. Impact of Smoking Status on CSM
eTable 6. Impact of Cumulative Smoking and Smoking Cessation on CSM
eFigure 1. Risk of Bias Assessment of Each Included Study with Summary (A) and Graph (B)
eFigure 2. Funnel Plots With Pseudo 95% CIs for Each Investigated Outcome
References
- 1.US Food and Drug Administration Harmful and potentially harmful constituents in tobacco products and tobacco smoke: established list. https://www.fda.gov/TobaccoProducts/GuidanceComplianceRegulatoryInformation/ucm297786.htm. Published April 2012. Accessed August 16, 2017.
- 2.How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: after treatment Report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention; 2010. [PubMed] [Google Scholar]
- 3.Cumberbatch MG, Rota M, Catto JW, La Vecchia C. The role of tobacco smoke in bladder and kidney carcinogenesis: a comparison of exposures and meta-analysis of incidence and mortality risks. Eur Urol. 2016;70(3):458-466. [DOI] [PubMed] [Google Scholar]
- 4.Rink M, Zabor EC, Furberg H, et al. Impact of smoking and smoking cessation on outcomes in bladder cancer patients treated with radical cystectomy. Eur Urol. 2013;64(3):456-464. [DOI] [PubMed] [Google Scholar]
- 5.Rink M, Xylinas E, Margulis V, et al. ; Upper Tract Urothelial Carcinoma Collaboration . Impact of smoking on oncologic outcomes of upper tract urothelial carcinoma after radical nephroureterectomy. Eur Urol. 2013;63(6):1082-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fajkovic H, Shariat SF, Klatte T, et al. Impact of smoking status on survival after cytoreductive nephrectomy for metastatic renal cell carcinoma. World J Urol. 2016;34(10):1411-1419. [DOI] [PubMed] [Google Scholar]
- 7.Huncharek M, Haddock KS, Reid R, Kupelnick B. Smoking as a risk factor for prostate cancer: a meta-analysis of 24 prospective cohort studies. Am J Public Health. 2010;100(4):693-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Islami F, Moreira DM, Boffetta P, Freedland SJ. A systematic review and meta-analysis of tobacco use and prostate cancer mortality and incidence in prospective cohort studies. Eur Urol. 2014;66(6):1054-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zapata DF, Howard LE, Aronson WJ, et al. Smoking is a predictor of adverse pathological features at radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital database. Int J Urol. 2015;22(7):658-662. [DOI] [PubMed] [Google Scholar]
- 10.Ngo TC, Lee JJ, Brooks JD, Nolley R, Ferrari M, Presti JC Jr. Smoking and adverse outcomes at radical prostatectomy. Urol Oncol. 2013;31(6):749-754. [DOI] [PubMed] [Google Scholar]
- 11.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration. http://handbook-5-1.cochrane.org/. Updated March 2011. Accessed March 31, 2017.
- 13.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188. [DOI] [PubMed] [Google Scholar]
- 14.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28(2):105-114. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deeks JJ, Dinnes J, D’Amico R, et al. ; International Stroke Trial Collaborative Group; European Carotid Surgery Trial Collaborative Group . Evaluating non-randomised intervention studies. Health Technol Assess. 2003;7(27):iii-x, 1-173. [DOI] [PubMed] [Google Scholar]
- 18.Reeves BC, Deeks JJ, Higgins JPT, Wells GA Chapter 13: including non-randomized studies. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Cochrane Collaboration. http://handbook-5-1.cochrane.org/. Updated March 2011. Accessed March 31, 2017.
- 19.Review Manager (RevMan) [computer program]. Version 5.3. Copenhagen, Denmark: Nordic Cochrane Centre, Cochrane Collaboration; 2014.
- 20.Hayashi N, Matsushima M, Kido M, et al. BMI is associated with larger index tumors and worse outcome after radical prostatectomy. Prostate Cancer Prostatic Dis. 2014;17(3):233-237. [DOI] [PubMed] [Google Scholar]
- 21.Joshu CE, Mondul AM, Meinhold CL, et al. Cigarette smoking and prostate cancer recurrence after prostatectomy. J Natl Cancer Inst. 2011;103(10):835-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenfield SA, Stampfer MJ, Chan JM, Giovannucci E. Smoking and prostate cancer survival and recurrence. JAMA. 2011;305(24):2548-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ly D, Reddy CA, Klein EA, Ciezki JP. Association of body mass index with prostate cancer biochemical failure. J Urol. 2010;183(6):2193-2199. [DOI] [PubMed] [Google Scholar]
- 24.Major JM, Klonoff-Cohen HS, Pierce JP, et al. Prostate cancer postoperative nomogram scores and obesity. PLoS One. 2011;6(2):e17382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moreira DM, Aronson WJ, Terris MK, et al. Cigarette smoking is associated with an increased risk of biochemical disease recurrence, metastasis, castration-resistant prostate cancer, and mortality after radical prostatectomy: results from the SEARCH database. Cancer. 2014;120(2):197-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh JJ, Hong SK, Jeong CW, Byun SS, Lee SE. Significance of smoking status regarding outcomes after radical prostatectomy. Int Urol Nephrol. 2012;44(1):119-124. [DOI] [PubMed] [Google Scholar]
