Key Points
Question
Do patients with moderate to severe obstructive sleep apnea have thinner calvaria and skull bases compared with those without obstructive sleep apnea?
Findings
In this cohort study, obstructive sleep apnea was independently associated with calvarial and skull base thinning compared with obese, age-matched patients without obstructive sleep apnea. The patients with obstructive sleep apnea had a higher rate of tegmen dehiscence than those without obstructive sleep apnea.
Meaning
Obstructive sleep apnea may mechanistically contribute to the development of disorders related to skull thinning, such as spontaneous cerebrospinal fluid leaks.
This cohort study examines the association of obstructive sleep apnea in adults with intracranial bone (calvaria and skull base) thickness.
Abstract
Importance
Spontaneous cerebrospinal fluid leaks (sCSF-L) of the temporal bone are associated with obesity, calvarial thinning, and obstructive sleep apnea (OSA), and the incidence has doubled in the past decade. It is currently unknown if OSA is independently associated with skull thinning.
Objective
To determine if patients with OSA have thinner skulls than patients without OSA.
Design, Setting, and Participants
A retrospective cohort study of patients who underwent a level 1 polysomnogram (PSG) and also had high-resolution computed tomographic (CT) imaging of the head from January 2010 to March 2017 at Indiana University was carried out. Patients with moderate to severe OSA (apnea-hypopnea index [AHI]≥25/h) and without OSA (AHI<5/h) were matched for age and body mass index (BMI, calculated as weight in kilograms divided by height in meters squared).
Interventions
Measurement of calvarial thickness, extracranial zygoma thickness, skull base height and tegmen dehiscence (>4 mm) when blinded to OSA status.
Main Outcomes and Measures
Primary outcomes were calvarial, skull base, and zygoma thickness differences between patients with OSA vs those without OSA.
Results
A total of 22 933 patients had a PSG and 1012 also had head CT imaging. Of the 1012 patients with both PSG and CT, the mean (SD) age was 50.8 (16.2) years and 624 (61.7%) were women. Those patients with moderate to severe OSA (56) and without OSA (58) were matched for mean (SD) age (50.3 [6.5] vs 49.8 [6.1] years]) and BMI (37.4 [8.1] vs 38.6 [6.8]). Patients with OSA had thinner mean (SD) calvaria (2.73 [0.67] vs 2.47 [0.52] mm; difference, −0.26 mm; 95% CI, −0.49 to −0.04; Cohen d, 0.44) and thinner skull bases (5.03 [1.40] vs 4.32 [1.28] mm; difference, −0.71; 95% CI, −1.23 to −0.19; Cohen d, 0.53). The mean (SD) extracranial zygoma thickness was the same (4.92 [0.87] vs 4.84 [0.84] mm; difference, −0.07 mm; 95% CI, −0.39 to 0.24). The tegmen mastoideum was dehiscent in nearly twice as many patients with OSA as those without (37% vs 20%; difference, 17%; 95% CI, 0.4-32).
Conclusions and Relevance
Obstructive sleep apnea was independently associated with intracranial bone (calvaria and skull base) thinning and not with extracranial (zygoma) thinning. These findings support a possible role of OSA in the pathophysiologic development of sCSF-L.
Introduction
Obstructive sleep apnea (OSA) is the most common sleep-related breathing disorder in adults.1 The estimated prevalence of moderate-to-severe OSA in the United States is 10% to 17% in men and 3% to 9% in women.2 The prevalence of OSA varies based on the defining criteria used and the population being studied.3 Obesity is the most common modifiable risk factor for OSA.2 Owing to rising obesity rates in the United States, the prevalence of OSA has risen by 14% to 55% over the past 2 decades.2 Polysomnography is the gold standard for diagnosing OSA. Obstructive sleep apnea is linked to multiple comorbid conditions,4 including cardiovascular disease,5 chronic fatigue,1 stroke,6 and increased all-cause mortality.7,8
Spontaneous cerebrospinal fluid leak (sCSF-L) occurs when there is a defect in the bone and dura of the skull base overlying pneumatized spaces. This process takes place in the absence of other causes such as tumor, trauma, or surgery.9 Spontaneous cerebrospinal fluid leak is associated with obesity, female sex, and middle age.9,10,11,12,13,14 Consistent with the rise in obesity in the United States, the rates of sCSF-L have more than doubled in the past decade.13
The exact mechanism of sCSF-L is still not well understood. It is known that patients with sCSF-L have isolated cranial bone defects of the skull base and global calvarial thinning without thinning of extracranial bones (zygoma).15 This suggests an intracranial process leads to skull thinning and the development of sCSF-L.15 Elevated intracranial pressure (ICP) on a constant or transient basis has been postulated to lead to skull thinning over time.10,11,13 Elevated ICP has been documented in some (approximately 36%) but not all patients with sCSF-L.10 In addition, OSA is associated with transient spikes in ICP during apnea episodes16 and the prevalence of OSA is 83% in patients with sCSF-L.17
Our study aimed to determine if OSA is independently associated with skull thinning by investigating the radiologic skull base and calvaria findings in non–sCSF-L patients with and without OSA based on formal PSG testing. To our knowledge, no studies have established a connection between OSA and skull thinning.
