Key Points
Question
Does use of a smartphone application (app) improve self-reported adherence to antihypertensive medications and blood pressure control?
Findings
In this randomized clinical trial of 411 adults with poorly controlled hypertension, patients randomized to receive a smartphone app had a small improvement in self-reported medication adherence with no difference in blood pressure compared with controls.
Meaning
Use of a smartphone app resulted in a small improvement in self-reported medication adherence but did not affect blood pressure; the benefit of this and other stand-alone mobile health interventions on clinical outcomes remains to be established.
Abstract
Importance
Medication nonadherence accounts for up to half of uncontrolled hypertension. Smartphone applications (apps) that aim to improve adherence are widely available but have not been rigorously evaluated.
Objective
To determine if the Medisafe smartphone app improves self-reported medication adherence and blood pressure control.
Design, Setting, and Participants
This was a 2-arm, randomized clinical trial (Medication Adherence Improvement Support App For Engagement—Blood Pressure [MedISAFE-BP]). Participants were recruited through an online platform and were mailed a home blood pressure cuff to confirm eligibility and to provide follow-up measurements. Of 5577 participants who were screened, 412 completed consent, met inclusion criteria (confirmed uncontrolled hypertension, taking 1 to 3 antihypertensive medications), and were randomized in a ratio of 1:1 to intervention or control.
Interventions
Intervention arm participants were instructed to download and use the Medisafe app, which includes reminder alerts, adherence reports, and optional peer support.
Main Outcomes and Measures
Co–primary outcomes were change from baseline to 12 weeks in self-reported medication adherence, measured by the Morisky medication adherence scale (MMAS) (range, 0-8, with lower scores indicating lower adherence), and change in systolic blood pressure.
Results
Participants (n = 411; 209 in the intervention group and 202 controls) had a mean age of 52.0 years and mean body mass index, calculated as weight in kilograms divided by height in meters squared, of 35.5; 247 (60%) were female, and 103 (25%) were black. After 12 weeks, the mean (SD) score on the MMAS improved by 0.4 (1.5) among intervention participants and remained unchanged among controls (between-group difference: 0.4; 95% CI, 0.1-0.7; P = .01). The mean (SD) systolic blood pressure at baseline was 151.4 (9.0) mm Hg and 151.3 (9.4) mm Hg, among intervention and control participants, respectively. After 12 weeks, the mean (SD) systolic blood pressure decreased by 10.6 (16.0) mm Hg among intervention participants and 10.1 (15.4) mm Hg among controls (between-group difference: −0.5; 95% CI, −3.7 to 2.7; P = .78).
Conclusions and Relevance
Among individuals with poorly controlled hypertension, patients randomized to use a smartphone app had a small improvement in self-reported medication adherence but no change in systolic blood pressure compared with controls.
Trial Registration
clinicaltrials.gov Identifier: NCT02727543
This 2-arm randomized clinical trial of individuals with uncontrolled hypertension determined if the Medisafe smartphone app improves self-reported medication adherence and blood pressure control.
Introduction
Hypertension is estimated to affect 34% of US adults 20 years or older, accounting for more than 73 000 deaths each year.1 Among modifiable risk factors, eliminating uncontrolled hypertension is estimated to have the single greatest potential to reduce cardiovascular mortality in women, and to have an effect that is second only to smoking cessation in men.1 While many factors contribute to poorly controlled hypertension, nonadherence is thought to account for nearly half of all such cases.2,3 In fact, among patients classified as having “medication-resistant” hypertension, more than 50% are actually nonadherent to their prescribed medications.3
Mobile health (mHealth) interventions, such as smartphone applications (apps), have been advocated as promising strategies to assist in the self-management of hypertension4,5 and other chronic conditions.6 These tools have the potential to address nonadherence by providing reminders for medication taking and refilling, tracking biometric results, offering education, and facilitating social interactions that provide support and motivation.7 From 2012 to 2015 there was a 515% increase in adherence apps available for download,8 and there are an estimated 107 apps currently available for hypertension alone.9
While the existing literature demonstrates improvements in blood pressure from smartphone technology,5,10,11,12 these studies have been conducted in clinic-based settings and have mainly tested the use of mHealth strategies that aim to improve communication between patients and physicians in the context of established therapeutic relationships. In contrast, a 2015 survey found that only 20% of smartphone health app users had received a recommendation by a physician to download an app13 and that most patients download and use apps without the active participation of their physician. Accordingly, we sought to evaluate the association of medication adherence and blood pressure control with a “stand-alone” smartphone app among patients with poorly controlled hypertension.
Methods
Study Design
The Medication adherence Improvement Support App For Engagement—Blood Pressure (MedISAFE-BP) Trial was a randomized clinical trial of individuals with uncontrolled hypertension in the United States. Enrollment began on April 25, 2016, and was completed on September 16, 2016. The full details of the trial design have been published previously,14 and the study has been registered on clinicaltrials.gov (NCT02727543). See the Trial Protocol in Supplement 1. The trial was reviewed and approved by the Chesapeake institutional review board, and all patients provided written informed consent prior to enrollment. All participants were provided compensation of up to $150 for their time to participate in the study. The academic authors were responsible for scientific oversight, including the design of the protocol. Patient recruitment, screening, randomization, follow-up, and data management were performed by Evidation Health without any involvement from the study sponsor. The academic authors used an independent copy of the study database to analyze the results and vouch for their analytic accuracy and completeness as well as the fidelity of the report to the study protocol.
