Key Points
Question
Is primary tumor location prognostic or predictive, according to BRAF, RAS, and microsatellite instability status, in patients with stage III colon cancer receiving adjuvant FOLFOX (folinic acid [leucovorin calcium], fluorouracil, and oxaliplatin) with or without cetuximab?
Findings
In this study of 1869 patients with tumor blocks of resected stage III colon cancer, for those patients with RAS mutant or BRAF mutant genotype, disease-free survival was better with right- vs left-sided tumors; for patients who had RAS and BRAF double wild type, disease-free survival was worse in those with right-sided tumors. No predictive effect of sidedness for cetuximab efficacy was found.
Meaning
The association between sidedness and disease recurrence varied between patients with RAS or BRAF wild type and those with a mutation; no beneficial effect of cetuximab on disease-free survival and overall survival in patients with left-sided tumors was seen in the adjuvant setting.
This post hoc analysis investigates the prognostic and predictive role of primary tumor location according to BRAF, RAS, and microsatellite instability status in patients with colon cancer.
Abstract
Importance
We know of no data on the prognostic value of primary tumor location (PTL) according to BRAF, RAS, and microsatellite instability (MSI) status in patients who have undergone resection for colon cancer (CC) and have been treated with current standard adjuvant chemotherapy.
Objective
To determine the prognostic and predictive value of PTL according to BRAF, RAS, and MSI status in patients with stage III CC receiving adjuvant treatment with FOLFOX (folinic acid [leucovorin calcium], fluorouracil, and oxaliplatin) with or without cetuximab.
Design, Setting, and Participants
This post hoc analysis included patients with available tumor blocks of resected stage III colon adenocarcinoma who participated in the Pan-European Trials in Alimentary Tract Cancer (PETACC)-8 phase 3 randomized trial. Among the 2559 patients who underwent randomization, 1900 were screened by next-generation sequencing, which showed that 1869 had full information concerning PTL. We categorized primary tumor site as located proximal (right) or distal (left) to the splenic flexure.
Main Outcomes and Measures
The associations between PTL (right- vs left-sided) and disease-free survival (DFS), survival after relapse (SAR), and overall survival (OS) were assessed by Cox models and adjusted for clinical and pathological features, treatment, and MSI, BRAF, and RAS status.
Results
Among the 1869 patients (1056 [57%] male; mean [SD] age, 59.4 [9.5] years) with full molecular data analyzed, 755 (40%) had a right-sided tumor, 164 (10%) had MSI, 942 (50%) had RAS mutations, and 212 (11%) had BRAF mutations. Right-sided tumor location was not prognostic for DFS in the whole population but was associated with a shorter SAR (hazard ratio [HR], 1.54; 95% CI, 1.23-1.93; P = .001) and OS (HR, 1.25; 95% CI, 1.02-1.54; P = .03). When looking at DFS in the different molecular subgroups, we found similar results for microsatellite-stable tumors and tumors with MSI; a better DFS in right-sided vs left-sided tumors in patients with RAS mutations (HR, 0.80; 95% CI, 0.64-1.00; P = .046); and a worse DFS in right-sided vs left-sided tumors in patients with RAS and BRAF double wild type (HR, 1.39; 95% CI, 1.01-1.92; P = .04). These results were found independently of the treatment received, and no beneficial effect of cetuximab on DFS or OS was observed in left-sided tumors.
Conclusions and Relevance
Although right-sided tumor location is associated with poor survival in patients with metastatic CC as previously reported, the association with disease recurrence appears to vary for patients with stage III CC and RAS or BRAF mutations vs those with double wild type.