Methods
Patient Selection
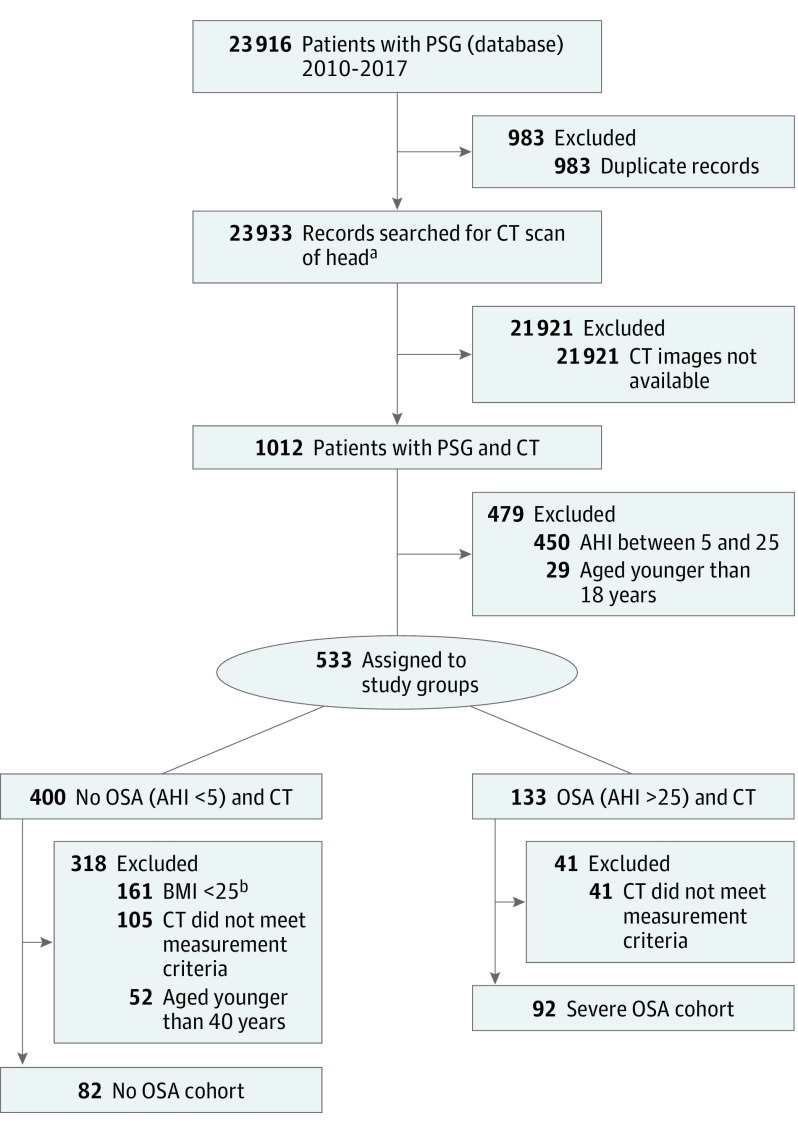
This study was approved by the institutional review board at Indiana University School of Medicine in Indianapolis (protocol 1702192365). Informed consent was waived by the board given the absence of patient-identifying information. The sleep disorders center database at Indiana University was queried for all patients who underwent a formal diagnostic polysomnogram (PSG) from January 1, 2010, to March 31, 2017 (22 933 unique patients). Patients who also had high-resolution (≤1-mm axial and coronal sections) CT of the head (inner ear, internal auditory canal, maxillofacial area/sinus, orbit, paranasal sinuses, petrous bone, and CT temporal bone) from January 1, 2001, to April 31, 2017, were evaluated (1012 patients) (Figure 1).
Figure 1. Patient Selection Flowchart.