Participants
We included individuals ages 18 to 75 years with a systolic blood pressure of 140 mm Hg or greater receiving treatment with at least 1, but no more than 3, first-line antihypertensive medications (thiazide, calcium channel blocker, β-blocker, angiotensin-converting enzyme inhibitor, or angiotensin receptor blocker). To identify participants with uncomplicated essential hypertension, we excluded patients prescribed 4 or more antihypertensive medications, those on dialysis, or those whose blood pressure was greater than 180/120 mm Hg on home blood pressure confirmation (see the Patient Recruitment and Randomization subsection for additional details). We also excluded individuals receiving chemotherapy at the time of screening as well as patients who were unable to understand written English, did not have a smartphone with compatible operating system (iOS or Android), were already using a smartphone medication adherence app, or did not live in the United States with a valid mailing address.
Patient Recruitment and Randomization
Participants were recruited through online patient communities, social media, pertinent mobile apps, and targeted advertisements.14 Potential participants were directed to a study website to assess eligibility and to provide informed consent. Eligible participants completed a baseline survey consisting of demographics and cardiovascular comorbidities, the 8-item Morisky medication adherence scale (MMAS-8),15 the hypertension knowledge questionnaire,16 and the Consumer Health Activation Index to assess patient activation (Michael Wolf, PhD, email communication, November 25, 2015). The MMAS is a widely used tool for self-reported medication adherence that was found to be reliable (α = .83), significantly associated with blood pressure control (P < .05), and to have 93% sensitivity as well as 53% specificity for low adherence in a validation study.15
Potentially eligible individuals were then mailed a Bluetooth-enabled blood pressure monitor (UA-651 BLE; A & D Medical) as well as instructions on how to set up the monitor and properly take a blood pressure reading. The A & D 651 blood pressure cuff has been approved by blood pressure associations for its accuracy in home use.17 Study participants were asked to provide 2 measurements that were taken 5 minutes apart, in accordance with professional society guidelines,18 and blood pressure was calculated as the average of these measurements. Patients were instructed to perform all blood pressure measurements in a seated position and at least 30 minutes after smoking, eating, drinking caffeinated drinks, or physical activity. Because of the pragmatic nature of this study, acceptable readings were considered to be 2 measurements that were at least 3 minutes apart but not more than 30 minutes apart. If, on this assessment, an individual’s average systolic blood pressure was confirmed to be greater than 140 mm Hg, but also less than 180/120 mm Hg, they were enrolled and randomized in a ratio of 1:1 to the intervention or control through the online portal using a random number generator.
Study participants and study staff interacting with patients were not blinded to group assignment. The study investigators and data analysts remained blinded until all follow-up data were obtained and the primary analytic strategies were finalized.
Intervention
Participants randomized to the intervention arm were provided with instructions on how to download and use the Medisafe app. This app aims to help individuals adhere to their prescribed therapies and has received the highest usability rating among medication adherence smartphone apps in several reviews.8,19 There are several key features of the app. First, medication lists are entered manually, along with their preferred timing of administration, or are autopopulated through a linkage with an existing medication record. In those cases in which this integration has been established, the app provides alerts to remind patients when it is time for them to take medications and generates weekly adherence reports. Second, the app also allows for tracking of blood pressure and other biometric measurements, although in the current study, the Medisafe app did not automatically sync with the home blood pressure cuff or interface with medical professionals in any way. Finally, users can designate a “Medfriend” who is granted access to the patient’s medication taking history, receives alerts when doses are missed, and can provide peer support.
Intervention arm participants who did not download the app and have 1 login within 2 days of randomization were contacted by email and telephone.14 If, after multiple follow-up attempts, a participant still did not log in, they were not contacted further but were followed for outcomes and analyzed in the intent-to-treat analysis.
The control arm did not receive any intervention.
Follow-up Assessment
Follow-up assessments were performed for participants in both study arms at 4, 8, and 12 weeks after enrollment based on intention-to-treat principles. As such, outcomes were evaluated in all randomized individuals, including those in the intervention arm who did not download the app. Each assessment included blood pressure measurement using the study-provided blood pressure monitor.18 The final study assessment at 12 weeks also included an exit survey that measured adherence, hypertension knowledge, and patient activation.
Outcomes
The co–primary outcomes were change in self-reported medication adherence and systolic blood pressure from randomization to 12 weeks. The secondary outcome was whether participants had well-controlled blood pressure, defined as 140/90 mm Hg or less.20
Statistical Analysis
We sought to enroll at least 390 patients to have 80% power to detect a 5-mm Hg difference in systolic blood pressure between treatment arms, with an α of .05, even with a 20% loss to follow up or a standard deviation of up to 17 mm Hg. A decrease in systolic blood pressure by 5 mm Hg correlates with clinically meaningful reductions in coronary heart disease and stroke.21,22,23 This sample size also provided 87% power to detect a 0.5-point difference in adherence between the groups assuming a standard deviation of 1.6,15 a similar loss to follow-up, and an α of .05.
