Abstract
This study examines the association between oncologists’ receipt of payments from pharmaceutical manufacturers and drug selection in 2 situations where there are multiple treatment options.
Physicians and teaching hospitals in the United States receive approximately $7 billion from the pharmaceutical industry annually.1 These payments have been associated with higher-cost, brand-name pharmaceutical prescribing.2,3,4 Whether industry payments are associated with physician treatment choice in oncology is uncertain. We examined the association between oncologists’ receipt of payments from pharmaceutical manufacturers and drug selection in 2 situations where there are multiple treatment options.
Methods
We linked the Centers for Medicare & Medicaid Services Open Payments data and the Medicare Part D Prescriber Public Use File for calendar years 2013 and 2014. The study used the Open Payments categorizations of general payments (eg, gifts, consultancy/speaker fees, meals, and travel) and research payments (eg, preclinical research, US Food and Drug Administration phase 1-4 trials, or investigator-initiated studies). We linked Part D Prescriber data to Open Payments using the National Provider Identifier and practice location via the National Plan and Provider Enumeration System. This study was exempted from review by the University of North Carolina institutional review board as not constituting human participants research.
We considered on-patent drugs that were within the same therapeutic class; had US Food and Drug Administration approval and National Comprehensive Cancer Network recommendation for treatment of a cancer of a given site, stage, and degree of previous treatment; and were prescribed by at least 10 physicians in 2014. This resulted in the following 2 sets of drugs: sorafenib, sunitinib malate, and pazopanib hydrochloride (metastatic renal cell cancer [mRCC] group) and dasatinib, imatinib mesylate, and nilotinib hydrochloride monohydrate (chronic myeloid leukemia [CML] group).
We included physicians with a provider type of oncologist and at least 20 filled prescriptions among the respective 3 drugs in 2014. For each physician, we included all general payments from each drug manufacturer. We attributed research payments to physicians identified as principal investigators. Our primary exposure was payments received during 2013 (yes or no), and the primary outcome was prescriptions filled during 2014; we also analyzed payments as a continuous variable. We used the conditional logit model by McFadden to test whether receipt of payments from a manufacturer was associated with increased relative prescribing of that manufacturer’s drug within the choice set of multiple drugs. Separately, we evaluated drug-specific results using multivariable logistic regression, controlling for physician age, region, practice size, and prescribing volume. Separate models were estimated for general payments and research payments.
Results
Among 354 physicians who prescribed mRCC drugs and 2225 physicians who prescribed CML drugs, we found increased odds of prescribing a manufacturer’s drug among physicians receiving general payments only or either payment type (Table). Of physicians prescribing the drugs, 9.0% (32 of 354) of those prescribing for mRCC and 3.8% (38 of 2225) of those prescribing for CML received research payments in both 2013 and 2014, compared with 25.1% (89 of 354) and 39.5% (879 of 2225) for general payments, respectively. Receipt of research payments was associated with increased prescribing for mRCC but not CML. Similarly, when treating payments as a continuous variable, increasing amounts of general payments were associated with increased prescribing.
Table. Association Between Pharmaceutical Industry Payments Received in 2013 and Drug Choice in 2014a.
| Overall Association | Metastatic Renal Cell Cancer | Chronic Myeloid Leukemia | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | |
| No general payments or research payments in 2013 | 1 [Reference] | NA | 1 [Reference] | NA |
| Any general payments and/or research payments in 2013 | 1.84 (1.25-2.70) | .002 | 1.31 (1.14-1.48) | <.001 |
| No general payments in 2013 | 1 [Reference] | NA | 1 [Reference] | NA |
| Any general payments in 2013 | 2.05 (1.34-3.14) | .001 | 1.29 (1.13-1.47) | <.001 |
| No research payments in 2013 | 1 [Reference] | NA | 1 [Reference] | NA |
| Any research payments in 2013 | 1.84 (1.25-2.70) | .02 | 1.16 (0.89-1.53) | .27 |
| Continuous measure of general payments in 2013b | 1.23 (1.12-1.35) | <.001 | 1.05 (1.03-1.08) | <.001 |
| Continuous measure of research payments in 2013b | 1.07 (1.01-1.14) | .03 | 1.01 (0.99-1.04) | .24 |
Abbreviation: NA, not applicable.
Results reflect differences in prescribing aggregated across drugs within each drug group, accounting for payments made by each manufacturer. Comparisons are for physicians who received any payment from company X and their relative prescribing of drug X vs other drugs within each cancer type. The model was able to accommodate physicians who received payments from more than one of the relevant companies; they were treated as having experienced the primary exposure for each company from which they received payments. All results are adjusted for physician region, practice size, prescribing volume, and year of medical school graduation.
Because payment data are skewed, we used a natural log transformation for estimating the association between increasing US dollar amounts of payments and prescribing.