Abbreviations: AHI, apnea-hypopnea index; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); OSA, obstructive sleep apnea; PSG, polysomnogram.
aPatients were identified by first searching the Sleep Disorders Database for all patients who underwent a PSG since 2010. There were 1012 patients with a PSG and a high-resolution computed tomographic (CT) scan of the head available for measurements. From these patients, 2 cohorts were isolated: A non-OSA cohort consisting of 82 patients, and a severe OSA cohort consisting of 92 patients.
bCalculated as weight in kilograms divided by height in meters squared.
Patients were divided into 2 groups based on apnea-hypopnea index (AHI): a non-OSA group (AHI<5), and a moderate to severe OSA group (AHI≥25). We chose this OSA group to obtain sufficient numbers of patients with the most severe degree of OSA, as we hypothesized that those with the highest AHI would have the largest possible effect on skull thinning compared with those without OSA. To adequately power the study we chose matched cohorts with AHI≥25. Patients with AHI of 5 to 24 or age younger than 18 years were excluded. In the non-OSA cohort, patients with a body mass index (BMI, calculated as weight in kilograms divided by height in meters squared) less than 25 and aged younger than 40 years were excluded to better match age and BMI between the groups. One patient with cranio-osteogenic pathology was excluded and 2 patients with sCSF-L were excluded (1 OSA and 1 non-OSA). These patients with sCSF-L, along with other patients with sCSF-L identified earlier in our patient selection process, were placed in a subgroup of their own for further analysis. For all patients, medical records were searched for medical history of smoking, diabetes, hypertension, and osteopenia to help match patients based on these comorbidities, which may be associated with bone thinning and obesity.8,18 In this initial patient selection process, our criteria yielded 82 patients in the non-OSA cohort and 92 patients in the OSA cohort (Figure 1).
Race data were obtained and assigned from the electronic medical record as documented during previous patient hospital visits; thus, the investigators had no role in classifying race. Owing to a relatively low number of black patients available for measurements and an unequal proportion of black patients in each subgroup, we could not isolate a well-matched group of black patients to adequately power a group comparison between OSA and non-OSA patients. As a result, black patients were excluded.
Our power calculation was based on our preliminary findings. We anticipated a mean (SD) difference in calvarial thickness of 0.3 (0.55) mm between patients with OSA vs those without OSA. The estimated sample size needed to achieve a power of 0.8 with an α of 0.05 for this comparison was 53 measurements per group. Accordingly, an appropriate number of white patients matched by age, sex, hemoglobin A1c levels, and BMI were then obtained for subgroup analysis (28 patients with OSA and 29 in patients without OSA).