We conducted our analyses using intention-to-treat principles. Means and frequencies of baseline characteristics were evaluated for any between-arm differences despite randomization. As prespecified in the published protocol,14 the co–primary outcomes were analyzed using univariate linear regression models, and missing data were accounted for using multiple imputation with PROC MI using SAS statistical software (version 9.4; SAS Institute Inc), after the creation of 25 imputed data sets.24,25 The secondary outcome was analyzed using univariate logistic regression. We defined statistical significance as P < .05 and we did not adjust our P value threshold for our 2 co–primary outcomes, which we assumed would be correlated.26,27,28
In secondary analyses, we fitted multivariable models to adjust for the following baseline characteristics that have been associated with medication adherence and/or blood pressure control: sex, race/ethnicity, body mass index (BMI, calculated as weight in kilograms divided by height in meters squared), physical activity, education, cigarette use, patient activation, and baseline adherence. In sensitivity analyses, we repeated our analyses among those individuals for whom complete outcome data were available. We also evaluated longitudinal changes in blood pressure measurements at baseline and each of the subsequent 3 follow-up assessments using generalized estimating equations with an identity link function and autoregressive errors.
In subgroup analyses, we evaluated differential effects of the intervention on the co–primary outcomes with respect to sex, age, baseline blood pressure, baseline adherence, and baseline patient activation based on the statistical significance of the interaction term for the subgroup of interest in the multivariable model. Finally, adherence was analyzed with respect to movement between adherence categories based on the MMAS-8 score, which is how this scale was originally described.15 In specific, an MMAS-8 score of less than 6 was classified as “low” adherence, 6 or 7 was classified as “moderate” adherence, and 8 was classified as “high” adherence. All analyses were performed using SAS software (version 9.4).
Results
Participants
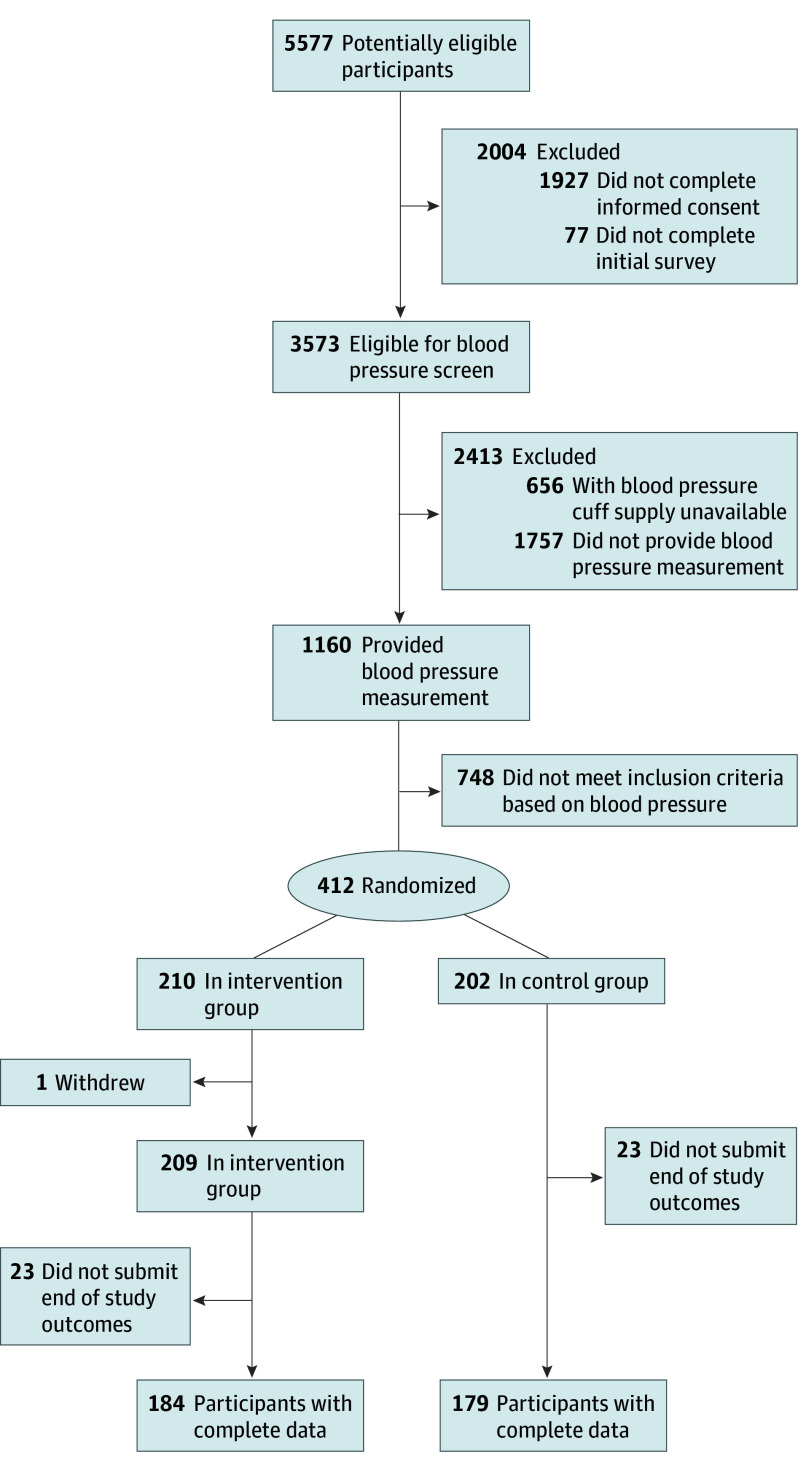
We screened 5577 individuals, of whom 1160 met eligibility criteria and provided a home blood pressure measurement (Figure 1). A total of 412 participants with poorly controlled blood pressure were randomized, 210 to the intervention arm and 202 to the control arm. One person withdrew from the intervention arm. Therefore, the intention-to-treat analysis included 209 patients in the intervention arm and 202 controls.