- 27.Pantarotto J, Malone S, Dahrouge S, Gallant V, Eapen L. Smoking is associated with worse outcomes in patients with prostate cancer treated by radical radiotherapy. BJU Int. 2007;99(3):564-569. [DOI] [PubMed] [Google Scholar]
- 28.Pickles T, Liu M, Berthelet E, Kim-Sing C, Kwan W, Tyldesley S; PROSTATE COHORT OUTCOMES INITIATIVE . The effect of smoking on outcome following external radiation for localized prostate cancer. J Urol. 2004;171(4):1543-1546. [DOI] [PubMed] [Google Scholar]
- 29.Rieken M, Shariat SF, Kluth LA, et al. Association of cigarette smoking and smoking cessation with biochemical recurrence of prostate cancer in patients treated with radical prostatectomy. Eur Urol. 2015;68(6):949-956. [DOI] [PubMed] [Google Scholar]
- 30.Solanki AA, Liauw SL. Tobacco use and external beam radiation therapy for prostate cancer: influence on biochemical control and late toxicity. Cancer. 2013;119(15):2807-2814. [DOI] [PubMed] [Google Scholar]
- 31.Steinberger E, Kollmeier M, McBride S, Novak C, Pei X, Zelefsky MJ. Cigarette smoking during external beam radiation therapy for prostate cancer is associated with an increased risk of prostate cancer-specific mortality and treatment-related toxicity. BJU Int. 2015;116(4):596-603. [DOI] [PubMed] [Google Scholar]
- 32.American Society for Therapeutic Radiology and Oncology Consensus Panel Consensus statement: guidelines for PSA following radiation therapy. Int J Radiat Oncol Biol Phys. 1997;37(5):1035-1041. [PubMed] [Google Scholar]
- 33.Thames H, Kuban D, Levy L, et al. Comparison of alternative biochemical failure definitions based on clinical outcome in 4839 prostate cancer patients treated by external beam radiotherapy between 1986 and 1995. Int J Radiat Oncol Biol Phys. 2003;57(4):929-943. [DOI] [PubMed] [Google Scholar]
- 34.Roach M III, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965-974. [DOI] [PubMed] [Google Scholar]
- 35.Gong Z, Agalliu I, Lin DW, Stanford JL, Kristal AR. Cigarette smoking and prostate cancer-specific mortality following diagnosis in middle-aged men. Cancer Causes Control. 2008;19(1):25-31. [DOI] [PubMed] [Google Scholar]
- 36.Jäger T, Eisenhardt A, Rübben H, Lümmen G. Does cigarette smoking influence the survival of patients with prostate cancer? [in German]. Urologe A. 2007;46(4):397-400. [DOI] [PubMed] [Google Scholar]
- 37.Tendulkar RD, Kattan MW, Yu C, et al. Development of a nomogram to predict for all-cause and cancer-specific mortality in high-risk prostate cancer treated by external beam radiotherapy and androgen deprivation. J Clin Oncol. 2013;31(15 suppl):e16004. doi: 10.1200/jco.2013.31.15_suppl.e16004 [DOI] [Google Scholar]
- 38.Bassett JC, Gore JL, Chi AC, et al. Impact of a bladder cancer diagnosis on smoking behavior. J Clin Oncol. 2012;30(15):1871-1878. [DOI] [PubMed] [Google Scholar]
- 39.Silva J, López-Huertas H, Cadillo-Chávez R, Sánchez-Ortiz R. Smoking associated with higher risk of pathologic upgrading in Hispanic men with low-risk prostate cancer who undergo surgery: implications for brachytherapy and active surveillance. J Urol. 2014;191(4):e473-e474. doi: 10.1016/j.juro.2014.02.1191 [DOI] [Google Scholar]
- 40.Oefelein MG, Resnick MI. Association of tobacco use with hormone refractory disease and survival of patients with prostate cancer. J Urol. 2004;171(6, pt 1):2281-2284. [DOI] [PubMed] [Google Scholar]
- 41.Moreira DM, Nickel JC, Gerber L, et al. Smoking Is associated with acute and chronic prostatic inflammation: results from the REDUCE Study. Cancer Prev Res (Phila). 2015;8(4):312-317. [DOI] [PubMed] [Google Scholar]
- 42.Lee PN, Hamling J. Systematic review of the relation between smokeless tobacco and cancer in Europe and North America. BMC Med. 2009;7:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Enokida H, Shiina H, Urakami S, et al. Smoking influences aberrant CpG hypermethylation of multiple genes in human prostate carcinoma. Cancer. 2006;106(1):79-86. [DOI] [PubMed] [Google Scholar]
- 44.Prueitt RL, Wallace TA, Glynn SA, et al. An immune-inflammation gene expression signature in prostate tumors of smokers. Cancer Res. 2016;76(5):1055-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Westmaas JL, Newton CC, Stevens VL, Flanders WD, Gapstur SM, Jacobs EJ. Does a recent cancer diagnosis predict smoking cessation? an analysis from a large prospective US cohort. J Clin Oncol. 2015;33(15):1647-1652. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. All Included Articles
eTable 2. Impact of Smoking Status on BCR
eTable 3. Impact of Cumulative Smoking and Smoking Cessation on BCR
eTable 4. Impact of Smoking Status on Metastasis
eTable 5. Impact of Smoking Status on CSM
eTable 6. Impact of Cumulative Smoking and Smoking Cessation on CSM
eFigure 1. Risk of Bias Assessment of Each Included Study with Summary (A) and Graph (B)
eFigure 2. Funnel Plots With Pseudo 95% CIs for Each Investigated Outcome