Finally, we also isolated a group of sCSF-L patients for further comparison. These were patients from Indiana University who had presented for repair of sCSF-L in the past 3 years. These patients had PSG studies available and also had high-resolution CT scans of the head, and we aimed to compare findings between our OSA, non-OSA, and sCSF-L groups.
Radiologic Analysis and Measurements
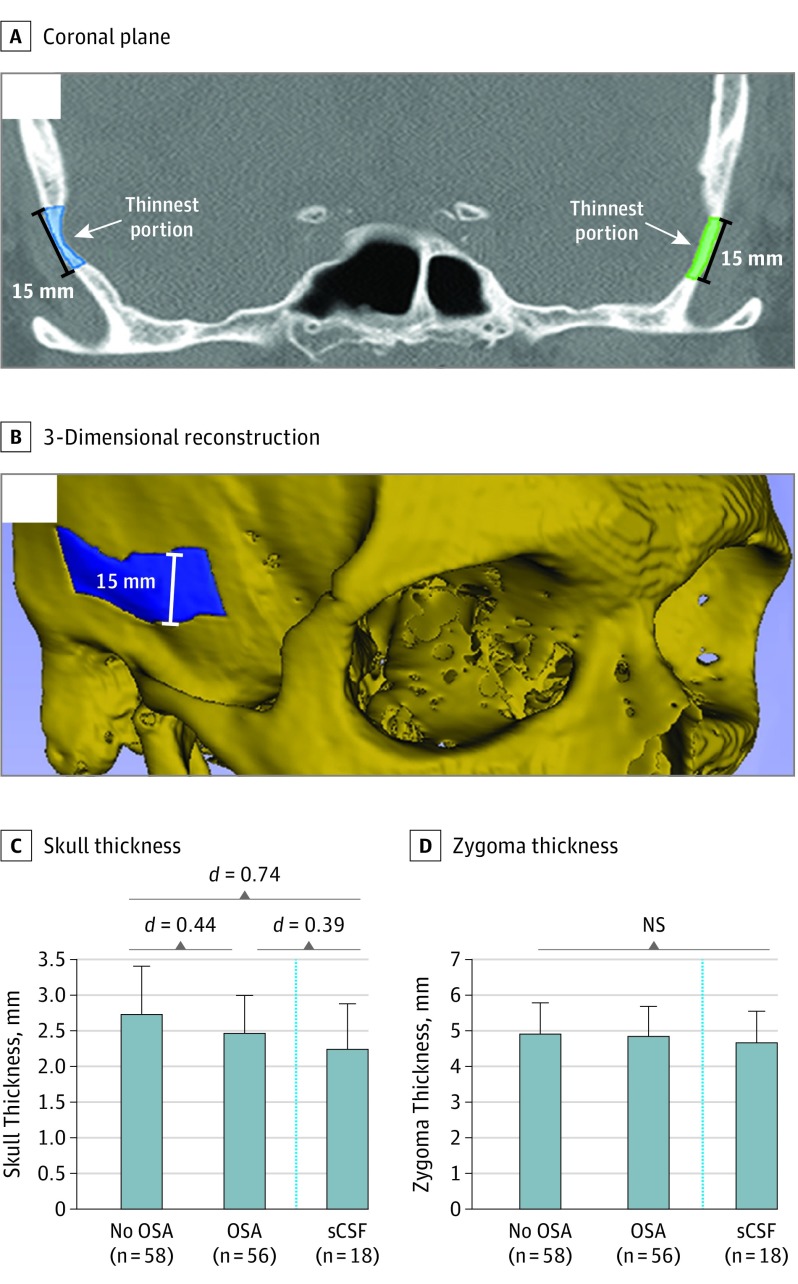
After our patient groups were determined, CT scans were obtained, deidentified, and randomized for analysis. Measurements were performed when blinded to OSA status. Measurements were performed using 3D Slicer (version 4.6.2, http://www.slicer.org) an open source, NIH-funded image analysis tool. We developed an accurate and consistent method of measuring calvarial and zygoma thickness (eMethods in the Supplement) (Figure 2A and B). Coronal imaging was used to determine the skull base thickness (height of the internal auditory canal) and the rate of tegmen dehiscence (eMethods in the Supplement). Zygoma thickness was used as an internal imaging control because the extracranial zygoma is not subject to intracranial forces. Measurements were performed and reported bilaterally, with n = number of measurements, yielding 2 measurements for each patient. A small number of CT scans did not extend posterior enough to adequately measure skull base height. Thus, the n for Figure 2 and Figure 3 differ slightly.
Figure 2. Calvarial and Zygoma Measurements in Non-OSA, OSA, and sCSF-L Patients.
Abbreviations: NS, not significant; OSA obstructive sleep apnea; sCSF, spontaneous cerebrospinal fluid leak. A, Measurements were taken in the coronal plane of a 15-mm (height) segment of the thinnest portion of the squamous temporal bone. Segments were highlighted bilaterally, starting at the level of the foramen rotundum anteriorly and extending posteriorly to the level of the upper extent of the superior semicircular canal. Volume was calculated using 3D Slicer’s volumetric analysis tool (version 4.6.2, http://www.slicer.org). B, A 3-Dimensional reconstruction illustrating the highlighted calvarial segment. C, Patients with OSA and patients with sCSF-L had statistically significantly thinner skulls than patients without OSA. Skull thickness was not statistically significantly different between patients with OSA and those with sCSF-L. D, There were no statistically significant differences in zygoma thickness between non-OSA, OSA, and sCSF-L patients. Dashed line indicates that patients with sCSF-L were not part of the original database search.
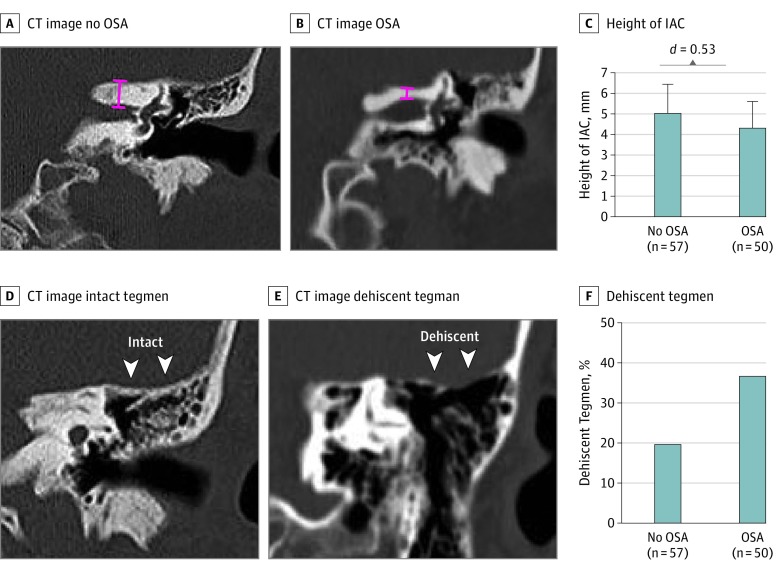
Figure 3. Skull Base Measurements in Non-OSA, OSA, and sCSF-L Patients.