Figure 1. CONSORT Diagram.
Baseline Characteristics
Participants were located across the United States in a mix of rural, suburban, and urban locations. Participants had a mean age of 52.0 years and a mean BMI of 35.5; 247 (60%) were female, and 103 (25%) were of black race/ethnicity. The baseline characteristics of the intervention arm were generally similar to those of the controls (Table 1). Sex, race/ethnicity, BMI, and baseline adherence were well balanced between the 2 arms. Intervention participants were more likely than controls to report being white (71.3% compared with 58.9%; P = .03), currently use cigarettes (18.7% compared with 10.9%; P = .03), and to have dyslipidemia (48.8% compared with 36.6%; P = .01). Among the intervention arm, 188 (90%) downloaded and launched the smartphone app.
Table 1. Baseline Characteristics by Treatment Group.
| Characteristic | Intervention (n = 209) | Control (n = 202) | P Value |
|---|---|---|---|
| Age, mean (SD), y | 51.7 (10.5) | 52.4 (10.1) | .51 |
| Female, No. (%) | 120 (57.4) | 127 (62.9) | .26 |
| Race/ethnicity, No. (%) | .03 | ||
| Black | 43 (20.6) | 60 (29.7) | |
| White | 149 (71.3) | 119 (58.9) | |
| Other | 17 (8.1) | 23 (11.4) | |
| BMI, mean (SD) | 35.38 (7.9) | 35.59 (8.6) | .79 |
| Physical activity, No. (%) | .49 | ||
| ≤2 h/wk | 127 (60.8) | 116 (57.4) | |
| >2 h/wk | 82 (39.2) | 86 (42.6) | |
| Education, No. (%) | .49 | ||
| Did not finish high school | 3 (1.4) | 5 (2.5) | |
| High school graduate | 31 (14.8) | 20 (9.9) | |
| Some college | 46 (22.0) | 56 (27.7) | |
| College graduate | 73 (34.9) | 68 (33.7) | |
| Vocational degree | 19 (9.1) | 22 (10.9) | |
| Graduate degree | 37 (17.7) | 31 (15.4) | |
| Current cigarette use, No. (%) | 39 (18.7) | 22 (10.9) | .03 |
| Comorbidities, No. (%) | |||
| History of heart attack | 3 (1.4) | 6 (3.0) | .33 |
| History of stroke | 11 (5.3) | 7 (3.5) | .37 |
| Diabetes | 46 (22.0) | 50 (24.8) | .51 |
| Dyslipidemia | 102 (48.8) | 74 (36.6) | .01 |
| Total prescription medications, No. (%) | 4.0 (2.6) | 4.0 (2.7) | .98 |
| Patient activation, No. (%)a | .25 | ||
| Low | 165 (80.5) | 150 (75.8) | |
| Moderate/high | 40 (19.5) | 48 (24.2) | |
| Phone attitude, No. (%) | .78 | ||
| Can’t live without phone | 159 (76.1) | 156 (77.2) | |
| Phone not always needed | 50 (23.9) | 46 (22.8) |
Abbreviation: BMI, body mass index, calculated as weight in kilograms divided by height in meters squared.
Measured by the Consumer Health Activation Index
Medication Adherence
At baseline, mean (SD) adherence as measured by the MMAS-8 was 6.0 (1.8) among the intervention arm and 5.7 (1.8) among controls. By 12 weeks of follow-up, the mean (SD) adherence increased by 0.4 (1.5) in the intervention arm and remained unchanged among controls (between-group difference, 0.4; 95% CI, 0.1-0.7; P = .01) (Table 2). The results remained unchanged after adjustment for differences in baseline characteristics in secondary analyses and in the complete case analysis (eTable in Supplement 2).
Table 2. Primary and Secondary Outcomesa.
| Variable | Intervention Group (n = 209) | Control Group (n = 202) | Unadjusted Effect Estimate | Adjusted Effect Estimate | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Wk 0 | Wk 12 | Change | Wk 0 | Wk 12 | Change | Absolute Difference | P Value | Absolute Difference | P Value | |
| Primary outcomes | ||||||||||
| Medication adherence,b mean (SD) | 6.0 (1.8) | 6.3 (1.6) | 0.4 (1.5) | 5.7 (1.8) | 5.7 (1.8) | −0.01 (1.5) | 0.4 (0.1 to 0.7) | .01 | 0.5 (0.2 to 0.7) | .001 |
| Systolic BP, mm Hg, mean (SD) | 151.4 (9.0) | 140.8 (15.7) | −10.6 (16.0) | 151.3 (9.4) | 141.2 (17.3) | −10.1 (15.4) | −0.5 (−3.7 to 2.7) | .78 | −0.1 (−3.2 to 3.1) | .97 |
| Secondary outcome | ||||||||||
| Controlled BP,c No. (%) | 0 | 67 (35.8) | 35.8 | 0 | 69 (37.9) | 37.9 | OR, 0.9 (95% CI, 0.6 to 1.4) | .68 | OR, 0.8 (95% CI, 0.5 to 1.3) | .34 |
Abbreviations: BP, blood pressure; OR, odds ratio.