Introduction
Biological and molecular factors such as microsatellite instability (MSI) and KRAS and BRAF mutational status have recently been proposed as prognostic factors in nonmetastatic colorectal cancers and may play a role as stratification factors in future adjuvant trials.1
While the prognostic value of primary tumor location (PTL) for overall survival (OS) in metastatic colorectal cancer (mCRC) seems clear and consistent in reports in recent decades, the prognostic impact of PTL for stage III nonmetastatic colorectal cancer remains unclear. Moreover, as many recent publications on PTL deal with patients with mCRC treated with anti–epidermal growth factor receptors, very few data sets report results in patients with RAS mutations. Finally, greater effectiveness of anti–epidermal growth factor receptors in left-sided tumors has also been suggested in patients with mCRC.2
We therefore examined the relationship between PTL and disease-free survival (DFS), OS, and survival after recurrence (SAR) in patients with stage III colon cancer (CC) who received adjuvant FOLFOX (folinic acid [leucovorin calcium], fluorouracil, and oxaliplatin) alone or combined with cetuximab. These data were then assessed according to MSI, RAS, and BRAF status and treatment received.
Methods
The Pan-European Trials in Alimentary Tract Cancer (PETACC-8) study was done in accordance with the Declaration of Helsinki (amended 2000) and the International Conference on Harmonization of Technical Requirements of Pharmaceuticals for Human Use (ICH) Note for Guidance on Good Clinical Practice and approved by the appropriate ethics committees. All patients analyzed gave their informed consent for translational research projects in addition to the informed consent given for the therapeutic trial. Patients with histologically proven stage III resected colon adenocarcinoma for the previously reported PETACC-8 trial were randomly assigned to receive 6 months of FOLFOX or FOLFOX plus cetuximab.3 We categorized PTL as on the right or the left of the splenic flexure.
Tumor samples were prospectively banked. Methods for MSI, KRAS, NRAS, and BRAF assessments were previously described.4,5
The DFS, OS, and SAR curves were estimated with the Kaplan-Meier method. Differences between groups of patients were analyzed using log-rank tests and Cox models with the SAS statistical software package version 9.4 (SAS Institute). A P value (1-sided) of less than .05 was considered significant.
Results
Study Population
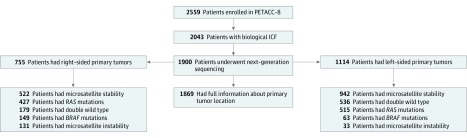
Of 2559 patients who underwent randomization, 1900 were screened by next-generation sequencing, which showed that 1869 patients had full information concerning PTL (Figure 1). Of those 1869 patients, 755 (40%) had right-sided tumors, 164 (10%) had MSI, 942 (50%) had RAS mutations, and 212 (11%) had BRAF mutations. Demographic and clinical characteristics of the patients in the molecular study (n = 1869) were not statistically different from those excluded from the molecular study (n = 690) (eTable 1 in the Supplement). All demographic and molecular characteristics according to sidedness are summarized in eTable 2 and eTable 3 in the Supplement.
Figure 1. Flow of Patients in PETACC-8 Trial Molecular Study .
Of 2559 patients who underwent randomization, 1900 were screened by next-generation sequencing, which showed that 1869 had full information concerning PTL. Of those 1869 patients, 755 (40%) had right-sided tumors, 164 (10%) had microsatellite instability, 942 (50%) had RAS mutations, and 212 (11%) had BRAF mutations.
Outcome in the Whole Population
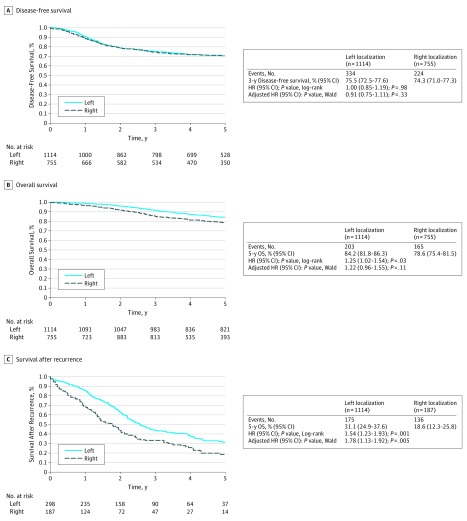
No difference was noted in DFS when comparing right-sided with left-sided stage III CC in the whole study population (Figure 2A). However, SAR (hazard ratio [HR], 1.54; 95% CI, 1.23-1.93; P = .001) and OS (HR, 1.25; 95% CI, 1.02-1.54; P = .03) were significantly better for left-sided tumors, and 5-year SAR and OS rates were 31.1% vs 18.5% and 84.2% vs 78.6% for left-sided vs right-sided tumors, respectively (Figure 2B and C).