Abbreviations: IAC, internal auditory canal; OSA, obstructive sleep apnea. A, Representative coronal computed tomographic (CT) image showing skull base (internal auditory canal) height measurement (red line) in a patient without OSA. B, Representative coronal CT showing skull base height measurement (red line) in a patient with OSA. C, Patients with OSA had significantly thinner skull base height compared with those without OSA. D, Representative coronal CT showing an intact tegmen mastoideum. E, Representative coronal CT showing a dehiscent tegmen mastoideum. F, Patients with OSA had nearly double the incidence of tegmen dehiscence compared with those who did not have OSA (37% vs 20%).
Statistical Analysis
Data was aggregated with Excel software (2013, Microsoft Corp). Statistical analysis was performed to determine the existence of significant differences between the measurements and demographic patient data obtained. All data analyses were carried out using SPSS statistical software (version 24, IBM Corp). Two-tailed independent samples t tests were applied. For all tests, 95% confidence intervals (CIs) were used to inform statistical and clinical significance. Cohen d was used as a measure of effect size where indicated. Based on Cohen d, we consider d = 0.2 to be a small effect, d = 0.5 to be moderate effect, and d≥0.8 to be large effect.
Results
Of the 23 916 patients first reviewed, 174 patients were initially included in this study based on the exclusion and inclusion criteria applied (Figure 1). Ninety-two patients were diagnosed with moderate to severe OSA and 82 did not have OSA based on PSG testing. These patients underwent calvarial and zygoma thickness measurements using 3D Slicer (Figure 2A and B). In this initial cohort of patients, our preliminary measurements suggested a difference in skull and skull-base thickness based on race, but owing to low patient numbers in the black subgroup, no definitive conclusions could be drawn. As a result, black patients were excluded from further analysis. As noted in the Methods section, we then sought to isolate a clinically relevant and well-matched cohort with enough patients to adequately power a group comparison between patients with OSA and those without OSA. Patients between the ages of 40 and 60 years were selected because they were the most well-matched, were the most common ages in the cohort, and were also clinically relevant given the high prevalence of sCSF-L in middle-aged patients.9
Our cohort of patients aged 40 to 60 years allowed for a total of 58 and 56 measurements in the non-OSA and OSA groups, respectively. The mean timeframe between PSG and CT imaging was less than 2 years and did not differ between groups (OSA, 21.7 months vs non-OSA, 20.9 months). The non-OSA and OSA groups were matched for mean (SD) age (49.8 [6.1] years vs 50.3 [6.5] years), average BMI (38.6 [6.8] vs 37.4 [8.1]), and average hemoglobin A1c levels (7.09% [2.22%] vs 7.03% [2.16%]) (Table). Sex did not have an effect on mean calvarial or zygoma thickness in this cohort. Compared with those without OSA, patients with OSA had significantly thinner calvaria (mean [SD], 2.73 [0.67] vs 2.47 [0.52] mm; difference, −0.26 mm; 95% CI, −0.49 to −0.04; Cohen d, 0.44) (Table) (Figure 2C). As an extracranial control, the zygoma thickness was not significantly different between the 2 groups (mean [SD], 4.92 [0.87] vs 4.85 [0.84] mm; difference, −0.07 mm; 95% CI, −0.39 to 0.24) (Table) (Figure 2D).