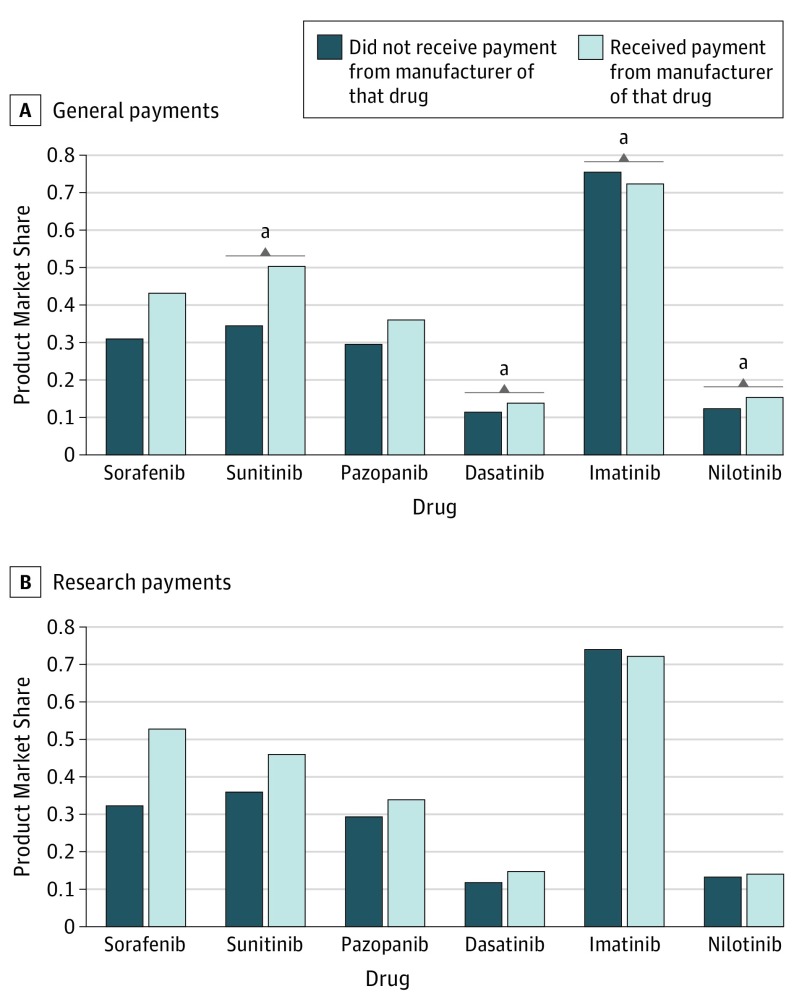
Considering individual drugs, we found increased prescribing when receiving vs not receiving general payments for sunitinib (50.5% vs 34.4%, P = .01), dasatinib (13.8% vs 11.4%, P = .02), and nilotinib (15.4% vs 12.5%, P = .01) (Figure) but found decreased prescribing of imatinib (72.4% vs 75.5%, P = .02). Differences for sorafenib and pazopanib were not statistically significant. Research payments were not associated with statistically significant differences in prescribing for any individual drug. Results were similar when including payments specifically attributed to the drug of interest rather than all payments from the corresponding manufacturer and when changing the exposure to receipt of payments in both 2013 and 2014 (vs 2013 without respect to 2014). Our study had some limitations. These include the observational design precluding causal assessment, potential inaccuracies with Open Payments data,5 lack of generalizability to other cancers, absence of information about the indications for the drugs, and small sample sizes for comparisons in the research payments analysis, notably for physicians receiving CML research payments.
Figure. Association Between Receipt of Pharmaceutical Industry Payments in 2013 and Drug Prescribing in 2014, by Individual Drug.
A and B, Multivariable logistic regression was used to estimate the association between receipt of manufacturer payments and physicians’ prescribing of that manufacturer’s drug. Each bar represents the market share for a specific drug (the probability of using the drug of interest relative to the other included treatments). Physicians who received payments from the manufacturer of the drug of interest are shown in light blue; physicians who did not are shown in dark blue. Results are adjusted for physician age, region, practice size, prescribing volume, and year of medical school graduation. Sunitinib was given as sunitinib malate; pazopanib as pazopanib hydrochloride; imatinib as imatinib mesylate; and nilotinib as nilotinib hydrochloride monohydrate.
aP < .05.
Conclusions
For 3 of the 6 cancer drugs studied, physicians who received general payments were more likely to prescribe the drug marketed by the company that made the payments. Imatinib was a notable exception; this may reflect a strategy by the manufacturer of imatinib (which also produces nilotinib) to promote switching to nilotinib before the patent expiration of imatinib in 2015.
References
- 1.Centers for Medicare & Medicaid Services The facts about Open Payments data. https://openpaymentsdata.cms.gov/summary. Published 2016. Accessed March 4, 2018.
- 2.Yeh JS, Franklin JM, Avorn J, Landon J, Kesselheim AS. Association of industry payments to physicians with the prescribing of brand-name statins in Massachusetts. JAMA Intern Med. 2016;176(6):763-768. [DOI] [PubMed] [Google Scholar]
- 3.DeJong C, Aguilar T, Tseng CW, Lin GA, Boscardin WJ, Dudley RA. Pharmaceutical industry–sponsored meals and physician prescribing patterns for Medicare beneficiaries. JAMA Intern Med. 2016;176(8):1114-1122. [DOI] [PubMed] [Google Scholar]
- 4.Perlis RH, Perlis CS. Physician payments from industry are associated with greater Medicare Part D prescribing costs. PLoS One. 2016;11(5):e0155474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ratain MJ. Forecasting unanticipated consequences of “The Sunshine Act”: mostly cloudy. J Clin Oncol. 2014;32(22):2293-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]