Figure 2. Disease-Free Survival, Overall Survival, and Survival After Recurrence in the Whole Population According to Primary Tumor Location.
A, No difference was noted in disease-free survival when comparing right-sided with left-sided stage III colon cancer in the whole study population. B, Overall survival was significantly better for left-sided tumors. C, Survival after recurrence was significantly better for left-sided tumors. HR indicates hazard ratio.
Multivariable Analysis
In multivariable analysis, the following were associated with shorter DFS: histopathology grades 3 and 4; TNM categories pT3, pT4, and pN2; RAS mutations; Eastern Cooperative Oncology Group Performance Status (ECOG PS) 1 and 2; and bowel obstruction and/or perforation. Overall survival was also worse in patients with histopathology grades 3 and 4; TNM categories pT3, pT4, and pN2; RAS mutations; ECOG PS 1 and 2; and bowel obstruction and/or perforation. Moreover, patients with MSI tumors had better OS. Only right-sided, grade 3 or 4, pN2, and BRAF-mutated tumors were associated with shorter SAR (eTable 4 in the Supplement).
Outcome in Different Molecular Subgroups
All molecular subgroups’ outcomes are summarized in eTable 5 in the Supplement.
No difference in DFS was reported for right-sided vs left-sided tumors in microsatellite-stable or MSI tumors (eFigure 1 in the Supplement).
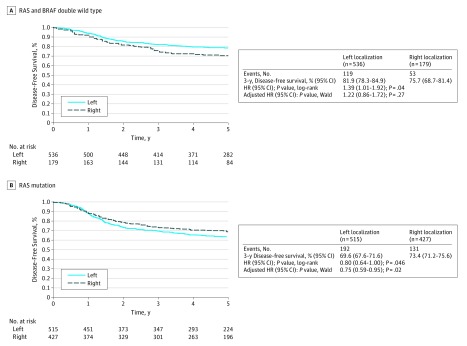
In patients with double wild-type genome, those with right-sided primary tumors had a shorter DFS compared with those with left-sided tumors (HR, 1.39; 95% CI, 1.01-1.92; P = .04), with 3-year DFS rates of 75.7% and 81.9%, respectively (Figure 3A). However, these results were not significant (HR, 1.22; 95% CI, 0.86-1.72; P = .26) when adjusted for histopathological grade, pT, pN, ECOG PS, and bowel obstruction and/or perforation.
Figure 3. Disease-Free Survival in Patients With RAS and BRAF Double Wild Type and RAS Mutations According to the Primary Tumor Location.
A, In patients with RAS and BRAF double wild type, those with right-sided primary tumors had a shorter disease-free survival compared with those with left-sided tumors. B, For RAS-mutated tumors, patients with right-sided primary tumors had a longer disease-free survival compared with those with left-sided tumors.
For RAS-mutated tumors, patients with right-sided primary tumors had a longer DFS compared with those with left-sided tumors (HR, 0.80; 95% CI, 0.64-1.00; P = .046), with 3-year DFS of 73.4% and 69.6%, respectively (Figure 3B). These results were still significant (HR, 0.75; 95% CI, 0.59-0.95; P = .02) when adjusted for histopathological grade, pT, pN, ECOG PS, and bowel obstruction and/or perforation in a multivariable model. The same trends were observed when looking at BRAF-mutated tumors (eTable 5 in the Supplement).
Treatment Outcomes in Right-Sided vs Left-Sided CC
When separately analyzing patients treated with FOLFOX alone or FOLFOX plus cetuximab, the association of PTL with DFS, OS, and SAR was comparable to that described in the general population (eFigure 2 in the Supplement).