Table. Patient Demographics and Clinical Data.
| Characteristic | No OSA (N = 58) |
OSA (N = 56) |
Mean Difference (95% CI) | Cohen d |
|---|---|---|---|---|
| Age, mean (SD), y | 49.76 (6.14) | 50.29 (6.53) | 0.53 (−1.83 to 2.88) | 0.08 |
| BMI, mean (SD)a | 38.58 (6.78) | 37.39 (8.09) | −1.18 (−3.59 to 1.59) | 0.16 |
| Sex, No. (%) | ||||
| Male | 10/36 (27.8) | 26/36 (72.2) | ||
| Female | 48/78 (61.5) | 30/78 (38.5) | ||
| HbA1c, mean, (SD) | 6.85 (1.93) | 6.95 (1.99) | 0.10 (−0.72 to 0.92) | 0.05 |
| Calvarial thickness, mean (SD), mm | 2.73 (0.67) | 2.47 (0.52) | −0.26 (−0.49 to −0.04) | 0.44 |
| Skull base height, mean, (SD), mm | 5.03 (1.40) | 4.32 (1.28) | −0.71 (−1.23 to −0.19) | 0.53 |
| Zygoma thickness, mean, (SD), mm | 4.92 (0.87) | 4.84 (0.84) | −0.07 (−0.39 to 0.24) | 0.09 |
| Tegmen dehiscence, % | 20 | 37 | 17 (0.4 to 32) | NA |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HbA1c, hemoglobin A1c; OSA, obstructive sleep apnea.
Next, we sought to compare our findings with a cohort of temporal bone sCSF-L patients we had identified during our patient selection process, a cohort that is known to have tegmen dehiscence and skull thinning.15 Nine patients with sCSF-L (18 measurements) were white, between the ages of 40 and 60 years, with a statistically similar mean (SD) age of 52 (5.2) years, and a mean (SD) BMI of 35.1 (5.9). Patients with sCSF-L had significantly thinner calvaria than patients without OSA, with a 17.9% difference in thickness (mean [SD], 2.73 [0.67] vs 2.24 [0.64] mm; difference, −0.49; 95% CI, −0.97 to −0.01; Cohen d = 0.74) (Figure 2C). Calvarial thickness was not significantly different between patients with OSA and patients with sCSF-L (mean [SD], 2.47 [0.52] vs 2.24 [0.64] mm; difference, −0.23; 95% CI, −0.62 to 0.15; Cohen, d = 0.39) (Figure 2C). The extracranial zygoma thickness was not statistically significantly different between the 3 groups (non-OSA: 4.92 [0.87] vs OSA: 4.85 [0.84] vs sCSF: 4.66 [0.89] mm) (Figure 2D).
In addition to the calvaria, the skull base was also thinner in patients with OSA. Among patients aged 40 to 60 years, those with OSA had significantly thinner mean (SD) skull bases than those without OSA (5.03 [1.40] vs 4.32 [1.28] mm; difference, −0.71; 95% CI, −1.23 to −0.19; Cohen d = 0.53) (Figure 3A-C). The tegmen mastoideum was dehiscent in nearly twice as many of the patients with OSA as those without OSA (37% vs 20%; difference, 17%; 95% CI, 0.4%-32%) (Figure 3D-F).
Discussion
In obese middle-aged white patients, moderate to severe OSA has been independently associated with calvarial and skull base thinning. The CIs for OSA vs non-OSA calvaria and skull base differences are both relatively narrow, suggesting precision in the measurements. The Cohen d values for both calvarium (0.44) and skull base (0.53) demonstrate a moderate effect size. The upper limit of the 95% CI for skull base height was 1.23 mm, a clinically meaningful finding because skull base erosion and thinning to a magnitude of 1 mm may be sufficient to cause a sCSF-L in inherently thin portions of the skull base.13
The strength of this study includes the nonbiased approach to evaluate a cohort of patients with objective in-laboratory PSG testing and the use of a highly accurate quantification method for skull measurement. This study suggests that, with further research, we may develop the ability to risk stratify patients for risk of OSA based on head imaging, and this has broad implications for the tens of thousands of patients who have head CT imaging every year.
Patients with sCSF-L by definition have bony defects in the skull base and are typically obese (mean BMI, 38), female (72%), and middle-aged (45-65 years).12 An isolated intracranial process is hypothesized to mechanistically cause sCSF-L because these patients have global calvarial thinning with no thinning in extracranial bones.15 Chronically elevated or transient spikes in ICP theoretically could result in calvarial and skull base thinning.12
Some studies have proposed that this increased ICP may be caused by obesity,9 as a positive linear relationship has been shown between CSF pressure and BMI.19 However, skull thinning was not seen in a control group of obese patients without sCSF-L.15 These data suggest that other obesity-related factors are implicated in calvarial and skull base thinning.12 Importantly, elevated ICP has only been observed in 10% to 36% of patients with sCSF-L.10,20 In addition, OSA, which is strongly associated with obesity,18,21 has been shown to cause transient elevations in ICP during apneic episodes.16,22 A recent prospective study found that the prevalence of OSA among patients with sCSF-L is 83%.17
We investigated a cohort of patients who were obese, yet did not have sCSF-L, and discovered that patients with moderate to severe OSA have thinning of the squamous portion of the temporal bone and skull base compared with non-OSA patients, a relationship independent of age, BMI, sex, and multiple comorbidities. We also found that patients with sCSF-L had nearly 20% thinner calvaria than those without OSA, similar to previous studies showing a mean 22% thinning of the skull in patients with sCSF-L.15 We used the thickness of the squamous temporal bone as a surrogate for global calvarial thickness because it is not feasible to measure the entire calvarium.15 Our data suggest that an intracranial process in patients with moderate to severe OSA leads to skull and skull base thinning.