The results presented herein reflect the primary analyses of the trial outcomes, which were conducted after multiple imputation. The adjusted models controlled for: sex, race/ethnicity, body mass index, physical activity, education, cigarette use, patient activation, and baseline adherence.
Measured by 8-item Morisky Medication Adherence Scale.
Defined as BP <140/90 mm Hg.
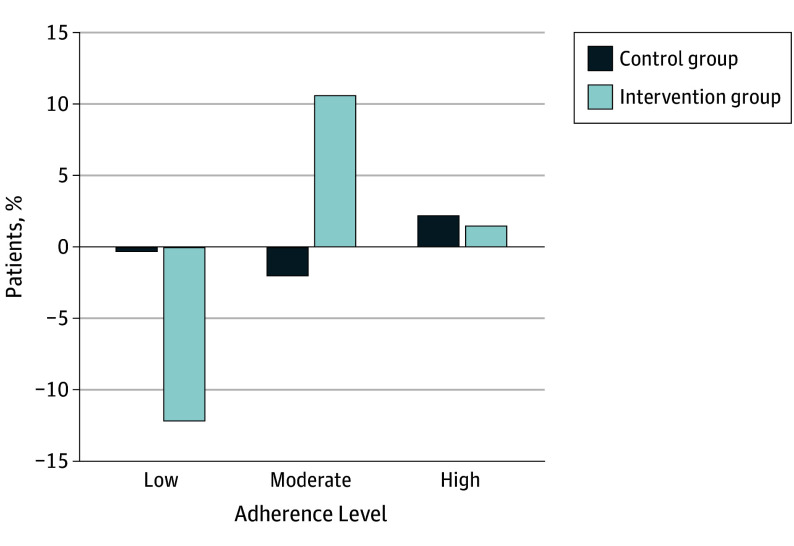
Subgroup analyses of the association of the intervention with adherence by sex, age, baseline systolic blood pressure, baseline adherence, and activation showed no significant between-group differences, although the magnitude of the improvement in adherence was largest for participants reporting low baseline adherence (Table 3). Consistent with this, the overall improvement in adherence from the intervention resulted from a reduction in the proportion of patients with low levels of adherence and a commensurate rise in the proportion of individuals with moderate adherence (Figure 2).
Table 3. Subgroup Analyses of the Difference Between Intervention and Control in Adherence and Blood Pressure From Baseline to 12 Weeksa.
| Subgroup | Adherence Difference Between Intervention and Control Groups (95% CI) | Interaction P Value | SBP Difference Between Intervention and Control Groups (95% CI) | Interaction P Value |
|---|---|---|---|---|
| Sex | .23 | .47 | ||
| Female | 0.54 (0.12 to 0.96) | 1.53 (−2.46 to 5.51) | ||
| Male | 0.15 (−0.29 to 0.59) | −0.91 (−6.29 to 4.47) | ||
| Age | .66 | .31 | ||
| At or below median | 0.30 (−0.14 to 0.74) | −1.32 (−5.83 to 3.20) | ||
| Above median | 0.44 (0.01 to 0.88) | 2.02 (−2.60 to 6.64) | ||
| Baseline SBP | .68 | .17 | ||
| <160 mm Hg | 0.41 (0.07 to 0.75) | −0.48 (−3.92 to 2.97) | ||
| ≥160 mm Hg | 0.25 (−0.45 to 0.94) | 5.31 (−2.92 to 13.54) | ||
| Adherenceb | .07 | .73 | ||
| Poor: <6 | 0.70 (0.24 to 1.15) | 1.63 (−2.97 to 6.22) | ||
| Moderate: ≥6 and <8 | 0.29 (−0.21 to 0.79) | −1.50 (−7.44 to 4.44) | ||
| Good: 8 | 0.03 (−0.43 to 0.49) | 0.74 (−6.14 to 7.62) | ||
| Activationc | .71 | .46 | ||
| Poor: <80 | 0.34 (−0.01 to 0.69) | −0.27 (−4.04 to 3.50) | ||
| Moderate/good: ≥80 | 0.48 (−0.17 to 1.13) | 2.70 (−3.63 to 9.03) |
Abbreviation: SBP, systolic blood pressure.
These subgroup analyses were conducted after multiple imputation and without any adjustment for baseline characteristics. The presented numbers reflect the difference between intervention and control groups in outcomes from baseline to 12 weeks. As a result, positive numbers reflect differences that were larger among intervention group than controls and negative numbers reflect differences that were smaller among the intervention group than controls.
Adherence was measured by the 8-item Morisky Medication Adherence Scale.
Activation was measured by the Consumer Health Activation Index.
Figure 2. Change in Adherence Category From Baseline to 12 Weeks of Follow-up.