In patients with double wild type, there was no statistical difference in DFS when comparing FOLFOX plus cetuximab vs FOLFOX alone in both right-sided tumors (HR, 0.89; 95% CI, 0.78-1.26; P = .94) and left-sided tumors (HR, 0.84; 95% CI, 0.59-1.21; P = .34) (eFigure 3 in the Supplement).
Discussion
These data describe the prognostic value of PTL in stage III CC treated with FOLFOX with full RAS, BRAF, and MSI assessment. We confirmed historical data that right-sided tumors were older and more likely to be poorly differentiated, exhibit vascular invasion or lymphatic infiltration, and have MSI and BRAF mutation.6,7 Similarly, at disease relapse, right-sided tumors had a worse prognosis. This result is similar in all molecular subgroups presently characterized and is in accordance with the currently published results of PTL prognostic value in the metastatic setting.2
However, when looking at disease recurrence, patients with RAS-mutated and/or BRAF-mutated right-sided tumors had a better DFS than left-sided tumors. Right-sided tumors remained of poor prognosis regarding DFS only in patients with double wild type.
Some recent studies demonstrated that PTL may be considered as a predictive factor in RAS wild-type mCRC treated with chemotherapy and cetuximab.2,8,9,10,11 In the present work, no benefit of adding cetuximab to FOLFOX was observed in our population of patients with stage III left-sided tumors; nor was any detrimental effect of adding cetuximab observed in right-sided tumors.
The major strengths of our study are that patients are coming from a randomized, prospective, registration-designed phase 3 trial; that all patients were treated with the current standard FOLFOX chemotherapy regimen (with or without cetuximab) in this setting; and that a full RAS and BRAF mutational profile using next-generation sequencing for all included patients (not limited to KRAS and BRAFV600E) was determined together with the MSI assessment.
Limitations
Our work also has limitations. The post hoc design of the present analysis and the limited number of patients with BRAF mutations and MSI tumors led to small subgroups for some of the analyses performed.
Conclusions
Although PTL does not seem to be associated with DFS in the whole study population, opposite sidedness prognostic values are observed for RAS and BRAF wild-type and mutant tumors. A larger analysis that uses all currently available adjuvant trials with complete molecular analysis and takes into account other prognostic molecular data from the consensus molecular subtypes12 is needed to confirm these first results and to better determine the molecular differences explaining them.
eTable 1. Demographic and clinical Characteristics - Analyzed Patients vs Not analyzed patients
eTable 2. Demographic and clinical characteristics according to the primary tumor location
eTable 3. RAS/BRAF mutations according to the primary tumor location
eTable 4. Multivariate Cox proportional hazards regression models for DFS, OS and SAR in the whole population
eTable 5. Unadjusted and Adjusted effect for the tumor for DFS, OS and SAR in the molecular subgroups population
eFigure 1. Kaplan-Meier curve for A) disease free-survival (DFS) in MSS patients according to the primary tumor location ; B) DFS in MSI patients according to the primary tumor location
eFigure 2. A) Kaplan-Meier curve for disease free-survival (DFS) in patients treated with FOLFOX ; B) Kaplan-Meier curve for DFS in patients treated with FOLFOX and cetuximab
eFigure 3. A) Kaplan-Meier curve for disease free-survival (DFS) in Double Wildtype patients treated with FOLFOX compared to FOLFOX and cetuximab in rightsided tumors ; B) Kaplan Meier curve for DFS in Double Wild-type patients treated with FOLFOX compared to FOLFOX and cetuximab in left-sided tumors.