Overall, our study suggests that OSA may independently increase the risk of developing sCSF-L. We propose that OSA, when untreated for prolonged periods of time, plays a role in progressive calvarial and skull base thinning, and this continuous thinning may eventually be sufficient to cause CSF fistulae. Our data show that patients without OSA, patients with OSA, and patients with sCSF-L fall on a continuum of skull thinning (Figure 2C and D), while their zygoma thickness measurements are not statistically significantly different (Figure 2C and D), supporting our theory of progressive calvarial thinning. These findings add to the discussion regarding the association of obesity and OSA with sCSF-L.
Limitations
A potential limitation of this study is the small sample size in our final groups relative to our initial large number of patients. However, we would argue that this relatively small sample size was inevitable; we aimed to control for numerous variables to investigate the independent effect of OSA on calvarial and zygoma thickness, and to do so required strict criteria for patient inclusion. Thus, our study required us to begin with a very large number of patients to maintain adequate power during our exclusion steps, and we always intended to isolate a very select cohort of patients. In future studies, increasing our study sample size would increase the precision of the CIs.
Also, our study is limited by the exclusion of black patients. However, we believe that this step was necessary because we found a possible effect of race on calvarial and zygoma thickness in preliminary measurements, and we were not able to isolate a well-matched cohort of black patients with adequate power for further analysis. We feel that our study is as representative as possible and clinically relevant, given that our subgroup of white patients aged 40 to 60 years parallels the demographic known to be at highest risk of sCSF-L.9 Of note, our need to stratify our data by race highlights the need for future studies to delineate race when looking at differences in skull thickness between various groups. Future studies investigating the prevalence of sCSF-L in white and black populations would be valuable and should aim to include an adequate number of black patients to allow meaningful conclusions to be drawn about the impact of race on skull measurements in patients with OSA.
Our study also included different types of high-resolution CT scans with small inherent differences. However, we accounted for this by using a correction factor (eMethods in the Supplement) to augment the CT internal auditory canal (IAC) measurements, and this likely strengthened our data. By augmenting the CT IAC measurements, the predominant study ordered for patients with sCSF-L, we were able to compensate for any intrinsic aspect of the scan results that could yield a lower measurement in this subgroup. Our data highlights a need for future studies investigating skull thickness to account for intrinsic differences between CT scan results.
Another potential limitation of our study is our inability to account for other causes of skull thinning that are yet to be discovered. We controlled for all known variables including age, race, sex, BMI, and comorbidities, but some variables were not possible to account for. For example, it was not possible to measure ICP in patients owing to the retrospective nature of our study and the reluctance of patients to prospectively sign up for nonindicated lumbar punctures. Thus, we were not able to delineate the relationship between OSA and increased ICP in these study patients, and so we deferred to the available literature, which suggests that OSA causes transient spikes in ICP during sleep.16,22 The development of sCSF-L is likely a multifactorial process and we believe that OSA is an important factor among others.
Conclusions
By analyzing the results of CT scans of patients with and without OSA, we have found a significant relationship between OSA and calvarial and skull base thinning. Based on our results, OSA likely plays a role in the pathophysiology of skull base thinning and may contribute to the development of sCSF-L. Future studies are needed to identify the mechanism of how OSA may lead to skull thinning and how this may increase the risk of sCSF-L. Patients with skull thinning on CT imaging may be at increased risk of OSA and those patients should be considered for OSA screening using tools such as the STOP-Bang questionnaire. Future studies may reveal the utility of radiologic skull measurements as predictors of OSA. Future studies may also reveal other factors contributing to the development of sCSF-L.
eMethods.