Blood Pressure
At baseline, the mean (SD) systolic blood pressure was 151.4 (9.0) mm Hg in the intervention arm and 151.3 (9.4) mm Hg among controls. After 12 weeks of follow-up, the mean (SD) systolic blood pressure decreased by 10.6 (16.0) mm Hg in the intervention group and by 10.1 (15.4) mm Hg in the control group (Table 2). There was no difference in the change in blood pressure between the groups (between-group difference, 0.5 mm Hg; 95% CI, −3.7 to 2.7 mm Hg; P = .78). Good blood pressure control (defined as blood pressure <140/90 mm Hg) at 12 weeks was achieved by 35.8% in the intervention group and 37.9% in the control group (odds ratio, 0.9; 95% CI, 0.6-1.4; P = .68). Analyses of the 2 blood pressure outcomes remained unchanged after multivariable adjustment in secondary analyses and in complete case analysis (see eTable in Supplement 2). Changes in blood pressure over time are shown in the eFigure in Supplement 2. A longitudinal model of blood pressure over time had an effect estimate of -0.5 mm Hg per month (95% CI, −3.2 to 2.1 mm Hg; P = .70).
Subgroup analyses of the association of the intervention with systolic blood pressure by sex, age, baseline systolic blood pressure, baseline adherence, and patient activation showed no significant between-group differences (Table 3).
Discussion
Among those with poorly controlled hypertension, patients randomized to the use of a stand-alone smartphone app had a small improvement in self-reported medication adherence but no change in systolic blood pressure at 12 weeks of follow-up compared with controls.
There are several potential explanations for finding a small improvement in self-reported medication adherence without corresponding reductions in blood pressure. Readings from home blood pressure monitoring devices were used to determine trial eligibility and evaluate outcomes. It is possible that the reductions in blood pressure from baseline to the end of follow-up that we observed in both the intervention and control arms may have resulted from fluctuations in these home blood pressure readings29 and/or regression to the mean, and that the magnitude of these changes was larger than the a priori hypothesized effect from the smartphone app. Furthermore, patients in both arms were required to submit home blood pressure readings periodically, which was done a median of 10 and 9 times by the intervention and control groups, respectively, over the 12 weeks of study follow-up. Thus, all patients were engaged in some level of self-monitoring. This has shown to have small positive effects on blood pressure control30 and medication adherence31 and may have been particularly motivating for the participants in our trial, who were a relatively small proportion of those who underwent initial screening for inclusion.
Alternatively, while we observed a statistically significant improvement in adherence from the intervention, the magnitude of this change was likely too small to translate into improvements in blood pressure. A change of 2 points in the MMAS-8 has been suggested as the minimum detectable difference for antihypertensive medication adherence,32 which is substantially larger than the mean 0.4-point change that we observed. Similarly, it seems that patients must be highly adherent to their prescribed antihypertensive medications to derive clinical benefit.33,34 In contrast, the improvement in adherence that we observed resulted primarily from patients with low levels of adherence at baseline becoming moderately adherent by the end of follow-up, with little difference between intervention and controls in the proportion of patients who were highly adherent. If becoming highly adherent from the intervention takes more time than the 12-week duration of our trial, it is possible that we would have observed larger adherence improvements and corresponding changes in blood pressure with longer follow-up. Finally, medication adherence was measured by self-report. While the tool we used has been validated and extensively used,15 self-reported questionnaires are subject to social desirability bias and may overestimate true adherence.35 As such, after exposure to an app that very clearly encouraged adherence, intervention arm participants may have been more likely to report being adherent without actually changing their medication-taking behavior.
How then could use of a smartphone app be enhanced to result in greater benefits for patients with hypertension? While the app we tested has received very high usability scores,8,19 it may be that individuals with hypertension have needs that are different from those of individuals with other conditions. Therefore, one solution is to offer more disease-specific customization of smartphone tools. An alternative idea is to link the app to clinical care. Several studies have shown greater effects on clinical outcomes when home self-monitoring of blood pressure is linked with additional support, mostly through connection to health care professionals.30,36 In keeping with this, prior trials of text messaging for individuals with hypertension had positive results compared with control.10,37 In these trials, participants were enrolled from a clinical setting, and therefore patients likely associated the messages they received with their primary care physicians, potentially leading to greater effect. Similarly, the only randomized clinical trial of an mHealth app for patients with hypertension relied heavily on nurse health coaches to provide treatment recommendations.12 In that study, Moore et al12 evaluated the use of CollaboRhythm, an interface that allows tracking of medications and pairs a patient with a coach to offer recommendations and reminders. After 12 weeks, those in the intervention arm decreased their blood pressure by 10 mm Hg more than the control group. It is important to note, however, that using apps in the context of clinical care and relying on physicians to interact with patients for them to work is resource intensive and undercuts the efficiencies of stand-alone tools to aid patients in self-management.