eAppendix. Supplementary data, PETACC-8 investigators
References
- 1.Taieb J, Le Malicot K, Shi Q, et al. . Prognostic value of BRAF and KRAS mutations in MSI and MSS stage III colon cancer. J Natl Cancer Inst. 2016;109(5):. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold D, Lueza B, Douillard JY, et al. . Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol. 2017;28(8):1713-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taieb J, Tabernero J, Mini E, et al. ; PETACC-8 Study Investigators . Oxaliplatin, fluorouracil, and leucovorin with or without cetuximab in patients with resected stage III colon cancer (PETACC-8): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(8):862-873. [DOI] [PubMed] [Google Scholar]
- 4.Taieb J, Zaanan A, Le Malicot K, et al. . Prognostic effect of BRAF and KRAS mutations in patients with stage III colon cancer treated with leucovorin, fluorouracil, and oxaliplatin with or without cetuximab: a post hoc analysis of the PETACC-8 trial. [Published online January 14, 2016]. JAMA Oncol. doi: 10.1001/jamaoncol.2015.5225. [DOI] [PubMed] [Google Scholar]
- 5.Taieb J, Balogoun R, Le Malicot K, et al. ; PETACC8 Investigators . Adjuvant FOLFOX +/- cetuximab in full RAS and BRAF wildtype stage III colon cancer patients. Ann Oncol. 2017;28(4):824-830. [DOI] [PubMed] [Google Scholar]
- 6.Benedix F, Kube R, Meyer F, Schmidt U, Gastinger I, Lippert H; Colon/Rectum Carcinomas (Primary Tumor) Study Group . Comparison of 17,641 patients with right- and left-sided colon cancer: differences in epidemiology, perioperative course, histology, and survival. Dis Colon Rectum. 2010;53(1):57-64. [DOI] [PubMed] [Google Scholar]
- 7.Jess P, Hansen IO, Gamborg M, Jess T; Danish Colorectal Cancer Group . A nationwide Danish cohort study challenging the categorisation into right-sided and left-sided colon cancer. BMJ Open. 2013;3(5):e002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venook AP, Niedzwiecki D, Innocenti F, et al. . Impact of primary (1°) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): analysis of CALGB/SWOG 80405 (Alliance). J Clin Oncol. 2016;34(15)(suppl):3504. doi: 10.1200/JCO.2016.34.15_suppl.3504 [DOI] [Google Scholar]
- 9.Heinemann V, Modest DP, von Weikersthal LF, et al. . Gender and tumor location as predictors for efficacy: influence on endpoints in first-line treatment with FOLFIRI in combination with cetuximab or bevacizumab in the AIO KRK 0306 (FIRE3) trial. J Clin Oncol. 2014;32(15)(suppl):3600.doi: 10.1200/JCO.2014.32.15_suppl.360025135994 [DOI] [Google Scholar]
- 10.Douillard JY, Siena S, Cassidy J, et al. . Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28(31):4697-4705. [DOI] [PubMed] [Google Scholar]
- 11.Schwartzberg LS, Rivera F, Karthaus M, et al. . PEAK: a randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol. 2014;32(21):2240-2247. [DOI] [PubMed] [Google Scholar]
- 12.Guinney J, Dienstmann R, Wang X, et al. . The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21(11):1350-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Demographic and clinical Characteristics - Analyzed Patients vs Not analyzed patients
eTable 2. Demographic and clinical characteristics according to the primary tumor location
eTable 3. RAS/BRAF mutations according to the primary tumor location
eTable 4. Multivariate Cox proportional hazards regression models for DFS, OS and SAR in the whole population
eTable 5. Unadjusted and Adjusted effect for the tumor for DFS, OS and SAR in the molecular subgroups population
eFigure 1. Kaplan-Meier curve for A) disease free-survival (DFS) in MSS patients according to the primary tumor location ; B) DFS in MSI patients according to the primary tumor location
eFigure 2. A) Kaplan-Meier curve for disease free-survival (DFS) in patients treated with FOLFOX ; B) Kaplan-Meier curve for DFS in patients treated with FOLFOX and cetuximab
eFigure 3. A) Kaplan-Meier curve for disease free-survival (DFS) in Double Wildtype patients treated with FOLFOX compared to FOLFOX and cetuximab in rightsided tumors ; B) Kaplan Meier curve for DFS in Double Wild-type patients treated with FOLFOX compared to FOLFOX and cetuximab in left-sided tumors.
eAppendix. Supplementary data, PETACC-8 investigators