References
- 1.Young T, Palta M, Dempsey J, Peppard PE, Nieto FJ, Hla KM. Burden of sleep apnea: rationale, design, and major findings of the Wisconsin Sleep Cohort Study. WMJ. 2009;108(5):246-249. [PMC free article] [PubMed] [Google Scholar]
- 2.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kapur VK, Auckley DH, Chowdhuri S, et al. . Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13(3):479-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291(16):2013-2016. [DOI] [PubMed] [Google Scholar]
- 5.Farrell PC, Richards G. Recognition and treatment of sleep-disordered breathing: an important component of chronic disease management. J Transl Med. 2017;15(1):114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson KG, Johnson DC. Frequency of sleep apnea in stroke and TIA patients: a meta-analysis. J Clin Sleep Med. 2010;6(2):131-137. [PMC free article] [PubMed] [Google Scholar]
- 7.Punjabi NM, Caffo BS, Goodwin JL, et al. . Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6(8):e1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bibbins-Domingo K, Grossman DC, Curry SJ, et al. ; US Preventive Services Task Force . Screening for obstructive sleep apnea in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2017;317(4):407-414. [DOI] [PubMed] [Google Scholar]
- 9.Wang EW, Vandergrift WA III, Schlosser RJ. Spontaneous CSF Leaks. Otolaryngol Clin North Am. 2011;44(4):845-856, vii. vii. [DOI] [PubMed] [Google Scholar]
- 10.Allen KP, Perez CL, Kutz JW, et al. . Elevated intracranial pressure in patients with spontaneous cerebrospinal fluid otorrhea. Laryngoscope. 2014;124(1):251-254. [DOI] [PubMed] [Google Scholar]
- 11.Fleischman GM, Ambrose EC, Rawal RB, et al. . Obstructive sleep apnea in patients undergoing endoscopic surgical repair of cerebrospinal fluid rhinorrhea. Laryngoscope. 2014;124(11):2645-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lobo BC, Baumanis MM, Nelson RF. Surgical repair of spontaneous cerebrospinal fluid (CSF) leaks: a systematic review. Laryngoscope Investig Otolaryngol. 2017;2(5):215-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson RF, Gantz BJ, Hansen MR. The rising incidence of spontaneous cerebrospinal fluid leaks in the United States and the association with obesity and obstructive sleep apnea. Otol Neurotol. 2015;36(3):476-480. [DOI] [PubMed] [Google Scholar]
- 14.Stucken EZ, Selesnick SH, Brown KD. The role of obesity in spontaneous temporal bone encephaloceles and CSF leak. Otol Neurotol. 2012;33(8):1412-1417. [DOI] [PubMed] [Google Scholar]
- 15.Nelson RF, Hansen KR, Gantz BJ, Hansen MR. Calvarium thinning in patients with spontaneous cerebrospinal fluid leak. Otol Neurotol. 2015;36(3):481-485. [DOI] [PubMed] [Google Scholar]
- 16.Jennum P, Børgesen SE. Intracranial pressure and obstructive sleep apnea. Chest. 1989;95(2):279-283. [DOI] [PubMed] [Google Scholar]
- 17.Rabbani CC, Saltagi MZ, Manchanda SK, et al. . Prevalence of obstructive sleep apnea (OSA) in spontaneous cerebrospinal fluid (CSF) leaks: a prospective observational study. Otol Neurotol. 2018. In press. [DOI] [PubMed] [Google Scholar]
- 18.Lee W, Nagubadi S, Kryger MH, Mokhlesi B. Epidemiology of obstructive sleep apnea: a population-based perspective. Expert Rev Respir Med. 2008;2(3):349-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berdahl JP, Fleischman D, Zaydlarova J, Stinnett S, Allingham RR, Fautsch MP. Body mass index has a linear relationship with cerebrospinal fluid pressure. Invest Ophthalmol Vis Sci. 2012;53(3):1422-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim L, Wisely CE, Dodson EE. Transmastoid approach to spontaneous temporal bone cerebrospinal fluid leaks: hearing improvement and success of repair. Otolaryngol Head Neck Surg. 2014;150(3):472-478. [DOI] [PubMed] [Google Scholar]
- 21.Li C, Ford ES, Zhao G, Croft JB, Balluz LS, Mokdad AH. Prevalence of self-reported clinically diagnosed sleep apnea according to obesity status in men and women: National Health and Nutrition Examination Survey, 2005-2006. Prev Med. 2010;51(1):18-23. [DOI] [PubMed] [Google Scholar]
- 22.Sugita Y, Iijima S, Teshima Y, et al. . Marked episodic elevation of cerebrospinal fluid pressure during nocturnal sleep in patients with sleep apnea hypersomnia syndrome. Electroencephalogr Clin Neurophysiol. 1985;60(3):214-219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.