Limitations
There are several limitations of this trial. Recruitment was performed entirely using online mechanisms. While it is estimated that more than 50% of patients use the internet for medical information,38 the results of our trial may not be generalizable to other populations of individuals with poorly controlled hypertension, who may have different sociodemographic and comorbidity characteristics than the patients in our study participants. Furthermore, because home blood pressure measurements tend to be lower than those obtained by health care professionals,39 our study population may, in fact, have had less well-controlled hypertension than patients for whom the same inclusion criteria had been applied in an office-based setting. In addition, we excluded those with extremely high blood pressure, for which immediate medical attention is recommended, and therefore this intervention may not be applicable to populations with blood pressures greater than 180/120 mm Hg. Many patients with this level of blood pressure elevation require careful medical supervision and therefore reliance on a stand-alone smartphone app may not be prudent. We also used home blood pressure monitors to evaluate our study outcomes, and these results were not independently verified for pragmatic reasons and to avoid the potential cointervention that would likely result from obtaining such external validation. Although patients were instructed on how to perform these measurements following guidance from the US Preventive Services Task Force, an organization that also endorses home blood pressure readings as a valid alternative to office-based assessments,18 any inaccuracies in these measurements would bias our results to the null. Our results are also subject to the possibility of contamination. In the survey that participants completed at the end of follow-up, 17 individuals in the control group (8.4%) reported downloading the Medisafe app during the course of the trial, even though the name of the app being evaluated was not mentioned in the patient consent form. Conversely, as many as 10% of individuals in the intervention group did not launch the app and thus could not have benefited from it. Because we followed intention-to-treat principles, both of these factors would bias our results to the null. The intervention lasted for 12 weeks; therefore, we will be unable to determine the effect of the smartphone app on longer-term outcomes, including stroke or myocardial infarction. Finally, we tested a single app, Medisafe, and our results may not generalize to other smartphone-based interventions.
Conclusions
The availability of smartphone health apps has expanded quickly, with a recent study showing 160 medication adherence–specific health apps.40 However, there has been a lack of rigorous evaluation to date, with most studies relying on self-report and not including a clinically important outcome.7,41 The MedISAFE-BP trial is, to our knowledge, the first randomized clinical trial reporting the effect of a stand-alone mHealth platform to increase medication adherence and improve blood pressure control. We found significant improvement in medication adherence, but no difference in systolic blood pressure between the intervention and control groups.
Trial protocol
eTable. Trial Outcomes Assessed Among Patients with Complete Data Only
eFigure. Blood Pressure Change Over Time by Treatment Group
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart Disease and Stroke Statistics-2017 Update: a report from the American Heart Association. Circulation. 2017;135(10):e146-e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Booth JN III, Muntner P, Abdalla M, et al. Differences in night-time and daytime ambulatory blood pressure when diurnal periods are defined by self-report, fixed-times, and actigraphy: Improving the Detection of Hypertension study. J Hypertens. 2016;34(2):235-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Oliveira-Filho AD, Costa FA, Neves SJ, de Lyra Junior DP, Morisky DE. Pseudoresistant hypertension due to poor medication adherence. Int J Cardiol. 2014;172(2):e309-e310. [DOI] [PubMed] [Google Scholar]
- 4.Rehman H, Kamal AK, Morris PB, Sayani S, Merchant AT, Virani SS. Mobile Health (mHealth) technology for the management of hypertension and hyperlipidemia: slow start but loads of potential. Curr Atheroscler Rep. 2017;19(3):12. [DOI] [PubMed] [Google Scholar]
- 5.Buis L, Hirzel L, Dawood RM, et al. Text messaging to improve hypertension medication adherence in African Americans from primary care and emergency department settings: results from two randomized feasibility studies. JMIR Mhealth Uhealth. 2017;5(2):e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Jongh T, Gurol-Urganci I, Vodopivec-Jamsek V, Car J, Atun R. Mobile phone messaging for facilitating self-management of long-term illnesses. Cochrane Database Syst Rev. 2012;12:CD007459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dayer L, Heldenbrand S, Anderson P, Gubbins PO, Martin BC. Smartphone medication adherence apps: potential benefits to patients and providers. J Am Pharm Assoc (2003). 2013;53(2):172-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dayer LE, Shilling R, Van Valkenburg M, et al. Assessing the medication adherence app marketplace from the health professional and consumer vantage points. JMIR Mhealth Uhealth. 2017;5(4):e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar N, Khunger M, Gupta A, Garg N. A content analysis of smartphone-based applications for hypertension management. J Am Soc Hypertens. 2015;9(2):130-136. [DOI] [PubMed] [Google Scholar]
- 10.Chow CK, Redfern J, Hillis GS, et al. Effect of lifestyle-focused text messaging on risk factor modification in patients with coronary heart disease: a randomized clinical trial. JAMA. 2015;314(12):1255-1263. [DOI] [PubMed] [Google Scholar]
- 11.Thakkar J, Kurup R, Laba TL, et al. Mobile telephone text messaging for medication adherence in chronic disease: a meta-analysis. JAMA Intern Med. 2016;176(3):340-349. [DOI] [PubMed] [Google Scholar]
- 12.Moore JO, Marshall MA, Judge DC, et al. Technology-supported apprenticeship in the management of hypertension: a randomized controlled trial. J Clin Outcomes Manag. 2014;21(3):110-122. [Google Scholar]
- 13.Krebs P, Duncan DT. Health app use among US mobile phone owners: a national survey. JMIR Mhealth Uhealth. 2015;3(4):e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morawski K, Ghazinouri R, Krumme A, et al. Rationale and design of the Medication adherence Improvement Support App For Engagement-Blood Pressure (MedISAFE-BP) trial. Am Heart J. 2017;186:40-47. [DOI] [PubMed] [Google Scholar]
- 15.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich). 2008;10(5):348-354. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Oliveria SA, Chen RS, McCarthy BD, Davis CC, Hill MN. Hypertension knowledge, awareness, and attitudes in a hypertensive population. J Gen Intern Med. 2005;20(3):219-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benetti E, Fania C, Palatini P. Validation of the A&D BP UA-651 device with a wide-range cuff for home blood pressure measurement according to the European Society of Hypertension International Protocol revision 2010. Blood Press Monit. 2015;20(3):164-167. [DOI] [PubMed] [Google Scholar]
- 18.Siu AL; U.S. Preventive Services Task Force . Screening for high blood pressure in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163(10):778-786. [DOI] [PubMed] [Google Scholar]
- 19.Santo K, Richtering SS, Chalmers J, Thiagalingam A, Chow CK, Redfern J. Mobile phone apps to improve medication adherence: a systematic stepwise process to identify high-quality apps. JMIR Mhealth Uhealth. 2016;4(4):e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520. [DOI] [PubMed] [Google Scholar]
- 21.Appel LJ, Moore TJ, Obarzanek E, et al. ; DASH Collaborative Research Group . A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med. 1997;336(16):1117-1124. [DOI] [PubMed] [Google Scholar]
- 22.Harsha DW, Bray GA. Weight loss and blood pressure control (Pro). Hypertension. 2008;51(6):1420-1425. [DOI] [PubMed] [Google Scholar]
- 23.Crim MT, Yoon SS, Ortiz E, et al. National surveillance definitions for hypertension prevalence and control among adults. Circ Cardiovasc Qual Outcomes. 2012;5(3):343-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horton J, Lipsitz R. Multiple imputation in practice. Am Stat. 2001;55(3):244-254. [Google Scholar]
- 25.Li P, Stuart EA, Allison DB. Multiple imputation: a flexible tool for handling missing data. JAMA. 2015;314(18):1966-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43-46. [PubMed] [Google Scholar]
- 27.Feise RJ. Do multiple outcome measures require p-value adjustment? BMC Med Res Methodol. 2002;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schulz KF, Grimes DA. Multiplicity in randomised trials, I: endpoints and treatments. Lancet. 2005;365(9470):1591-1595. [DOI] [PubMed] [Google Scholar]
- 29.Mancia G. Short- and long-term blood pressure variability: present and future. Hypertension. 2012;60(2):512-517. [DOI] [PubMed] [Google Scholar]
- 30.Uhlig K, Patel K, Ip S, Kitsios GD, Balk EM. Self-measured blood pressure monitoring in the management of hypertension: a systematic review and meta-analysis. Ann Intern Med. 2013;159(3):185-194. [DOI] [PubMed] [Google Scholar]
- 31.Fletcher BR, Hartmann-Boyce J, Hinton L, McManus RJ. The effect of self-monitoring of blood pressure on medication adherence and lifestyle factors: a systematic review and meta-analysis. Am J Hypertens. 2015;28(10):1209-1221. [DOI] [PubMed] [Google Scholar]
- 32.Muntner P, Joyce C, Holt E, et al. Defining the minimal detectable change in scores on the eight-item Morisky medication adherence scale. Ann Pharmacother. 2011;45(5):569-575. [DOI] [PubMed] [Google Scholar]
- 33.Choudhry NK, Glynn RJ, Avorn J, et al. Untangling the relationship between medication adherence and post-myocardial infarction outcomes: medication adherence and clinical outcomes. Am Heart J. 2014;167(1):51-58.e5. [DOI] [PubMed] [Google Scholar]
- 34.Haynes RB, Sackett DL, Gibson ES, et al. Improvement of medication compliance in uncontrolled hypertension. Lancet. 1976;1(7972):1265-1268. [DOI] [PubMed] [Google Scholar]
- 35.Shi L, Liu J, Koleva Y, Fonseca V, Kalsekar A, Pawaskar M. Concordance of adherence measurement using self-reported adherence questionnaires and medication monitoring devices. Pharmacoeconomics. 2010;28(12):1097-1107. [DOI] [PubMed] [Google Scholar]
- 36.Margolis KL, Asche SE, Bergdall AR, et al. A successful multifaceted trial to improve hypertension control in primary care: why did it work? J Gen Intern Med. 2015;30(11):1665-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bobrow K, Farmer AJ, Springer D, et al. Mobile phone text messages to support treatment adherence in adults with high blood pressure (SMS-Text Adherence Support [StAR]): a single-blind, randomized trial. Circulation. 2016;133(6):592-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diaz JA, Griffith RA, Ng JJ, Reinert SE, Friedmann PD, Moulton AW. Patients’ use of the internet for medical information. J Gen Intern Med. 2002;17(3):180-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verberk WJ, Kroon AA, Kessels AG, de Leeuw PW. Home blood pressure measurement: a systematic review. J Am Coll Cardiol. 2005;46(5):743-751. [DOI] [PubMed] [Google Scholar]
- 40.Heldenbrand S, Dayer L, Renna C, Shilling R, Martin B. Navigating the Flooded Adherence App Marketplace: Rating the Quality of Medication Adherence Apps. Presented at the American Pharmacist Association Annual Meeting and Exposition, San Diego, CA; 2015. [Google Scholar]
- 41.Linn AJ, Vervloet M, van Dijk L, Smit EG, Van Weert JC. Effects of eHealth interventions on medication adherence: a systematic review of the literature. J Med Internet Res. 2011;13(4):e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eTable. Trial Outcomes Assessed Among Patients with Complete Data Only
eFigure. Blood Pressure Change Over Time by Treatment Group